A Comprehensive Review on Hydrogen Absorption Behaviour of Metal Alloys Prepared through Mechanical Alloying
Abstract
1. Introduction
2. Non-Equilibrium Preparation Techniques
3. Mechanical Alloying
3.1. Background
3.2. Mg-Based Alloys
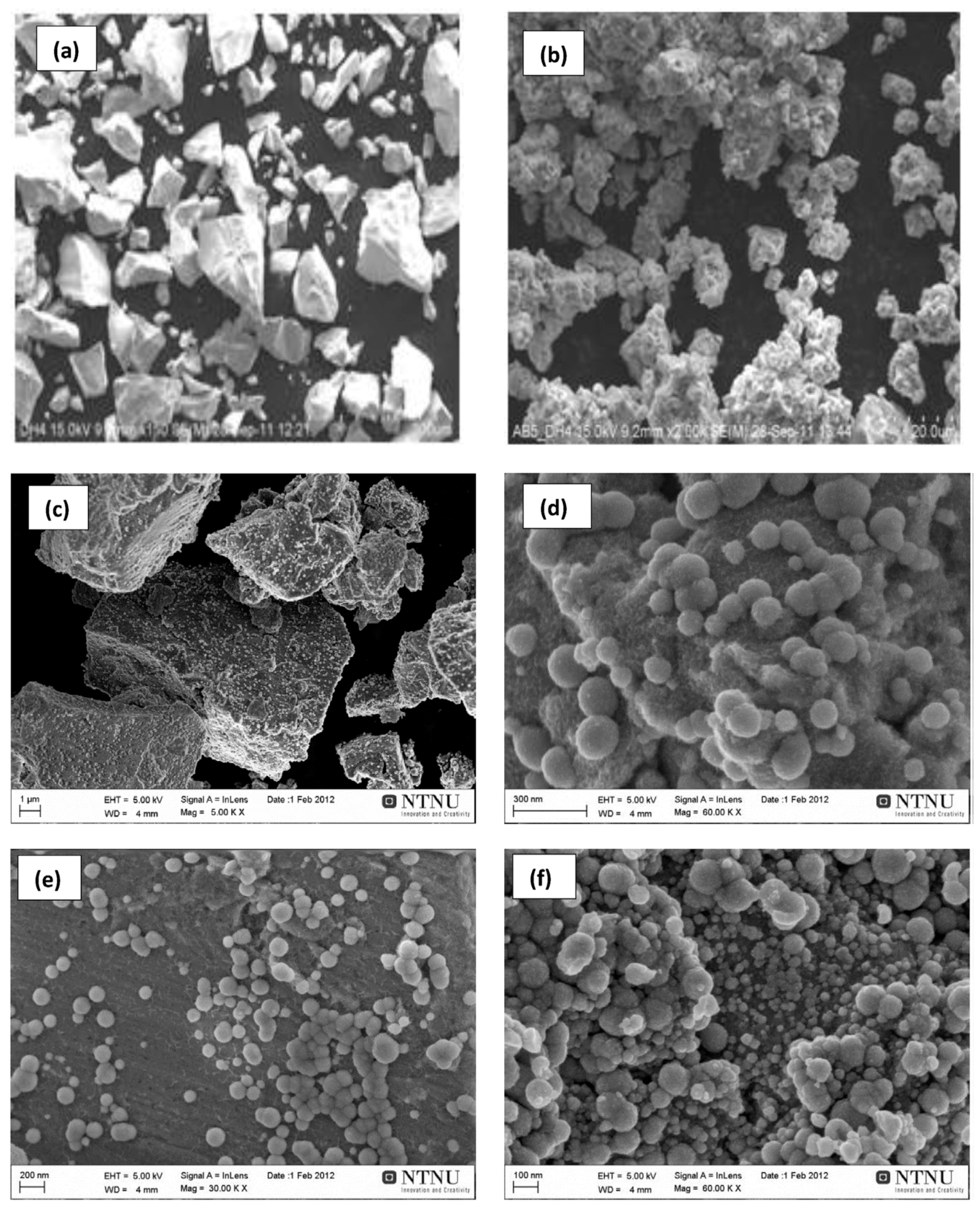
3.2.1. Structural Characteristics of Mechanically Milled Mg-Based Alloys
3.2.2. Hydrogen Absorption Behaviour of Mechanically Alloyed Mg-Based Alloys
3.2.3. Ball-Milled MgH2 Doped with Catalytic Additives
3.3. AB5-Type Alloys
3.3.1. Structural Characteristics of Mechanically Milled AB5-Type Alloys
3.3.2. Hydrogen Absorption Behaviour of Mechanically Alloyed AB5-Type Alloys
3.4. AB2-Type Alloys
3.4.1. Structural Characteristics of Mechanically Milled AB2-Type Alloys
3.4.2. Hydrogen Absorption Behaviour of Mechanically Alloyed AB2-Type Alloys
3.5. Comparison between Hydriding Kinetics of Materials Prepared by MA with Other Techniques
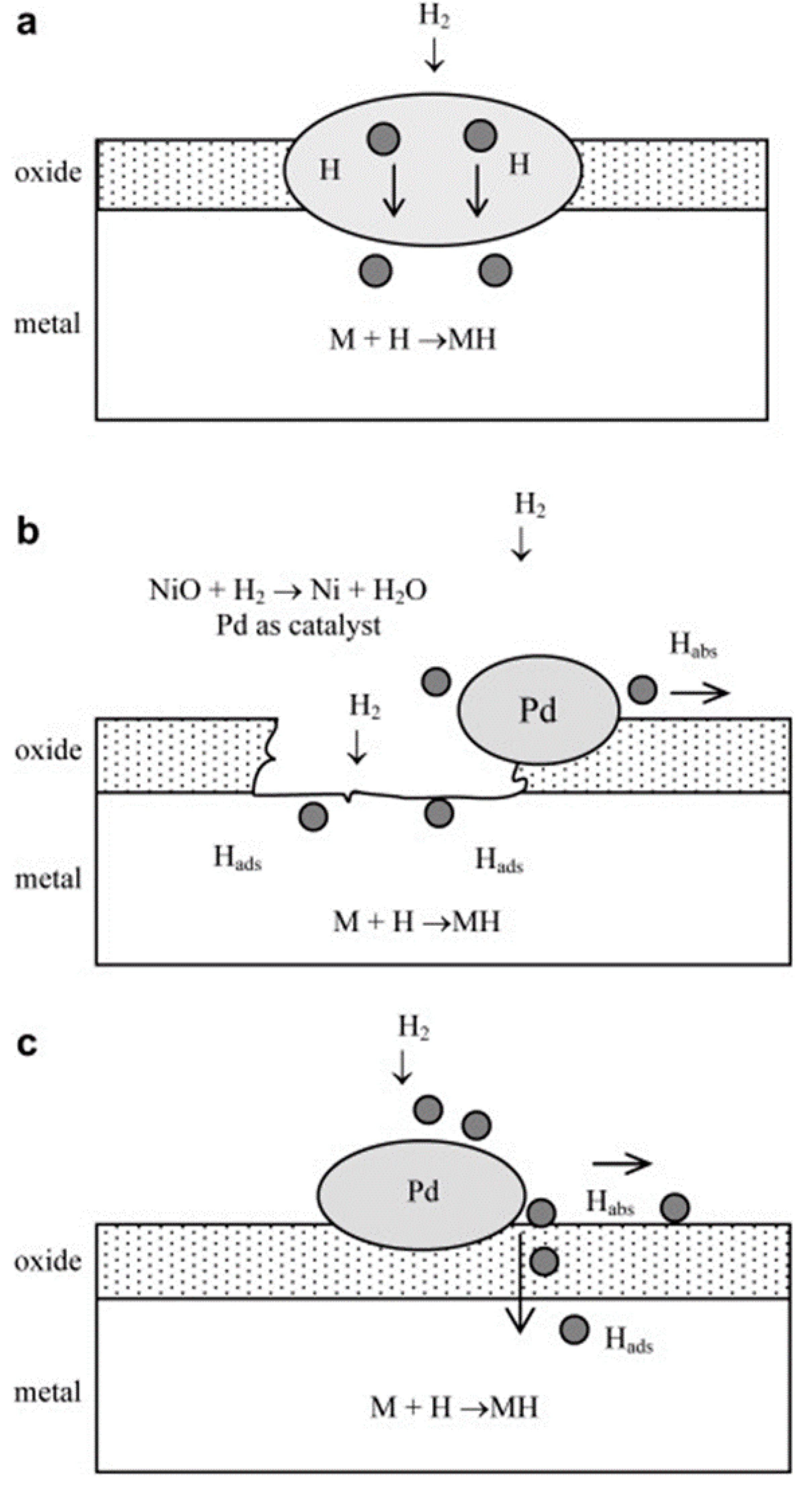
3.6. Surface Modification of Ball Milled Metal Alloys
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andreas, R. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Mater. Sustain. Energy 2011, 414, 265–270. [Google Scholar]
- Berlouis, L.E.A.; Cabrera, E.; Hall, P.J. Thermal analysis investigation of hydriding properties of nanocrystalline Mg–Ni–and Mg–Fe-based alloys prepared by high-energy ball milling. J. Alloys Compd. 2000, 305, 82–89. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Crivello, J.C.; Dam, B.; Yartys, V.A. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–20. [Google Scholar] [CrossRef]
- Liu, T.; Chen, C.; Qin, C.; Li, X. Improved hydrogen storage properties of Mg-based nanocomposite by addition of LaNi5 nanoparticles. Int. J. Hydrogen Energy 2014, 39, 18273–18279. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V. An overview of hydrogen storage methods. In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Springer: Dordrecht, The Netherland, 2004; pp. 75–104. [Google Scholar]
- Berube, V.; Chen, G.; Dresselhaus, M.S. Impact of nanostructuring on the enthalpy of formation of metal hydrides. Int. J. Hydrog. Energy 2008, 33, 4122–4131. [Google Scholar] [CrossRef]
- Pandey, S.K.; Srivastava, A.; Srivastava, O.N. Improvement in hydrogen storage capacity in LaNi5 through substitution of Ni by Fe. Int. J. Hydrog. Energy 2007, 32, 2461–2465. [Google Scholar] [CrossRef]
- Kazakov, A.N.; Dunikov, D.O.; Mitrokhin, S.V. AB5-type intermetallic compounds for biohydrogen purification and storage. Int. J. Hydrog. Energy 2016, 41, 21774–21779. [Google Scholar] [CrossRef]
- Takeda, H.; Kabutomori, T.; Ohnishi, K. Metal hydride air-conditioning. Encycl. Life Support Syst. 2009, 2, 249–263. [Google Scholar]
- Davids, W.; Lototskyy, M.V.; Linkov, V. Chemical surface modification for the improvement of the hydrogenation kinetics and poisoning resistance of TiFe. J. Alloys Compd. 2011, 509S, S770–S774. [Google Scholar]
- Semboshi, S.; Masahashi, N.; Hanada, S. Degradation of hydrogen absorbing capacity in cyclically hydrogenated TiMn2. Acta Metall. Sin. 2001, 49, 927–935. [Google Scholar] [CrossRef]
- Andreasen, A. Predicting formation enthalpies of metal hydrides. Nature 2001, 414, 353–358. [Google Scholar]
- Chebab, S.; Abdellaoui, M.; Latroche, M.; Paul-boncour, V. LaCaMgNi9 synthesized by mechanical alloying: Structural and electrochemical characterization. J. Tunis. Chem. Soc. 2016, 18, 52–59. [Google Scholar]
- Young, K. Increase in the Surface Catalytic Ability by Addition of Palladium in C14 Metal Hydride Alloy. Batteries. 2017, 3, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ren, H.; Ding, X.; Liu, X.; Chen, L. An investigation on hydrogen storage kinetics of nanocrystalline and amorphous Mg2Ni1−xCox(x = 0 – 0.4) alloy prepared by melt spinning. J. Alloys Compd. 2011, 509, 2808–2814. [Google Scholar] [CrossRef]
- Simchi, H.; Kaflou, A.; Simchi, A. Synergetic effect of Ni and Nb2O5 on dehydrogenation properties of nanostructured MgH2 synthesized by high-energy mechanical alloying. Int. J. Hydrog. Energy 2009, 34, 7724–7730. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suñol, J.J.; Fort, J. Materials developed by mechanical alloying and melt spinning. Int. Rev. Phys. 2008, 2, 31–35. [Google Scholar]
- Scudino, S.; Sakaliyska, M.; Surreddi, K.B. Mechanical alloying and milling of Al-Mg alloys. J. Alloys Compd. 2009, 483, 2–7. [Google Scholar] [CrossRef]
- Poondi, D.; Singh, J. Synthesis of metastable silver-nickel alloys by a novel laser-liquid-solid interaction technique. J. Mater. Res. 2000, 35, 2467–2476. [Google Scholar]
