Compositional Optimization and Structural Properties of the Filled Skutterudite Smy(FexNi1−x)4Sb11.5Sn0.5
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Scanning Electron Microscopy–Energy-Dispersive X-Ray Spectroscopy (SEM-EDS)
2.3. High Temperature Powder X-ray Diffraction
3. Results
3.1. Compositional and Morphological Characterization
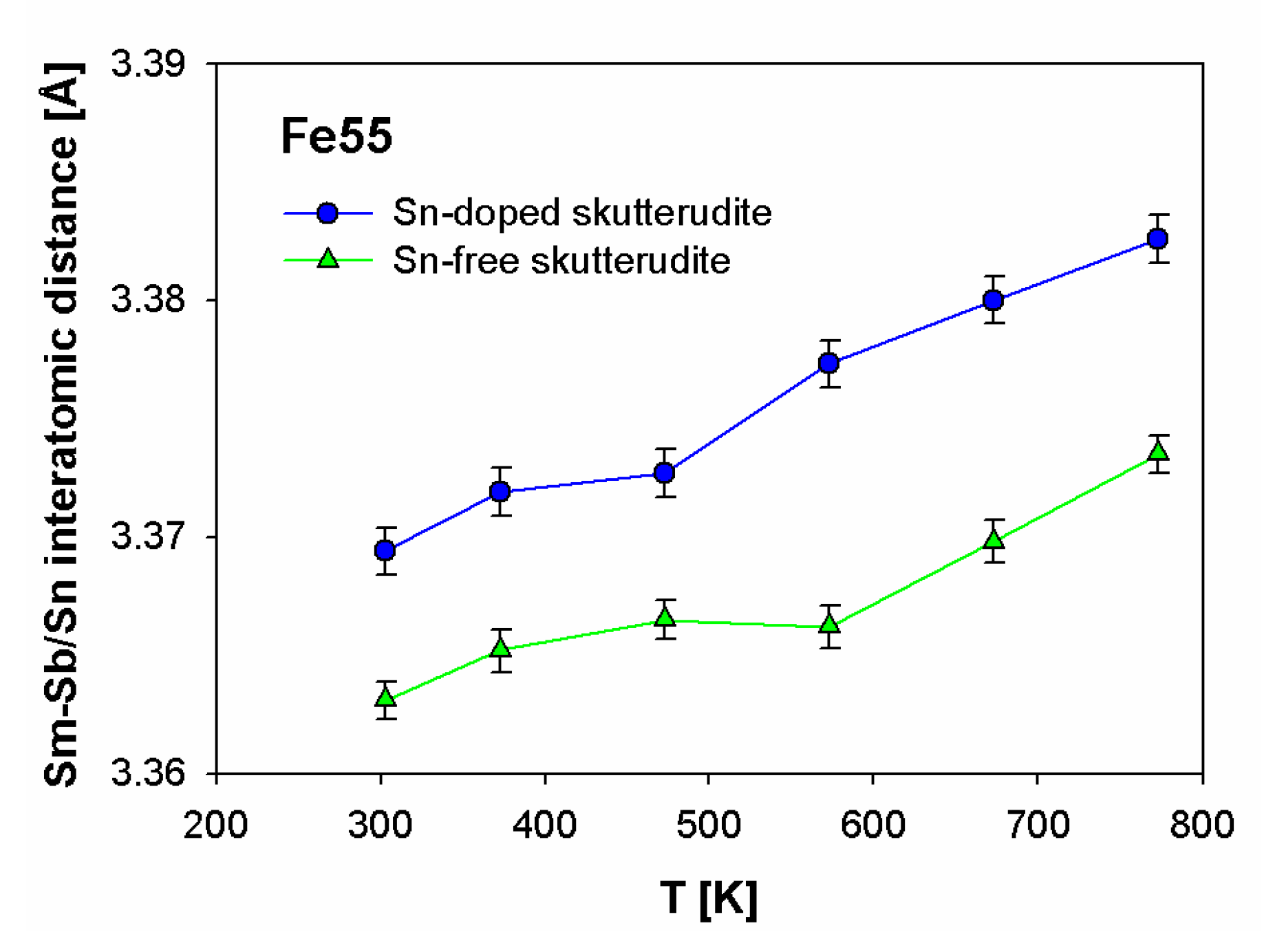
3.2. Structural Characterization
4. Discussion
5. Conclusions
- -
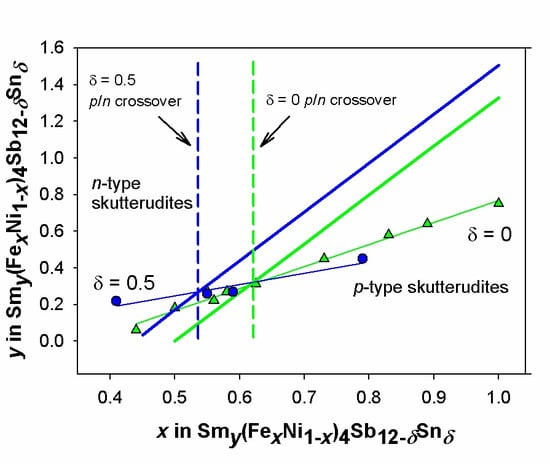
- Relying on the refined Sm amounts, the p/n crossover is expected to be located at x ~ 0.53, meaning that the p-region is enlarged with respect to the Sn-free system.
