High Temperature Dielectric Properties of Iron- and Zinc-Bearing Products during Carbothermic Reduction by Microwave Heating
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
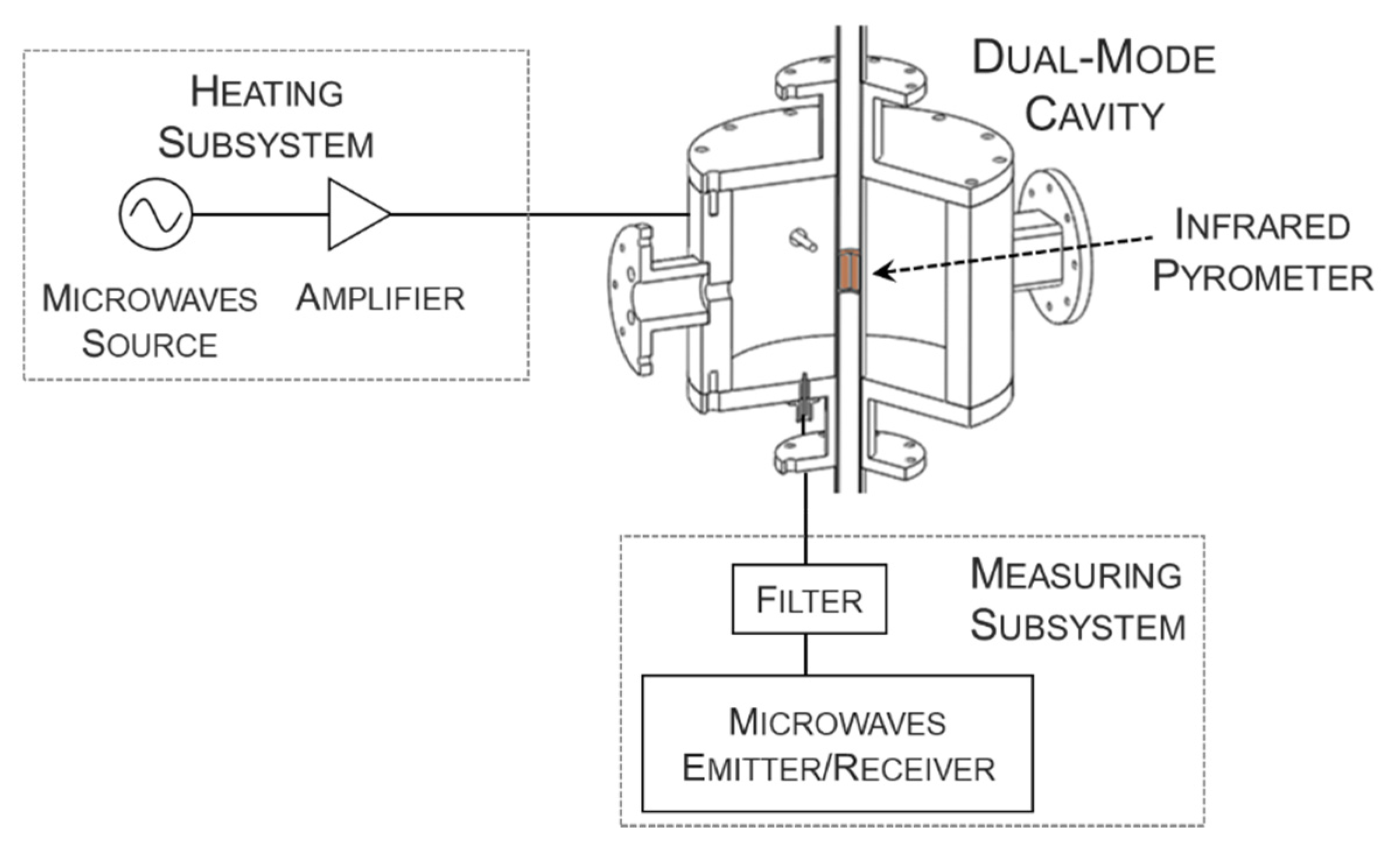
2.2. Dielectric Characterization as a Function of Temperature
2.3. Dielectric Characterization as a Function of Density
2.4. Thermogravimetry (TG) and Differential Scanning Calorimetry (DSC)
2.5. Mass Spectrometry
2.6. X-Ray Diffraction
3. Results and Discussion
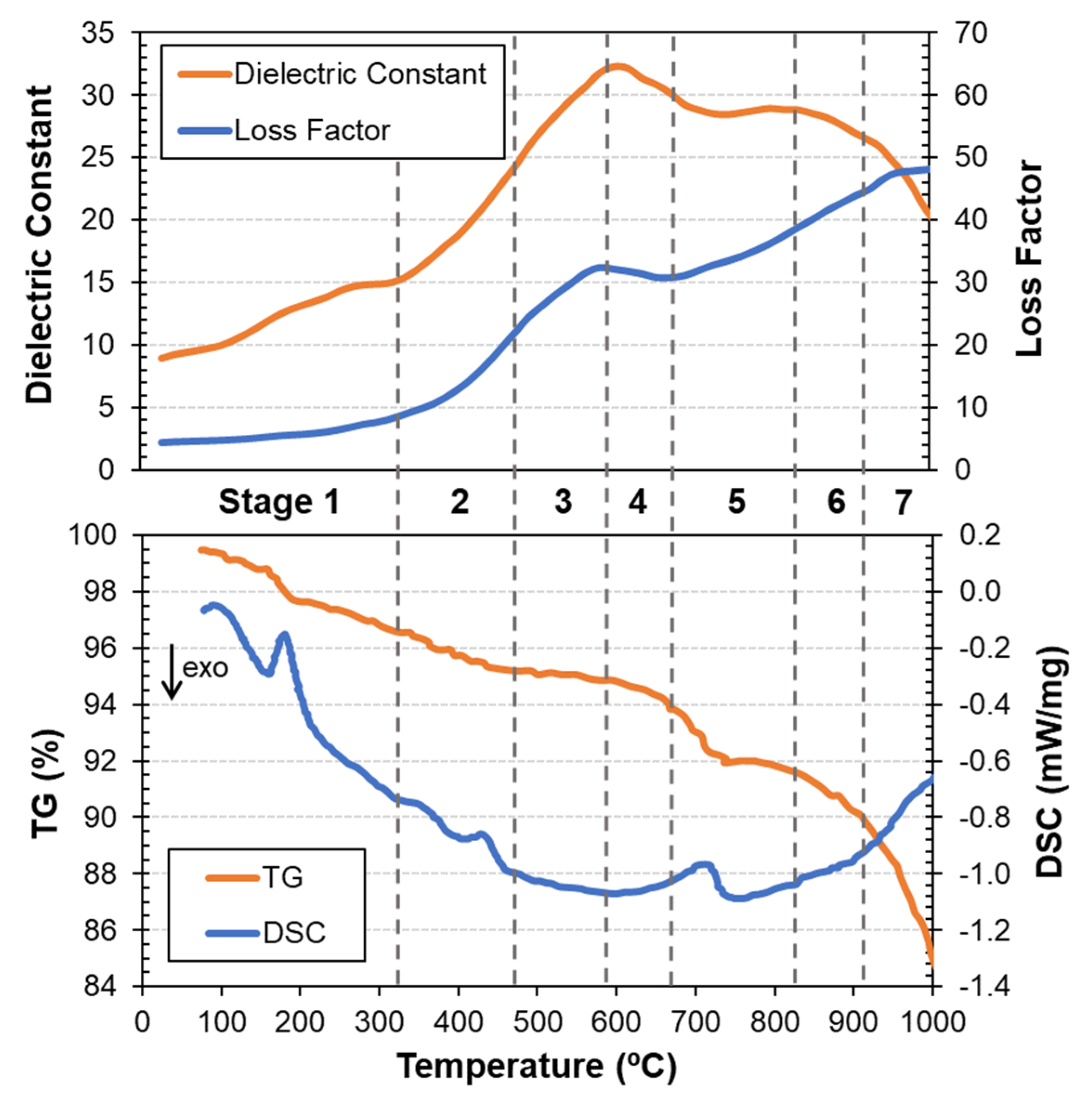
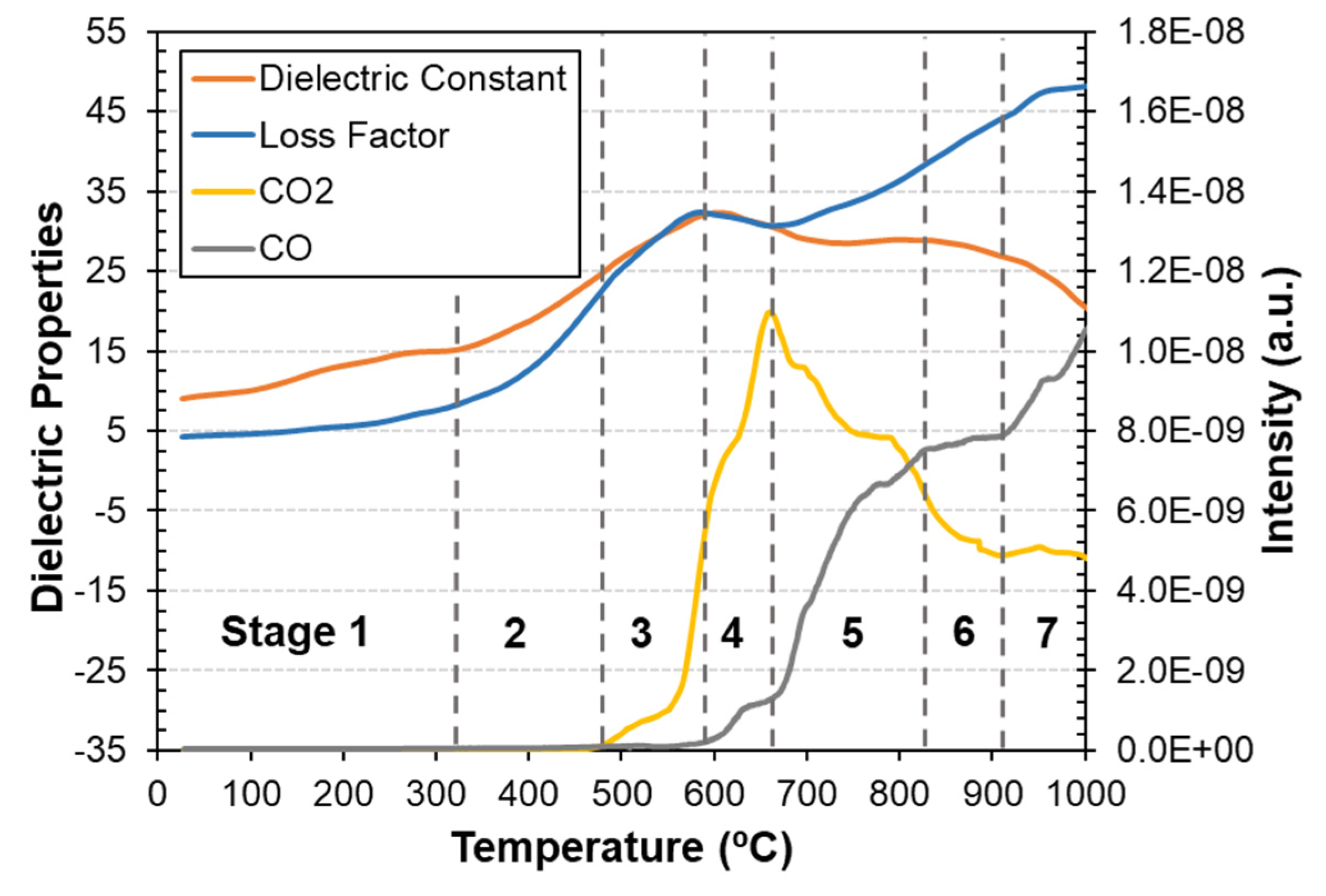
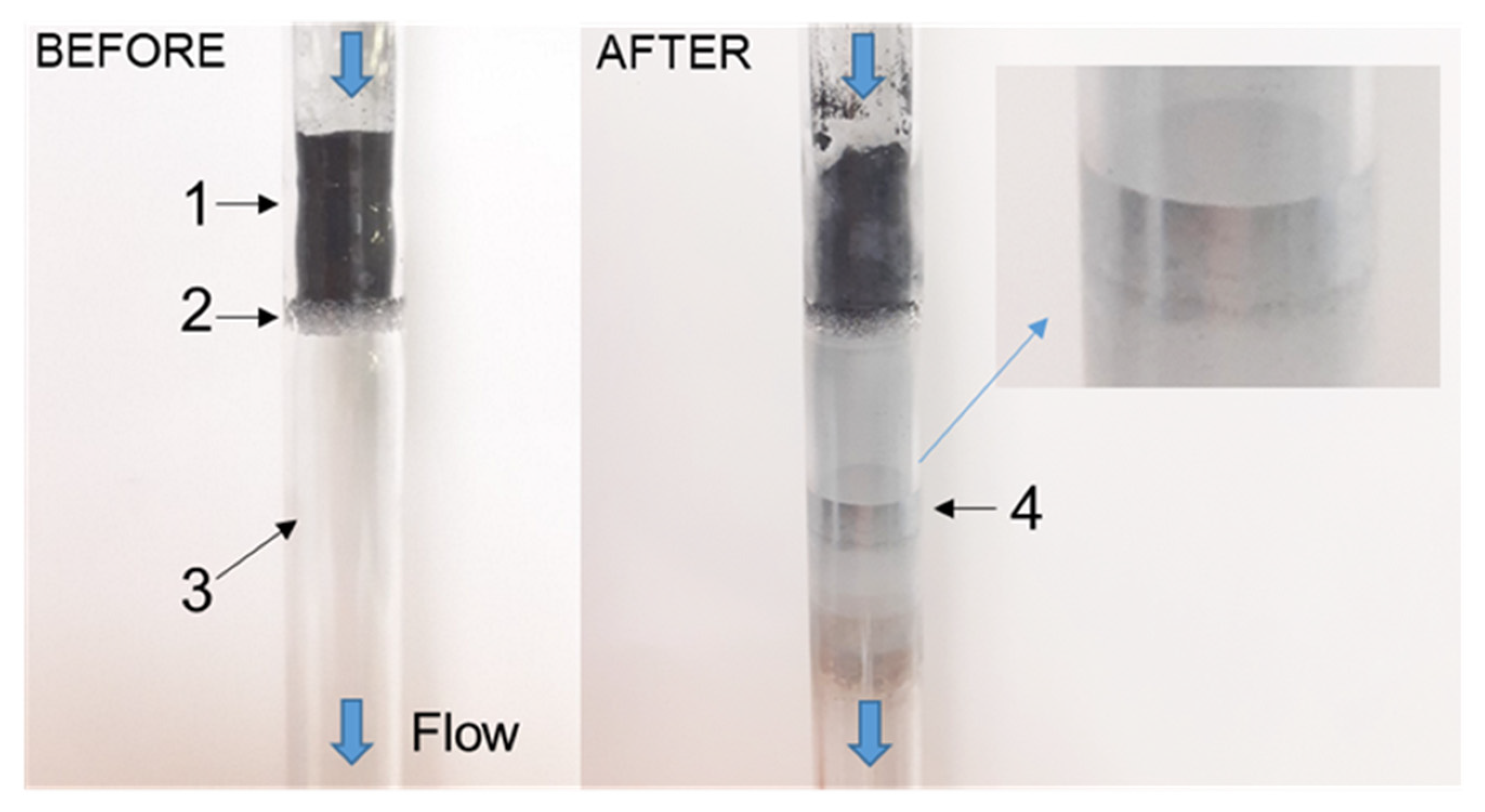
3.1. Dielectric Properties, Thermal Analysis and Mass Spectrometry during Carbothermic Reduction
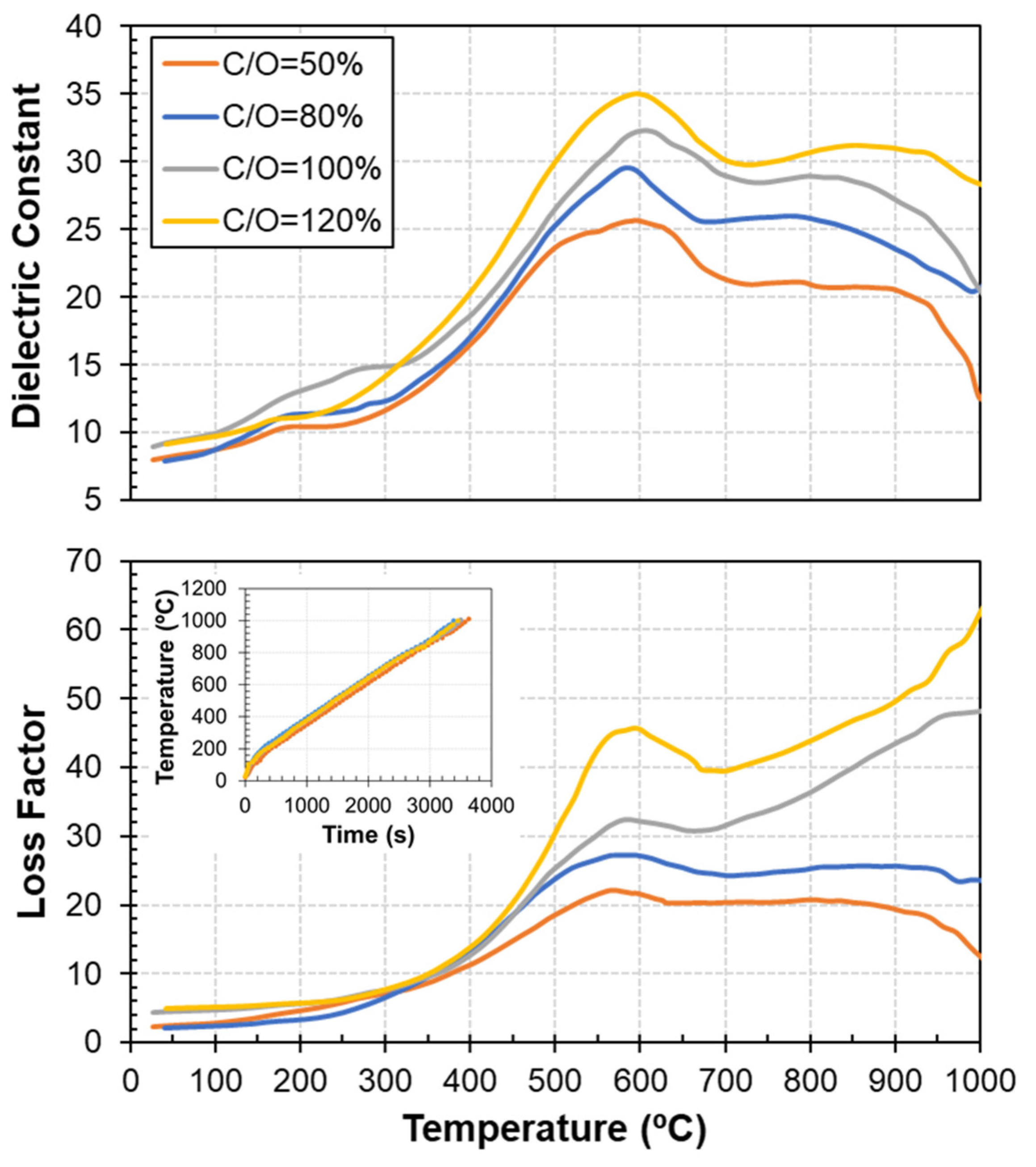
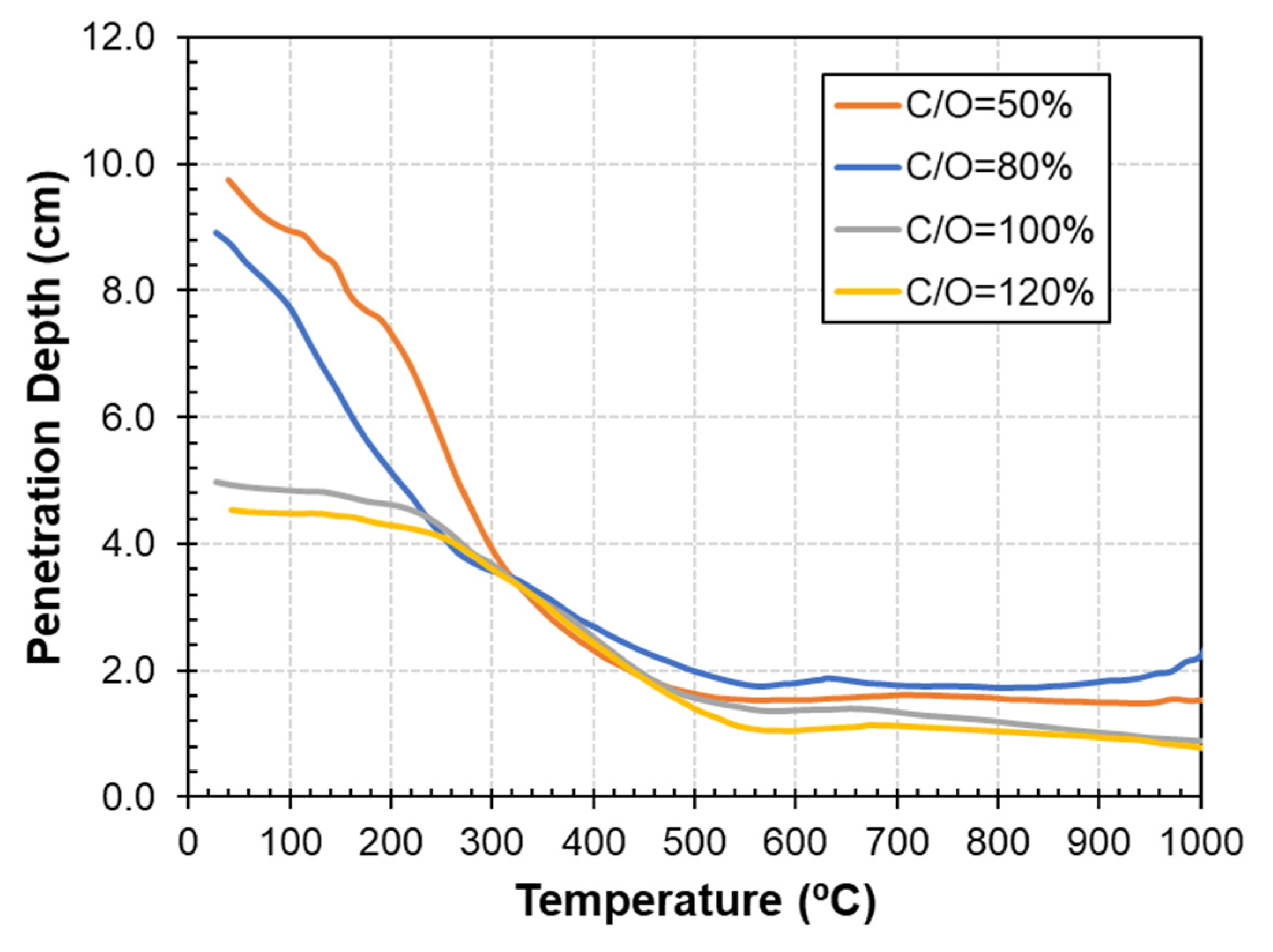
3.2. Dielectric Properties as a Function of Carbon Content
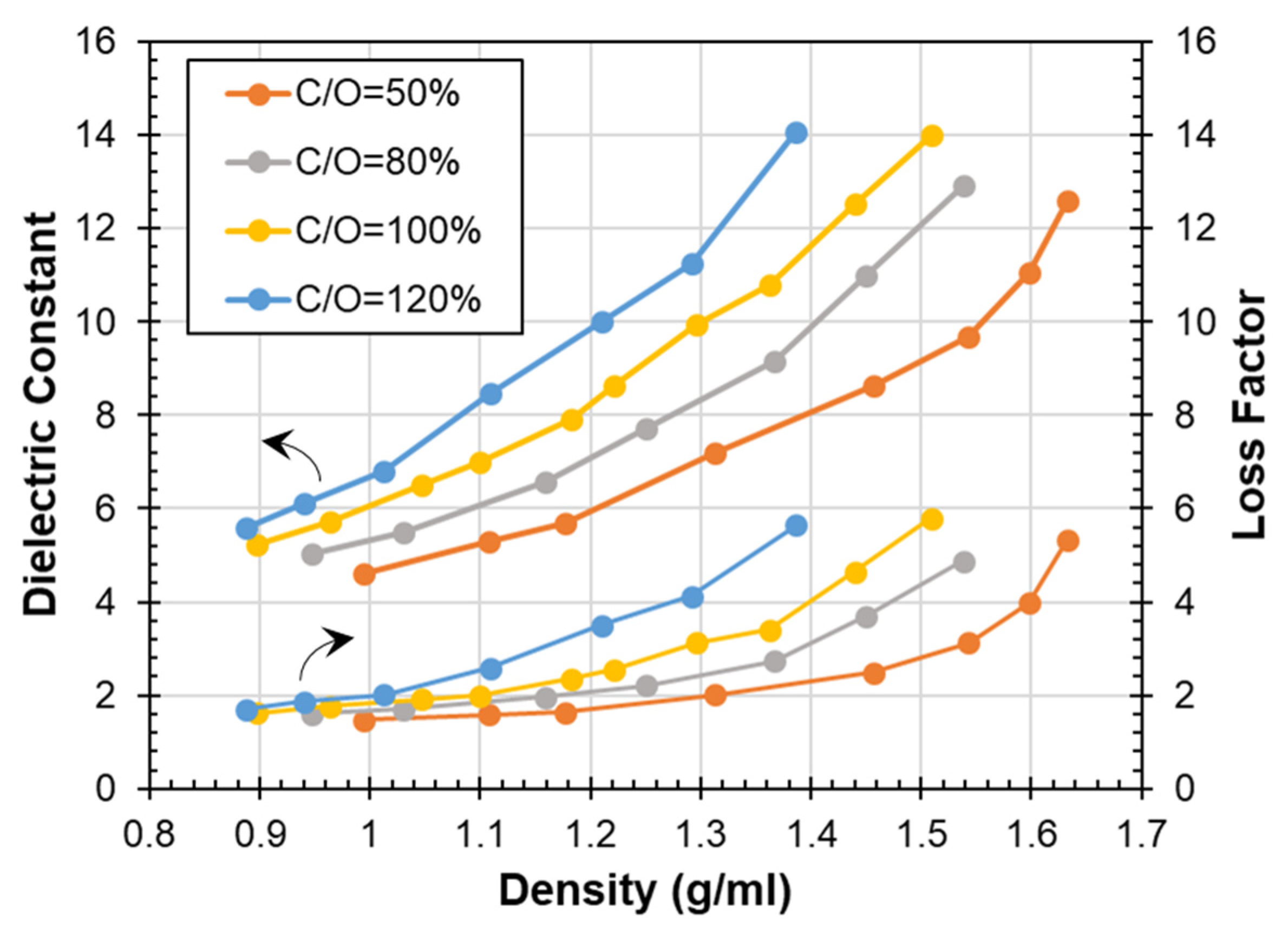
3.3. Dielectric Properties as a Function of Compaction Density
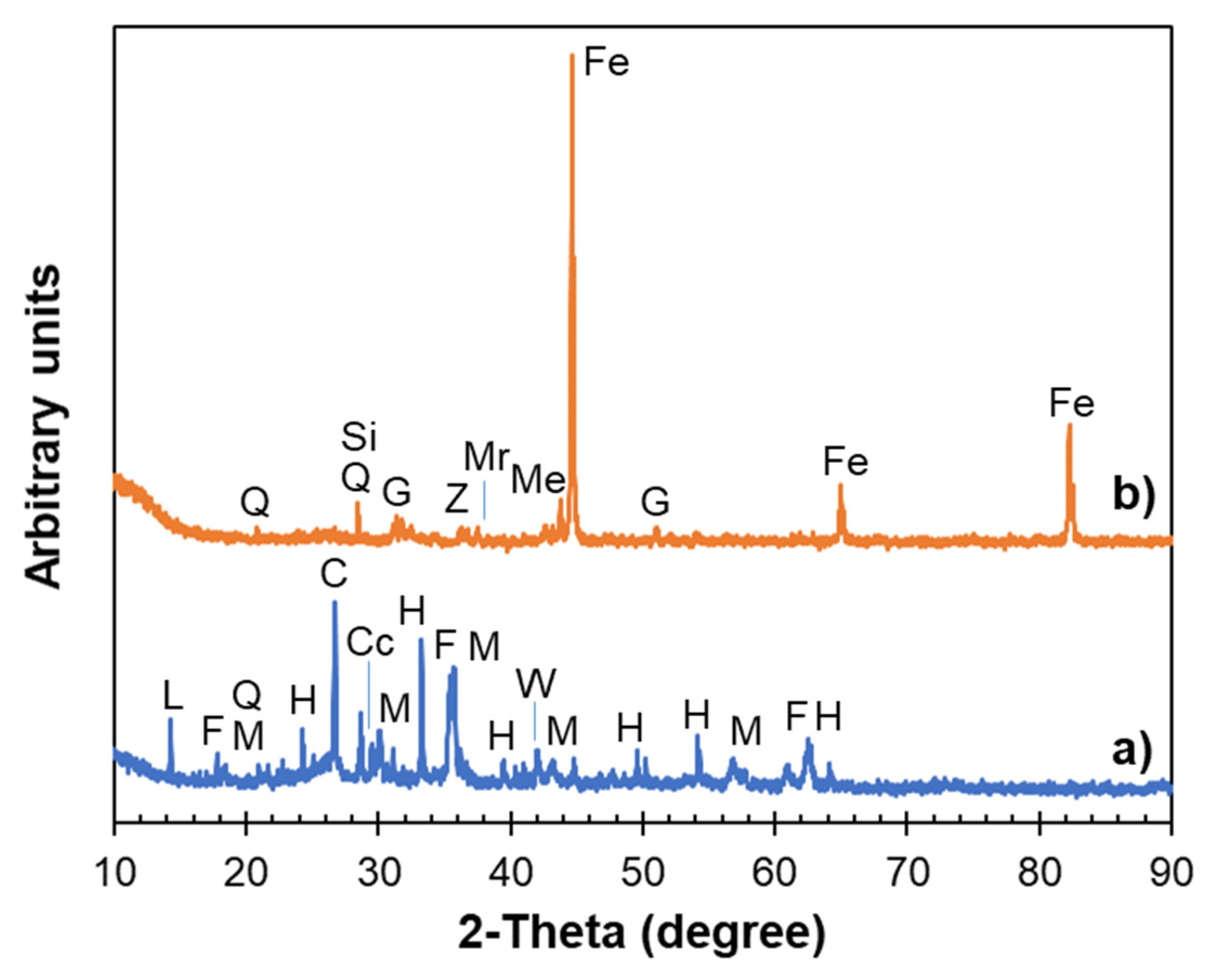
3.4. XRD Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agrawal, D. Microwave sintering of metal powders. In Advances in Powder Metallurgy; Chang, I., Zhao, Y., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 361–379. [Google Scholar]
