Precise Control of Copper-Localized Surface Plasmon Resonance in the Near Infrared Region for Enhancement of Up-Conversion Luminescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Substrate
2.3. Sample Fabrication
2.4. Characterization
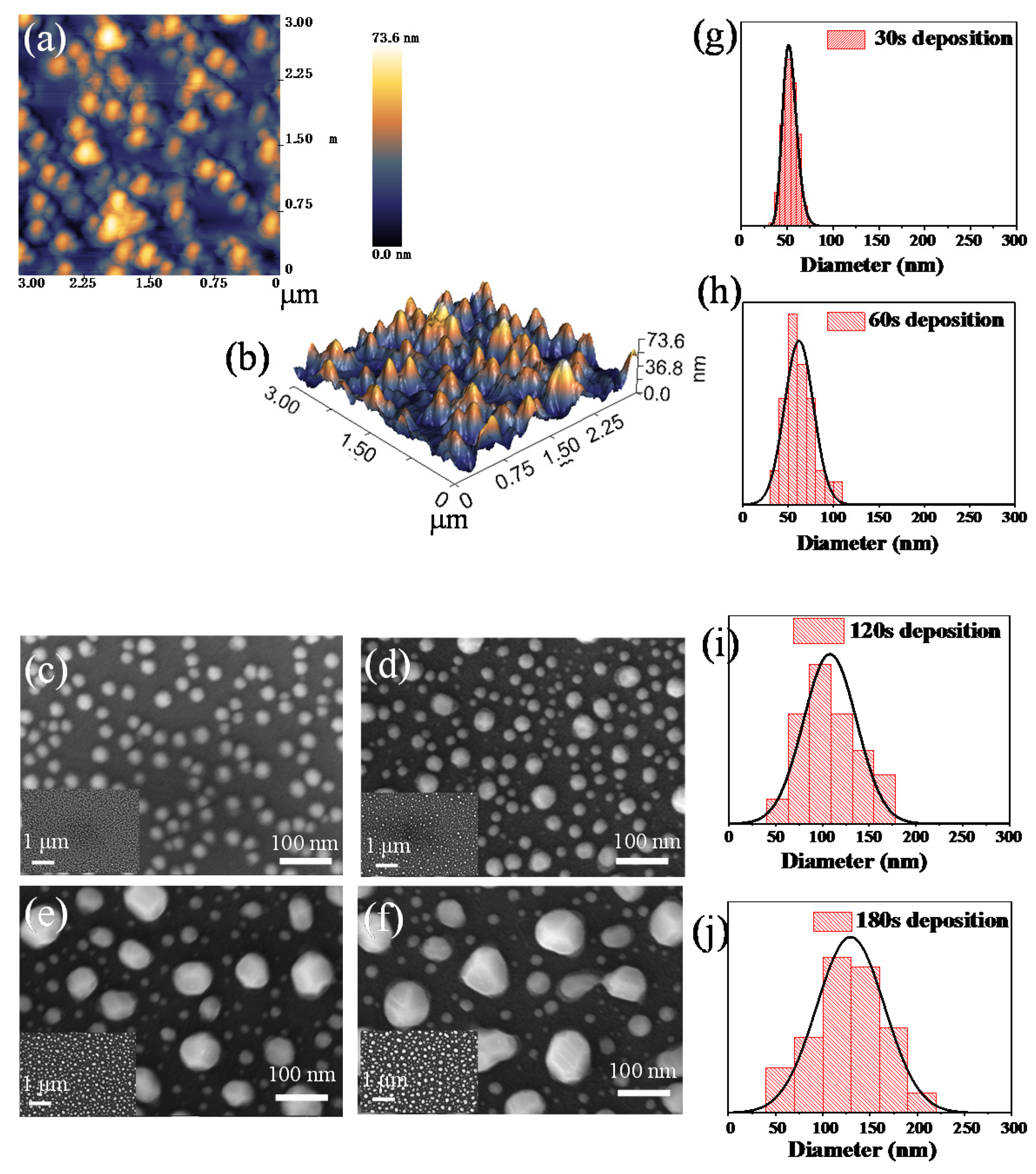
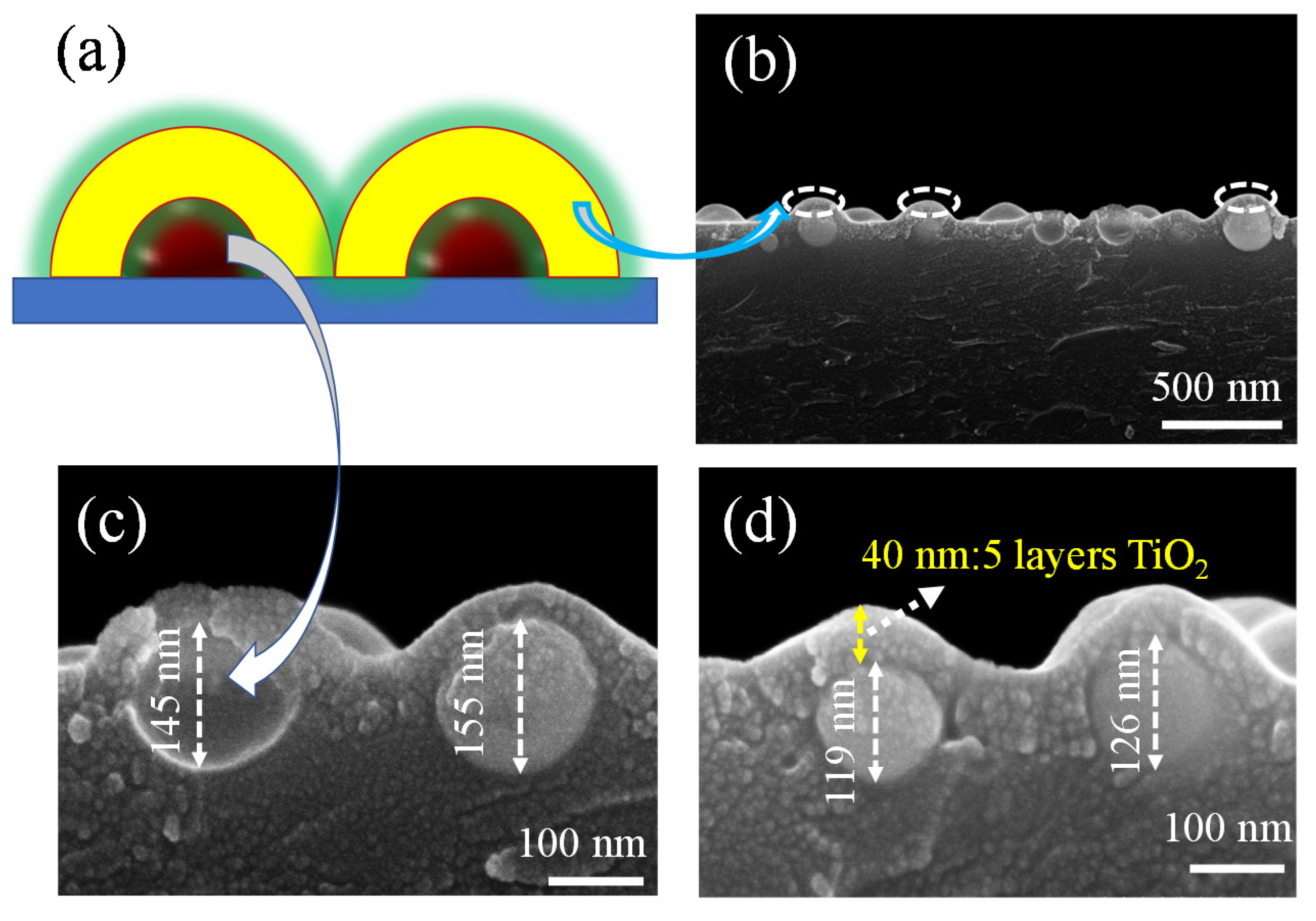
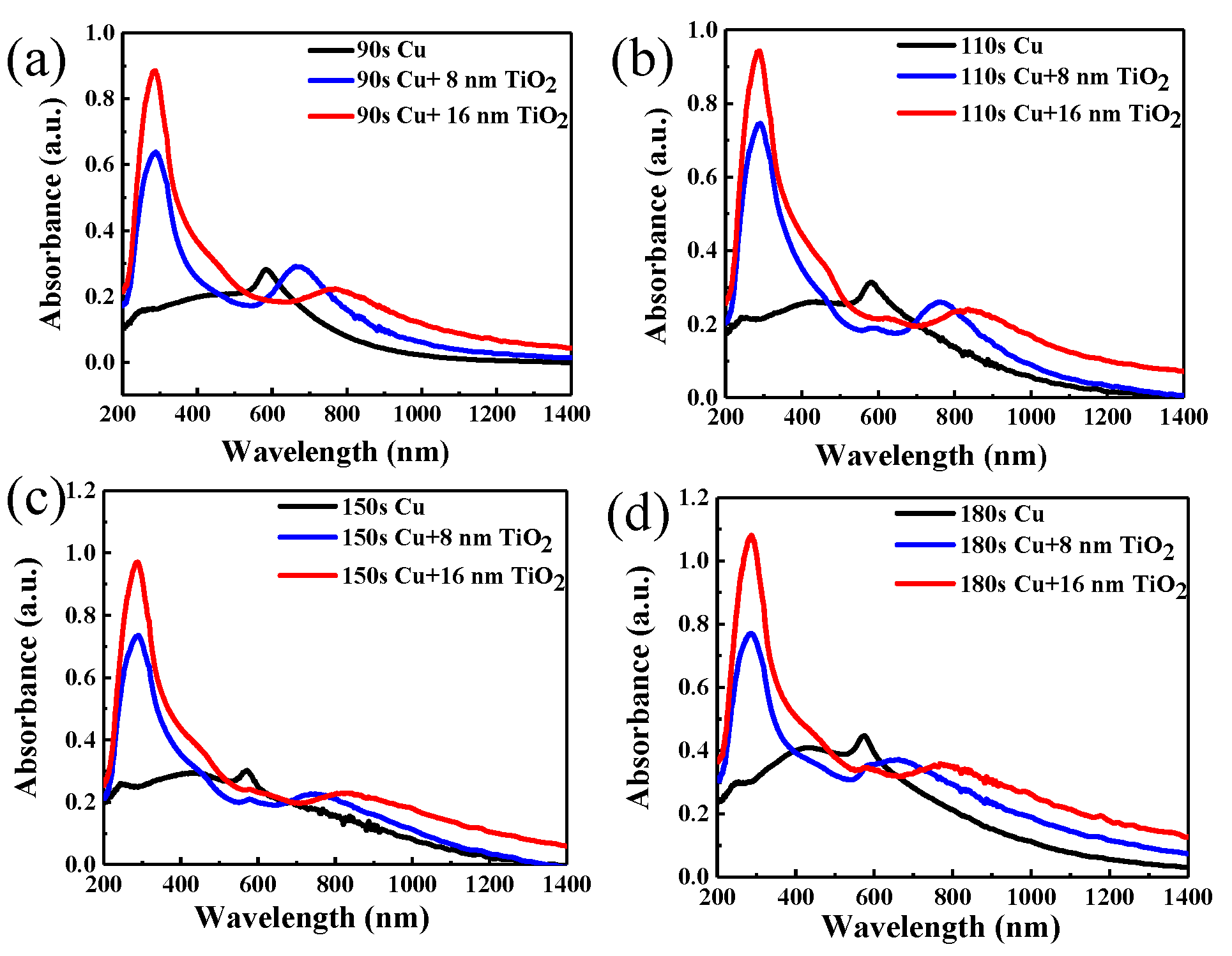
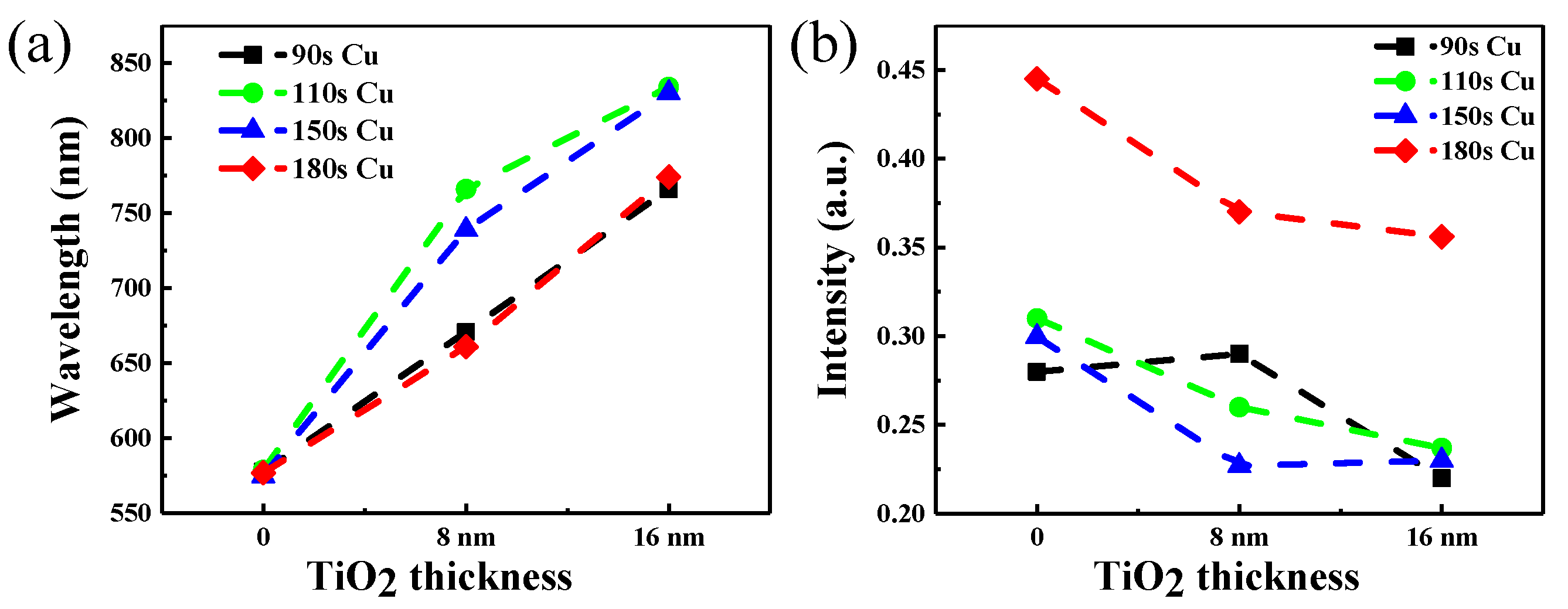
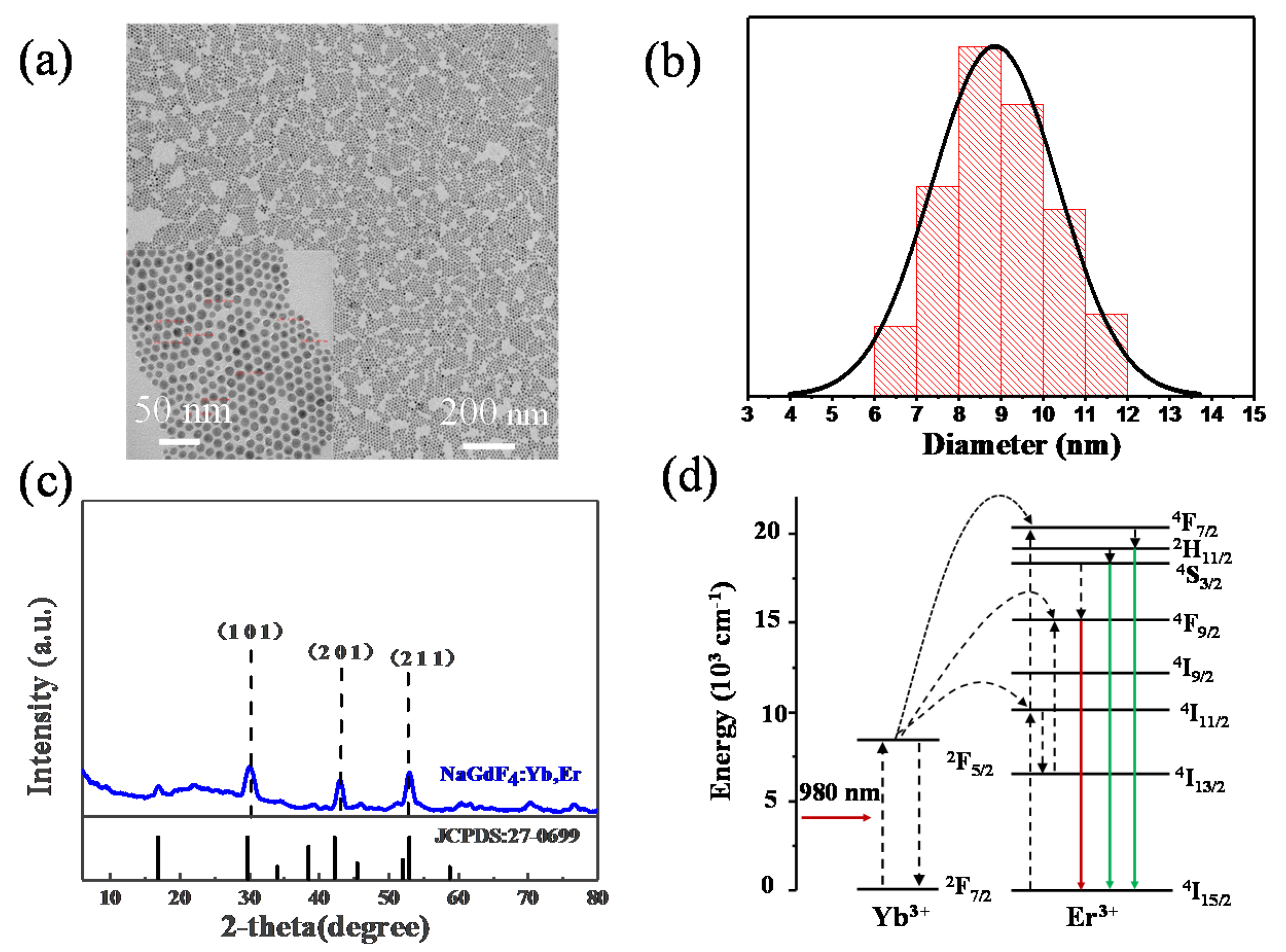
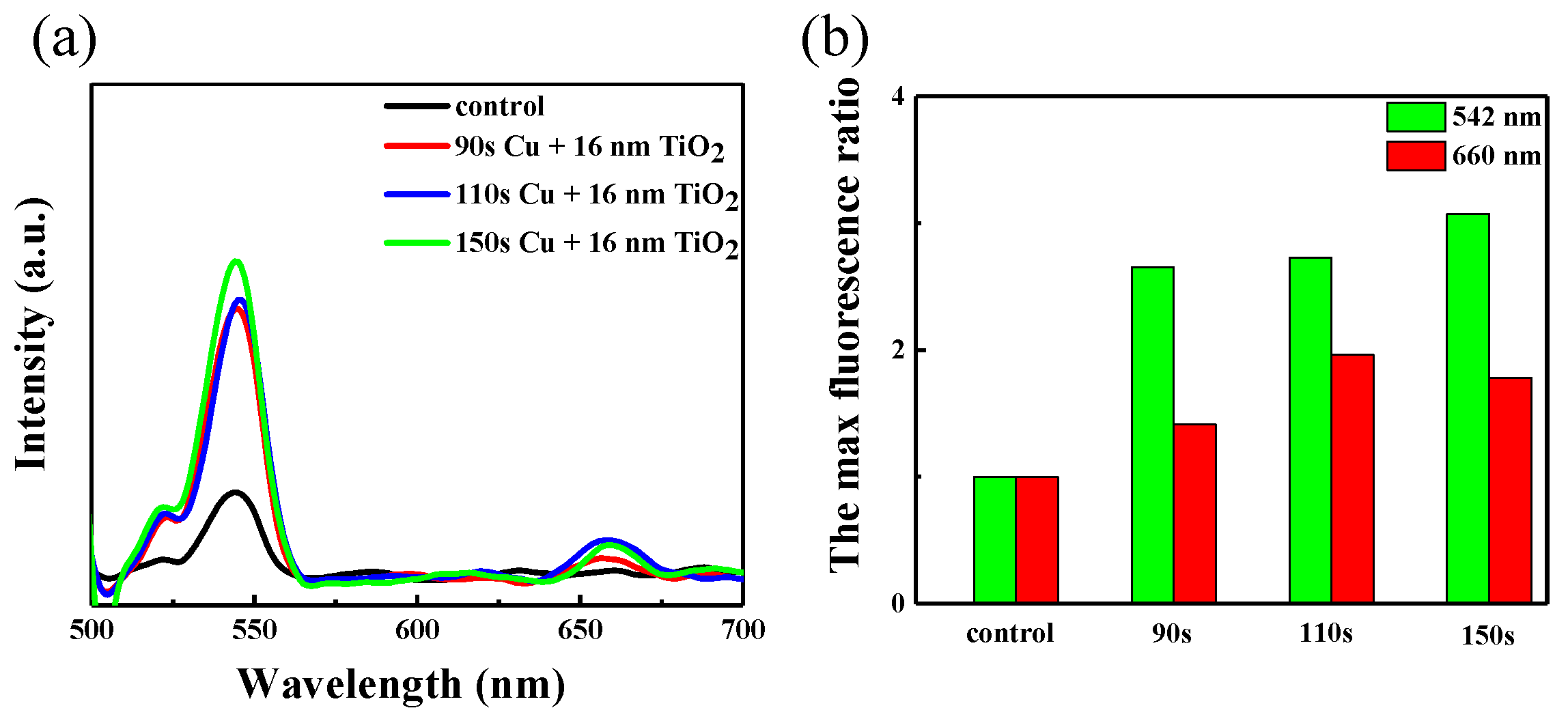
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fang, Z.; Zhen, Y.-R.; Fan, L.; Zhu, X.; Nordlander, P. Tunable wide-angle plasmonic perfect absorber at visible frequencies. Phys. Rev. B 2012, 85, 1–7. [Google Scholar] [CrossRef]
- Lu, C.Y.; Browne, D.E.; Yang, T.; Pan, J.W. Demonstration of a compiled version of Shor’s quantum factoring algorithm using photonic qubits. Phys. Rev. Lett. 2007, 99, 250504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aslan, K.; Previte, M.J.R.; Geddes, C.D. Metal-enhanced fluorescence from copper substrates. Appl. Phys. Lett. 2007, 90, 1–3. [Google Scholar] [CrossRef]
- Jing, Z.; Zhuang, L.; Li, F. Upconversion Nanophosphors for Small-Animal Imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Y.; Shen, W.; Gai, S.; Tang, J.; Wang, Y.; Huang, L.; Liu, J.; Wang, W.; Belfiore, L.A. Fabrication and luminescence of KGdF4:Yb3+/Er3+ nanoplates and their improving performance for polymer solar cells. Sci. Bull. 2018, 63, 216–218. [Google Scholar] [CrossRef]