- -
- No slope change is observed in the trend of the skutterudite cell parameter vs. x in correspondence of the p/n crossover at any considered temperature.
- -
- A much smaller CTE mismatch is revealed between p- and n-compositions in the present system than in the Sn-free one, which makes Smy(FexNi1−x)4Sb11.5Sn0.5 a promising thermoelectric material in terms of mechanical properties.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uher, C. Skutterudite-Based Thermoelectrics. In Thermoelectrics Handbook—Macro to Nano, 1st ed.; Rowe, D.M., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 1–17. [Google Scholar]
- Morelli, D.T.; Meisner, G.P. Low temperature properties of the filled skutterudite CeFe4Sb12. J. Appl. Phys. 1995, 77, 3777. [Google Scholar] [CrossRef]
- Nolas, G.S.; Slack, G.A.; Morelli, D.T.; Tritt, T.M.; Ehrlich, A.C. The effect of rare-earth filling on the lattice thermal conductivity of skutterudites. J. Appl. Phys. 1996, 79, 4002. [Google Scholar] [CrossRef]
- Rull-Bravo, M.; Moure, A.; Fernàndez, J.F.; Martìn-Gonzàlez, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2015, 5, 41653–41667. [Google Scholar] [CrossRef]
- Rogl, G.; Rogl, P. Skutterudites: Progress and challenges. In Novel Thermoelectric Materials and Device Design Concepts; Skipidarov, S., Nikitin, M., Eds.; Springer: Cham/Basel, Switzerland, 2019; pp. 177–201. [Google Scholar]
- Nazibul Hasan, M.; Wahid, H.; Nayan, N.; Sultan Mohamed Ali, M. Inorganic thermoelectric materials: A review. Int. J. Energ. Res. 2020. [Google Scholar] [CrossRef]
- Sales, B.C. Filled skutterudites. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Gschneidner, K.A., Jr., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; North Holland: Amsterdam, The Netherlands, 2003; Volume 33, pp. 1–34. [Google Scholar]
- Oftedal, I. Die Kristallstruktur von Skutterudit und Speiskobalt-Chloanthit. Z. Kristallogr. A 1928, 66, 517–546. [Google Scholar] [CrossRef]
- Toprak, M.S.; Stiewe, C.; Platzek, D.; Williams, S.; Bertini, L.; Müller, E.; Gatti, C.; Zhang, Y.; Rowe, M.; Muhammed, M. The impact of nanostructuring on the thermal conductivity of thermoelectric CoSb3. Adv. Funct. Mater. 2004, 14, 1189–1196. [Google Scholar] [CrossRef]
- Slack, G.A. The thermal conductivity of nonmetallic crystals. In Solid State Physics; Ehrenreich, H., Seitz, F., Turnbull, D., Eds.; Academic Press: New York, NY, USA, 1979; Volume 34, pp. 1–71. [Google Scholar]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Nolas, G.S.; Takizawa, H.; Endo, T.; Sellinschegg, H.; Johnson, D.C. Thermoelectric properties of Sn-filled skutterudites. Appl. Phys. Lett. 2000, 77, 52. [Google Scholar] [CrossRef]