- Xing, N.; Wu, Y.; Zhou, S. Improved hydrogenation-dehydrogenation characteristics of nanostructured melt-spun Mg-10Ni-2Mm alloy processed by rapid solidification. Prog. Nat. Sci. Mater. Int. 2010, 20, 49–53. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Qin, F.X.; Zhu, M. The hydrogen storage behavior of Mg3La and Mg3LaNi0.1. Scr. Mater. 2006, 55, 1075–1078. [Google Scholar] [CrossRef]
- Ares, J.R.; Cuevas, F. Influence of thermal annealing on the hydrogenation properties of mechanically milled AB5-type alloys. Mater. Sci. Eng. 2004, 108, 76–80. [Google Scholar] [CrossRef]
- Guevara, L.; Welsh, R.; Atwater, M.A. Parametric Effects of Mechanical Alloying on Carbon Nanofiber Catalyst Production in the Ni-Cu System. Metals (Basel) 2018, 8, 286. [Google Scholar] [CrossRef]
- Shang, C.X.; Bououdina, M.; Guo, Z.X. Mechanical alloying and electronic simulations of (MgH2 + M) systems (M = Al, Ti, Fe, Ni, Cu and Nb) for hydrogen storage. Int. J. Hydrog. Energy 2004, 29, 73–80. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Strrm-olsen, J.O.; Schulz, R. Catalytic effect of Pd on hydrogen absorption in mechanically alloyed Mg2Ni, LaNi5 and FeTi. J. Alloys Compd. 1995, 217, 295–300. [Google Scholar] [CrossRef]
- Iturbe-garcía, J.L.; García-núñez, M.R.; López-muñoz, B.E. Synthesis of the Mg2Ni Alloy Prepared by Mechanical Alloying Using a High Energy Ball Mill. J. Mex. Chem. Soc. 2010, 54, 46–50. [Google Scholar] [CrossRef]
- Kumar, S.; Tiwari, G.P.; Krishnamurthy, N. High performance FeTi-3.1 mass% V alloy for on board hydrogen storage solution. Energy 2014, 75, 520–524. [Google Scholar] [CrossRef]
- Andreasen, A. Hydrogenation properties of Mg–Al alloys. Int. J. Hydrog. Energy 2008, 33, 7489–7497. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostrctured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Friedrichs, O.; Kolodziejczyk, L.; Fernandez, A. Synthesis of nanocrystalline MgH2 powder by gas-phase condensation and in situ hydridation: TEM, XPS and XRD study. J. Alloys Compd. 2007, 435, 721–724. [Google Scholar] [CrossRef]
- Lopez-Suarez, A. Effect of absorption and desorption of hydrogen in Ti and Ti alloys. In New Advances in Hydrogenation Processes: Fundamental and Applications; Iran Polymer and Petrochemical Institute: Teheran, Iran, 2017. [Google Scholar] [CrossRef]
- Parker, S.F.; Konrad, M.; Wieland, S.D. The effect of particle size, morphology and support commercial catalysts. Chem. Sci. 2019, 10, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Mustafa, N.S.; Yap, A.H. Catalytic effect of CeCl3 on the hydrogen storage properties of MgH2. Mater. Chem. Phys. 2016, 170, 77–82. [Google Scholar] [CrossRef]
- Lototskyy, M.; Davids, M.W.; Pollet, B.G. Magnesium-based hydrogen storage nanomaterials prepared by high energy reactive ball milling in hydrogen at the presence of mixed titanium—iron oxide. J. Alloys Compd. 2015, 645, S454–S459. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Schulz, R. Hydriding behavior of Mg–Al and leached Mg–Al compounds prepared by high-energy ball-milling. J. Alloys Compd. 2000, 297, 282–293. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH 2–V nanocomposite. J. Alloys Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]
- Huang, H.X.; Huang, K.L.; Liu, S.Q. Microstructures and electrochemical properties of Mg0.9Ti0.1Ni1-xMx (M = Co, Mn; x = 0, 0.1, 0.2) hydrogen storage alloys. Powder Technol. 2010, 198, 144–148. [Google Scholar] [CrossRef]
- Vermeulen, P.; Niessen, R.A.H.; Notten, P.H.L. Hydrogen storage in metastable MgyTi(1-y) thin films. Electrochem. Commun. 2006, 8, 27–32. [Google Scholar] [CrossRef]
- Luo, W. (LiNH2-MgH2): A viable hydrogen storage system. J. Alloys Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Castro, F.J.; Fuster, V.; Urretavizcaya, G. Hydrogen sorption properties of a MgH2-10wt. % graphite mixture. J. Alloys Compd. 2011, 509, S595–S598. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Qi, W.B.Y. A Comparison Study of Hydrogen Storage Thermodynamics and Kinetics of YMg11Ni Alloy Prepared by Melt Spinning and Ball Milling. Acta Metall. Sin. 2017, 30, 1040–1048. [Google Scholar] [CrossRef]
- Imamura, H.; Hashimoto, Y.; Sakata, Y. Preparation and Properties of Ball-Milled MgH2/Al Nanocomposites for Hydrogen Storage. Mater. Trans. 2014, 55, 572–576. [Google Scholar] [CrossRef]
- CRYSTMET Database, version 5.0.0; Toth Information Systems Inc.: Ottawa, ONT, Canada, 2013.