- Deng, R.; Qin, F.; Chen, R.; Huang, W.; Hong, M.; Liu, X. Temporal full-colour tuning through non-steady-state upconversion. Nat. Nanotechnol. 2015, 10, 237–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Deng, R.; Tian, J.; Zong, Y.; Jin, D.; Liu, X. Multicolor Barcoding in a Single Upconversion Crystal. J. Am. Chem. Soc. 2014, 136, 4893–4896. [Google Scholar] [CrossRef]
- Haase, P.D.M.; Schfer, D.H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, J.; Yan, P.; Liu, L.; Wang, J.; Wang, Y.; Huang, L.; Liu, J.; Belfiore, L.A.; Tang, J. Synthesis and tunable photoresponse for core-shell structured NaGdF4: Yb, Er@SiO2@Eu(TTA)3Phen nanocomplexes. Scr. Mater. 2018, 152, 1–5. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Qian, H.; Hu, Y.; Niagara Muhammad, I. Seed-mediated synthesis of NaYF4:Yb, Er/NaGdF4 nanocrystals with improved upconversion fluorescence and MR relaxivity. Nanotechnology 2010, 21, 125602. [Google Scholar] [CrossRef]
- Liu, F.Y.; He, X.X.; Liu, L.; You, H.P.; Zhang, H.M.; Wang, Z.X. Conjugation of NaGdF4 upconverting nanoparticles on silica nanospheres as contrast agents for multi-modality imaging. Biomaterials 2013, 34, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Law, W.C.; Ågren, H.; Prasad, P.N. Monodisperse