Effect of Titanium Addition on As-Cast Structure and High-Temperature Tensile Property of 20Cr-8Ni Stainless Steel for Heavy Castings
Abstract
1. Introduction
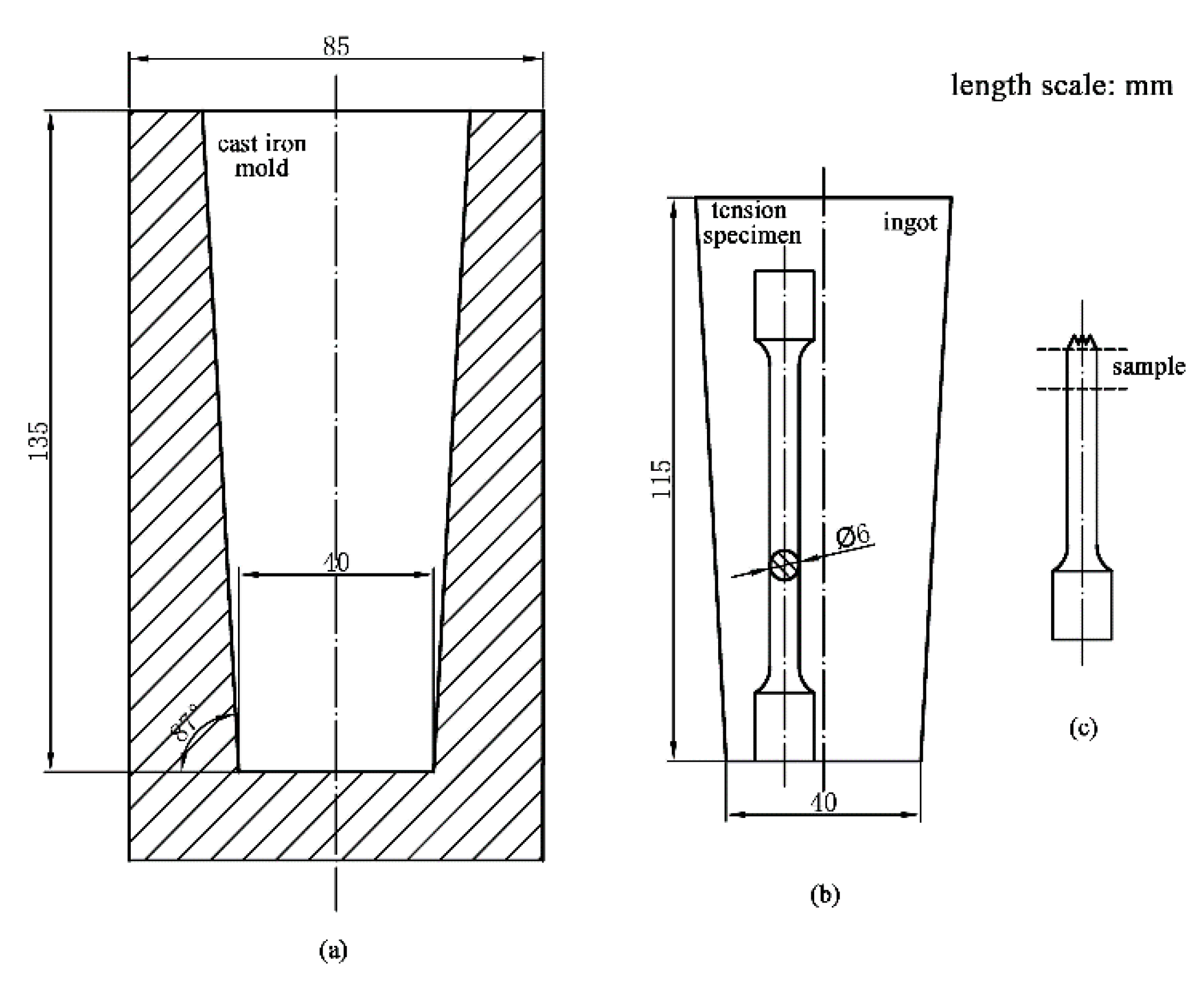
2. Materials and Methods
3. Results
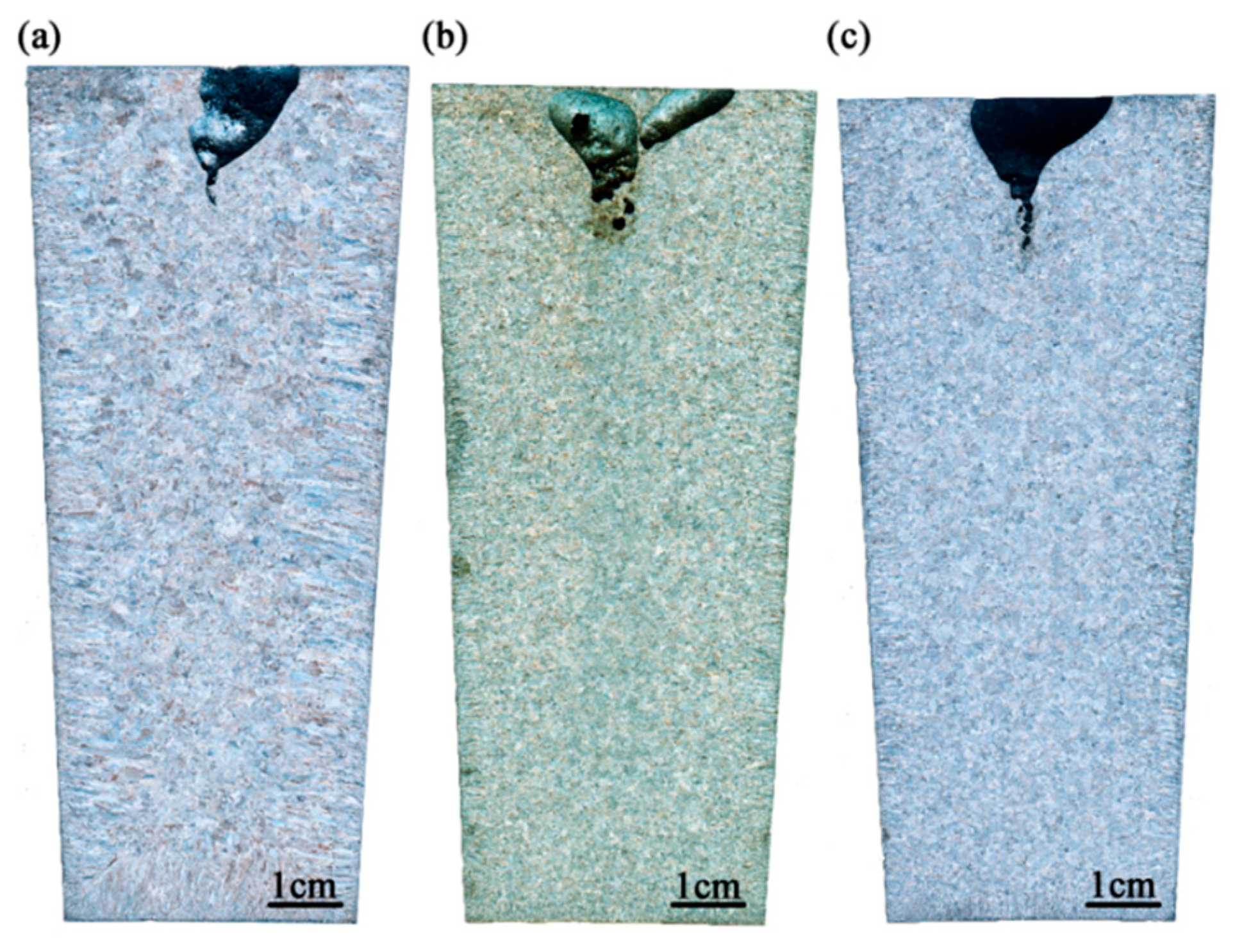
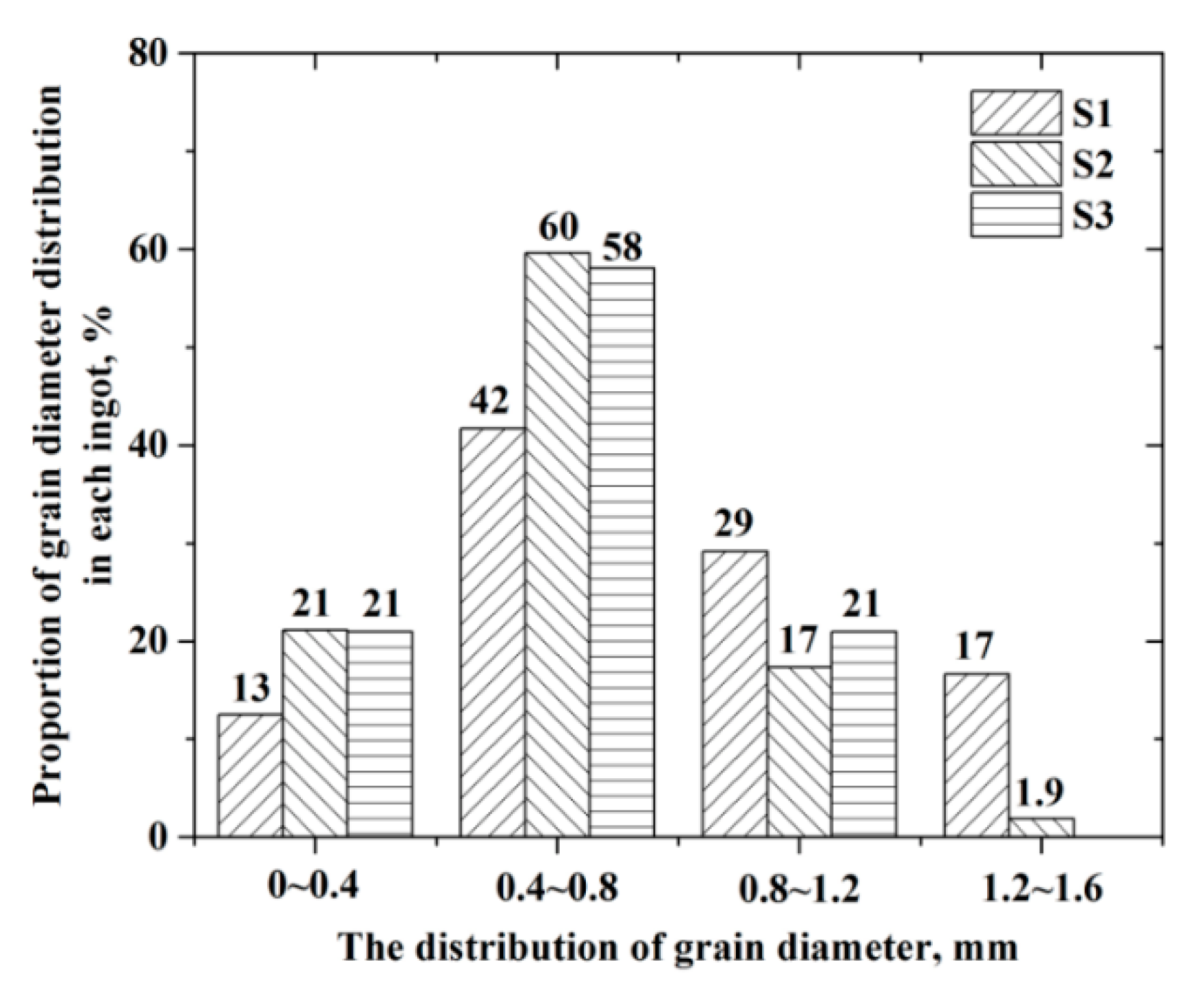
