Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium
Abstract
1. Introduction
2. Materials and Methods
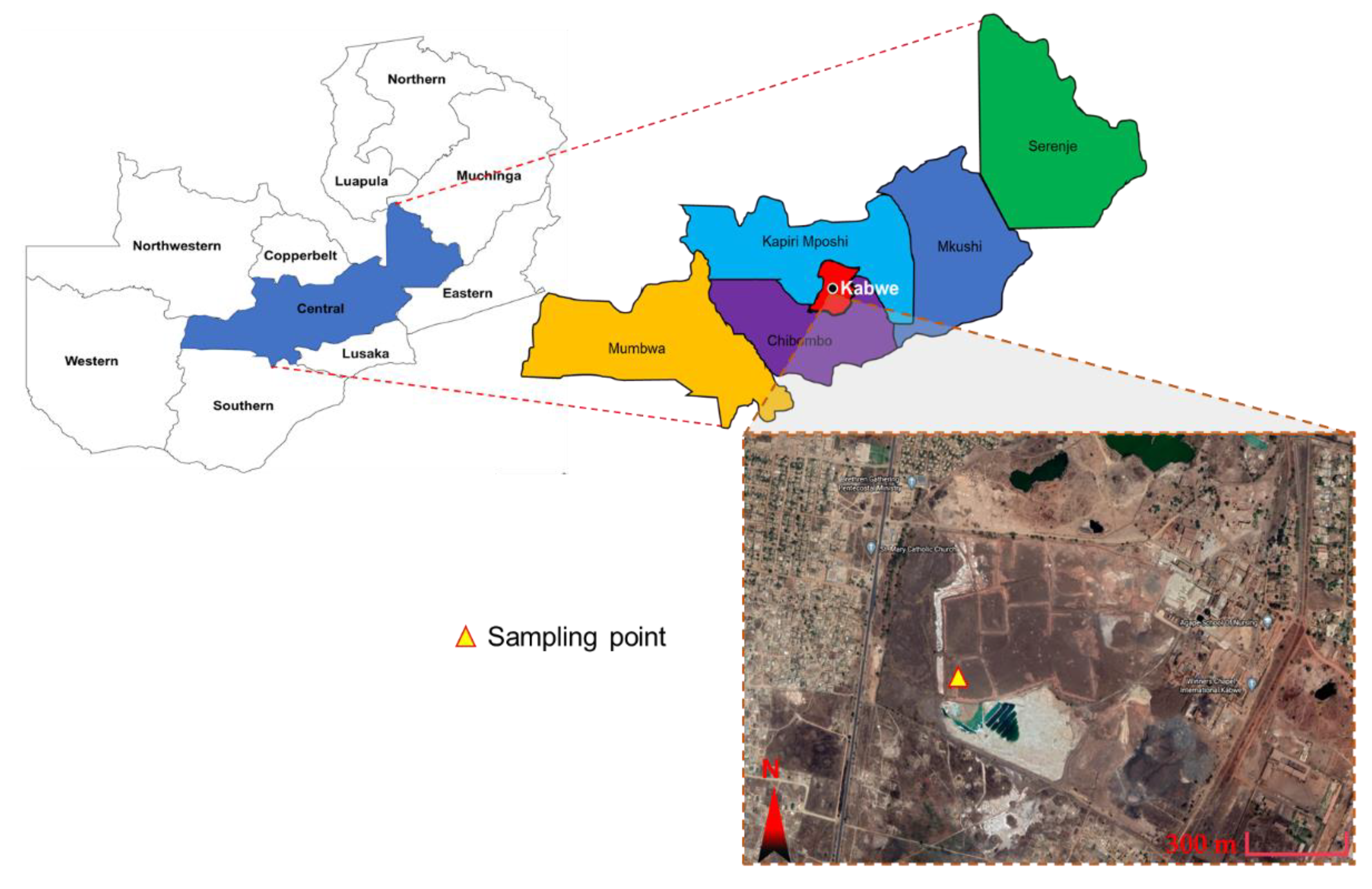
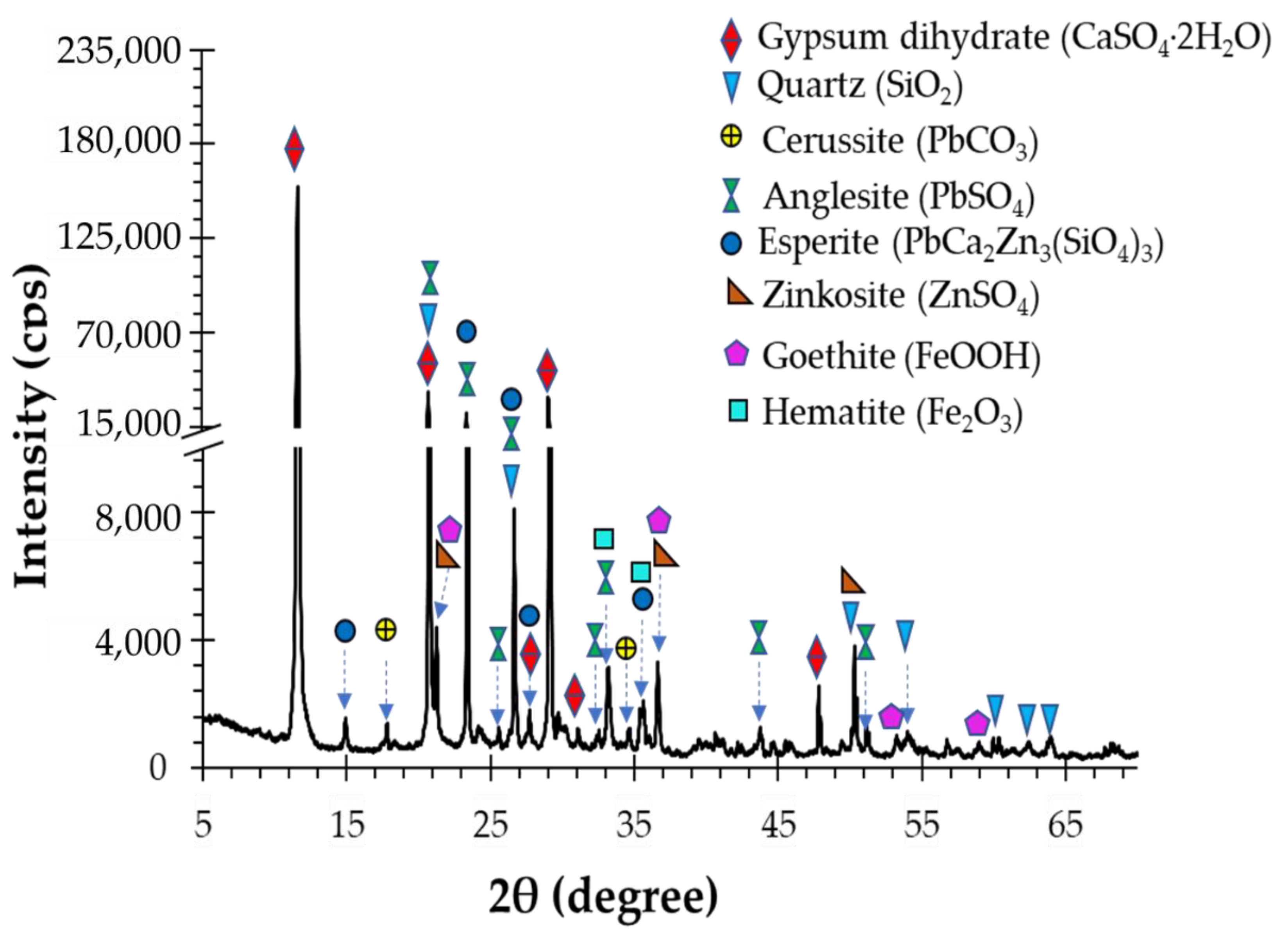
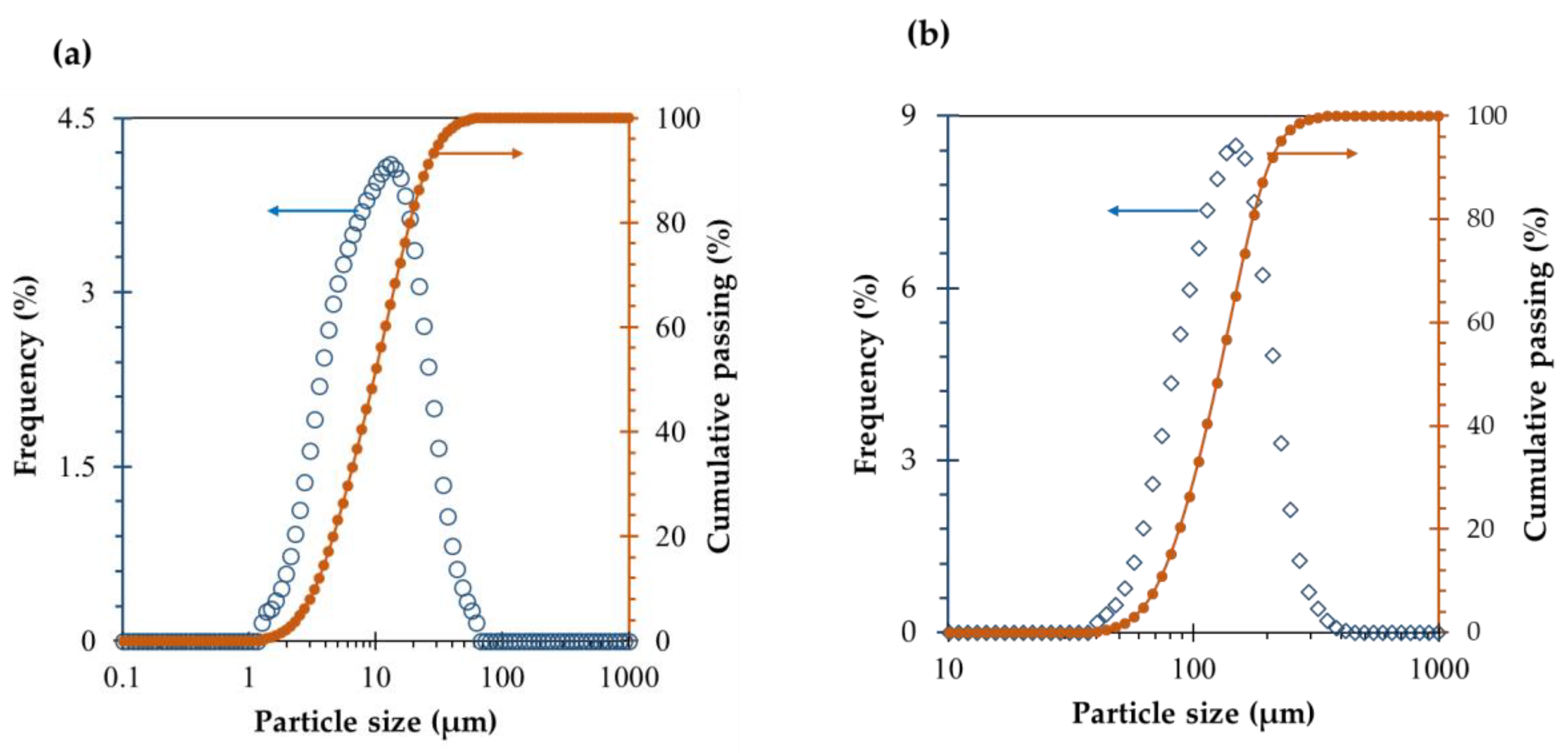
2.1. Materials
2.2. Methods
2.2.1. Leaching-Cementation Experiments in Chloride Solution
2.2.2. Leachability of Lead and Zinc after Concurrent Dissolution-Cementation
3. Results and Discussion
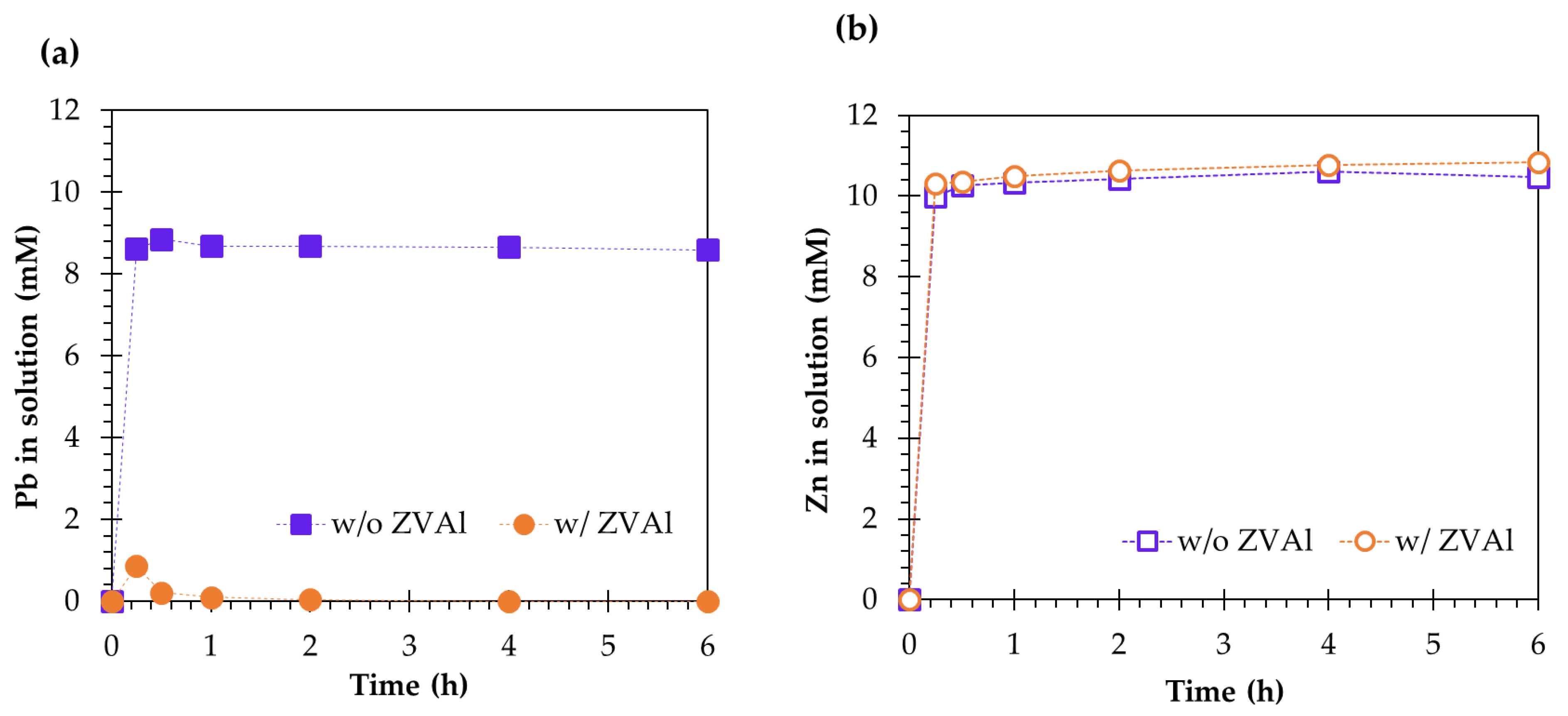
3.1. Concurrent Dissolution-Cementation of Pb and Zn from Zinc Plant Leach Residues
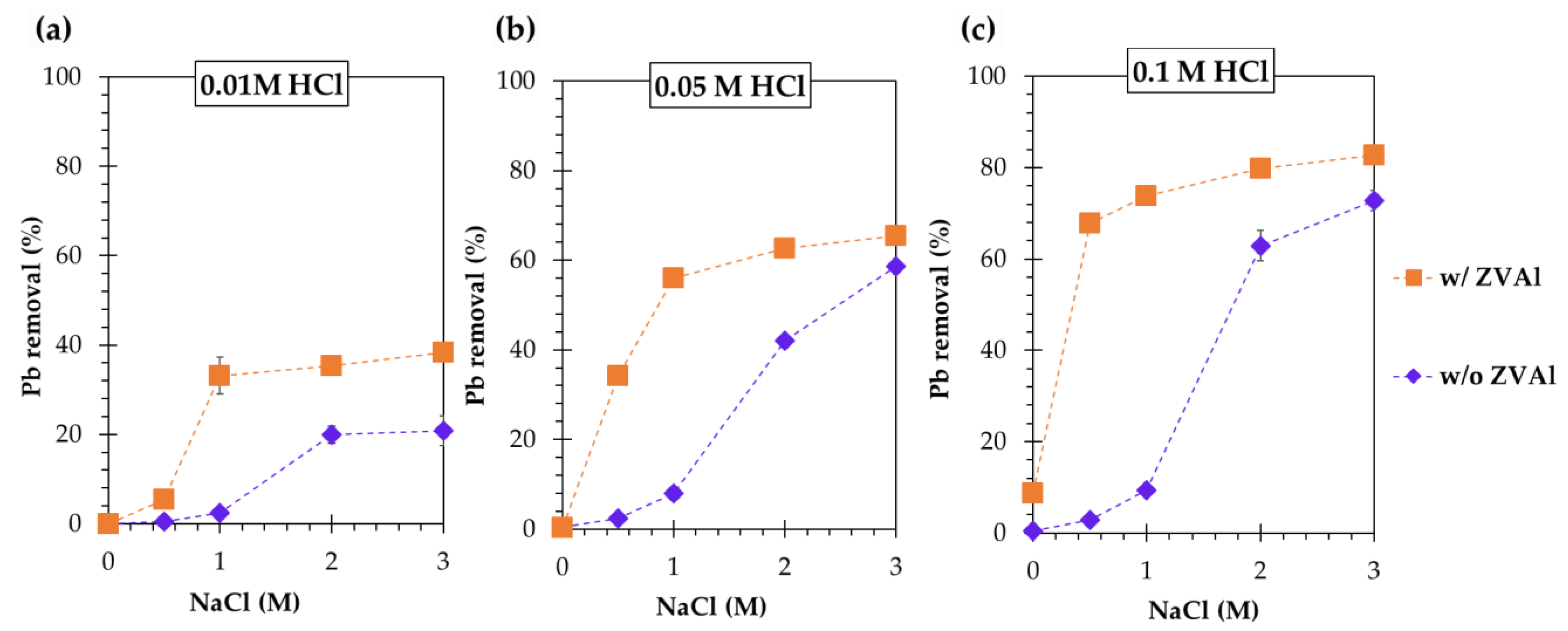
3.2. Effects of Solution Composition on Pb and Zn Removal from Zinc Plant Leach Residues
3.3. Leachability of Lead and Zinc after Concurrent Dissolution-Cementation
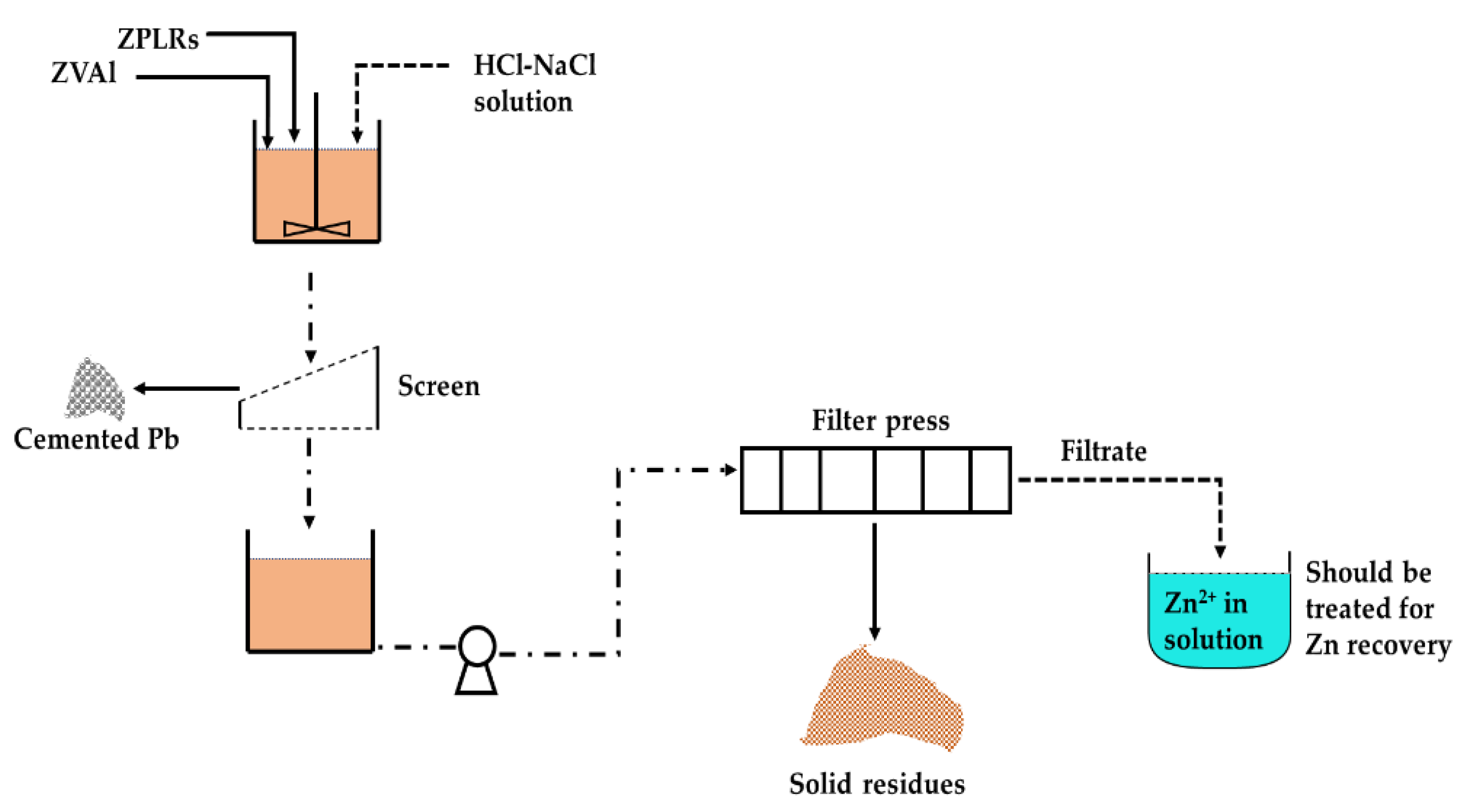
3.4. Conceptual Flowsheet
4. Conclusions
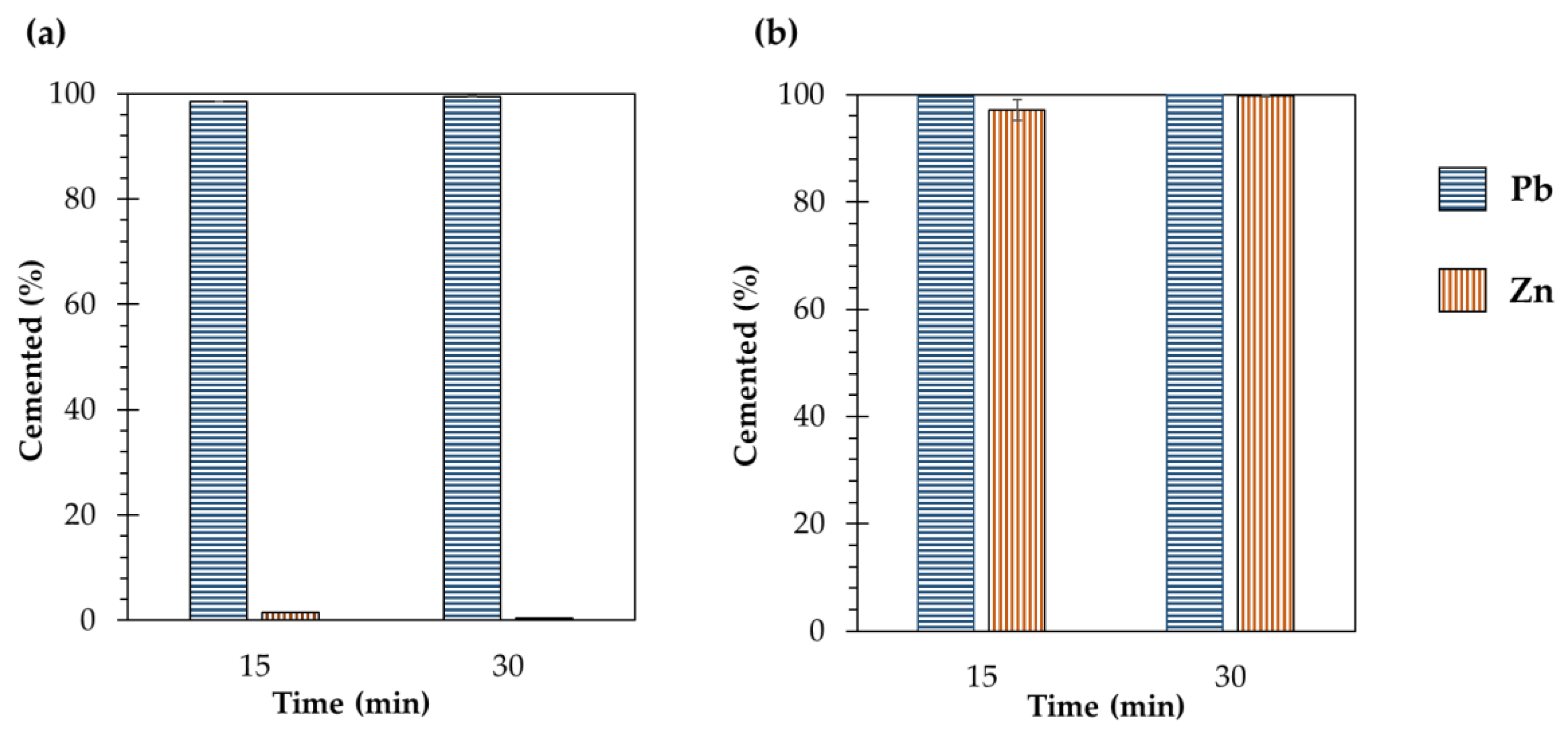
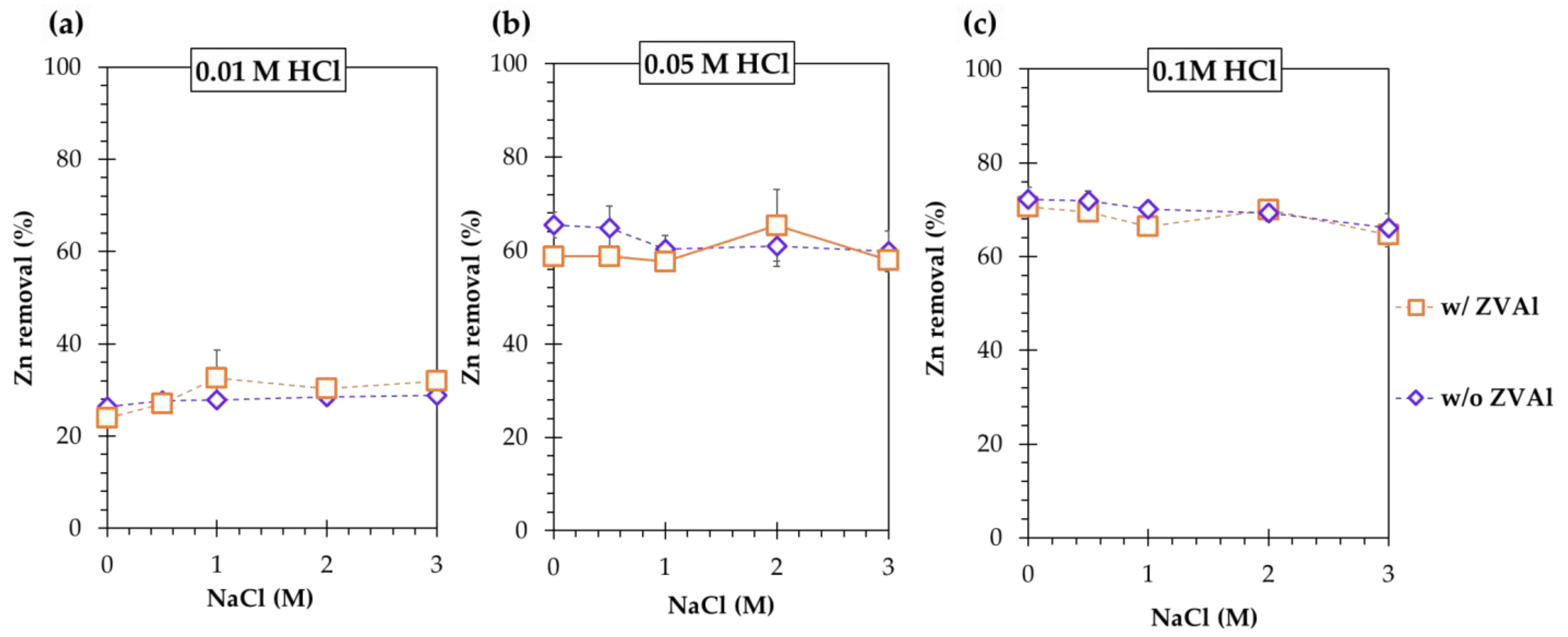
- Zinc removal from ZPLRs increased with increasing the HCl concentration (i.e., increased from 27% to 60% and 70% when the HCl concentration increased from 0.01 to 0.05 and 0.1 M, respectively) but it was neither affected by the increase of NaCl concentration nor the addition of ZVAl during leaching;