NaYbF4:Tm3+/NaGdF4 core/shell nanocrystals with near-infrared to near-infrared up-conversion photoluminescence and magnetic resonance properties. Nanoscale 2011, 3, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Yoo, J.R.; Kim, Y.S.; Heo, J. Mechanism of the blue up-conversion in Tm3+/Nd3+-doped calcium aluminate glasses. J. Am. Ceram. Soc. 2010, 80, 1485–1490. [Google Scholar] [CrossRef]
- Ren, G.; Zeng, S.; Hao, J. Tunable Multicolor Upconversion Emissions and Paramagnetic Property of Monodispersed Bifunctional Lanthanide-Doped NaGdF4 Nanorods. J. Phys. Chem. C 2011, 115, 20141–20147. [Google Scholar] [CrossRef]
- Li, H.; Deng, Q.; Liu, B.; Yang, J.; Wu, B. Fabrication of core@spacer@shell Aunanorod@mSiO2@Y2O3: Er nanocomposites with enhanced upconversion fluorescence. RSC Adv. 2016, 6, 13343–13348. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, K. Enhanced Up-Conversion Emission in Al3+ Co-Doped ZnGa2O4:Yb3+, Tm3+ Powder Phosphors. J. Fluoresc. 2018, 28, 801–808. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Liu, B.; Wang, Y.; Sekino, T.; Lee, S.W.; Sato, T. UV, visible and near-infrared lights induced NOx destruction activity of (Yb, Er)-NaYF4/C-TiO2 composite. Sci. Rep. 2013, 3, 2918. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.; Shi, Y.; Xie, S.; Li, Y.; Xu, L.; Tu, B.; Zhao, D. Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence. Angew. Chem. Int. Ed. 2007, 46, 7976–7979. [Google Scholar] [CrossRef]
