Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Commercial Al-7Si-Mg Alloys
2.2. Unmodified Al-7Si-Mg Alloys
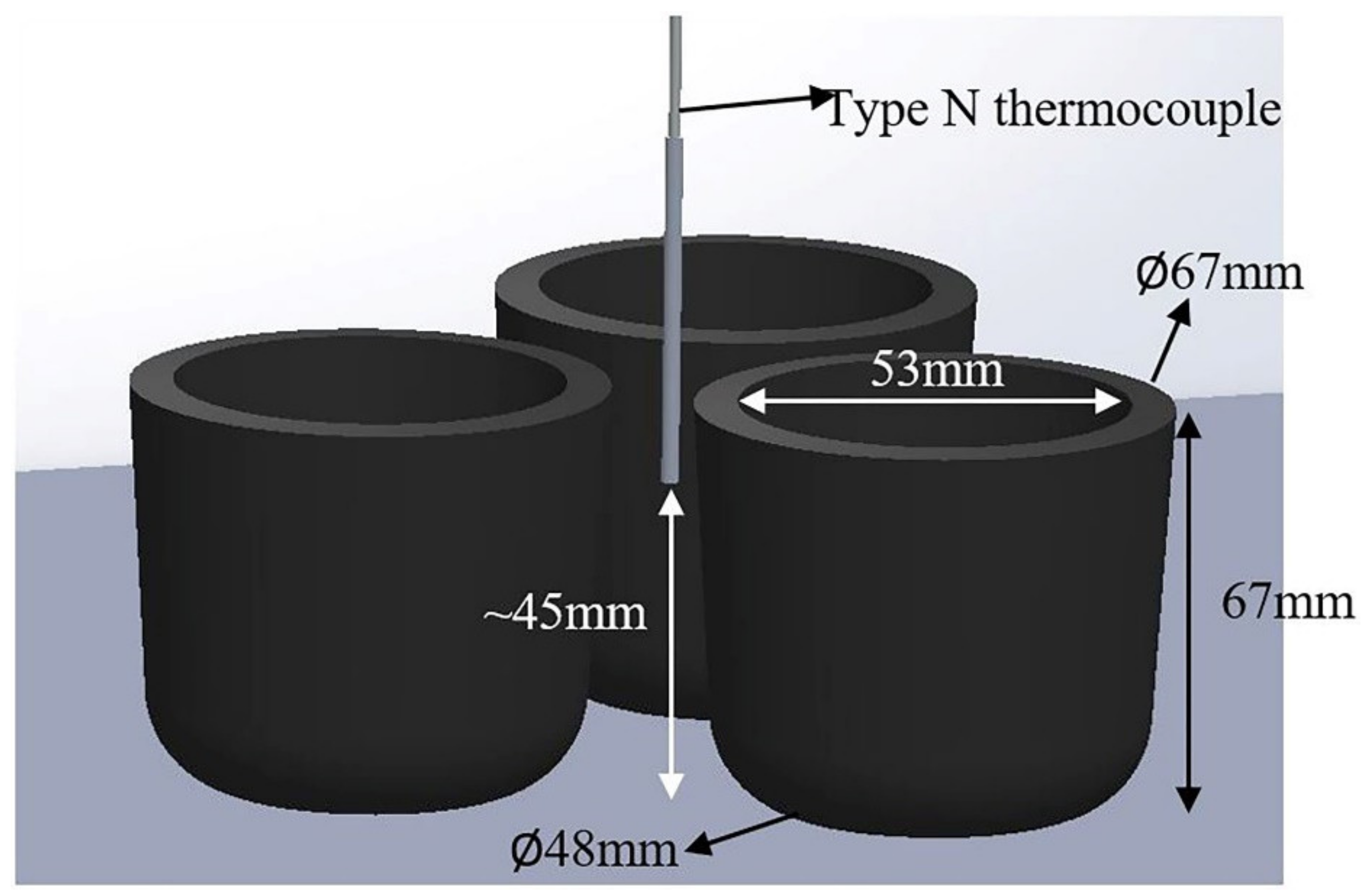
2.3. Isothermal Holding Treatments
2.4. SSM Al-7Si-Mg Castings
2.5. T6 and T5 Heat Treatments of the SSM Al-7Si-Mg Castings
2.6. Microstructural Characterization
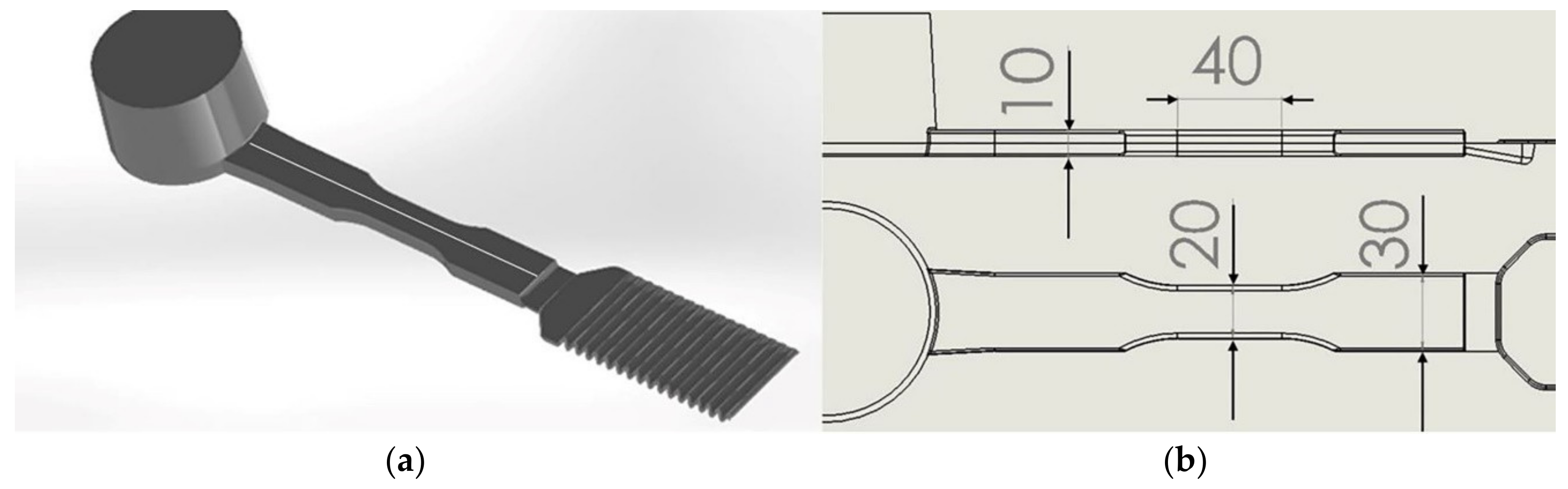
2.7. Tensile Testing
3. Results
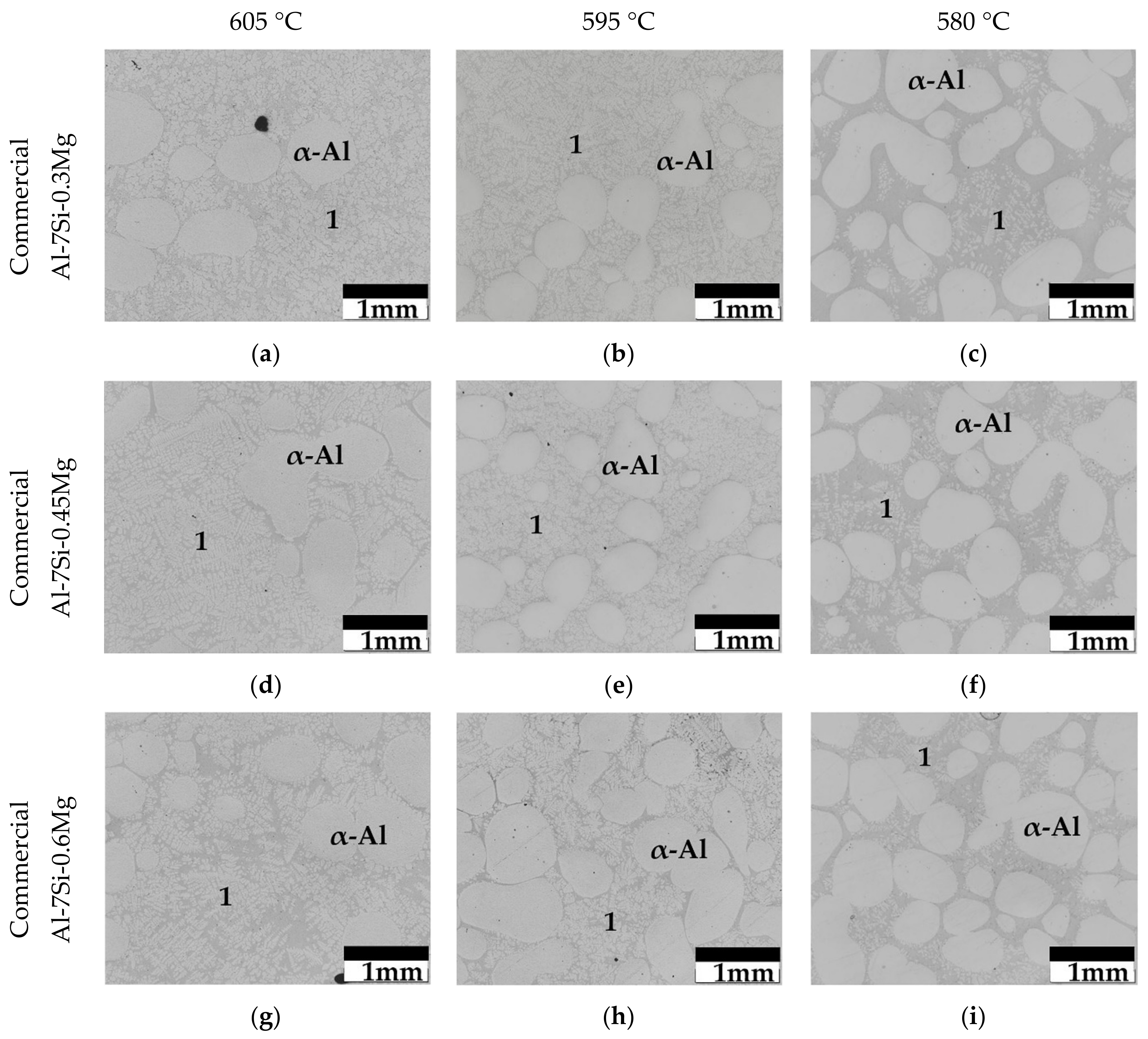
3.1. Isothermal Holding Treatments
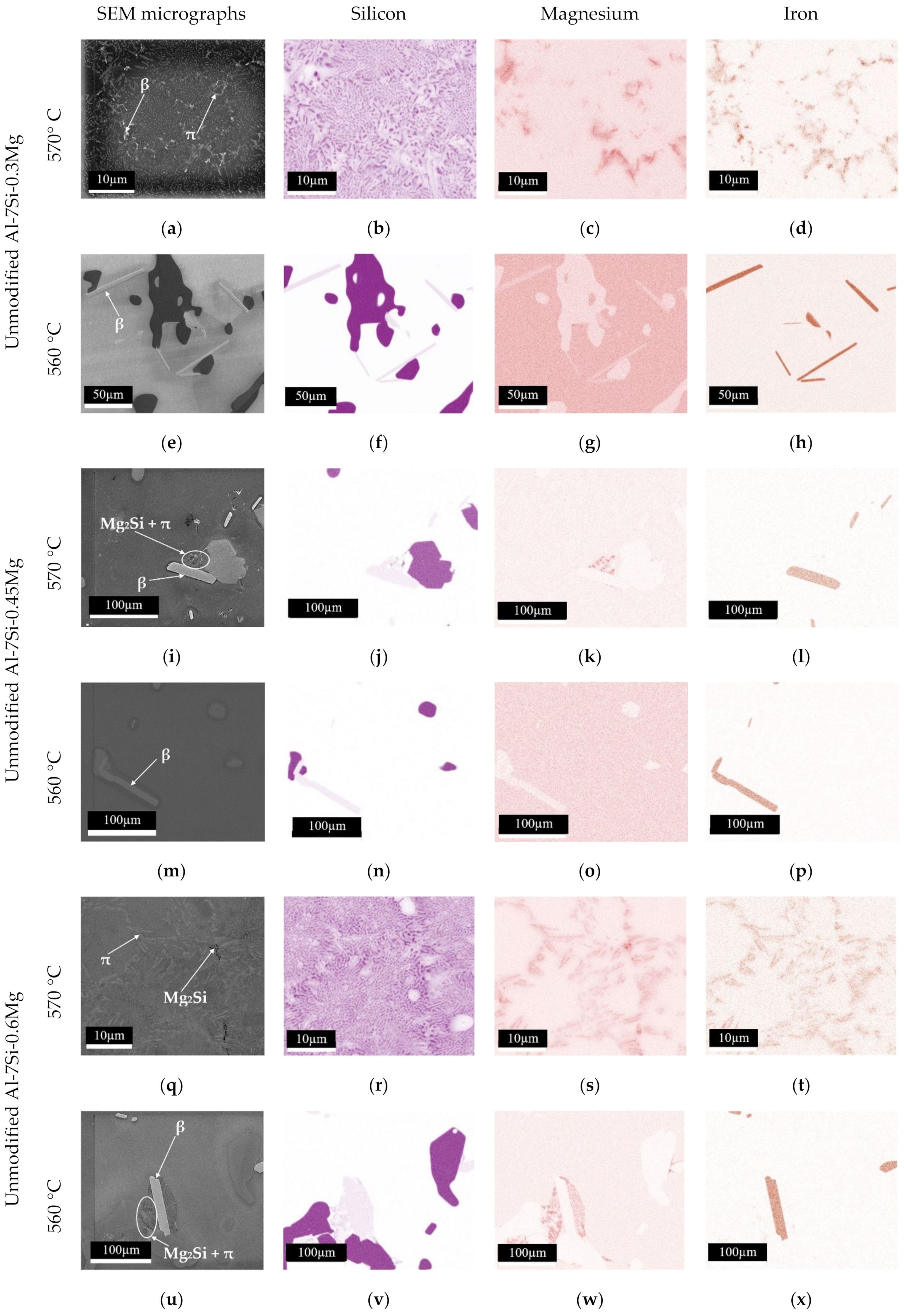
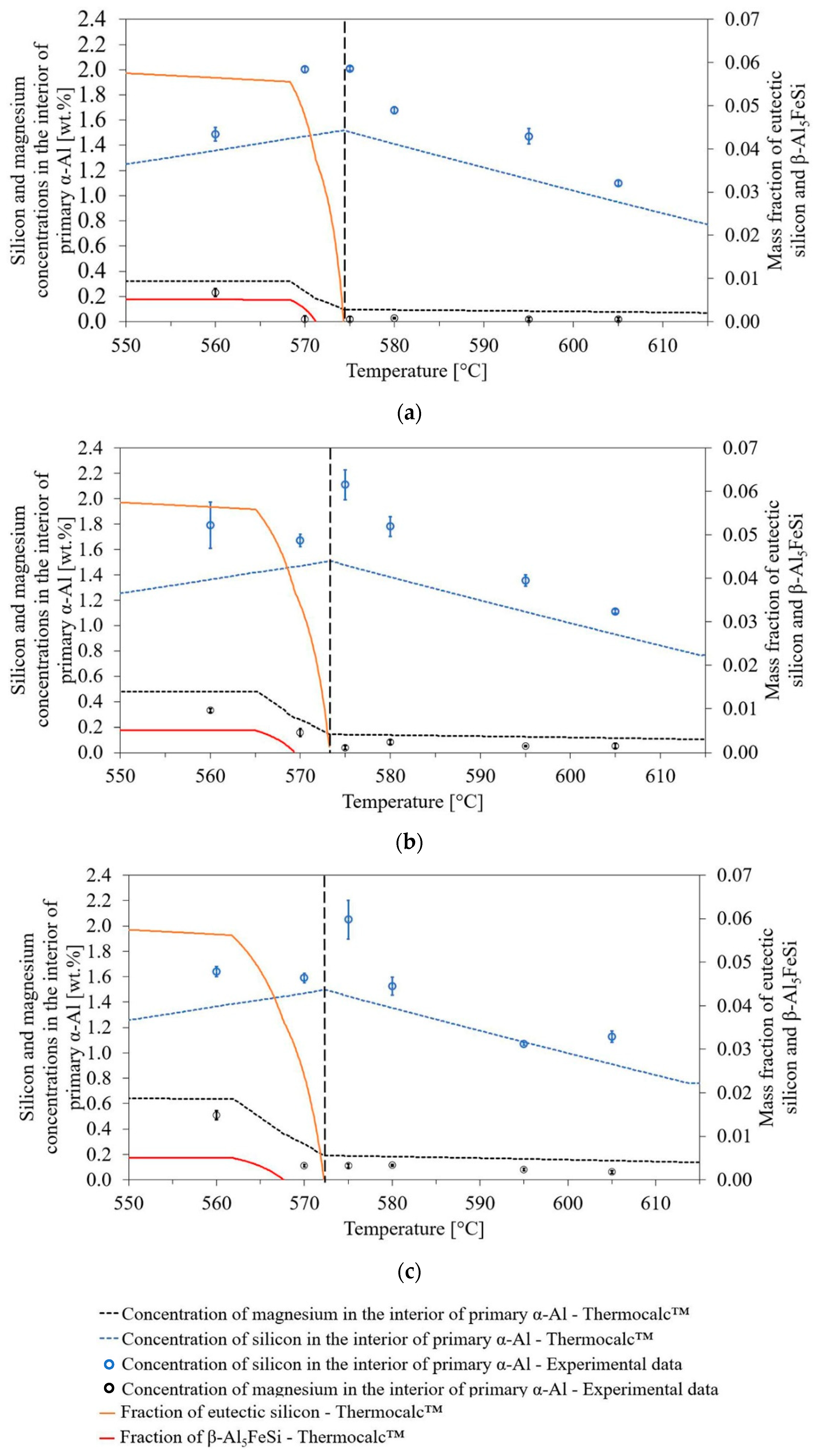
3.2. Solute Concentrations in Primary α-Al Solid-Solution after Isothermal Holding Treatments
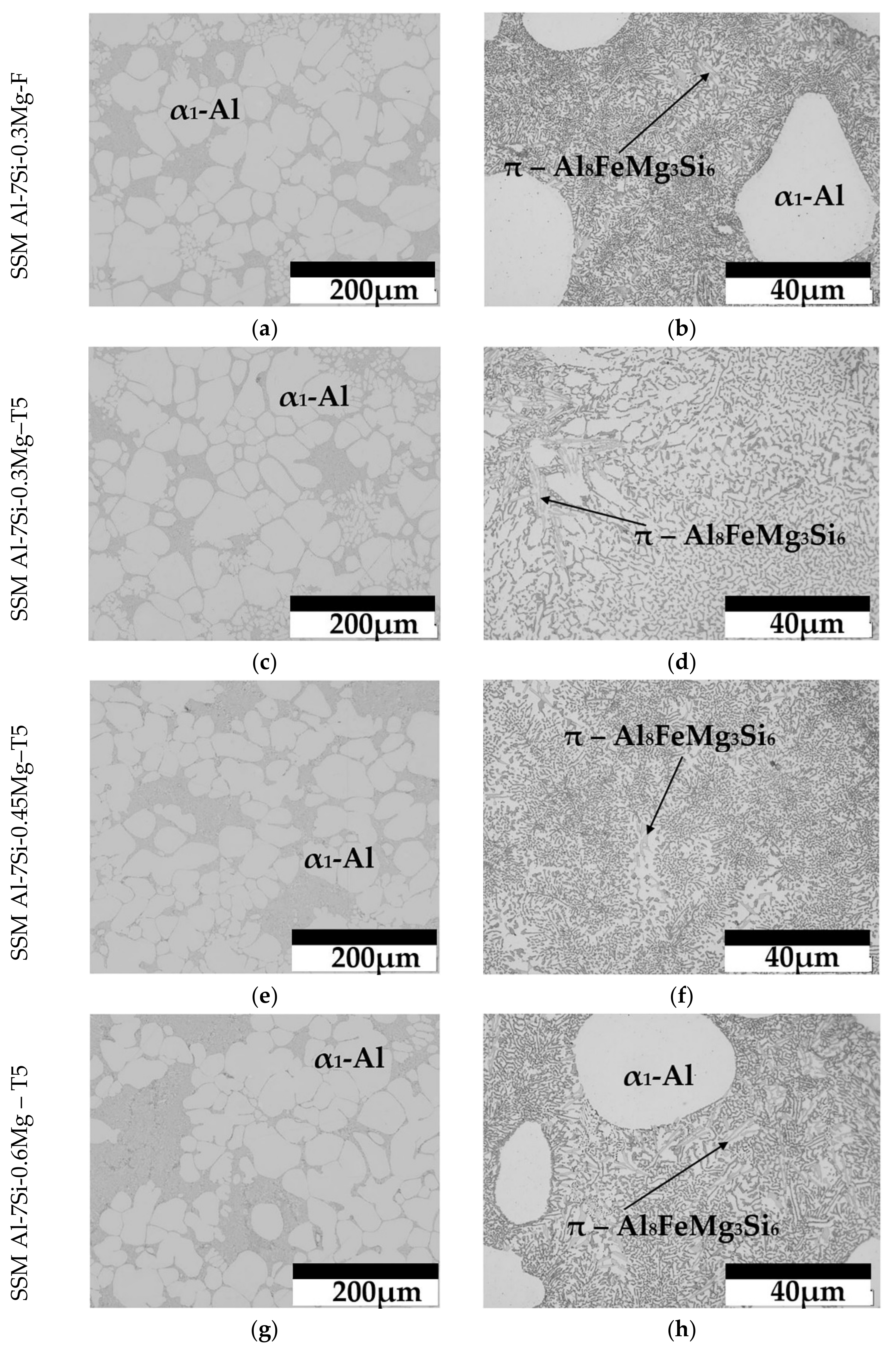
3.3. SSM Castings
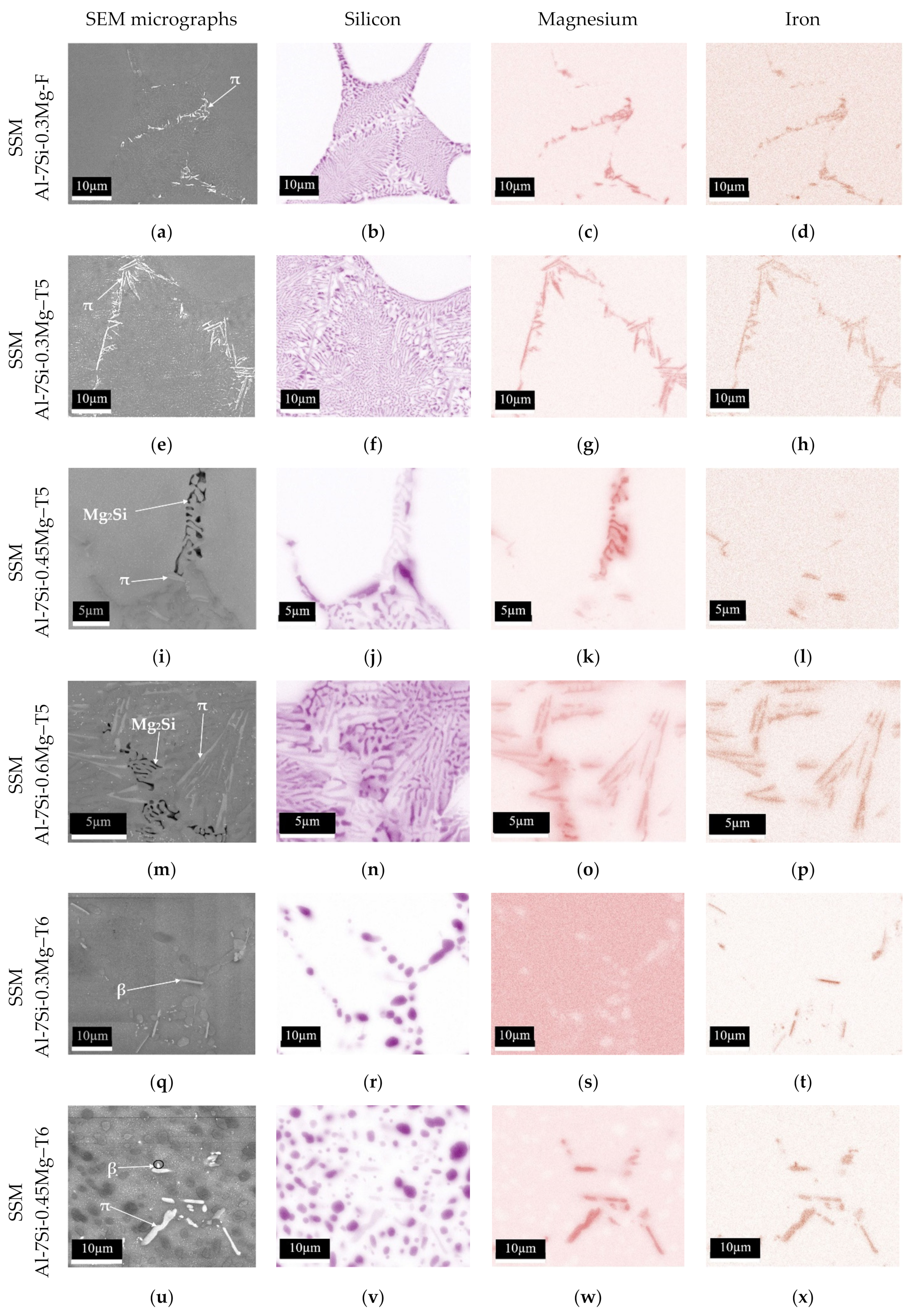
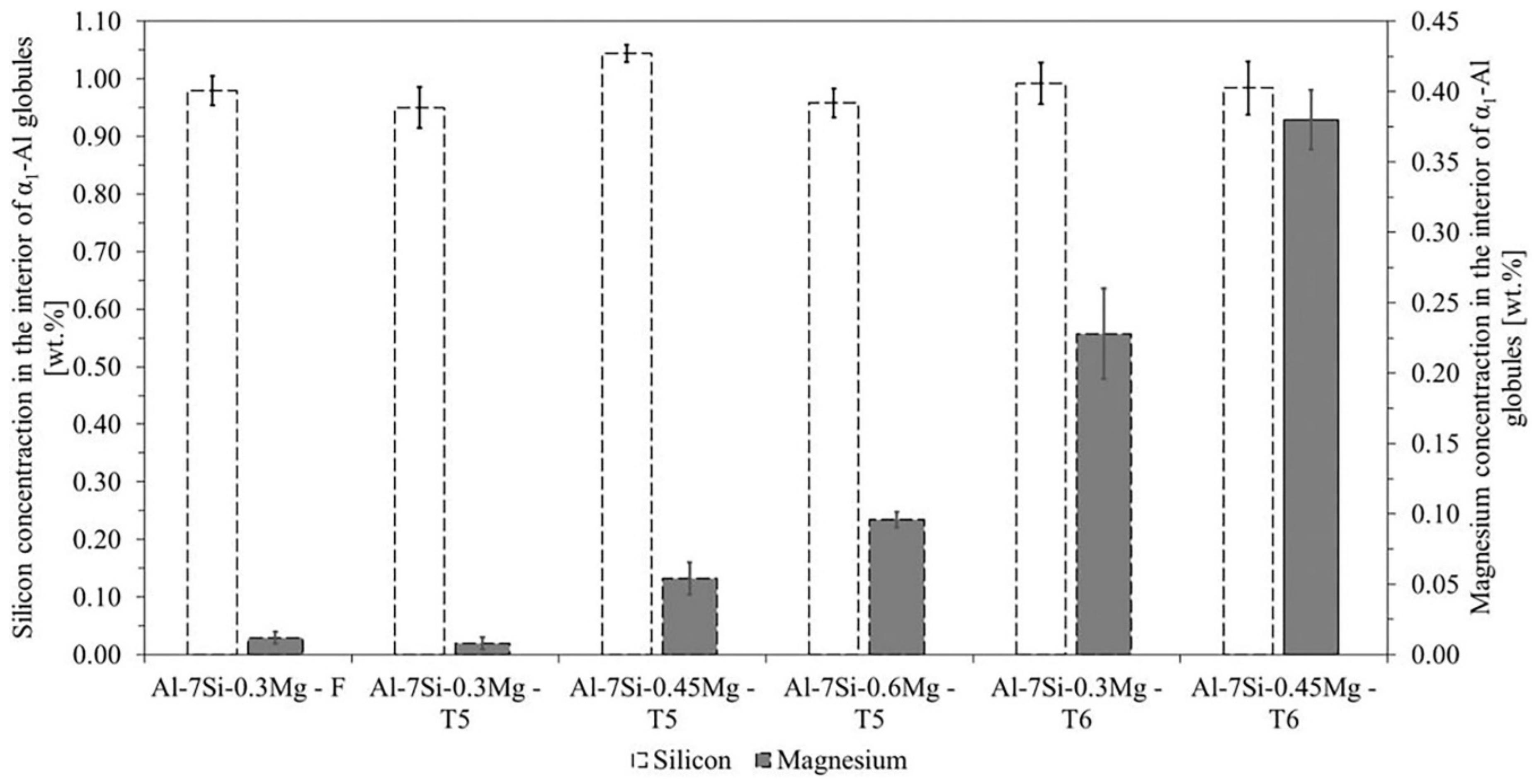
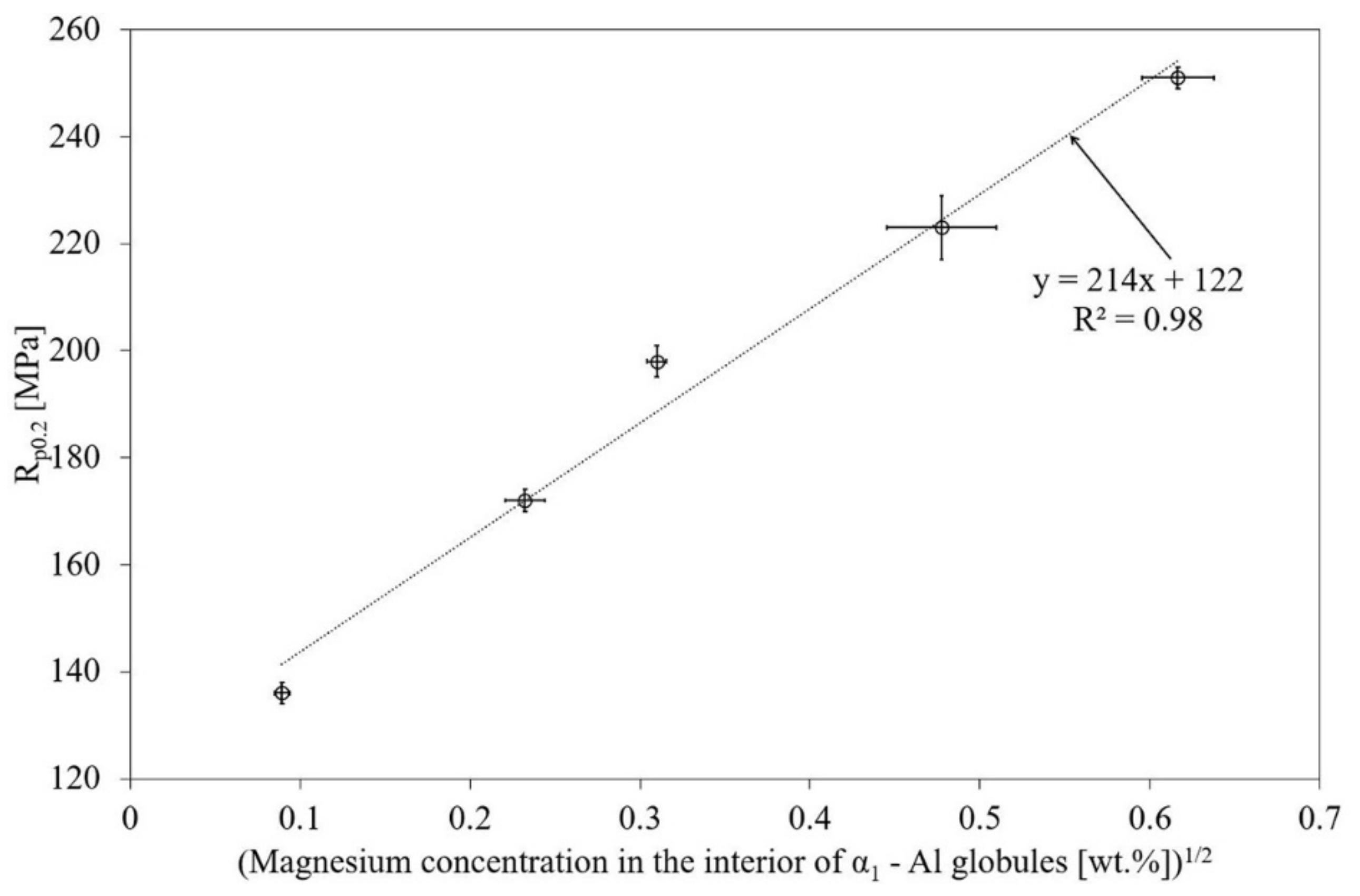
3.4. Silicon and Magnesium Concentrations in the Interior of Primary α1-Al Globules of SSM Castings
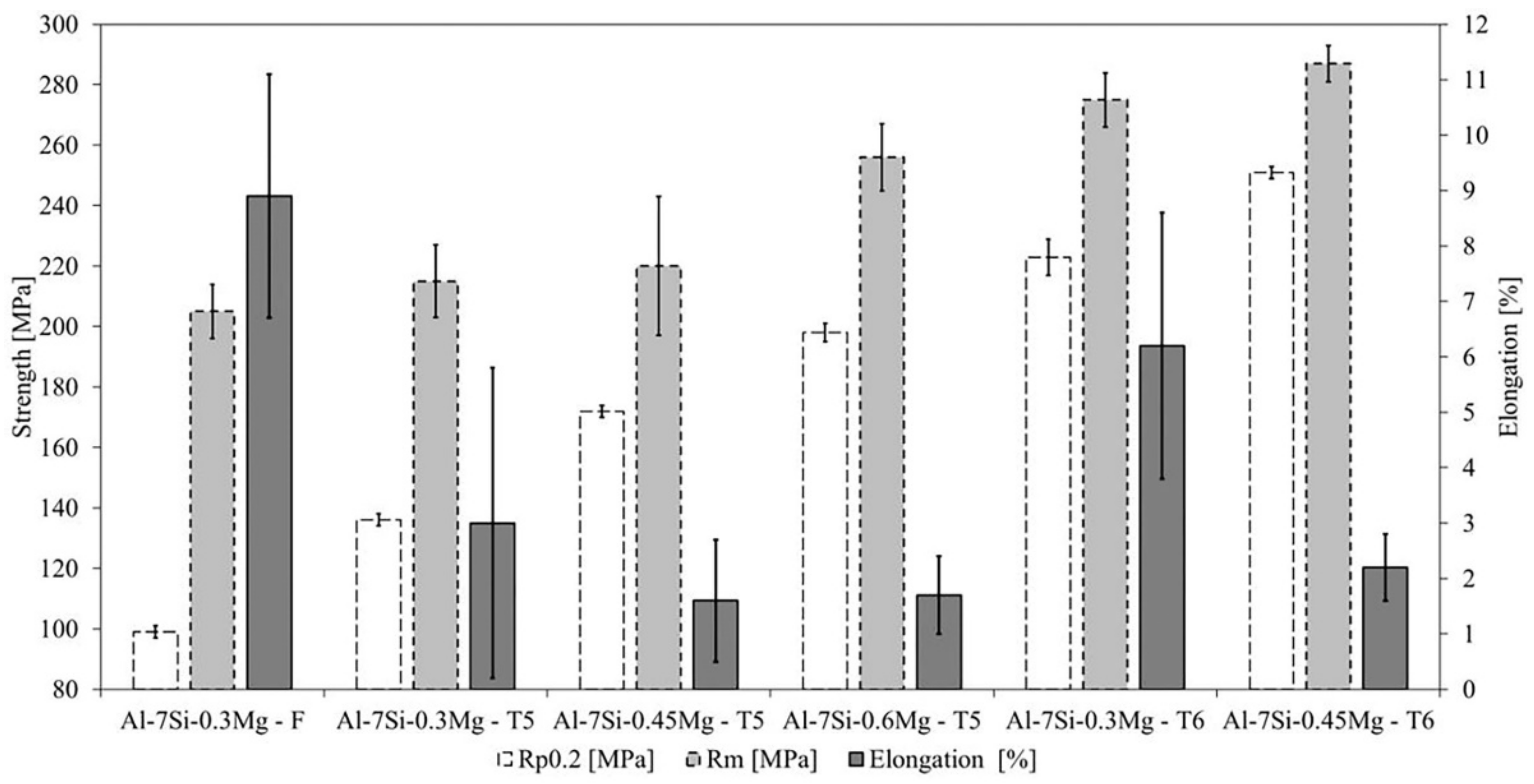
3.5. Mechanical Properties
4. Discussion