- Nolas, G.S.; Yang, J.; Takizawa, H. Transport properties of germanium-filled CoSb3. Appl. Phys. Lett. 2004, 84, 5210. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, P.; Duan, H.; Chen, J.; Snyder, G.J.; Shi, X.; Brummerstedt Iversen, B.; Chen, L. Enhanced thermoelectric performance in rare earth-filled skutterudites. J. Mater. Chem. C 2016, 4, 4374–4379. [Google Scholar] [CrossRef]
- Khan, A.U.; Kobayashi, K.; Tan, D.-M.; Yamauchi, Y.; Hasegawa, K.; Mitome, M.; Xue, Y.; Jiang, B.; Tsuchiya, K.; Golberg, D.; et al. Nano-micro porous skutterudites with 100% enhancement in ZT for high performance thermoelectricity. Nano Energy 2017, 31, 152–159. [Google Scholar] [CrossRef]
- Rogl, G.; Rogl, P. Skutterudites, a most promising group of thermoelectric materials. Curr. Opin. Green Sust. Chem. 2017, 4, 50–57. [Google Scholar] [CrossRef]
- Benyahia, M.; Ohorodniichuk, V.; Leroy, E.; Dauscher, A.; Lenoir, B.; Alleno, E. High thermoelectric figure of merit in mesostructured In0.25Co4Sb12 n-type skutterudite. J. Alloy Compd. 2018, 73, 1096–1104. [Google Scholar] [CrossRef]
- Rogl, G.; Rogl, P. How nanoparticles can change the figure of merit, ZT, and mechanical properties of skutterudites. Mater. Today Phys. 2017, 3, 48–69. [Google Scholar] [CrossRef]
- Slack, G.A.; Tsoukala, V.G. Some properties of semiconducting IrSb3. J. Appl. Phys. 1994, 76, 1665. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M. New and old concepts in thermoelectric materials. Angew. Chem. Int. Edit. 2009, 48, 8616–8639. [Google Scholar] [CrossRef]
- Mi, J.L.; Christensen, M.; Nishibori, E.; Brummerstedt Iversen, B. Multitemperature crystal structures and physical properties of the partially filled thermoelectric skutterudites M0.1Co4Sb12 (M = La, Ce, Nd, Sm, Yb, and Eu). Phys. Rev. B 2011, 84, 064114. [Google Scholar] [CrossRef]
- Artini, C.; Carlini, R.; Spotorno, R.; Failamani, F.; Mori, T.; Mele, P. Structural properties and thermoelectric performance of the double filled skutterudite (Sm,Gd)y(FexNi1−x)4Sb12. Materials 2019, 12, 2451. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Royanian, E.; Heinrich, P.; Bauer, E.; Rogl, P.; Zehetbauer, M.; Puchegger, S.; Reinecker, M.; Schranz, W. New p- and n-type skutterudites with ZT > 1 and nearly identical thermal expansion and mechanical properties. Acta Mater. 2013, 61, 4066–4079. [Google Scholar] [CrossRef]
- Dahal, T.; Jie, Q.; Lan, Y.; Guo, C.; Ren, Z. Thermoelectric performance of Ni compensated cerium and neodymium double filled p-type skutterudites. Phys. Chem. Chem. Phys. 2014, 16, 18170–18175. [Google Scholar] [CrossRef]