- Zinc was not to be sequestered from the acidified chloride leaching pulp by cementation using ZVAl and was attributed to the dissolution of cemented Zn or preferential reduction of H+ to H2 by ZVAl over Zn2+ to Zn;
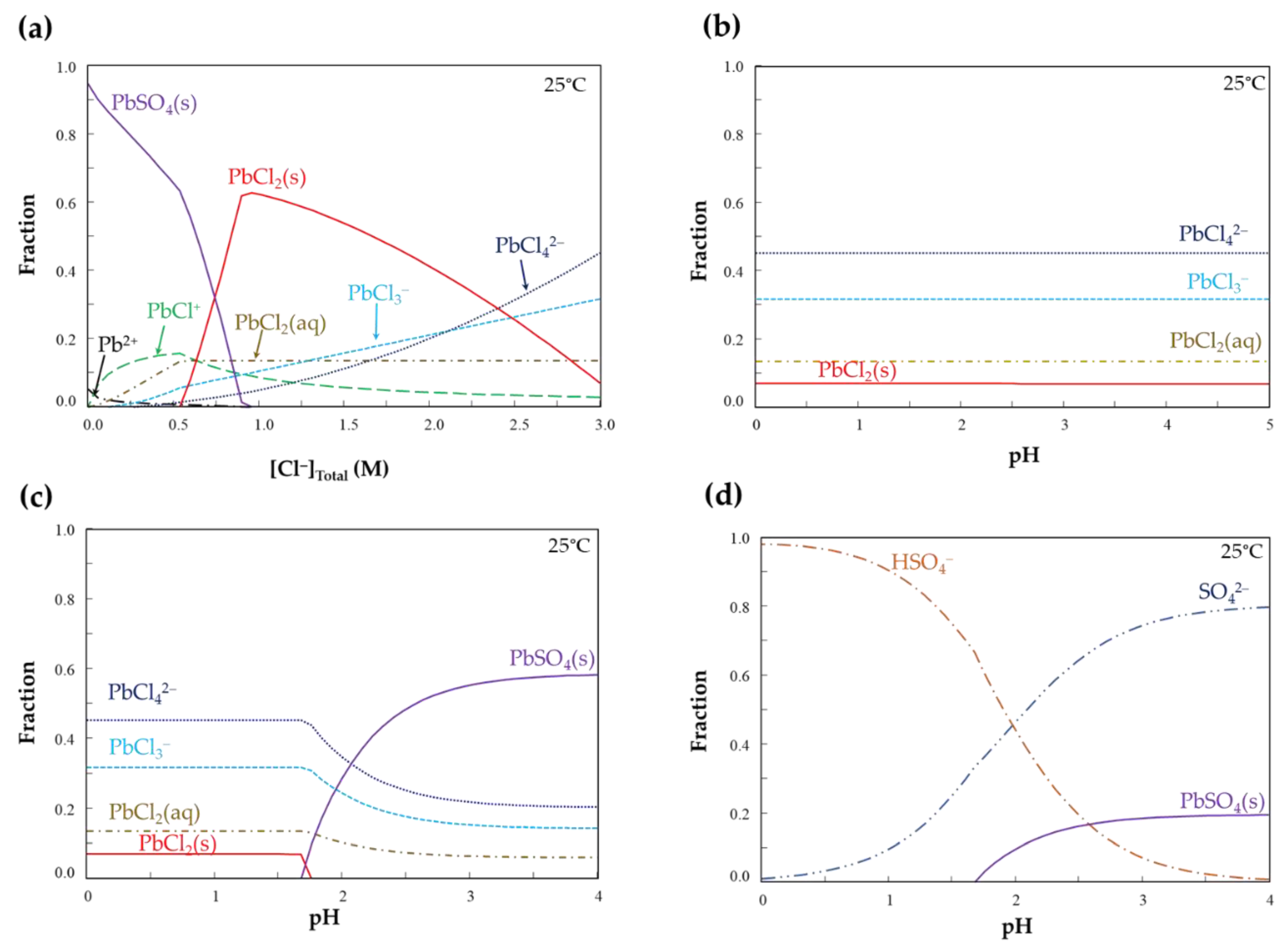
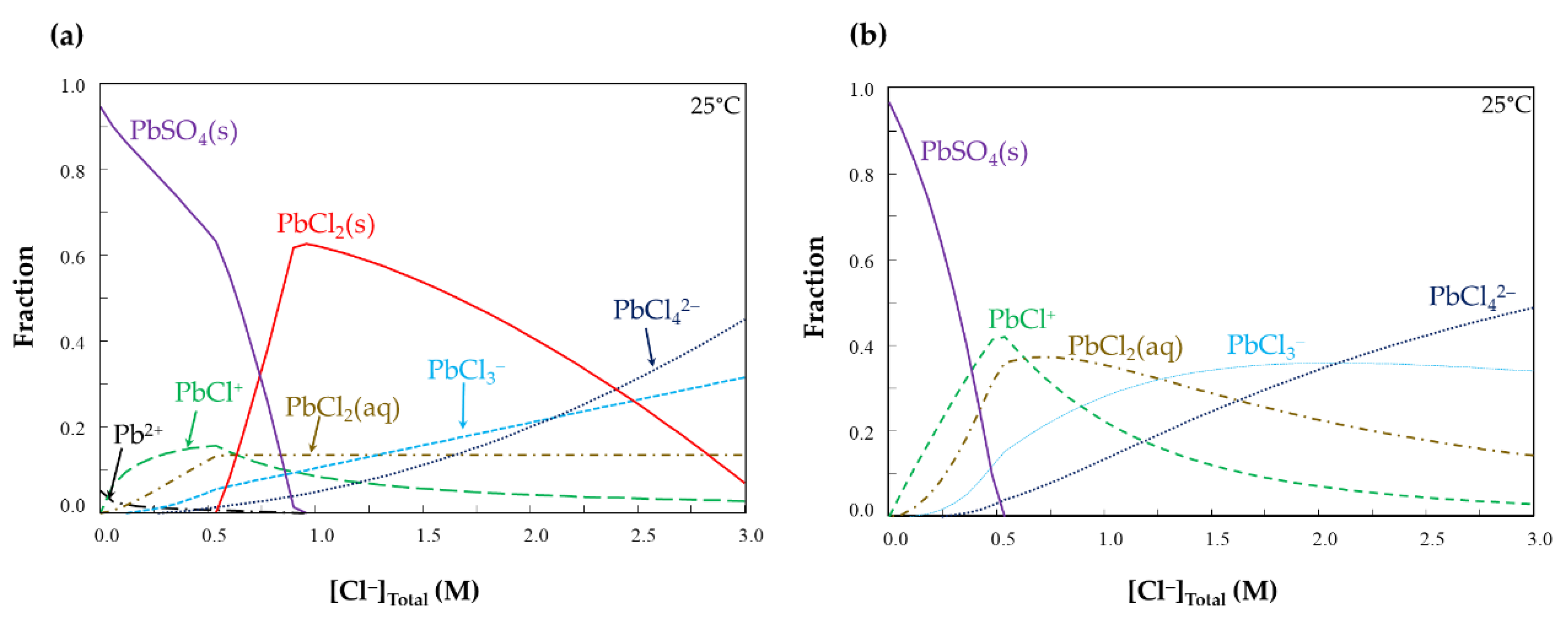
- Lead removal from ZPLRs without the addition of ZVAl increased with increasing NaCl and HCl concentrations. Pb removal steadily increased from around 0% to 28%, 0.5% to 58%, and 0.5% to 72% for 0.01, 0.05, and 0.1 M HCl, respectively, when NaCl increased from 0 to 3 M, respectively. The increase of Pb removal with HCl concentration was attributed to an H+ attack to dissolve Pb from carbonates, as well as fixing free SO42− as HSO4−, thereby, limiting the precipitation/formation of solid PbSO4. Meanwhile, Pb removal increased at higher NaCl concentrations because of the formation of more soluble Pb-Cl complexes;
- The addition of ZVAl during ZPLRs leaching (concurrent dissolution-cementation technique) dramatically increased the Pb removal even at low chloride concentration. Pb removal at 0.05 M HCl increased from 2.5% to 35.5% and 8% to 57% for 0.5 and 1 M NaCl concentration, respectively. Meanwhile, for 0.1 M HCl, the addition of ZVAl during ZPLRs leaching increased the Pb removal from 3% to 69% and 9% to 72% for 0.5 and 1 M NaCl concentration, respectively. The increase was attributed to shifting the equilibrium as the result of sequestration of dissolved Pb, thereby, enhancing dissolution of lead host minerals and dissolution of intermediate sparling soluble solid, PbCl2; and