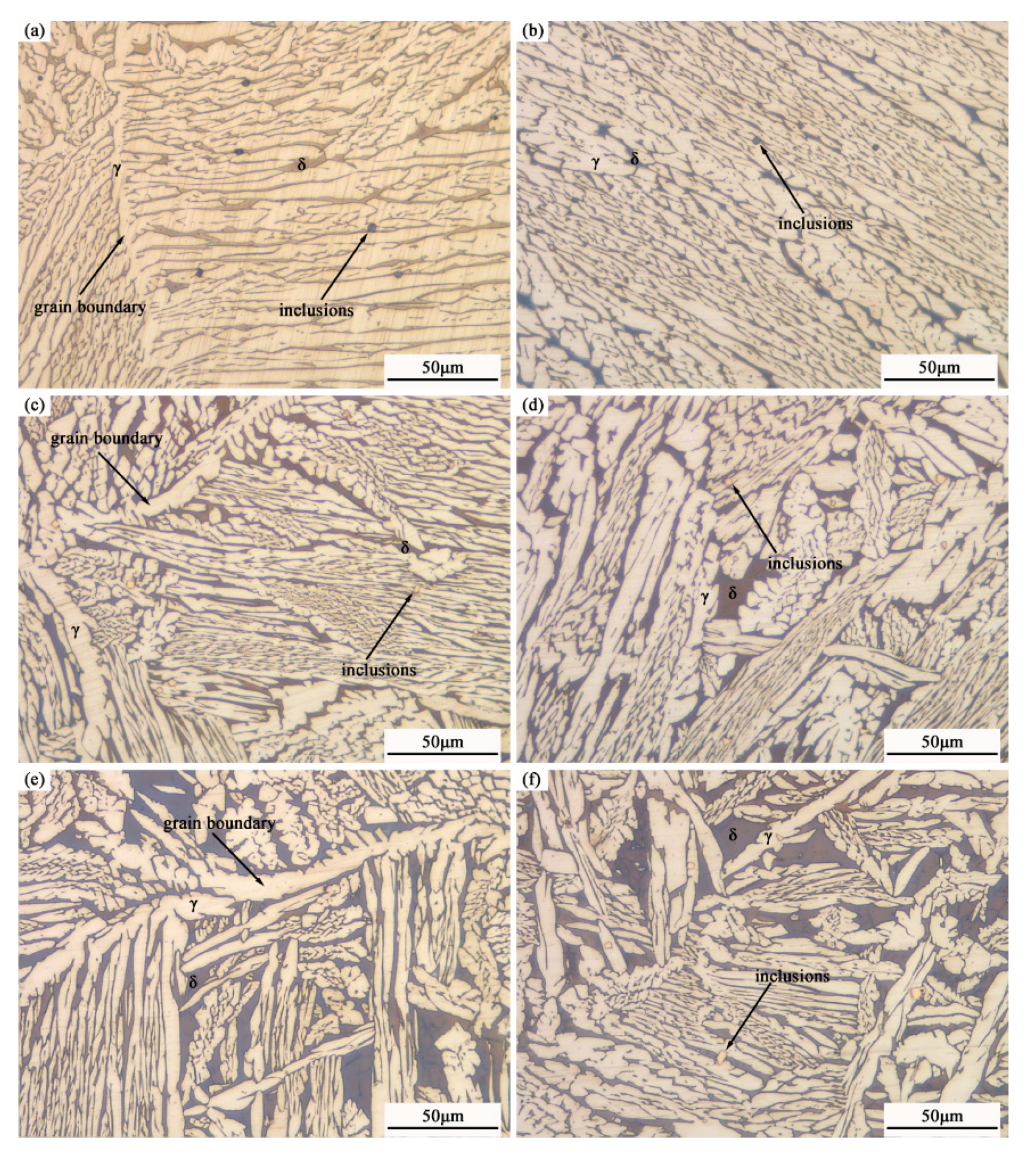
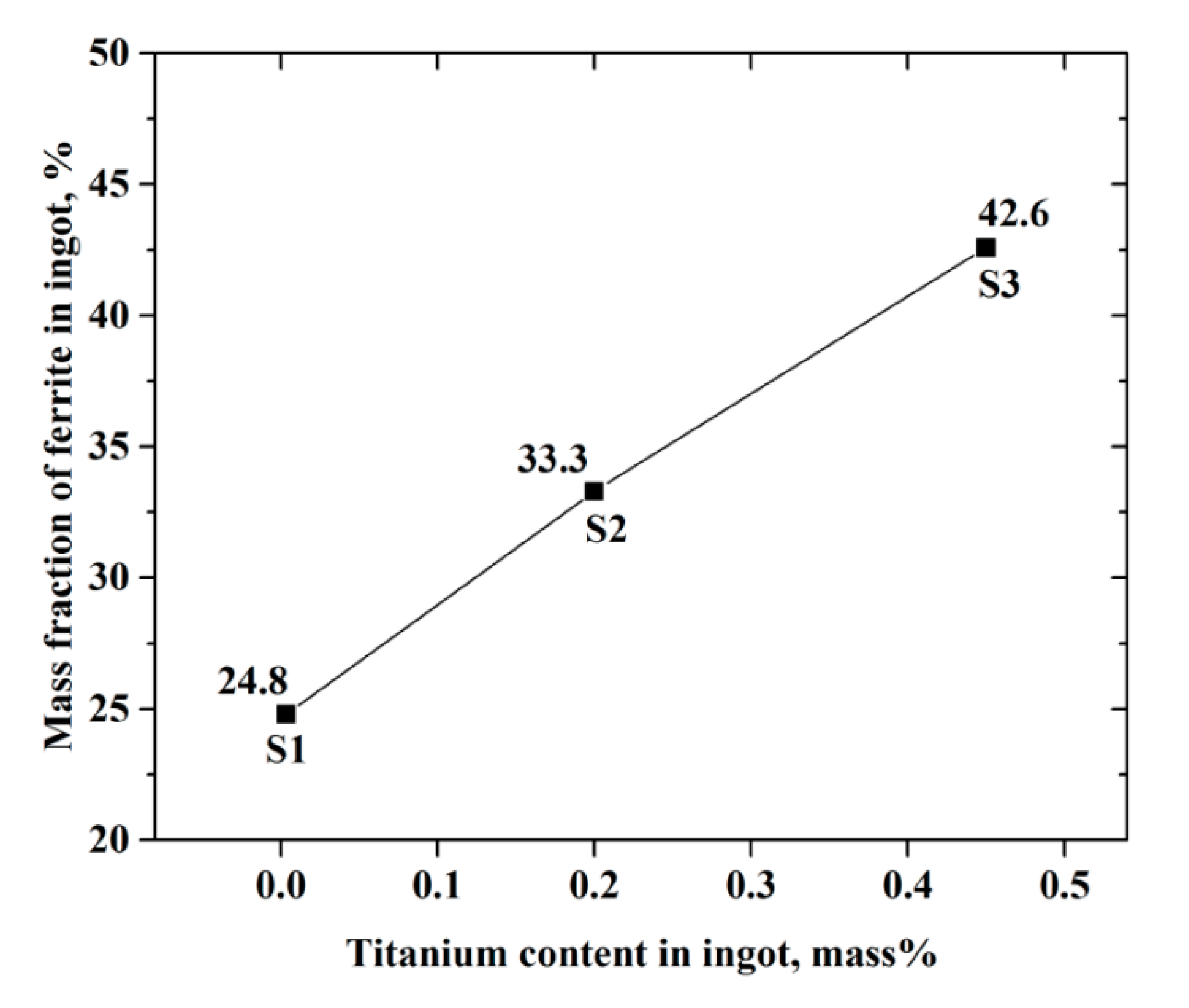
3.1. As-Cast Structure of Ingots
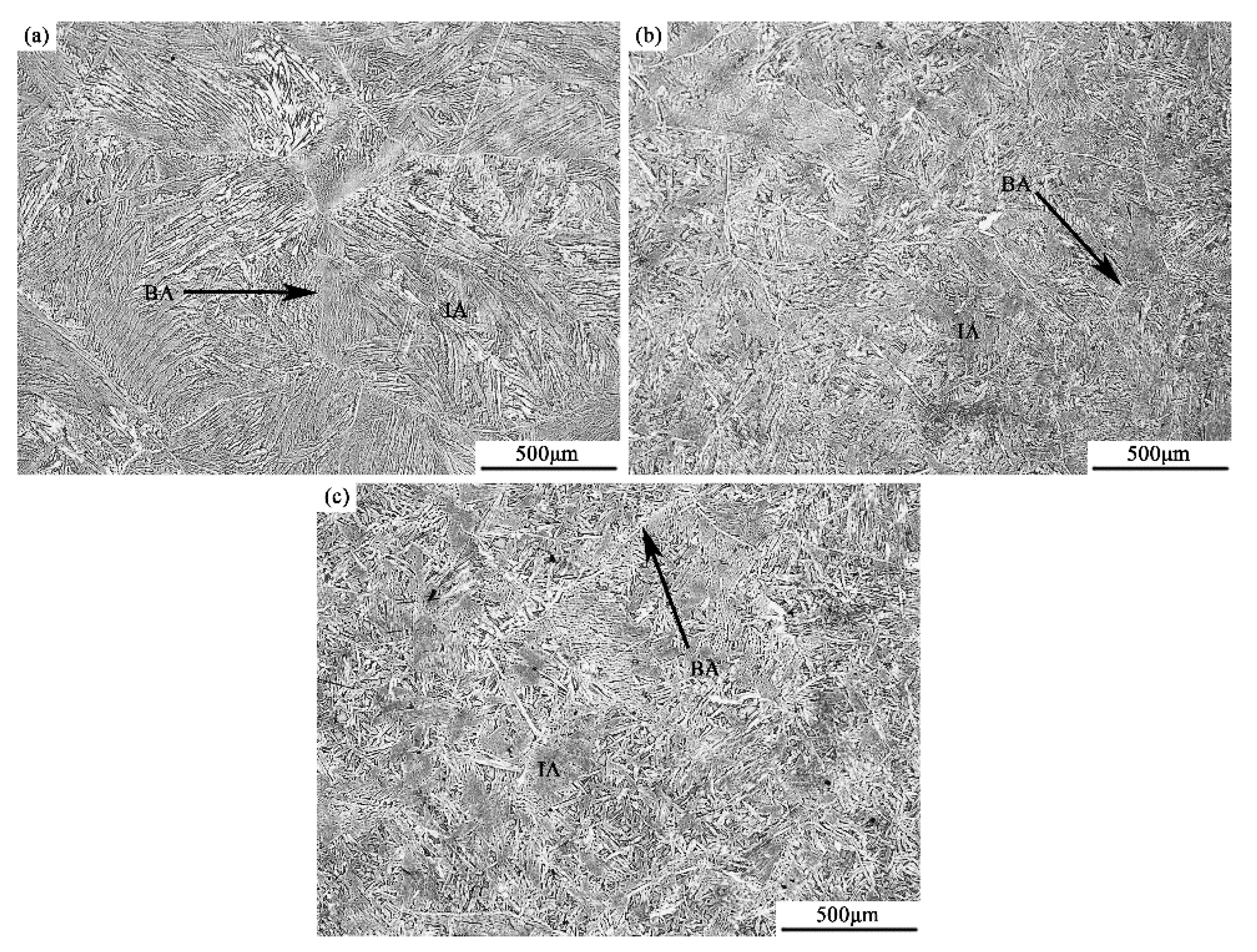