- Veres, J.; Lovas, M.; Hredzak, S.; Zubrick, A.; Dolinska, S.; Skrinsky, J. Application of Microwave energy in waste treatment. J. Pol. Min. Eng. Soc. 2017, 1, 39–44. [Google Scholar]
- Panias, D.; Krestou, A. Use of microwave energy in metallurgy. In Proceedings of the 1st International Conference on Advances in Mineral Resources Management and Environmental Geotechnology, AMIREG, Hania, Greece, 7–9 June 2004; pp. 215–220. [Google Scholar]
- World Steel Association. Steel Statistical Yearbook 2019; World Steel Association: Brussels, Belgium, 2019. [Google Scholar]
- Omran, M.; Fabritius, T.; Mattila, R. Thermally assisted liberation of high phosphorus oolitic iron ore: A comparison between microwave and conventional furnaces. Powder Technol. 2015, 269, 7–14. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Heikkinen, E.; Chen, G. Dielectric properties and carbothermic reduction of zinc oxide and zinc ferrite by microwave heating. R. Soc. Open Sci. 2017, 4, 170710. [Google Scholar] [CrossRef] [PubMed]
- Mourão, M.B.; Carvalho, I.P.; Takano, C. Carbothermic reduction by microwave heating. ISIJ Int. 2001, 41, S27–S30. [Google Scholar] [CrossRef]