- Yang, J.; Meisner, G.P.; Rawn, C.J.; Wang, H.; Chakoumakos, B.C.; Martin, J.; Nolas, G.S.; Pedersen, B.L.; Stalick, J.K. Low temperature transport and structural properties of misch-metal-filled skutterudites. J. Appl. Phys. 2007, 102, 083702. [Google Scholar] [CrossRef]
- Shi, X.; Kong, H.; Li, C.-P.; Uher, C.; Yang, J.; Salvador, J.R.; Wang, H.; Chen, L.; Zhang, W. Low thermal conductivity and high thermoelectric figure of merit in n-type BaxYbyCo4Sb12 double-filled skutterudites. Appl. Phys. Lett. 2008, 92, 182101. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Vaqueiro, P.; Knight, K.S.; Powell, A.V. Synthesis, characterization and physical properties of the skutterudites YbxFe2Ni2Sb12 (0 x 0.4). J. Solid State Chem. 2012, 193, 36–41. [Google Scholar] [CrossRef]
- Choi, S.; Kurosaki, K.; Ohishi, Y.; Muta, H.; Yamanaka, S. Thermoelectric properties of Tl-filled Co-free p-type skutterudites: Tlx(Fe,Ni)4Sb12. J. Appl. Phys. 2014, 115, 023702. [Google Scholar] [CrossRef]
- Artini, C.; Zanicchi, G.; Costa, G.A.; Carnasciali, M.M.; Fanciulli, C.; Carlini, R. Correlations between structural and electronic properties in the filled skutterudite Smy(FexNi1−x)4Sb12. Inorg. Chem. 2016, 55, 2574–2583. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.F.; Liu, R.H.; Yang, J.; Shi, X.; Huang, X.Y.; Zhang, W.; Chen, L.D.; Yang, J.; Singh, D.J. Thermoelectric properties of Ni-doped CeFe4Sb12 skutterudites. J. Appl. Phys. 2012, 111, 023705. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Falmbigl, M.; Bauer, E.; Rogl, P.; Zehetbauer, M.; Gelbstein, Y. Thermoelectric properties of p-type didymium (DD) based skutterudites DDy(Fe1−xNix)4Sb12 (0.13⩽x⩽0.25, 0.46⩽y⩽0.68). J. Alloy Compd. 2012, 537, 242–249. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Heinrich, P.; Bauer, E.; Kumar, P.; Peranio, N.; Eibl, O.; Hork, J.; Zehetbauer, M.; Rogl, P. New bulk p-type skutterudites DD0.7Fe2.7Co1.3Sb12−xXx (X = Ge, Sn) reaching ZT > 1.3. Acta Mater. 2015, 91, 227–238. [Google Scholar] [CrossRef]
- Duan, F.; Zhang, L.; Dong, J.; Sakamoto, J.; Xu, B.; Li, X.; Tian, Y. Thermoelectric properties of Sn substituted p-type Nd filled skutterudites. J. Alloy Compd. 2015, 639, 68–73. [Google Scholar] [CrossRef]
- Bérardan, B.; Alleno, E.; Godart, C.; Rouleau, O.; Rodriguez-Carvajal, J. Preparation and chemical properties of the skutterudites (Ce–Yb)yFe4−x(Co/Ni)xSb12. Mater. Res. Bull. 2005, 40, 537–551. [Google Scholar] [CrossRef]
- Liu, T.; Tang, X.; Xie, W.; Yan, Y.; Zhang, Q. Crystal structures and thermoelectric properties of Sm-filled skutterudite compounds SmyFexCo4−xSb12. J. Rare Earth 2007, 25, 739–743. [Google Scholar]