- Lototskyy, M.; Wafeeq, M.; Yartys, V.A. Nanostructured hydrogen storage materials prepared by high-energy reactive ball milling of magnesium and ferrovanadium. Int. J. Hydrog. Energy 2019, 44, 6687–6701. [Google Scholar] [CrossRef]
- Williams, M.; Lototskyy, M.V.; Pollet, B.G. Hydrogen absorption study of high-energy reactive ball milled Mg composites with palladium additives. J. Alloys Compd. 2013, 580, s144–s148. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pandey, S.K.; Srivastava, O.N. Catalytic effect of carbon nanostructures on the hydrogen storage properties of MgH2-NaAlH4 composite. Int. J. Hydrog. Energy 2014, 39, 14240–14246. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2. J. Energy Chem. 2019, 28, 46–53. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. J. Alloys Compd. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Juahir, N.; Mustafa, N.S.; Sinin, A.M. Improved hydrogen storage properties of MgH2 by addition of Co2NiO nanoparticles. RSC Adv. 2015, 5, 60983–60989. [Google Scholar] [CrossRef]
- Malka, I.E.; Pisarek, M.; Bystrzycki, J. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH2 sorption properties. Int. J. Hydrog. Energy 2011, 36, 12909–12917. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Cheng, H.M. Hydrogen storage properties of MgH2/SWNT composite prepared by ball milling. J. Alloys Compd. 2006, 420, 278–282. [Google Scholar] [CrossRef]
- El-eskandarany, M.S.; Shaban, E.; Al-duweesh, A. Superior catalytic effect of nanocrystalline big-cube Zr2 Ni metastable phase for improving the hydrogen sorption / desorption kinetics and cyclability of MgH2 powders. Energy 2015, 91, 274–282. [Google Scholar] [CrossRef]
- Santos, S.F.; Ishikawa, T.T.; Huot, J. MgH2 + FeNb nanocomposites for hydrogen storage. Mater. Chem. Phys. 2014, 147, 557–562. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage in mechanically milled Mg–LaNi5 and MgH2–LaNi5 composites. J. Alloys Compd. 2000, 297, 261–265. [Google Scholar] [CrossRef]
- Chen, X.Q.; Podloucky, R.; Rogl, P. Computational and experimental study of phase stability, cohesive properties, magnetism and electronic structure of TiMn2. Acta Mater. 2003, 51, 1239–1247. [Google Scholar] [CrossRef]
- Erika, T.; Sebastian, C.; Fernando, Z. Temperature performance of AB5 hydrogen storage alloy for Ni-MH batteries. Int. J. Hydrog. Energy 2016, 41, 19684–19690. [Google Scholar] [CrossRef]
- Hayakawa, H.; Akiba, E.; Kohno, T. Crystal Structures of La–Mg–Nix (x = 3-4) System Hydrogen Storage Alloys. Mater. Trans. 2005, 46, 1393–1401. [Google Scholar] [CrossRef][Green Version]
- Boeije, M.F.J.; Delczeg-czirjak, E.K.; Brück, E. On the phase stability of CaCu 5 -type compounds. J. Alloys Compd. 2017, 722, 549–554. [Google Scholar] [CrossRef]
- Singh, A.; Singh, B.K.; Srivastava, O.N. Studies on improvement of hydrogen storage capacity of AB5 type: MmNi4.6Fe0.4 alloy. Int. J. Hydrog. Energy 2004, 29, 1151–1156. [Google Scholar]
- Roberts, M.; Smith, R.I.; Hull, S. In situ investigation of commercial Ni(OH)2 and LaNi5-based electrodes by neutron powder diffraction. J. Mater. Res. 2015, 30, 407–416. [Google Scholar]
- Cermak, J.; David, B. Catalytic effect of Ni, Mg2Ni and Mg2NiH4 upon hydrogen desorption from MgH2. Int. J. Hydrog. Energy 2011, 6, 3–9. [Google Scholar] [CrossRef]