- Standish, N.; Huang, W. Microwave application in carbothermic reduction of iron ores. ISIJ Int. 1991, 3, 241–245. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.; Ju, S.; Peng, J.; Duan, X. Thermodynamics and kinetics of carbothermal reduction of zinc ferrite by microwave heating. Trans. Nonferrous Met. Soc. China 2013, 23, 3808–3815. [Google Scholar] [CrossRef]
- Quing, Y.; Guanghui, L.; Zhiwei, P.; Joonho, L.; Xiaolong, L.; Mingjun, R.; Yuanbo, Z.; Tao, J. Microwave-assisted self-reduction of composite briquettes of zinc ferrite and carbonaceous materials. Powder Technol. 2019, 342, 224–232. [Google Scholar]
- Quing, Y.; Zhiwei, P.; Guanghui, L.; Joonho, L.; Yong, L.; Mundan, L.; Liancheng, W.; Mingjun, R.; Yuanbo, Z.; Tao, J. Microwave-assisted reduction of electric arc furnace dust with biochar: An examination of transition of heating mechanism. ACS Sustain. Chem. Eng. 2019, 7, 9515–9524. [Google Scholar]
- Quing, Y.; Guanghui, L.; Zhiwei, P.; Augustine, R.; Perez, M.D.; Yong, L.; Mudan, L.; Mingjun, R.; Yuanbo, Z.; Tao, J. Microwave-assisted self-reduction of EAF dust-biochar composite briquettes for production of direct reduced iron. Powder Technol. 2020, 362, 781–789. [Google Scholar]