- Benyahia, M.; Vaney, J.B.; Leroy, E.; Rouleau, O.; Dauscher, A.; Lenoir, B.; Alleno, E. Thermoelectric properties in double-filled Ce0.3InyFe1.5Co2.5Sb12 p-type skutterudites. J. Alloy Compd. 2017, 696, 1031–1038. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Bauer, E.; Zehetbauer, M. A new generation of p-type didymium skutterudites with high ZT. Intermetallics 2011, 19, 546–555. [Google Scholar] [CrossRef]
- Alleno, E.; Zehani, E.; Gaborit, M.; Orodniichuk, V.; Lenoir, B.; Benyahia, M. Mesostructured thermoelectric Co1−yMySb3 (M = Ni, Pd) skutterudites. J. Alloy Compd. 2017, 692, 676–686. [Google Scholar] [CrossRef]
- Trivedi, V.; Battabyal, M.; Balasubramanian, P.; Mohan Muralikrishna, G.; Kumar Jain, P.; Gopalan, R. Microstructure and doping effect on the enhancement of the thermoelectric properties of Ni doped Dy filled skutterudites. Sustain. Energ. Fuels 2018, 2, 2687–2697. [Google Scholar] [CrossRef]
- Kong, L.; Jia, X.; Zhang, Y.; Sun, B.; Liu, B.; Liu, H.; Wang, C.; Liu, B.; Chen, J.; Ma, H. N-type Ba0.3Ni0.15Co3.85Sb12 skutterudite: High pressure processing technique and thermoelectric properties. J. Alloy Compd. 2018, 734, 36–42. [Google Scholar] [CrossRef]
- Nolas, G.S.; Cohn, J.L.; Slack, G.A. Effect of partial void filling on the lattice thermal conductivity of skutterudites. Phys. Rev. B 1998, 58, 164. [Google Scholar] [CrossRef]
- Qiu, P.; Shi, X.; Chen, X.; Huang, X.; Liu, R.; Chen, L. Effects of Sn-doping on the electrical and thermal transport properties of p-type cerium filled skutterudites. J. Alloy Compd. 2011, 509, 1101–1105. [Google Scholar] [CrossRef]
- Shaheen, N.; Sufyan Javed, M.; Ullah Shah, H.; Hussain, S.; Ashfaq Ahmad, M.; Raza, R.; Saleem, M.; Zhou, X. Enhanced thermoelectric properties in Ge-doped and single-filled skutterudites prepared by unique melt-spinning method. Cer. Int. 2018, 44, 12610–12614. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, F.; Li, X.; Yan, X.; Hu, W.; Wang, L.; Liu, Z.; Tian, Y.; Xu, B. Intensive suppression of thermal conductivity in Nd0.6Fe2Co2Sb12−xGex through spontaneous precipitates. J. Appl. Phys. 2013, 114, 083715. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, W.; Li, Y.; Wan, R.; Chen, W.; Zheng, R.; Ma, L.; Zhou, H. Structure and thermoelectric performance of Ti-filled and Te-doped skutterudite TixCo4Sb11.5Te0.5 bulks fabricated by combination of microwave synthesis and spark plasma sintering. Mater. Lett. 2018, 233, 166–169. [Google Scholar] [CrossRef]
- Dong, J.; Yang, K.; Xu, B.; Zhang, L.; Zhang, Q.; Tian, Y. Structure and thermoelectric properties of Se- and Se/Te-doped CoSb3 skutterudites synthesized by high-pressure technique. J. Alloy Compd. 2015, 647, 295–302. [Google Scholar] [CrossRef]