- Khajavi, S.; Rajabi, M.; Huot, J. Effect of cold rolling and ball milling on first hydrogenation of Ti0.5Zr0.5 (Mn1-xFex) Cr1, x = 0, 0.2, 0.4. J. Alloys Compd. 2019, 775, 912–920. [Google Scholar] [CrossRef]
- Gkanas, E.I.; Khzouz, M.; Makridis, S.S. Synthesis and Hydrogen Sorption Characteristics of Mechanically Alloyed Mg(NixMn1-x)2 Intermetallics. Mater. Sci. 2017, 12, 257–268. [Google Scholar]
- Srivastava, S.; Panwar, K. Effect of transition metals on ball-milled MmNi5 hydrogen storage alloy. Mater. Renew. Sustain. Energy 2015, 4, 19. [Google Scholar] [CrossRef]
- Broz, M.E.; Cook, R.F.; Whitney, D.L. Microhardness, toughness, and modulus of Mohs scale minerals. Am. Mineral. 2006, 91, 2006. [Google Scholar] [CrossRef]
- Chen, Y.; Sequeira, C.A.C.; Song, X.; Neto, R.; Wang, Q. Polytypism of La–Ni phases in multicomponent AB5 type hydride electrode alloys. Int. J. Hydrog. Energy 2002, 27, 63–68. [Google Scholar] [CrossRef]
- Dobrovolsky, V.D. The correlation between ionicity of metal-hydrogen bonds in hydrides and their thermal firmness. In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Springer: Dordrecht, The Netherlands, 2007; pp. 421–428. [Google Scholar]
- Joseph, B.; Schiavo, B. Effects of ball-milling on the hydrogen sorption properties of LaNi5. J. Alloys Compd. 2009, 480, 912–916. [Google Scholar] [CrossRef]
- Koultoukis, E.D.; Christodoulou, C.N.; Fruchart, D. High-Temperature Activated AB2 Nanopowders for Metal Hydride Hydrogen Compression. Int. J. energy Res. 2014, 38, 477–486. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability. Intermetallics 2004, 12, 713–720. [Google Scholar] [CrossRef]
- Hammerschmidt, T.; Ladines, A.N.; Drautz, R. Crystal-Structure Analysis with Moments of the Density-of-States: Application to Intermetallic Topologically Close-Packed Phases. Crystals 2016, 6, 18. [Google Scholar] [CrossRef]
- Ioannidou, A.; Makridis, S.; Kikkinides, E.S. Structural and Hydrogenation Properties of Zr0.9Ti0.1Cr1.2-xV0.8Nix (x=0, 0.4) Compounds. Mater. Sci. Forum. 2010, 636, 26–31. [Google Scholar]
- Liu, P.; Xie, X.; Liu, T. Hydrogen storage properties of (Ti0.85Zr0.15)1.05Mn1.2Cr0.6V0.1M0.1 (M=Ni, Fe, Cu) alloys easily activated at room temperature. Prog. Nat. Sci. Mater. Int. 2017, 27, 652–657. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Zhao, D. Hydrogen storage kinetics of nanocrystalline and amorphous Cu−Nd-added Mg2Ni-type alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 3524–3533. [Google Scholar] [CrossRef]
- Liu, J.; Song, X.P.; Chen, G.L. Hydrogen storage performance of Mg-based composites prepared by spark plasma sintering. J. Alloys Compd. 2009, 486, 338–342. [Google Scholar] [CrossRef]
- Becker, H. Processing of bulk Al7075 alloy by spark plasma sintering. Mater. Sci. Eng. 2017, 179, 2050. [Google Scholar]
- Pei, P.; Song, X.; Liu, J.; Song, A.; Zhang, P.; Chen, G. Study on the hydrogen desorption mechanism of a Mg-V composite prepared by SPS. Int. J. Hydrog. Energy 2011, 37, 984–989. [Google Scholar] [CrossRef]
- Huang, H.; Huang, K. Effect of Fluorination Treatment on Electrochemical Properties of M1Ni3.5 Co0.6Mn0.4Al0.5 Hydrogen Storage Alloy. J. Braz. Chem. Soc. 2012, 23, 951–957. [Google Scholar]