- Ishizaki, K.; Nagata, K.; Hayashi, T. Production of pig iron from magnetite ore-coal composite pellets by microwave heating. ISIJ Int. 2006, 46, 1403–1409. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Peng, J.; Liu, B.; Xia, H.; Gu, X.; Shi, Y. Effect of temperature on dielectric property and microwave heating behaviour of low grade Panzhihua ilmenite ore. Trans. Nonferrous Met. Soc. China 2013, 23, 3462–3469. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Guo, C.; Aoxi, H. Microwave absorption properties of steelmaking dusts: Effects of temperature on the dielectric constant and loss factor at 1064 MHz and 2423 MHz. RSC Adv. 2019, 9, 6859–6870. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, A.; Liu, C.; Qu, W.; Peng, J.; Luo, Y.; Zuo, Y. Dielectric properties and temperature increase characteristics of zinc oxide dust from fuming furnace. Trans. Nonferrous Met. Soc. China 2014, 24, 4004–4011. [Google Scholar] [CrossRef]
- Al-harahsheh, M.; Kingman, S.; Al-Makhadmah, L.; Hamilton, I.E. Microwave treatment of electric furnace dust with PVC: Dielectric characterization and pyrolysis-leaching. J. Hazard Mater. 2014, 274, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.N.; Gregory, A.P.; Cannell, D.; Patrick, M.; Wylie, S.; Youngs, I.; Hill, G. A Guide to the Characterisation of Dielectric Materials at RF and Microwave Frequencies; Technical Report; Institute of Measurement and Control, National Physical Laboratory: London, UK, 2003; pp. 115–123. [Google Scholar]
- García-Baños, B.; Catalá-Civera, J.M.; Peñaranda-Foix, F.; Plaza-González, P.; Llorens-Valles, G. In Situ Monitoring of Microwave Processing of Materials at High Temperatures through Dielectric Properties Measurement. Materials 2016, 9, 349. [Google Scholar] [CrossRef]
- García-Baños, B.; Reinosa, J.J.; Peñaranda-Foix, F.; Fernández, J.F.; Catalá-Civera, J.M. Temperature assessment of microwave-enhanced heating processes. Sci. Rep. 2019, 9, 10809. [Google Scholar] [CrossRef]
- Catalá-Civera, J.M.; Canós-Marín, A.J.; Plaza-González, P.; Gutiérrez-Cano, J.D.; García-Baños, B.; Peñaranda-Foix, F. Dynamic Measurement of Dielectric Properties of Materials at High Temperature During Microwave Heating in a Dual Mode Cylindrical Cavity. IEEE Trans. Microw. Theory Technol. 2015, 63, 2905–2914. [Google Scholar] [CrossRef]