- Duan, B.; Zhai, P.; Liu, L.; Zhang, Q. Enhanced thermoelectric performance in sulfur-doped Co4Sb11.9−xTexS0.1 skutterudites. Mater. Lett. 2012, 79, 69–71. [Google Scholar] [CrossRef]
- Sun, H.; Jia, X.; Deng, L.; Wang, C.; Lv, P.; Guo, X.; Sun, B.; Zhang, Y.; Liu, B.; Ma, H. Beneficial effect of high pressure and double-atom-doped skutterudite compounds Co4Sb11.5−xTe0.5Snx by HPHT. J. Alloy Compd. 2014, 612, 16–19. [Google Scholar] [CrossRef]
- Hui, S.; Nielsen, M.D.; Homer, M.R.; Medlin, D.L.; Tobola, J.; Salvador, J.R.; Heremans, J.P.; Pipe, K.P.; Uher, C. Influence of substituting Sn for Sb on the thermoelectric transport properties of CoSb3-based skutterudites. J. Appl. Phys. 2014, 115, 103704. [Google Scholar] [CrossRef]
- Chakoumakos, B.C.; Sales, B.C. Skutterudites: Their structural response to filling. J. Alloy Compd. 2006, 407, 87–93. [Google Scholar] [CrossRef]
- Phuangyod, A.; Hayashi, J.; Kawamura, Y.; Artini, C.; Latronico, G.; Carlini, R.; Saini, S.; Mele, P.; Sekine, C. Thermoelectric properties of p-type and n-type filled skutterudite compounds Smy(FexNi1−x)4Sb12 prepared under high pressure. Jpn. J. Appl. Phys. 2020, 59, 061004. [Google Scholar] [CrossRef]
- Kim, I.-H.; Park, K.-H.; Ur, S.-C. Thermoelectric properties of Sn-doped CoSb3 prepared by encapsulated induction melting. J. Alloy Compd. 2007, 442, 351–354. [Google Scholar] [CrossRef]
- Artini, C.; Carlini, R. Influence of composition and thermal treatments on microhardness of the filled skutterudite Smy(FexNi1−x)4Sb12. J. Nanosci. Nanotechnol. 2017, 17, 1634–1639. [Google Scholar] [CrossRef]
- Artini, C.; Castellero, A.; Baricco, M.; Buscaglia, M.T.; Carlini, R. Structure, microstructure and microhardness of rapidly solidified Smy(FexNi1−x)4Sb12 (x = 0.45, 0.50, 0.70, 1) thermoelectric compounds. Solid State Sci. 2018, 79, 71–78. [Google Scholar] [CrossRef]
- Artini, C.; Parodi, N.; Latronico, G.; Carlini, R. Formation and decomposition process of the filled skutterudite Smy(FexNi1−x)4Sb12 (0.40 ≤ x ≤ 1) as revealed by differential thermal analysis. J. Mater. Eng. Perform. 2018, 27, 6259–6265. [Google Scholar] [CrossRef]
- Carlini, R.; Parodi, N.; Soggia, F.; Latronico, G.; Carnasciali, M.; Artini, C. Corrosion behavior of Smy(FexNi1−x)4Sb12 (0.40 ≤ x ≤ 0.80) in sodium chloride solutions studied by electron microscopy and ICP-AES. J. Mater. Eng. Perform. 2018, 27, 6266–6273. [Google Scholar] [CrossRef]
- Spotorno, R.; Ghiara, G.; Carlini, R.; Latronico, G.; Mele, P.; Artini, C. Corrosion of the filled skutterudite Smy(FexNi1−x)4Sb12 (x=0.40, 0.80) by NaCl solutions: An electrochemical study. J. Electron. Mater. 2020, 49, 2872–2880. [Google Scholar] [CrossRef]