- Schwars, R.B.; Johnson, W.L. Formation of an amorphous alloy by solid-state reaction of the pure polycrystalline metals. Phys. Rev. Lett. 1983, 51, 415. [Google Scholar] [CrossRef]
- Termsuksawad, P.; Niyomsoan, S.; Gavra, Z. Measurement of hydrogen in alloys by magnetic and electronic techniques. J. Alloys Compd. 2004, 373, 86–95. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Milovzorov, G.S.; Kaloshkin, S.D. Preparation and hydrogen storage properties of nanocrystalline TiFe synthesized by mechanical alloying. Prog. Nat. Sci. Mater. Int. 2017, 27, 149–155. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Song, X.; Zhang, P.; Chen, G. The role of spark plasma sintering on the improvement of hydrogen storage properties of Mg-based composites. Int. J. Hydrog. Energy 2010, 35, 8080–8087. [Google Scholar] [CrossRef]
- Pei, P.; Song, X.P.; Chen, G.L. Improving hydrogen storage properties of Laves phase related BCC solid solution alloy by SPS preparation method. Int. J. Hydrog. Energy 2009, 34, 8597–8602. [Google Scholar] [CrossRef]
- Lv, P.; Guzik, M.N.; Huot, J. Effect of ball milling and cryomilling on the microstructure and first hydrogenation properties of TiFe + 4 wt.% Zr alloy. J. Mater. Res. Technol. 2019, 10, 1016. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Zhang, L.X.; Wang, Q.Q. Crystallization behavior and hydrogen storage kinetics of amorphous Mg11Y2Ni2 alloy. J. Alloys Compd. 2013, 551, 376–381. [Google Scholar] [CrossRef]
- Zhang, D.Z.Y.; Li, B.; Ren, H.; Li, X.; Qi, T. Enhanced Hydrogen Storage Kinetics of Nanocrystalline and Amorphous Mg2Ni-type Alloy by Melt Spinning. Materials (Basel) 2011, 4, 274–287. [Google Scholar] [CrossRef]
- Wu, Y.; Lototsky, M.V.; Yartys, V.A. Microstructure and hydrogenation behavior of ball-milled and melt-spun Mg–10Ni–2Mm alloys. J. Alloys Compd. 2008, 466, 176–181. [Google Scholar] [CrossRef]
- Lass, E.A. Hydrogen storage measurements in novel Mg-based nanostructured alloys produced via rapid solidification and devitrification. Int. J. Hydrog. Energy 2011, 36, 10787–10796. [Google Scholar] [CrossRef]
- Modibane, K.D.; Williams, M.; Lototskyy, M. Poisoning-tolerant metal hydride materials and their application for hydrogen separation from CO2/CO containing gas mixtures. Int. J. Hydrog. Energy 2013, 38, 9800–9810. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares Fernandez, J.R.; Klassen, T. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrog. Energy 2007, 32, 221–233. [Google Scholar]
- Williams, M.; Nechaev, A.N.; Lototskyy, M.V. Influence of aminosilane surface functionalization of rare earth hydride-forming alloys on palladium treatment by electroless deposition and hydrogen sorption kinetics of composite materials. Mater. Chem. Phys. 2009, 115, 136–141. [Google Scholar] [CrossRef]
- Yeung, K.L.; Christiansen, S.C.; Varma, A. Palladium composite membranes by electroless plating technique Relationships between plating kinetics, film microstructure and membrane performance. J. Memb. Sci. 1999, 159, 107–122. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef]
- Charbonnier, M.; Romand, M.; Goepfert, Y. Palladium reduction: A key step for the electroless Ni metallization of insulating substrates by a tin-free process. Thin Solid Film. 2006, 515, 1623–1633. [Google Scholar] [CrossRef]