- The most toxic metal, Pb, from ZPLRs was recovered and separated before solid-liquid separation, which simplifies the treatment flowsheet, as well as eliminates the need for extensive washing of the solid residues generated.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Backman, C.-M. Global Supply and Demand of Metals in the Future. J. Toxicol. Environ. Health Part A 2008, 71, 1244–1253. [Google Scholar] [CrossRef]
- Halada, K.; Shimada, M.; Ijima, K. Forecasting the Consumption of Metals up to 2050. J. Jpn. Inst. Met. 2007, 71, 831–839. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Mohr, S.; Giurco, D.; Retamal, M.; Mason, L.; Mudd, G. Global Projection of Lead-Zinc Supply from Known Resources. Resources 2018, 7, 17. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of lead-activated sphalerite by pyrite via galvanic interactions: Implications to the selective flotation of complex sulfide ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Behnajady, B.; Moghaddam, J.; Behnajady, M.A.; Rashchi, F. Determination of the optimum conditions for the leaching of lead from zinc plant residues in NaCl–H2SO4–Ca(OH)2 media by the Taguchi method. Ind. Eng. Chem. Res. 2012, 51, 3887–3894. [Google Scholar] [CrossRef]
- Hyk, W.; Kitka, K.; Rudnicki, D. Selective recovery of zinc from metallurgical waste materials from processing zinc and lead ores. Molecules 2019, 24, 2275. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, F.; Xiao, X.; Zhang, L.; Jiang, K. Optimization of brine leaching of metals from hydrometallurgical residue. Trans. Nonferr. Met. Soc. China 2010, 20, 2000–2005. [Google Scholar] [CrossRef]
- Sethurajan, M.; Huguenot, D.; Jain, R.; Lens, P.N.L.; Horn, H.A.; Figueiredo, L.H.A.; van Hullebusch, E.D. Leaching and selective zinc recovery from acidic leachates of zinc metallurgical leach residues. J. Hazard. Mater. 2017, 324, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ruşen, A.; Sunkar, A.S.; Topkaya, Y.A. Zinc and lead extraction from Çinkur leach residues by using hydrometallurgical method. Hydrometallurgy 2008, 93, 45–50. [Google Scholar] [CrossRef]
- Ru, Z.; Pan, C.; Liu, G.; Wang, X.; Dou, G.; Zhu, K. Leaching and recovery of zinc from leaching residue of zinc calcine based on membrane filter press. Trans. Nonferr. Met. Soc. China 2015, 25, 622–627. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Hashimoto, A.; Igarashi, T.; Yoneda, T. Leaching of boron, arsenic and selenium from sedimentary rocks: I. Effects of contact time, mixing speed and liquid-to-solid ratio. Sci. Total Environ. 2014, 472, 620–629. [Google Scholar] [CrossRef]