- Latronico, G.; Valenza, F.; Carlini, R.; Mele, P.; Artini, C. Interfacial reactivity of the filled skutterudite Smy(FexNi1−x)4Sb12 in contact with liquid In-based alloys and Sn. Metals 2020, 10, 364. [Google Scholar] [CrossRef]
- Artini, C.; Latronico, G.; Carlini, R.; Saini, S.; Takeuchi, T.; Choi, S.; Baldini, A.; Anselmi-Tamburini, U.; Valenza, F.; Mele, P. Effect of different processing routes on the power factor of the filled skutterudite Smy(FexNi1−x)4Sb12 (x = 0.50–0.80; y = 0.12–0.53). ES Mater. Manuf. 2019, 5, 29–37. [Google Scholar] [CrossRef]
- Artini, C.; Cingolani, A.; Anselmi Tamburini, U.; Valenza; Latronico, G.; Mele, P. Effect of sintering parameters on structure and microstructure of the filled skutterudite Smy(FexNi1−x)4Sb12. Mater. Res. Bull. submitted.
- Artini, C.; Fanciulli, C.; Zanicchi, G.; Costa, G.; Carlini, R. Thermal expansion and high temperature structural features of the filled skutterudite Smβ(FeαNi1−α)4Sb12. Intermetallics 2017, 87, 31–37. [Google Scholar] [CrossRef]
- Carlini, R.; Ullah Khan, A.; Ricciardi, R.; Mori, T.; Zanicchi, G. Synthesis, characterization and thermoelectric properties of Sm filled Fe4-xNixSb12 skutterudites. J. Alloy Compd. 2016, 655, 321–326. [Google Scholar] [CrossRef]
- Uher, C. In search of efficient n-type skutterudite thermoelectrics. In Twenty-First International Conference on Thermoelectrics, Proceedings of the 21st Conference on Thermoelectrics, Long Beach, CA, USA, 29 August 2002; IEEE: New York, NY, USA, 2002; pp. 35–41. [Google Scholar]
- Rogl, G.; Zhang, L.; Rogl, P.; Grytsiv, A.; Falmbigl, M.; Rajs, D.; Kriegisch, M.; Müller, H.; Bauer, E.; Koppensteiner, J.; et al. Thermal expansion of skutterudites. J. Appl. Phys. 2010, 107, 043507. [Google Scholar] [CrossRef]
- Rogl, G.; Bursik, J.; Grytsiv, A.; Puchegger, S.; Soprunyuk, V.; Schranz, W.; Yan, X.; Bauer, E.; Rogl, P. Nanostructuring as a tool to adjust thermal expansion in high ZT skutterudites. Acta Mater. 2018, 145, 359–368. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Singh, H.P. Determination of thermal expansion of germanium, rhodium and iridium by X-rays. Acta Cryst. A 1968, 24, 469–471. [Google Scholar] [CrossRef]
- Chakoumakos, B.C.; Sales, B.C.; Mandrus, D.; Keppens, V. Disparate atomic displacements in skutterudite-type LaFe3CoSb12, a model for thermoelectric behaviour. Acta Cryst. B 1999, 55, 341–347. [Google Scholar] [CrossRef] [PubMed]
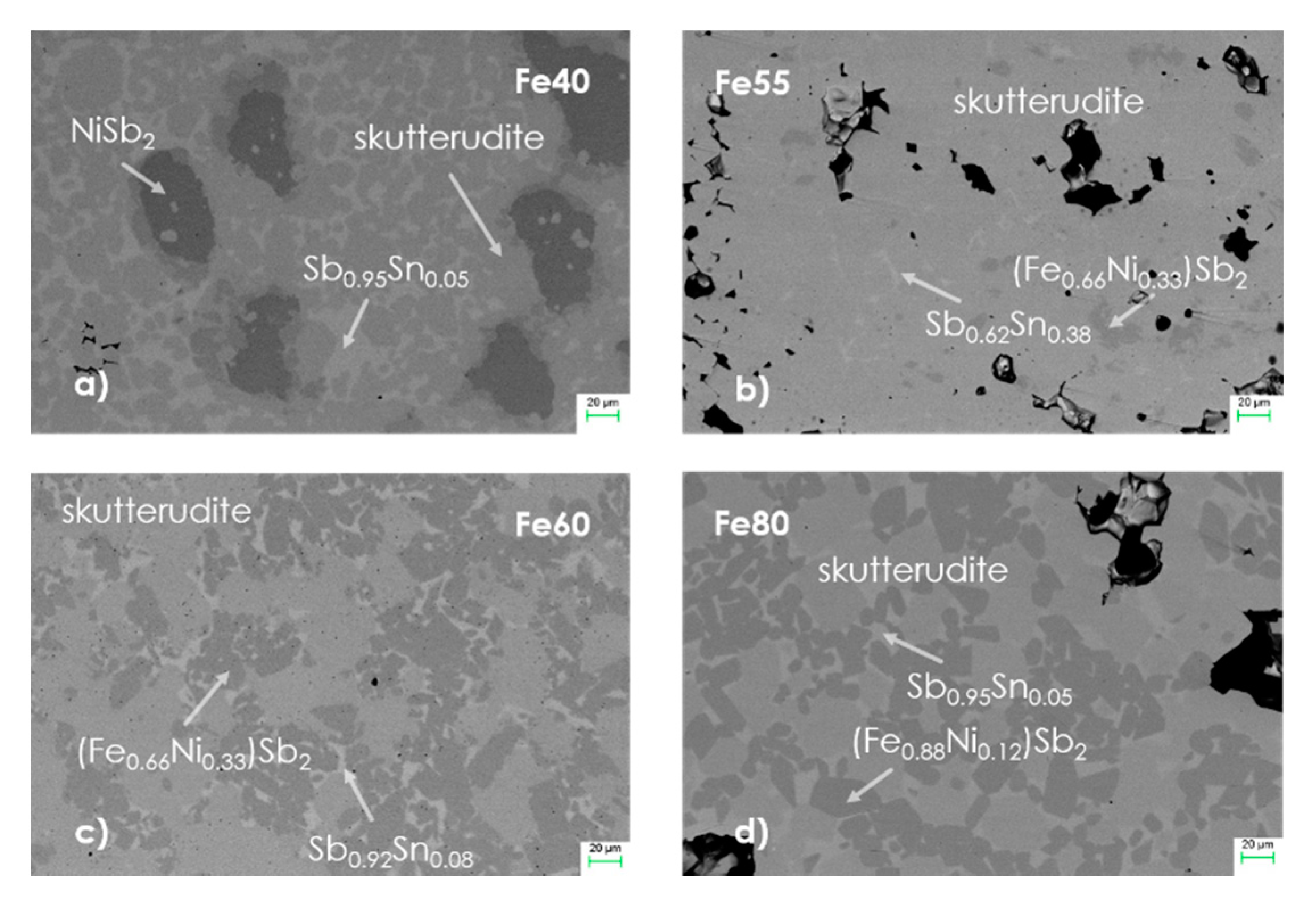





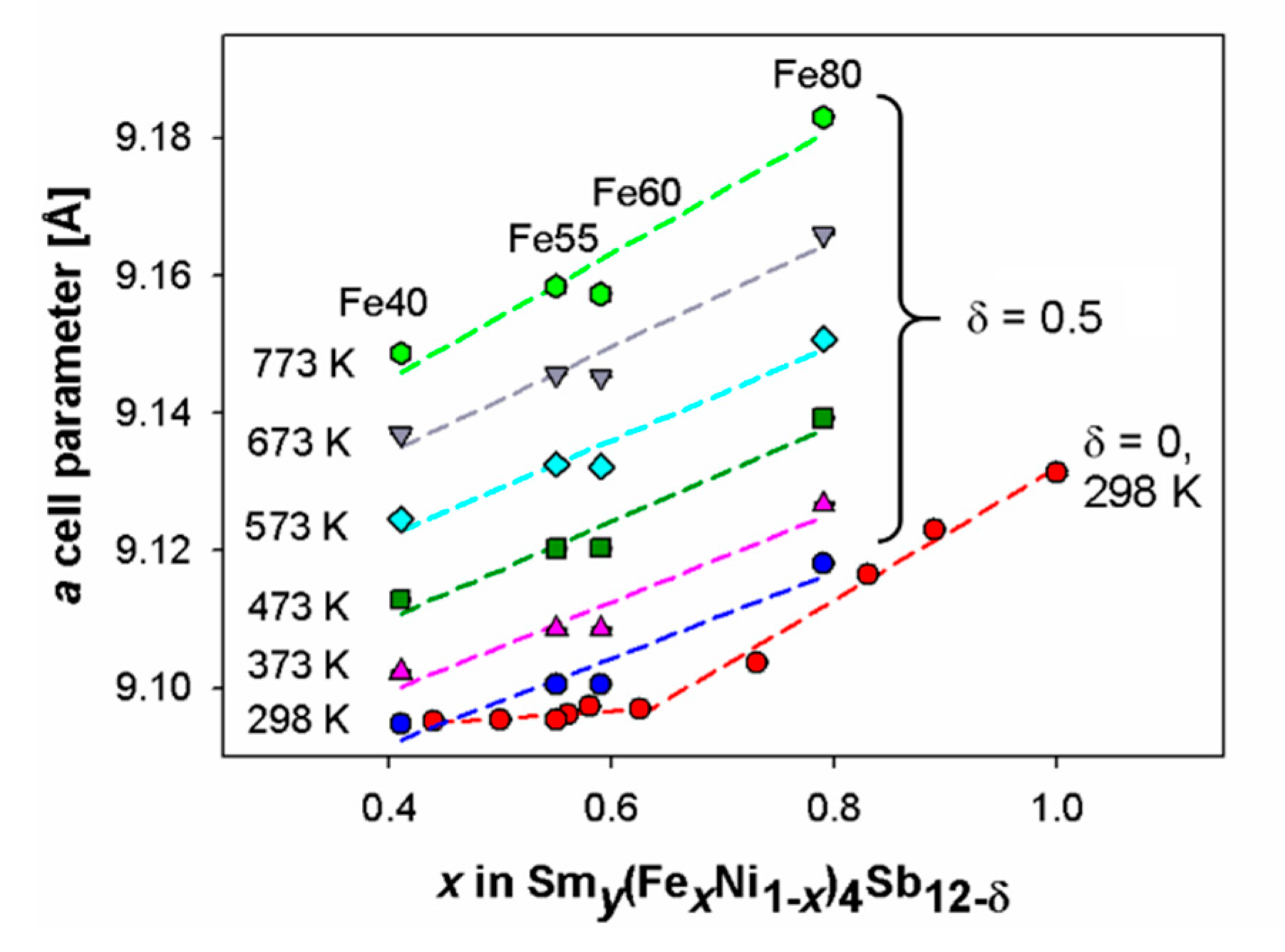

| Sample | Refined Composition | Additional Phases (wt.%) | Skutterudite a Cell Parameter [Å] | ||