- Avnir, D.; Kaufman, V.R.; Reisfeld, R. Organic fluorescent dyes trapped in silica and silica-titania thin films by the sol-gel method. Photophysical, film and cage properties. J. Non Cryst. Solids 1985, 74, 395–406. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Wilson, L.R.; Richards, B.S. Measurement method for photoluminescent quantum yields of fluorescent organic dyes in polymethyl methacrylate for luminescent solar concentrators. Appl. Opt. 2009, 48, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, S.K.W.; Ivaturi, A.; Marques-Hueso, J.; Krämer, K.W.; Richards, B.S. Ultra-high photoluminescent quantum yield of β-NaYF4: 10% Er3+ via broadband excitation of upconversion for photovoltaic devices. Opt. Express 2012, 20, A879. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, B.; Lundström, I.; Stenberg, E. Principles of biosensing with an extended coupling matrix and surface plasmon resonance. Sens. Actuators, B 1993, 11, 63–72. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Arppe, R.; Sørensen, T.J. Physical unclonable functions generated through chemical methods for anti-counterfeiting. Nat. Rev. Chem. 2017, 1, 0031. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature. 2003, 424, 824–830. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Anal. Bioanal. Chem. 1999, 377, 528–539. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Futamata, M.; Maruyama, Y.; Ishikawa, M. Local Electric Field and Scattering Cross Section of Ag Nanoparticles under Surface Plasmon Resonance by Finite Difference Time Domain Method. J. Phys. Chem. B 2003, 107, 7607–7617. [Google Scholar] [CrossRef]
- Suyver, J.F.; Aebischer, A.; Biner, D.; Gerner, P.; Grimm, J.; Heer, S.; Krämer, K.W.; Reinhard, C.; Güdel, H.U. Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Opt. Mater. 2005, 27, 1111–1130. [Google Scholar] [CrossRef]
- Hong, Y.P.; Dwight, K. Crystal structure and fluorescence lifetime of a laser material NdNa5(WO4)4. Mater. Res. Bull. 1974, 9, 775–780. [Google Scholar] [CrossRef]
- Manurung, R.V.; Wu, C.T.; Roy, P.K.; Chattopadhyay, S. A plasmon-tuned ‘gold sandwich’ for metal enhanced fluorescence in silica coated NaYF4:Yb,Er upconversion nanoparticles. RSC Adv. 2016, 6, 87088–87095. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, D.; Xu, W.; Zhu, J.; Pan, G.; Yin, Z.; Wang, H.; Zhu, Y.; Shaobo, C.; Song, H. Fabrication of Au-Ag nanocage@NaYF4@NaYF4:Yb,Er Core-Shell Hybrid and its Tunable Upconversion Enhancement. Sci. Rep. 2017, 7, 41079. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Yin, Z.; Zhang, T.; Chen, X.; Zhou, D.; Zhu, J.; Xu, W.; Cui, H.; Song, H. Remarkable Enhancement of Upconversion Luminescence on Cap-Ag/PMMA Ordered Platform and Trademark Anticounterfeiting. ACS Appl. Mater. Interfaces 2017, 9, 37128–37135. [Google Scholar] [CrossRef]