- Shan, X.; Payer, J.H.; Jennings, W.D. Mechanism of increased performance and durability of Pd-treated metal hydriding alloys. Int. J. Hydrog. Energy 2009, 34, 363–369. [Google Scholar] [CrossRef]
- Denys, R.V.; Lototskyy, M.V.; Linkov, V.M.; Williams, M. Palladium mixed-metal surface-modified AB5-type intermetallides enhance hydrogen sorption kinetics. S. Afr. J. Sci. 2010, 106, 1–6. [Google Scholar]
- Lototskyy, M.V.; Williams, M.; Yartys, V.A. Surface-modified advanced hydrogen storage alloys for hydrogen separation and purification. J. Alloys Compd. 2011, 509, 555–561. [Google Scholar] [CrossRef]
- Davids, W.; Lototskyy, M.V.; Williams, M. Surface modification of TiFe hydrogen storage alloy by metal-organic chemical vapour deposition of palladium. Int. J. Hydrog. Energy 2011, 36, 9743–9750. [Google Scholar] [CrossRef]
- Pasquini, L.; Callini, E.; Maurizio, C. Magnesium nanoparticles with transition metal decoration for hydrogen storage. J. Nanoparticle Res. 2011, 13, 5727–5737. [Google Scholar] [CrossRef]
- Mark, I.; Doyle, L.; Benjamin, D. Hydrogen storage materials. United States Pat. 2000, 6, 165. [Google Scholar]
- Modibane, K.D.; Lototskyy, M.; Davids, M.W.; Williams, M.; Hato, M.J.; Molapo, K.M. Influence of co-milling with palladium black on hydrogen sorption performance and poisoning tolerance of surface modified AB5-type hydrogen storage alloy. J. Alloys Compd. 2018, 750, 523–529. [Google Scholar] [CrossRef]
- Ceramics, M.; Skorokhod, V.V.; Klimenko, V.P. Reversable hydriding of LaNi5−xAlx−Pd composite in the presence of carbon monoxide. Powder Metall. Met. Ceram. 2001, 39, 575–583. [Google Scholar]
- Willey, D.B.; Pederzolli, D.; Harris, I.R. Low temperature hydrogenation properties of platinum group metal treated, nickel metal hydride electrode alloy. J. Alloys Compd. 2002, 332, 806–809. [Google Scholar] [CrossRef]
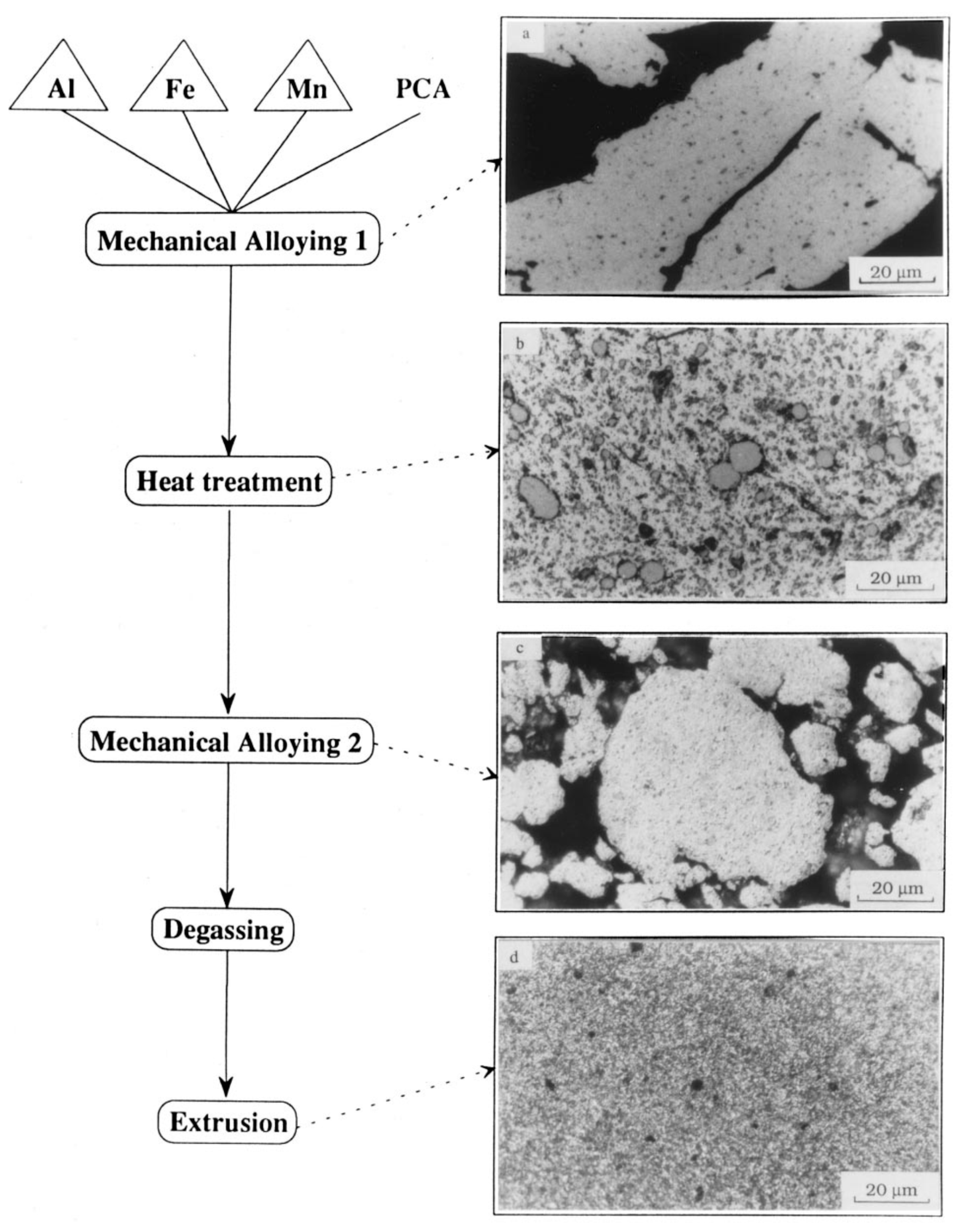
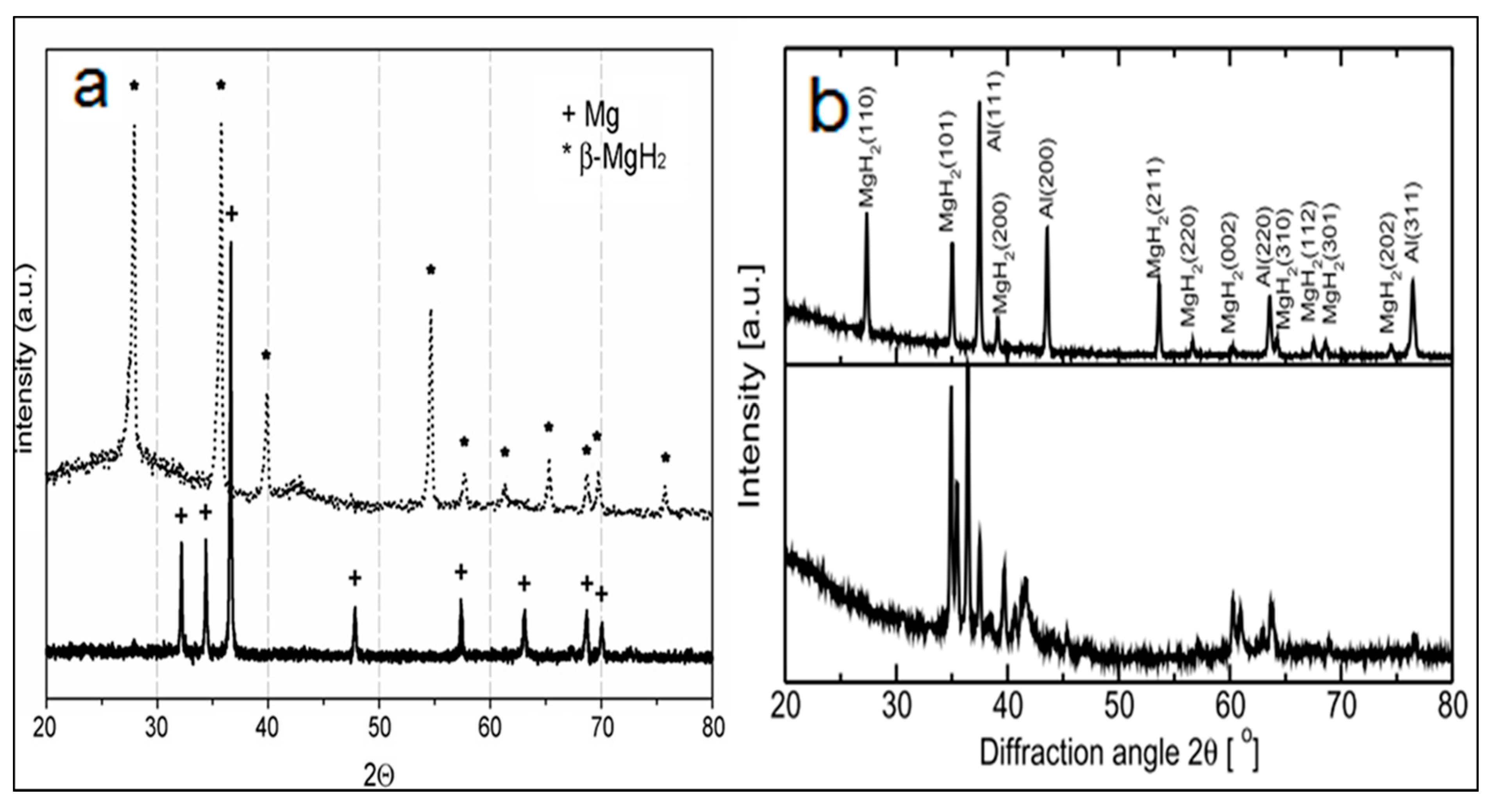

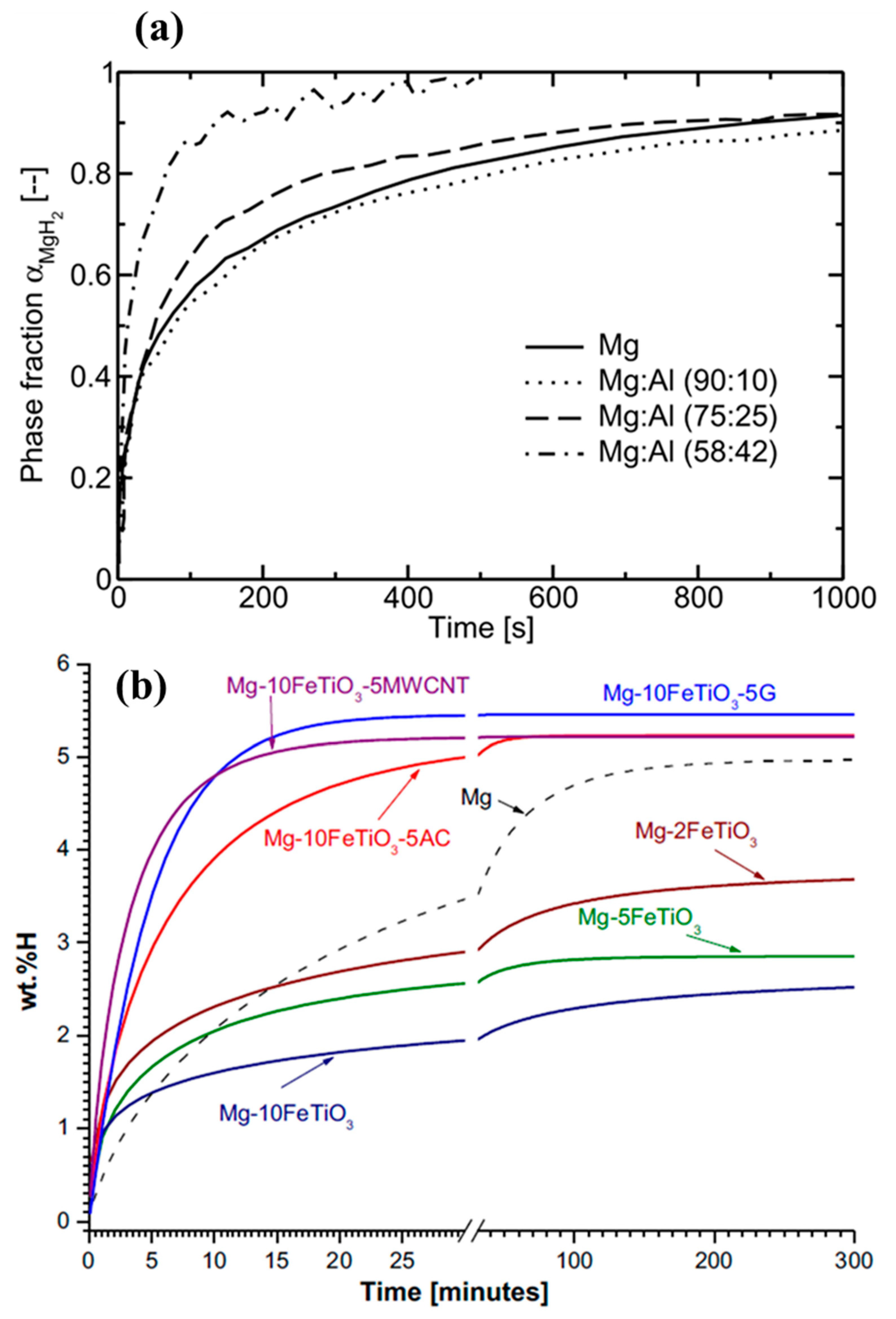
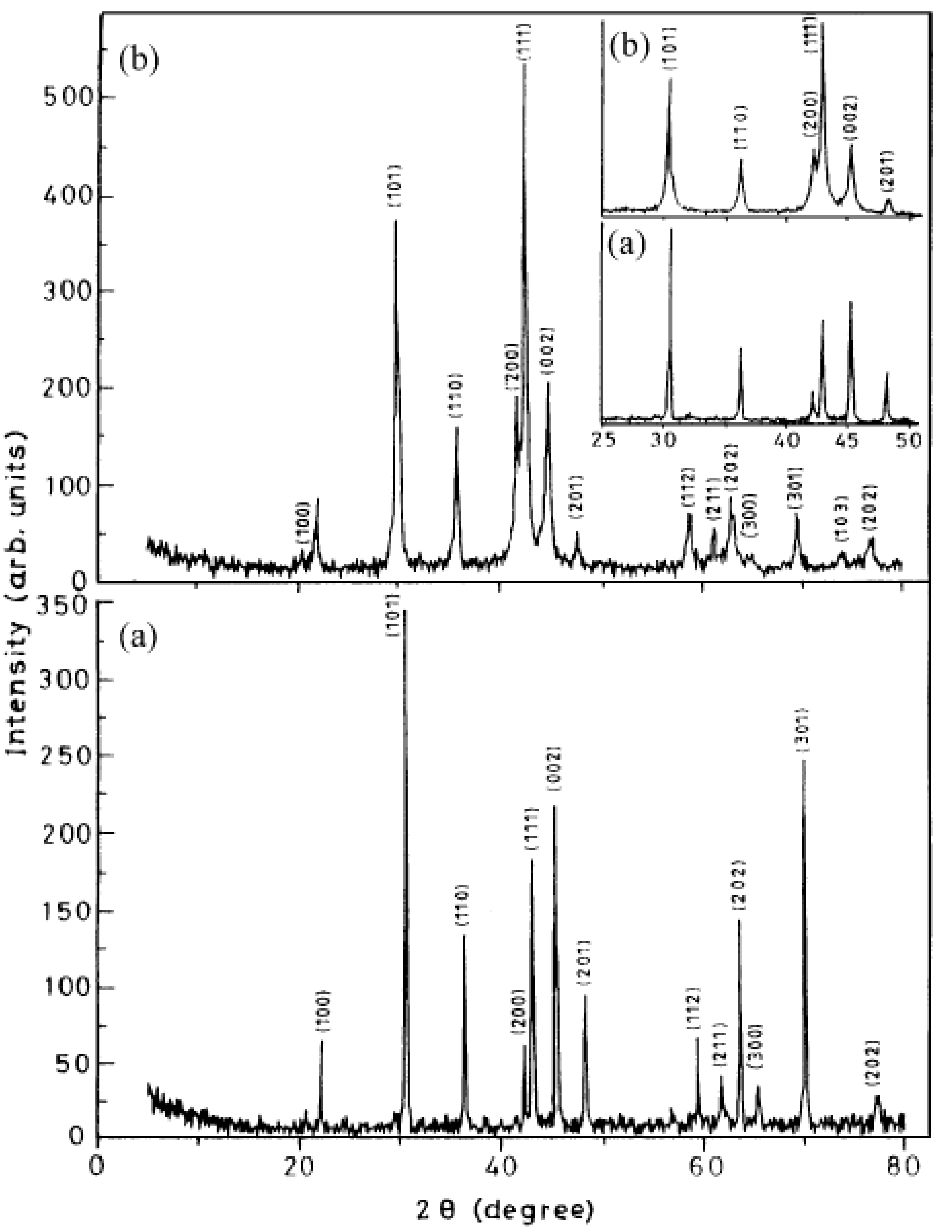

| Type of Alloy | Structure | Alloy Lattice Parameter, a, Å | Hydride Lattice Parameter, a, Å | Storage Capacity, wt. % | References |
|---|---|---|---|---|---|
| AB | BCC | 2.976 | 7.029 | 1.75 | [12] |
| AB2 | C14 | 4.866 | 4.902 | 1.70 | [13] |
| C15 | 6.939 | 7.158 | 2.01 | [11] | |
| AB5 | CaCu5 | 5.003 | 5.395 | 1.43 | [10] |
| A2B | (P6222) | 5.205 | 5.463 | 3.75 | [11] |
| (Fddd) | 5.284 | 5.411 | 3.04 | [11] |
| Catalyst Family | Catalyst Additive | ΔHdes, kJ/mol H2 | Hydriding Kinetics, min−1 | Hydrogen Capacity, wt. % | References |
|---|---|---|---|---|---|
| No catalyst | Undoped MgH2 | 76 | 1.4 × 10−3 | 7.66 | [52,57] |
| Metal | MgH2+10 wt. % Ni | 75 | – | 6.89 | [49] |
| MgH2+10 wt. % Co | 71 | – | 5.62 | [49] | |
| Metal halides | MgH2+10 wt. % CeCl3 | 75.7 | 0.05 | 6.32 | [36] |
| MgH2+NbF5 | 79.7 | 3.42 | 6.40 | [53] | |
| MgH2+ZrF4 | 76.4 | 3.2 | 6.44 | [53] | |
| Hydrides | MgH2+NaAlH4 | – | 0.28 | 3.10 | [49] |
| Metal oxide | MgH2+SrTiO3 | – | 0.02 | 6.63 | [50] |
| MgH2+1 mol. % Nb2O5 | 74 | 0.22 | 5.45 | [51] | |
| Nano-sized alloys | MgH2+10 wt. % Zr2Ni | – | 0.52 | 5.12 | [55] |
| MgH2+FeNb | – | 0.35 | 5.55 | [56] | |