|---|---|---|---|---|---|
| Fe40_303 | Sm0.22(Fe0.41Ni0.59)3.9Sb11.8Sn0.2 | Sb0.95Sn0.05 (11%) | 9.0948(1) | 18.5 | 9.39 |
| Fe40_373 | 9.1023(1) | 15.0 | 9.91 | ||
| Fe40_473 | 9.1128(1) | 12.5 | 10.5 | ||
| Fe40_573 | 9.1245(1) | 12.7 | 9.43 | ||
| Fe40_673 | 9.1370(1) | 12.1 | 11.0 | ||
| Fe40_773 | 9.1487(1) | 12.8 | 9.88 | ||
| Fe55_303 | Sm0.26(Fe0.55Ni0.45)3.7Sb11.5Sn0.5 | Fe0.66Ni0.33Sb2 (traces); SbSn (traces) | 9.1004(1) | 7.30 | 6.44 |
| Fe55_373 | 9.1085(1) | 6.85 | 6.11 | ||
| Fe55_473 | 9.1203(1) | 7.63 | 6.18 | ||
| Fe55_573 | 9.1326(1) | 6.58 | 6.19 | ||
| Fe55_673 | 9.1457(1) | 5.81 | 5.79 | ||
| Fe55_773 | 9.1585(1) | 6.03 | 5.18 | ||
| Fe60_303 | Sm0.27(Fe0.59Ni0.41)3.7Sb11.7Sn0.3 | Sb0.92Sn0.08 (9%); Fe0.66Ni0.33Sb2 (29%) | 9.1005(1) | 8.39 | 8.26 |
| Fe60_373 | 9.1086(1) | 8.03 | 9.26 | ||
| Fe60_473 | 9.1203(1) | 7.27 | 7.48 | ||
| Fe60_573 | 9.1322(1) | 6.08 | 7.90 | ||
| Fe60_673 | 9.1453(1) | 6.90 | 8.25 | ||
| Fe60_773 | 9.1573(1) | 4.66 | 5.14 | ||
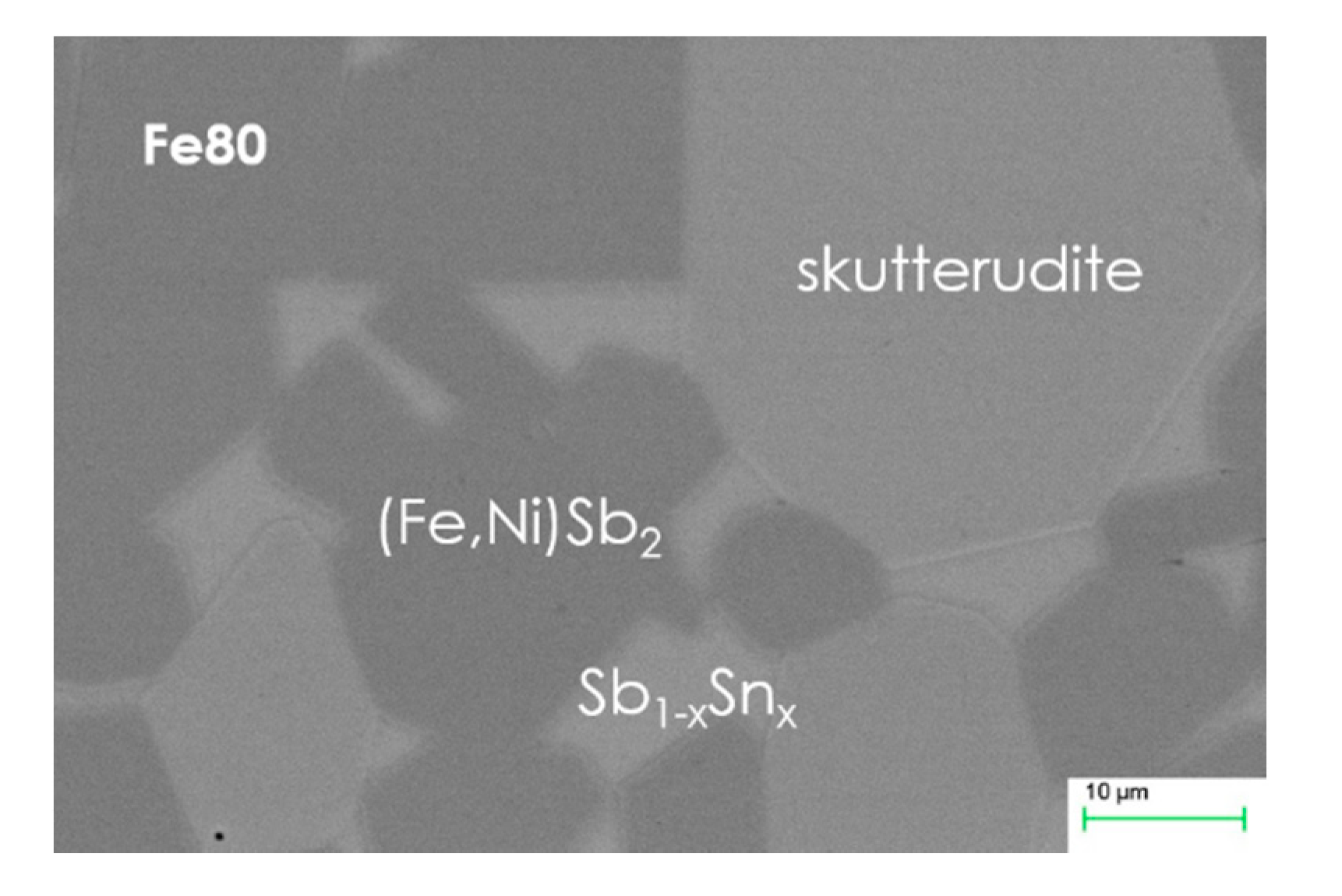
| Fe80_303 | Sm0.61(Fe0.79Ni0.21)3.9Sb11.8Sn0.2 | Sb; FeSb2; SbSn (traces) | 9.1181(1) | -- | -- |
| Fe80_373 | 9.1267(1) | -- | -- | ||
| Fe80_473 | 9.1392(1) | -- | -- | ||
| Fe80_573 | 9.1508(1) | -- | -- | ||
| Fe80_673 | 9.1661(1) | -- | -- | ||
| Fe80_773 | 9.1831(1) | -- | -- |
| Sample | Equation of the Regression Line | Expected a Value at 303 K [Å] | |
|---|---|---|---|
| Fe41 | a = 1.1520 × 10−4 T + 9.0592 | 9.0941 | 0.999 |
| Fe55 | a = 1.2370 × 10−4 T + 9.0623 | 9.0998 | 0.999 |
| Fe59 | a = 1.2135 × 10−4 T + 9.0633 | 9.1001 | 0.999 |
| Fe79 | a = 1.3595 × 10−4 T + 9.0756 | 9.1168 | 0.994 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artini, C.; Carlini, R.; Gigli, L.; Fanciulli, C. Compositional Optimization and Structural Properties of the Filled Skutterudite Smy(FexNi1−x)4Sb11.5Sn0.5. Metals 2020, 10, 692. https://doi.org/10.3390/met10050692
Artini C, Carlini R, Gigli L, Fanciulli C. Compositional Optimization and Structural Properties of the Filled Skutterudite Smy(FexNi1−x)4Sb11.5Sn0.5. Metals. 2020; 10(5):692. https://doi.org/10.3390/met10050692
Chicago/Turabian StyleArtini, Cristina, Riccardo Carlini, Lara Gigli, and Carlo Fanciulli. 2020. "Compositional Optimization and Structural Properties of the Filled Skutterudite Smy(FexNi1−x)4Sb11.5Sn0.5" Metals 10, no. 5: 692. https://doi.org/10.3390/met10050692
APA StyleArtini, C., Carlini, R., Gigli, L., & Fanciulli, C. (2020). Compositional Optimization and Structural Properties of the Filled Skutterudite Smy(FexNi1−x)4Sb11.5Sn0.5. Metals, 10(5), 692. https://doi.org/10.3390/met10050692