- Sui, M.; Zhang, Q.; Kunwar, S.; Pandey, P.; Li, M.-Y.; Lee, J. Study on the dimensional, configurational and optical evolution of palladium nanostructures on c-plane sapphire by the control of annealing temperature and duration. Appl. Surf. Sci. 2017, 416, 1–13. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Zhao, X.; Lee, U.-J.; Lee, K.-H. Dewetting behavior of Au films on porous substrates. Thin Solid Films 2010, 519, 706–713. [Google Scholar] [CrossRef]
- Pandey, P.; Sui, M.; Zhang, Q.; Li, M.-Y.; Kunwar, S.; Lee, J. Systematic control of the size, density and configuration of Pt nanostructures on sapphire (0 0 0 1) by the variation of deposition amount and dwelling time. Appl. Surf. Sci. 2016, 368, 198–207. [Google Scholar] [CrossRef]
- Yao, J.H.; Elder, K.R.; Guo, H.; Grant, M. Theory and simulation of Ostwald ripening. Phys. Rev. B 1993, 47, 14110–14125. [Google Scholar] [CrossRef] [PubMed]
- Marqusee, J.A.; Ross, J. Theory of Ostwald ripening: Competitive growth and its dependence on volume fraction. J. Chem. Phys. 1984, 80, 536–543. [Google Scholar] [CrossRef]