- Needleman, H. Lead poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Tamoto, S.; Takahashi, R. The roles of pyrite and calcite in the mobilization of arsenic and lead from hydrothermally altered rocks excavated in Hokkaido, Japan. J. Geochem. Explor. 2012, 119, 17–31. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Lin, W.; Taskinen, P.; He, J.; Wang, Y.; Chen, Y.; Tang, C.; Jokilaakso, A. Cleaner extraction of lead from complex lead-containing wastes by reductive sulfur-fixing smelting with low SO2 emission. Minerals 2019, 9, 119. [Google Scholar] [CrossRef]
- Rämä, M.; Nurmi, S.; Jokilaakso, A.; Klemettinen, L.; Taskinen, P.; Salminen, J. Thermal processing of jarosite leach residue for a safe disposable slag and valuable metals recovery. Metals 2018, 8, 744. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Jie, Y.; Tang, C.; He, J.; Chen, Y. Hydrometallurgical process for zinc recovery from C.Z.O. generated by the steelmaking industry with ammonia–ammonium chloride solution. Metals 2019, 9, 83. [Google Scholar] [CrossRef]
- Farahmand, F.; Moradkhani, D.; Safarzadeh, M.S.; Rashchi, F. Brine leaching of lead-bearing zinc plant residues: Process optimization using orthogonal array design methodology. Hydrometallurgy 2009, 95, 316–324. [Google Scholar] [CrossRef]
- Thao, N.T.; Tsuji, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, M. Redox potential-dependent chalcopyrite leaching in acidic ferric chloride solutions: Leaching experiments. Hydrometallurgy 2020. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, K.; Alorro, R.D.; Tabelin, C.B. Cementation of Co ion in leach solution using Zn powder followed by magnetic separation of cementation-precipitate for recovery of unreacted Zn powder. Miner. Eng. 2020, 145, 106061. [Google Scholar] [CrossRef]
- Calderon, A.R.M.; Alorro, R.D.; Tadesse, B.; Yoo, K.; Tabelin, C.B. Evaluation of maghemite-rich iron oxide composite prepared from magnetite as adsorbent for gold from chloride solution. JOM 2019, 71, 4639–4646. [Google Scholar] [CrossRef]
- Bodas, M.G. Hydrometallurgical treatment of zinc silicate ore from Thailand. Hydrometallurgy 1996, 40, 37–49. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Yan, J. Pressure leaching of synthetic zinc silicate in sulfuric acid medium. Hydrometallurgy 2011, 108, 171–176. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Toxicity Characteristic Leaching Procedure (TCLP), Test Method 1311- TCLP; US Environmental Protection Agency: Washington, DC, USA, 1991.