- Gutiérrez-Cano, J.D.; Plaza-González, P.; Canós-Marín, A.J.; García-Baños, B.; Catalá-Civera, J.M.; Peñaranda-Foix, F. A new standalone microwave instrument for measuring the complex permittivity of materials at microwave frequencies. IEEE Trans. Instrum. Meas. 2019, 69, 3595–3605. [Google Scholar] [CrossRef]
- Yucel, O.; Demirci, F.; Turan, A.; Alkan, M. Determination of direct reduction conditions of mil scale. High Temp. Mater. Process. 2013, 32, 405–412. [Google Scholar] [CrossRef]
- Zhu-Cheng, H.; Kai, W.; Bing, H.; Peng, H.; Tao, J. Non-Isothermal Kinetics of Reduction Reaction of Oxidized Pellet under Microwave Irradiation. J. Iron Steel Res. Int. 2012, 19, 1–4. [Google Scholar]
- He, G.; Li, S.; Yang, K.; Liu, J.; Liu, P.; Zhang, L.; Peng, J. Dielectric properties of zinc sulphide concentrate during the roasting at microwave frequencies. Minerals 2017, 7, 31. [Google Scholar] [CrossRef]
| Compound | wt% | Compound | wt% |
|---|---|---|---|
| Fetot * | 47.32 | Mn | 0.73 |
| C | 13.70 | S | 0.73 |
| Zn | 13.41 | Rb | 0.63 |
| SiO2 | 12.37 | K2O | 0.59 |
| CaO | 4.53 | Pb | 0.53 |
| MgO | 3.18 | NaO | 0.16 |
| Al2O3 | 2.06 | LOI | n.a. ** |
| Mineral Phase | Formula | Abundance |