4.1. Variation of Solute Concentration in the Interior of the Primary α-Al Phase after Isothermal Holding Treatments
4.2. Silicon and Magnesium Concentrations in the Interior of Primary α1-Al of SSM Castings
4.3. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Möller, H.; Govender, G.; Stumpf, W.E.; Pistorius, P.C. Comparison of heat treatment response of semisolid metal processed alloys A356 and F357. Int. J. Cast Met. Res. 2010, 23, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.A.; Barresi, J.; Couper, M.J.; StJohn, D.H. Influence of Mg content on the microstructure and solid solution chemistry of Al-7%Si-Mg casting alloys during solution treatment. In Aluminium Alloys-Their Physical and Mechanical Properties; Trans Tech Publications: Baech, Switzerland, 2000; Volume 331, pp. 277–282. [Google Scholar]
- Yao, J.Y.; Taylor, J.A. Characterisation of intermetallic particles formed during solution treatment of an Al–7Si–0.4Mg–0.12Fe alloy. J. Alloys Compd. 2012, 519, 60–66. [Google Scholar] [CrossRef]
- Kaufman, J.G.; Rooy, L.E. Aluminum Alloy Castings_ Properties, Processes, and Applications; ASM International: Cleveland, OH, USA, 2004. [Google Scholar]
- Blad, M.; Johannesson, B.; Nordberg, P.; Winklhofer, J. Manufacturing and fatigue verification of two different components made by semi-solid processing of aluminium TX630 alloy. In Semi-Solid Processing of Alloys and Composites XIV; Trans Tech Publications: Salt Lake, UT, USA, 2016; Volume 256, pp. 328–333. [Google Scholar]
- Menargues, S.; Martín, E.; Baile, M.T.; Picas, J.A. New short T6 heat treatments for aluminium silicon alloys obtained by semisolid forming. Mater. Sci. Eng. A 2015, 621, 236–242. [Google Scholar] [CrossRef]
- Major, J.; Hartlieb, M. Advances in aluminum foundry alloys for permanent and semi-permanent mold casting. Int. J. Met. 2009, 3, 43–53. [Google Scholar] [CrossRef]
- ASTM. ASTM B917/B917M-12-Standard Practice for Heat Treatment of Aluminum-Alloy Castings from All Processes; ASTM International: West Conshohocken, PA, USA, 2012; Volume 1, pp. 1–11. [Google Scholar]