- Zhan, Q.; Zhang, X.; Zhao, Y.; Liu, J.; He, S. Tens of thousands-fold upconversion luminescence enhancement induced by a single gold nanorod. Laser Photonics Rev. 2015, 9, 479–487. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Shao, L.; Shu, Y.; Wang, J.; Wu, H. Growth of Monodisperse Gold Nanospheres with Diameters from 20 nm to 220 nm and Their Core/Satellite Nanostructures. Adv. Opt. Mater. 2014, 2, 65–73. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Smirnov, V.V.; Kozhevin, V.M.; Yavsin, D.A.; Zabelin, M.A.; Yassievich, I.N.; Gurevich, S.A. New size effect in the catalysis by interacting copper nanoparticles. Appl. Catal. A 2005, 296, 70–79. [Google Scholar] [CrossRef]
- Mao, S.; Liu, J.; Pan, Y.; Lee, J.; Yao, Z.; Pandey, P.; Kunwar, S.; Zhu, Z.; Shen, W.; Belfiore, L.A.; et al. Morphological and optical evolution of metallic oxide/Au nanoparticle hybrid thin film: High absorption and reflectance by plasmonic enhancement. Appl. Surf. Sci. 2019, 495, 143575. [Google Scholar] [CrossRef]
- Lv, B.; Jiao, J.; Liu, Y.; Liu, L.; Zhang, J.; Li, Y.; Wang, J.; Tang, J. Heterostructure NaGdF4:Yb,Er anchored on MIL-101 for promoting photoelectronic response and photocatalytic activity. Nanoscale 2019, 11, 22730–22733. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, E.; Liu, Z.; Zhang, Z.; Zeng, J.; Ruan, W.; Li, G.; Cao, W. Electric field induced upconversion fluorescence enhancement and its mechanism in Er3+ doped 0.75Pb(Mg1/3Nb2/3)O3-0.25PbTiO3 transparent ceramic. Appl. Phys. Lett. 2016, 109, 132904. [Google Scholar] [CrossRef]