|---|---|---|
| Franklinite | ZnFe2O4 | xxxx 1 |
| Magnetite | Fe3O4 | xxx |
| Ca-Zn Hydroxide Hydrate | Ca(Zn2(OH)6)(H2O)2 | xxx |
| Quartz | SiO2 | xxx |
| Hematite | Fe2O3 | xxx |
| Calcite | CaCO3 | xx |
| Carbon | C | xx |
| Wüstite | FeO | x |
| Metallic Iron | Fe | x |
| Zincite | ZnO | x |
| Stage | Temperature | Main Reactions |
|---|---|---|
| 1 | 23 °C–330 °C | Free water vaporization Dehydration of Ca-Zn hydroxide hydrate Dehydration of coke |
| 2 | 330 °C–480 °C | Dehydroxylation of calcium hydroxide Decomposition of light components of coke |
| 3 | 480 °C–590 °C | Decomposition of zinc ferrite Decomposition of magnetite to metallic iron Decomposition of coke |
| 4 | 590 °C–680 °C | Reduction of magnetite to wüstite Boudouard reaction |
| 5 | 680 °C–840 °C | Wüstite reduction to metallic iron Decomposition of calcium carbonate Boudouard reaction |
| 6 | 840 °C–910 °C | Reduction of zinc oxide to zinc Reduction of remaining iron oxides to metallic iron Boudouard reaction |
| 7 | 910 °C–1000 °C | Vaporization of zinc Reduction of remaining iron oxides to metallic iron Boudouard reaction |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Baños, B.; Catalá-Civera, J.M.; Sánchez, J.R.; Navarrete, L.; López-Buendía, A.M.; Schmidt, L. High Temperature Dielectric Properties of Iron- and Zinc-Bearing Products during Carbothermic Reduction by Microwave Heating. Metals 2020, 10, 693. https://doi.org/10.3390/met10050693
García-Baños B, Catalá-Civera JM, Sánchez JR, Navarrete L, López-Buendía AM, Schmidt L. High Temperature Dielectric Properties of Iron- and Zinc-Bearing Products during Carbothermic Reduction by Microwave Heating. Metals. 2020; 10(5):693. https://doi.org/10.3390/met10050693
Chicago/Turabian StyleGarcía-Baños, Beatriz, Jose M. Catalá-Civera, Juan R. Sánchez, Laura Navarrete, Angel M. López-Buendía, and Lukas Schmidt. 2020. "High Temperature Dielectric Properties of Iron- and Zinc-Bearing Products during Carbothermic Reduction by Microwave Heating" Metals 10, no. 5: 693. https://doi.org/10.3390/met10050693
APA StyleGarcía-Baños, B., Catalá-Civera, J. M., Sánchez, J. R., Navarrete, L., López-Buendía, A. M., & Schmidt, L. (2020). High Temperature Dielectric Properties of Iron- and Zinc-Bearing Products during Carbothermic Reduction by Microwave Heating. Metals, 10(5), 693. https://doi.org/10.3390/met10050693