| Carbon materials | MgH2+5 wt. %SWNT | – | 1.34 | 6.72 | [54] |
| Mg-based alloys | ||||||
|---|---|---|---|---|---|---|
| Alloy type | Alloy material | Milling conditions (rotation speed and milling time) | Particle size distribution, µm | Hydriding kinetics, min−1 | Hydrogen capacity, wt. % | References |
| As-cast alloy | Mg | _ | 48–70 | 1.4 × 10−3 | 7.66 | [57] |
| As-milled alloys | Mg Mg-10FeTiO3 Mg-10FeTiO3-5MWCNT | 500 rpm for 120 min 500 rpm for 120 min 500 rpm for 120 min | <0.05 <0.05 <0.05 | 0.04 0.07 0.31 | 4.98 2.71 5.22 | [37] |
| Mg-10(FeV) Mg-10(FeV)-5MWCNT | 500 rpm, for 300 min 500 rpm, for 300 min | 0.001–0.01 ~0.01 | 0.02 0.02 | 6.96 6.69 | [47] | |
| Mg-0.5Pd Mg-5Pd | 500 rpm, for 360 min 500 rpm, for 360 min | 0.05–0.1 <0.01 | 0.04 0.02 | 5.13 5.56 | [48] | |
| AB2-type alloys | ||||||
| Alloy type | Alloy material | Milling conditions (rotation speed and milling time) | Particle size distribution, µm | Hydriding kinetics, min−1 | Hydrogen capacity, wt. % | References |
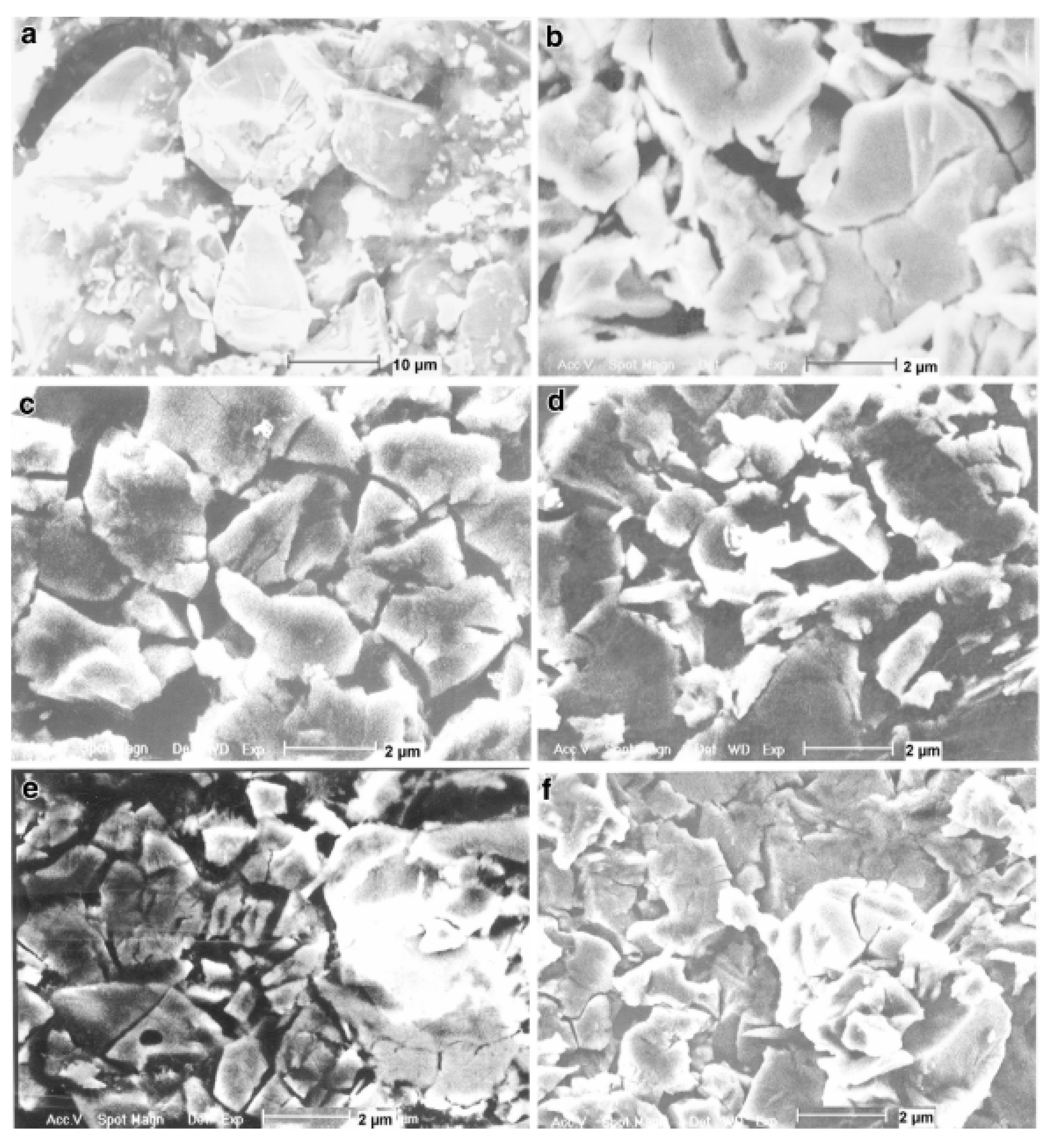
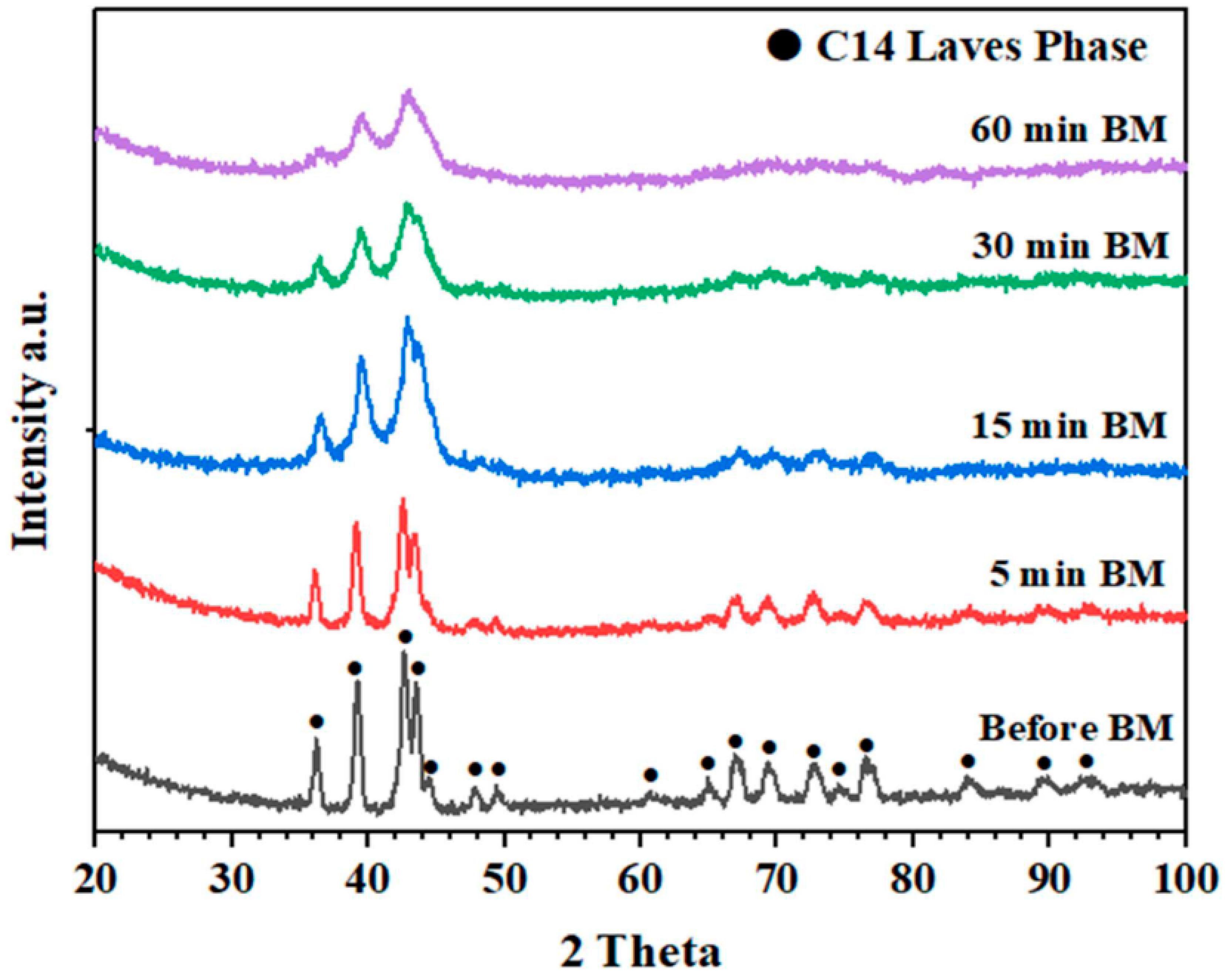
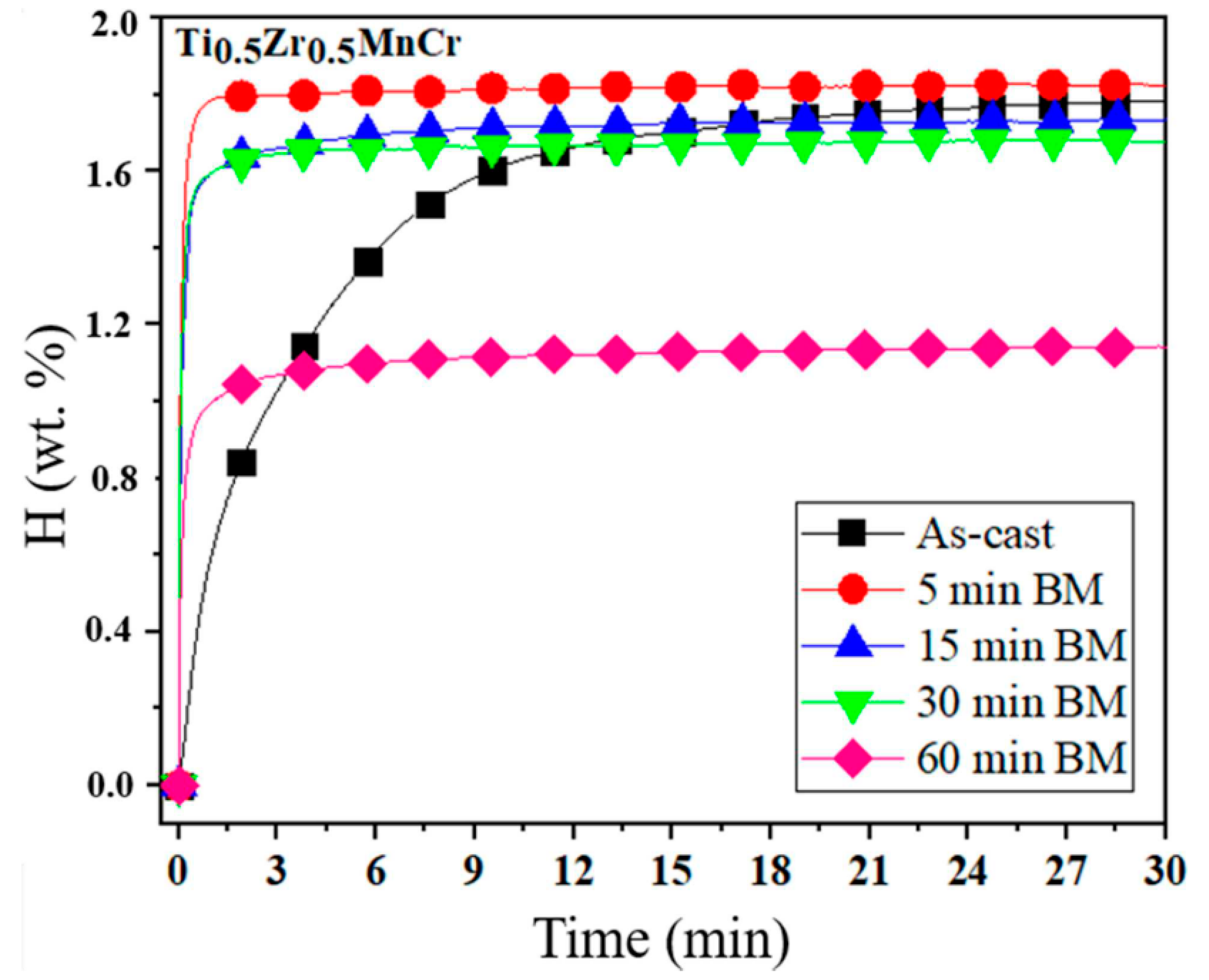
| As-cast alloy | Ti0.5Zr0.5MnCr | _ | 15–29 | 0.04 | 1.79 | [65] |
| As-milled alloys | Ti0.5Zr0.5MnCr | Milled for 5 min Milled for 30 min Milled for 60 min | ~8 3–6 0.5–1.7 | 0.13 0.05 0.06 | 1.82 1.65 1.15 | |
| As-milled alloys | Mg(Ni0.75Mn0.25)2 Mg(Ni0.1Mn0.9)2 MgMn2 | 350 rpm for 600 min 350 rpm for 600 min 350 rpm for 600 min | <10 | 0.25 0.14 0.1 | 0.43 0.93 1.21 | [66] |
| AB5-type alloys | ||||||
| Alloy type | Alloy material | Milling conditions (rotation speed and milling time) | Particle size distribution, µm | Hydriding kinetics, min−1 | Hydrogen capacity, wt. % | References |
| As-cast alloy | MmNi5 | _ | 5–50 | 0.24 | 1.20 | [67] |
| As-milled alloy | MmNi5 | 200 rpm for 180 min | 1–2 | 0.28 | 1.42 | |
| As-cast alloy | MnNi4.6Fe0.4 | _ | ~9.58 | _ | 1.50 | [62] |
| As-milled alloys | MnNi4.6Fe0.4 | 200 rpm for 10 min 200 rpm for 30 min 300 rpm for 10 min 300 rpm for 30 min | _ ~1.62 _ _ | _ _ _ _ | 2.00 1.82 1.91 1.21 | |
| Mechanical Alloying/Milling | ||||||
|---|---|---|---|---|---|---|
| Material | Rate constant (min−1) | Hydrogen absorption capacity (wt. %) | Conditions | References | ||
| Metal alloy material | As-cast | As-milled | As-cast | As-milled | ||
| Mg2Ni | 0.06 | 0.15 | 2.91 | 2.60 | 473 K, 1.0 MPa | [66] |
| TiFe+4 wt. % Zr (60 min milling) | 9.0 × 10−3 | 0.01 | 1.62 | 1.27 | 273 K, 4.5 MPa | [89] |
| TiFe+4 wt. % Zr (5 min milling) | 9.0 × 10−3 | 8.5 × 10−3 | 1.62 | 1.41 | 273 K, 4.5 MPa | [89] |
| Ti0.5Zr0.5MnCr (60 min milling) | 0.04 | 0.05 | 1.75 | 1.05 | 273 K, 2.0 MPa | [65] |
| Ti0.5Zr0.5MnCr (5 min milling) | 0.04 | 0.05 | 1.75 | 1.82 | 273 K, 2.0 MPa | [65] |
| Melt-spinning technique | ||||||
| Material | Rate constant (min−1) | Hydrogen absorption capacity (wt. %) | Conditions | References | ||
| Metal alloy material | As-cast | Melt-spun | As-cast | Melt-spun | ||
| Mg11Y2Ni2 | 0.90 | 0.52 | 3.61 | 3.89 | 523 K, 3.0 MPa | [90] |
| Mg3LaNi0.1 | 0.25 | 0.60 | 2.73 | 2.90 | 573 K, 4.0 MPa | [24] |
| Mg-10Ni-2Mn | 0.01 | 0.02 | 4.67 | 5.09 | 598 K, 1.0 MPa | [23] |
| Mg2Ni0.9Co0.1 | 9.0 × 10−3 | 0.02 | 2.38 | 3.00 | 473 K, 1.5 MPa | [91] |
| Spark plasma sintering | ||||||