- Möller, H.; Govender, G.; Stumpf, W. The T5 Heat Treatment of semi-solid metal processed aluminium alloy F357. Mater. Sci. Forum 2009, 618–619, 365–368. [Google Scholar] [CrossRef]
- Rosso, M.; Peter, I.; Villa, R. Effects of T5 and T6 heat treatments applied to rheocast A356 parts for automotive applications. Solid State Phenom. 2008, 141–143, 237–242. [Google Scholar] [CrossRef]
- Pedersen, L.; Arnberg, L. Anomalous microsegregation in Al–Si foundry alloys. Mater. Sci. Eng. A 1998, 241, 285–289. [Google Scholar] [CrossRef]
- Dons, A.L.; Pedersen, L.; Arnberg, L. The origin of “anomalous” microsegregation in Al-Si foundry alloys-Modelling and experimental verification. Mater. Sci. Eng. A 1999, 271, 91–94. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. Optimization of solution treatment of cast Al-7Si-0.3Mg and Al-8Si-3Cu-0.5Mg alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 1916–1927. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Schaffer, G.B.; Taylor, J.A. Mass balance characterisation of Al-7Si-Mg alloy microstructures as a function of solution treatment time. Int. J. Cast Met. Res. 2001, 14, 59–69. [Google Scholar] [CrossRef]