- Feng, Q.; Wen, S.; Wang, Y.; Zhao, W.; Liu, J. Dissolution kinetics of cerussite in acidic sodium chloride solutions. Bull. Korean Chem. Soc. 2015, 36, 1100–1107. [Google Scholar]
- Liu, W.; Yang, T.; Xia, X. Behavior of silver and lead in selective chlorination leaching process of gold-antimony alloy. Trans. Nonferr. Met. Soc. China 2010, 20, 322–329. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, L.; Li, H.; Li, H.; Koppala, S.; Yin, S.; Li, S.; Yang, K.; Zhu, F. Efficient recycling of Pb from zinc leaching residues by using the hydrometallurgical method. Mater. Res. Express 2019, 6, 075505. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Kojima, M.; Hiroyoshi, N.; Ito, M. Galvanic microencapsulation (GME) using zero-valent aluminum and zero-valent iron to suppress pyrite oxidation. Mater. Trans. 2019, 60, 277–286. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced cementation of gold via galvanic interactions using activated carbon and zero-valent aluminum: A novel approach to recover gold ions from ammonium thiosulfate medium. Hydrometallurgy 2020, 191, 105165. [Google Scholar] [CrossRef]
- St-Pierre, J.; Piron, D.L. Electrowinning of zinc from alkaline solutions. J. Appl. Electrochem. 1986, 16, 447–456. [Google Scholar] [CrossRef]
- Puigdomenech, I. Make Equilibrium Diagrams Using Sophisticated Algorithms (MEDUSA), Inorganic Chemistry; Royal Institute of Technology: Stockholm, Sweden, 2010. [Google Scholar]
- Silwamba, M.; Ito, M.; Fukushima, T.; Tabelin, C.B.; Takakuwa, S.; Hiroyoshi, N.; Chirwa, M.; Banda, K.; Nyambe, I. Characterization of zinc leaching residue in Kabwe area, Zambia. In Proceedings of the 2nd International Kabwe Mine Pollution Amelioration Initiative (KAMPAI)/JST, Lusaka, Zambia, 12 August 2018; p. 22. [Google Scholar]
- Ye, M.; Li, G.; Yan, P.; Zheng, L.; Sun, S.; Huang, S.; Li, H.; Chen, Y.; Yang, L.; Huang, J. Production of lead concentrate from bioleached residue tailings by brine leaching followed by sulfide precipitation. Sep. Purif. Technol. 2017, 183, 366–372. [Google Scholar] [CrossRef]