- Maurya, S.K.; Tiwari, S.P.; Kumar, A.; Kumar, K. Plasmonic enhancement of upconversion emission in Ag@NaYF4:Er3+/Yb3+ phosphor. J. Rare Earths 2018, 36, 903–910. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Chu, L.; Liu, J.; Lv, B.; Belfiore, L.A.; Tang, J. Precise Control of Copper-Localized Surface Plasmon Resonance in the Near Infrared Region for Enhancement of Up-Conversion Luminescence. Metals 2020, 10, 628. https://doi.org/10.3390/met10050628
Pan Y, Chu L, Liu J, Lv B, Belfiore LA, Tang J. Precise Control of Copper-Localized Surface Plasmon Resonance in the Near Infrared Region for Enhancement of Up-Conversion Luminescence. Metals. 2020; 10(5):628. https://doi.org/10.3390/met10050628
Chicago/Turabian StylePan, Yuyong, Lingling Chu, Jiliang Liu, Baize Lv, Laurence A. Belfiore, and Jianguo Tang. 2020. "Precise Control of Copper-Localized Surface Plasmon Resonance in the Near Infrared Region for Enhancement of Up-Conversion Luminescence" Metals 10, no. 5: 628. https://doi.org/10.3390/met10050628
APA StylePan, Y., Chu, L., Liu, J., Lv, B., Belfiore, L. A., & Tang, J. (2020). Precise Control of Copper-Localized Surface Plasmon Resonance in the Near Infrared Region for Enhancement of Up-Conversion Luminescence. Metals, 10(5), 628. https://doi.org/10.3390/met10050628