- Yildirim, M.; Özyürek, D. The effects of Mg amount on the microstructure and mechanical properties of Al-Si-Mg alloys. Mater. Des. 2013, 51, 767–774. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Correlation of solidification microstructure refining scale, Mg composition and heat treatment conditions with mechanical properties in Al-7Si-Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 391–402. [Google Scholar] [CrossRef]
- Caceres, C.H.; Davidson, C.J.; Wang, Q.G.; Griffiths, J.R. The effect of Mg on the microstructure and mechanical behavior of Al-Si-Mg casting alloys. Metall. Mater. Trans. A 1999, 30, 2611–2618. [Google Scholar] [CrossRef]
- Motzfeldt, K. High Temperature Experiments in Chemistry and Materials Science; John Wiley 6 Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Granath, O.; Wessén, M.; Cao, H. Determining effect of slurry process parameters on semisolid A356 alloy microstructures produced by RheoMetal process. Int. J. Cast Met. Res. 2008, 21, 349–356. [Google Scholar] [CrossRef]
- Wessén, M.; Cao, H. The RSF technology; A possible breakthrough for semi-solid casting processes. In Proceedings of the 3rd International Conference High Tech Die Casting, AIM, Vicenza, Italy, 21 September 2006. [Google Scholar]
- European Committee for Standardization. SS-EN-ISO 6892-1:2016 Metallic Materials-Tensile Testing-Part 1: Method of Test at Room Temperature; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- Jarfors, A.E.W. Melting and coarsening of A356 during preheating for semisolid forming. Int. J. Cast Met. Res. 2004, 17, 229–237. [Google Scholar] [CrossRef]
- Wang, Q.G.; Davidson, C.J. Solidification and precipitation behaviour of Al-Si-Mg casting alloys. J. Mater. Sci. 2001, 36, 739–750. [Google Scholar] [CrossRef]
- Bäckerud, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminum Alloys, Volume 2: Foundry Alloys; AFS/SKANALUMINIUM: Stockholm, Sweden, 1990. [Google Scholar]