| Material | Rate constant (min−1) | Hydrogen absorption capacity (wt. %) | Conditions | References | ||
| Metal alloy material | As-cast | Sintered | As-cast | Sintered | ||
| Mg sintered with 77% V77.8Zr7.4Ti7.4Ni7.4 | 1.7 × 10−3 | 6.7 × 10−3 | 7.26 | 2.52 | 573 K, 3.0 MPa | [87] |
| V35(Ti,Cr)65 sintered with ZrMn2 | 0.01 | 0.04 | 2.86 | 2.89 | 303 K, 0.6 MPa | [88] |
| Mg sintered with 30% ZrMn2 | 1.7 × 10−3 | 3.3 × 10−3 | 7.26 | 6.34 | 573 K, 3.0 MPa | [87] |
| Material | Rate constant, min−1 | Absorption Capacity, wt. % | Conditions | References | ||
|---|---|---|---|---|---|---|
| Alloy material | As-milled | As-spun | As-milled | As-spun | ||
| Mg–10Ni–2Mm | 0.04 | 0.01 | 3.21 | 4.22 | 573 K, 2.0 MPa | [92] |
| YMg11Ni | 0.05 | 0.03 | 4.72 | 4.07 | 593 K, 3.0 MPa | [44] |
| Alloy Type | Metal Alloy | Rate Constant, min−1 | Hydrogen Capacity, wt. % | Hydrogenation Conditions | References |
|---|---|---|---|---|---|
| As-milled | LaNi5 | 0.04 | 5.30 | 313 K, 1.5 MPa | [28] |
| Pd deposition | Pd-LaNi5 | 2.38 | 5.18 | 313 K, 1.5 MPa | |
| As-milled | Mg2Ni | 0.06 | 3.55 | 673 K, 2.0 MPa | [28] |
| Pd deposition | Pd-Mg2Ni | 0.59 | 2.64 | 573 K, 2.0 MPa | |
| As-milled | LaNi4.25Al0.75 | 4.8 × 10−1 | 3.21 | 293 K, 1.5 MPa | [107] |
| Pd deposition | Pd-LaNi4.25Al0.75 | 1.33 | 3.89 | 293 K, 1.5 MPa | |
| As-milled | La0.9Pr0.05Nd0.05Al0.3Mn0.4Co0.65Ni3.5 | 2.4 × 10−3 | 0.45 | 293 K, 0.5 MPa | [108] |
| Pt deposition | Pt-La0.9Pr0.05Nd0.05Al0.3Mn0.4 Co0.65Ni3.5 | 4.5 × 10−2 | 0.83 | 298 K, 0.1 MPa | |
| Ru deposition | Ru-La0.9Pr0.05Nd0.05Al0.3Mn0.4 Co0.65Ni3.5 | 3.3 × 10−2 | 0.34 | 298 K, 0.1 MPa | |
| Pt-Ru co-deposition | Pt-Ru-La0.9Pr0.05Nd0.05Al0.3 Mn0.4Co0.65Ni3.5 | 2.9 × 10−2 | 0.79 | 298 K, 0.1 MPa | |
| As-milled | La0.40Ce0.48(Nd,Pr)0.16Ni3.34Co0.64Al0.63Mn0.58 | 1.6 × 10−4 | 1.24 | 293 K, 0.5 MPa | [101] |
| Pd deposition | Pd-La0.40Ce0.48(Nd,Pr)0.16 Ni3.34Co0.64Al0.63Mn0.58 | 6.2 × 10−4 | 2.75 | 293 K, 0.5 MPa | |
| Pd-Ni co-deposition | Pd-Ni-La0.40Ce0.48(Nd,Pr)0.16 Ni3.34Co0.64Al0.63Mn0.58 | 1.1 × 10−3 | 3.60 | 293 K, 0.5 MPa |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somo, T.R.; Maponya, T.C.; Davids, M.W.; Hato, M.J.; Lototskyy, M.V.; Modibane, K.D. A Comprehensive Review on Hydrogen Absorption Behaviour of Metal Alloys Prepared through Mechanical Alloying. Metals 2020, 10, 562. https://doi.org/10.3390/met10050562
Somo TR, Maponya TC, Davids MW, Hato MJ, Lototskyy MV, Modibane KD. A Comprehensive Review on Hydrogen Absorption Behaviour of Metal Alloys Prepared through Mechanical Alloying. Metals. 2020; 10(5):562. https://doi.org/10.3390/met10050562
Chicago/Turabian StyleSomo, Thabang Ronny, Thabiso Carol Maponya, Moegamat Wafeeq Davids, Mpitloane Joseph Hato, Mykhaylo Volodymyrovich Lototskyy, and Kwena Desmond Modibane. 2020. "A Comprehensive Review on Hydrogen Absorption Behaviour of Metal Alloys Prepared through Mechanical Alloying" Metals 10, no. 5: 562. https://doi.org/10.3390/met10050562
APA StyleSomo, T. R., Maponya, T. C., Davids, M. W., Hato, M. J., Lototskyy, M. V., & Modibane, K. D. (2020). A Comprehensive Review on Hydrogen Absorption Behaviour of Metal Alloys Prepared through Mechanical Alloying. Metals, 10(5), 562. https://doi.org/10.3390/met10050562