- Tuffrey, N.E.; Jiricny, V.; Evans, J.W. Fluidized bed electrowinning of zinc from chloride electrolytes. Hydrometallurgy 1985, 15, 33–54. [Google Scholar] [CrossRef]
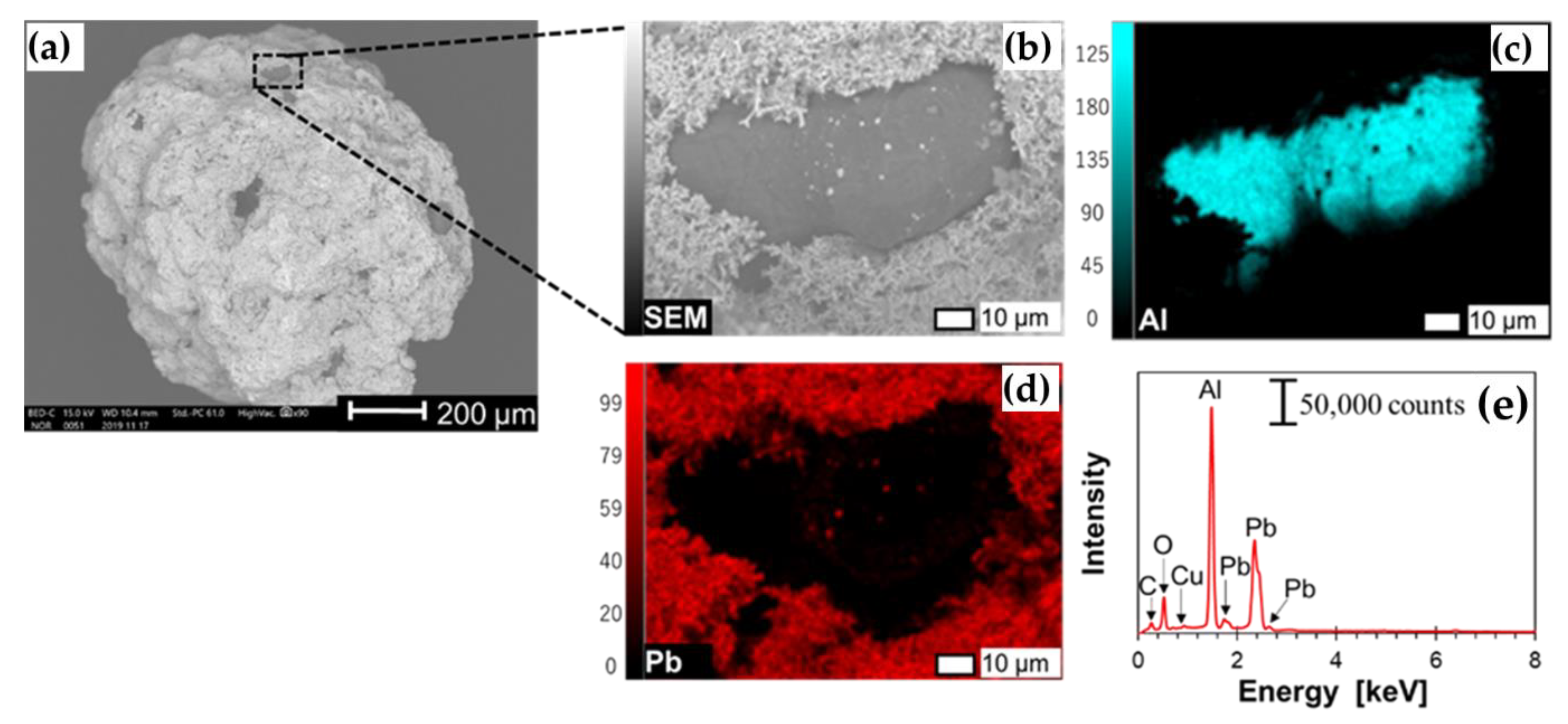
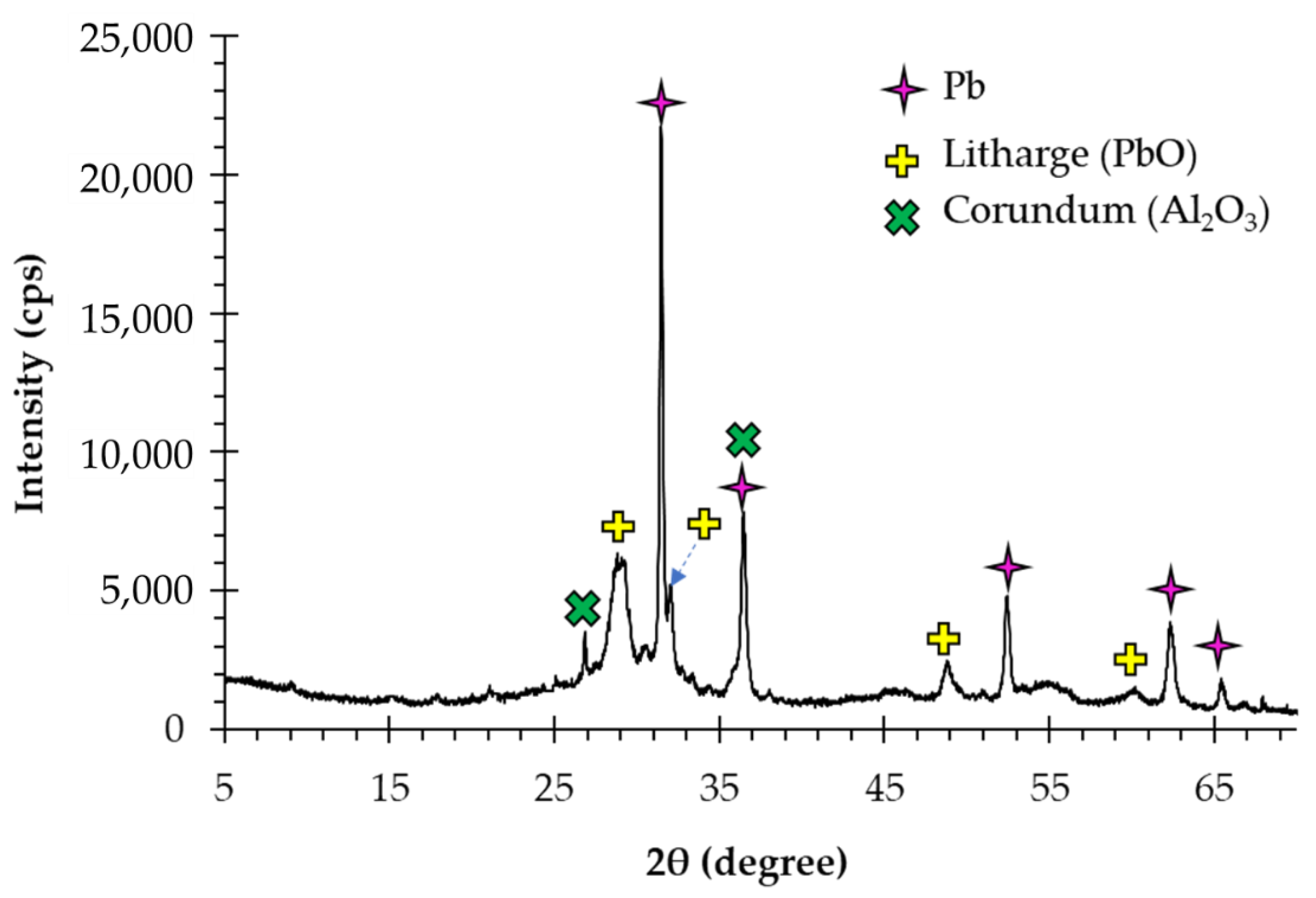
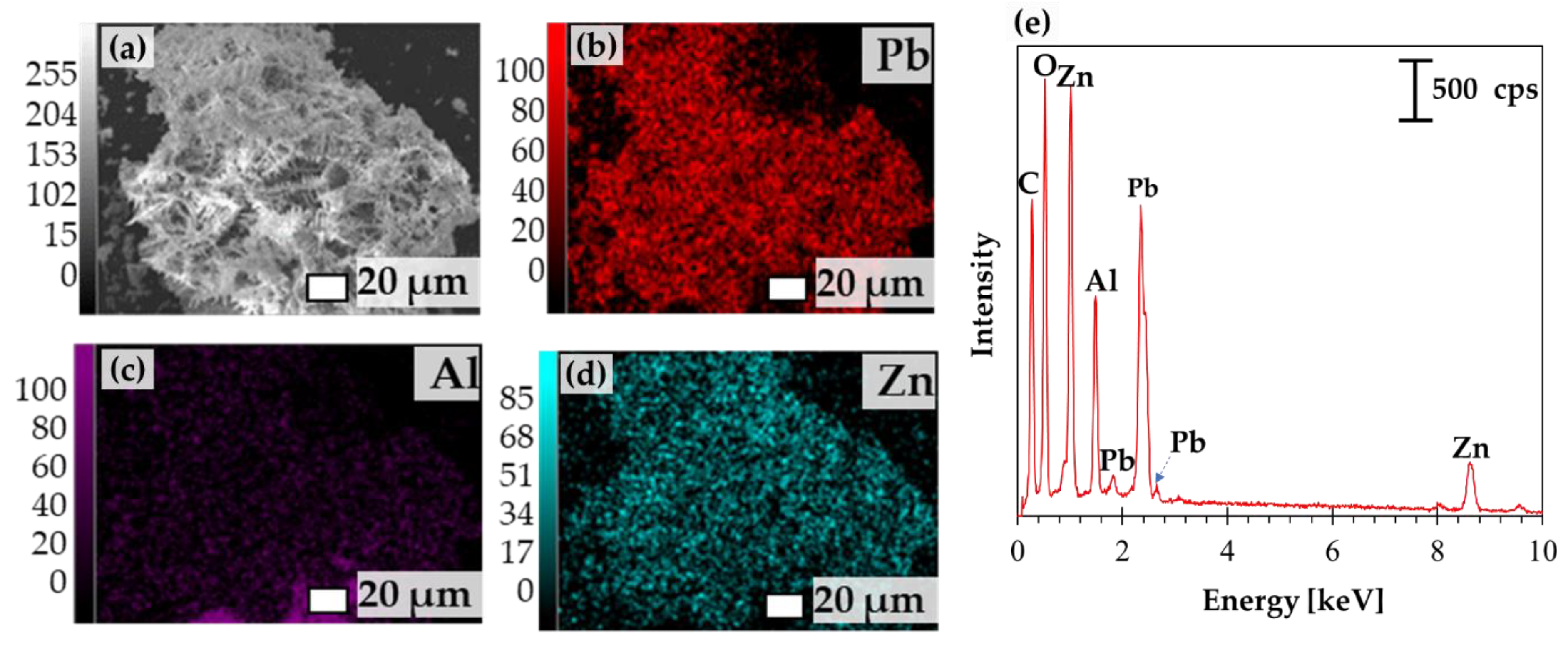
| Elements/Oxides | Pb * | Zn * | Fe * | Cu * | CaO | SiO2 | Al2O3 | SO3 | V2O5 | MnO | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass % | 6.2 | 2.5 | 17.0 | 0.2 | 10.6 | 31.4 | 2.9 | 18.2 | 0.7 | 0.3 | 1.1 |
| Element | Untreated ZPLRs | Treated Residues | Threshold (USEPA) |
|---|---|---|---|
| Pb | 12.95 mg/L | 0.12 mg/L | 5 mg/L |
| Zn | 473.5 mg/L | 21.5 mg/L | –* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Hashizume, R.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; et al. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium. Metals 2020, 10, 531. https://doi.org/10.3390/met10040531
Silwamba M, Ito M, Hiroyoshi N, Tabelin CB, Hashizume R, Fukushima T, Park I, Jeon S, Igarashi T, Sato T, et al. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium. Metals. 2020; 10(4):531. https://doi.org/10.3390/met10040531
Chicago/Turabian StyleSilwamba, Marthias, Mayumi Ito, Naoki Hiroyoshi, Carlito Baltazar Tabelin, Ryota Hashizume, Tomoki Fukushima, Ilhwan Park, Sanghee Jeon, Toshifumi Igarashi, Tsutomu Sato, and et al. 2020. "Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium" Metals 10, no. 4: 531. https://doi.org/10.3390/met10040531
APA StyleSilwamba, M., Ito, M., Hiroyoshi, N., Tabelin, C. B., Hashizume, R., Fukushima, T., Park, I., Jeon, S., Igarashi, T., Sato, T., Chirwa, M., Banda, K., Nyambe, I., Nakata, H., Nakayama, S., & Ishizuka, M. (2020). Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium. Metals, 10(4), 531. https://doi.org/10.3390/met10040531