- Payandeh, M.; Jarfors, A.E.W.; Wessén, M. Solidification sequence and evolution of microstructure during rheocasting of four Al-Si-Mg-Fe alloys with low Si content. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 1215–1228. [Google Scholar] [CrossRef]
- Wang, Q. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2887–2899. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams: Applications for Commercial Aluminum Alloys; Elsevier B.V.: Amsterdam, The Netherlands, 2005. [Google Scholar]


| Type | Alloys | Si | Mg | Fe | Ti | Sr | Al |
|---|---|---|---|---|---|---|---|
| Commercial | Al-7Si-0.3Mg | 6.94–7.10 | 0.30–0.35 | 0.13–0.16 | 0.072–0.099 | 0.012–0.027 | Bal. |
| Al-7Si-0.45Mg | 7.24–7.39 | 0.47–0.50 | 0.11 | 0.096–0.11 | 0.024–0.025 | Bal. | |
| Al-7Si-0.6Mg | 6.94–7.28 | 0.59–0.60 | 0.11–0.12 | 0.11–0.12 | 0.023–0.025 | Bal. | |
| Unmodified | Al-7Si-0.3Mg | 6.58 | 0.31 | 0.20 | <0.0004 | <0.0001 | Bal. |
| Al-7Si-0.45Mg | 7.02 | 0.44 | 0.18 | <0.0004 | <0.0001 | Bal. | |
| Al-7Si-0.6Mg | 7.03 | 0.58 | 0.20 | <0.0004 | <0.0001 | Bal. |
| Casting | Condition | Temperatures and Holding Times |
|---|---|---|
| SSM Al-7Si-0.3Mg | T5 | AA at 175 °C for 4.5 h |
| T6 | ST at 510 °C for 4 h + AA at 190 °C for 2 h | |
| SSM Al-7Si-0.45Mg | T5 | AA at 175 °C for 4.5 h |
| T6 | ST at 510 °C for 4 h + AA 190 °C for 2 h | |
| SSM Al-7Si-0.6Mg | T5 | AA at 180 °C for 4.5 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Dahle, A.K.; Jarfors, A.E.W. Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg. Metals 2020, 10, 614. https://doi.org/10.3390/met10050614
Santos J, Dahle AK, Jarfors AEW. Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg. Metals. 2020; 10(5):614. https://doi.org/10.3390/met10050614
Chicago/Turabian StyleSantos, Jorge, Arne K. Dahle, and Anders E. W. Jarfors. 2020. "Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg" Metals 10, no. 5: 614. https://doi.org/10.3390/met10050614
APA StyleSantos, J., Dahle, A. K., & Jarfors, A. E. W. (2020). Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg. Metals, 10(5), 614. https://doi.org/10.3390/met10050614