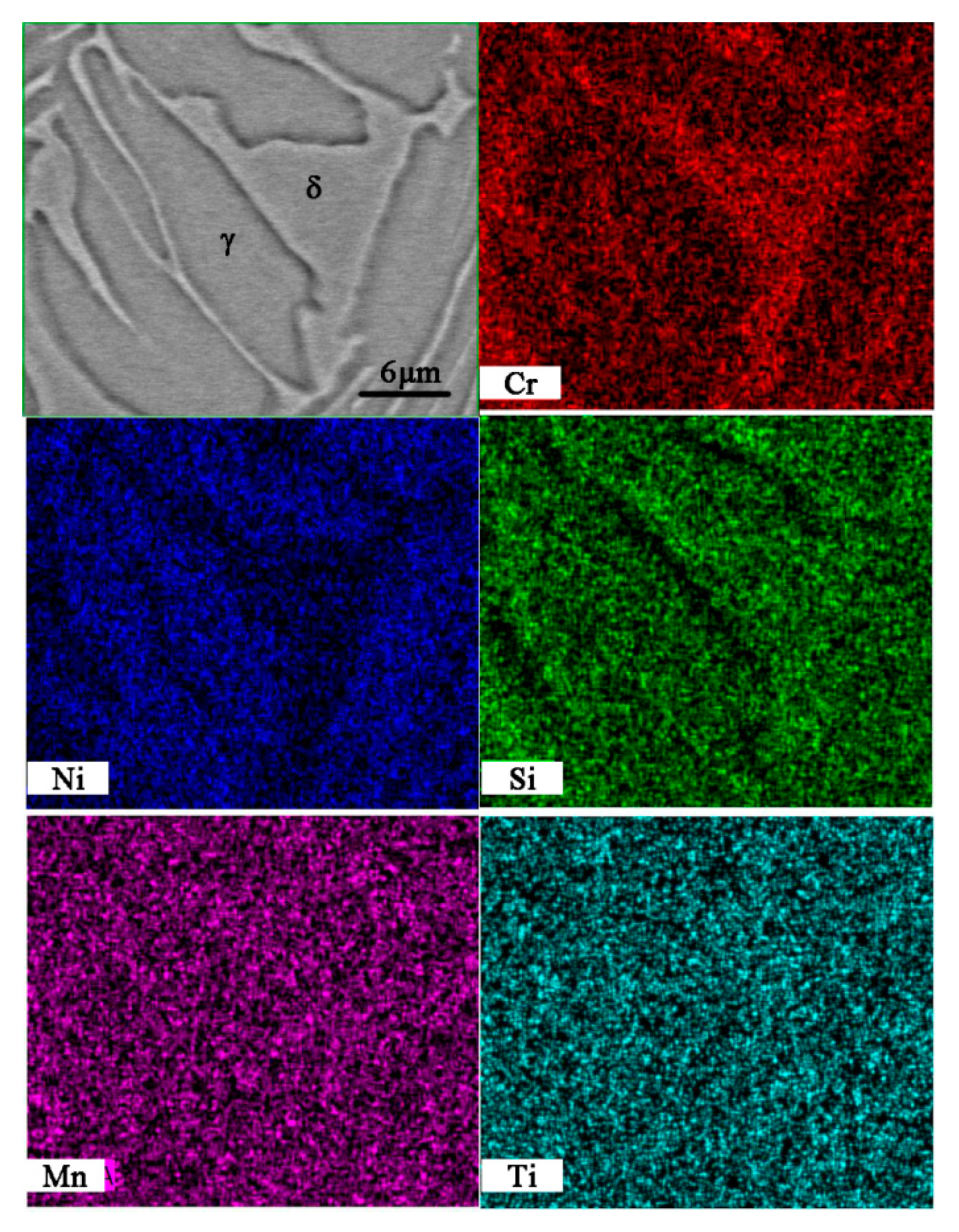
3.2. Microstructure and Element Distribution
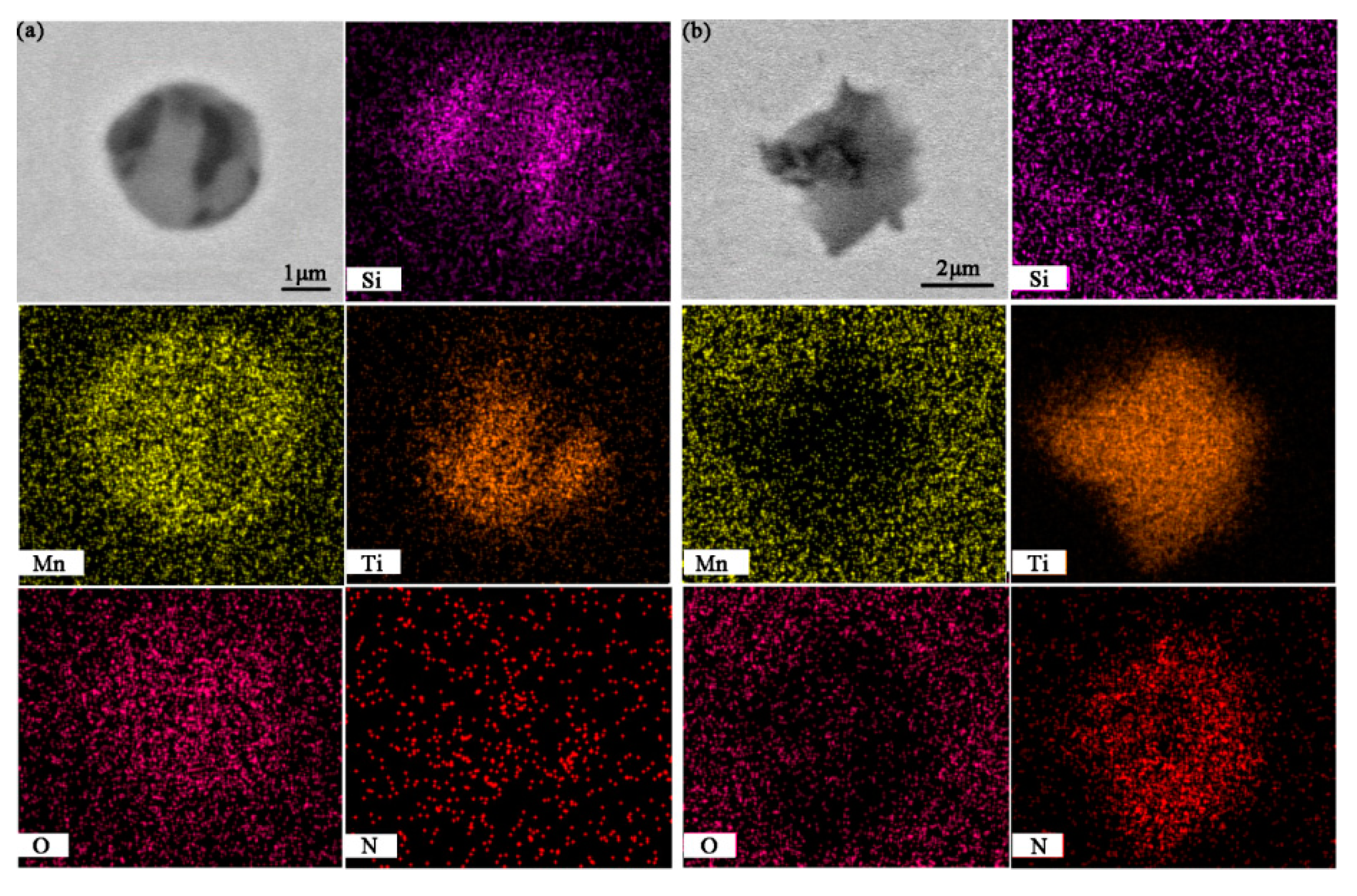
3.3. Typical Inclusions
3.4. Tensile Properties
4. Discussion
4.1. Formation of TiN and Effect on As-Cast Structure
4.2. Effect of Titanium on Microstructure
4.3. Improvement of Tensile Property
5. Conclusions
- (1)
- With the increasing titanium content, the typical inclusions changed from dual phase Si-Mn-Ti oxides to pure TiN or complex Ti2O3 + TiN. Meanwhile, the equiaxed grain ratio of ingot increased from 51 percent to nearly 100 percent, and the size of equiaxed grain decreased owing to heterogeneous nucleation of δ-Fe;
- (2)
- With the increasing titanium content, the soluble titanium content in ferrite phase increased, retarded the formation of austenite phase, and changed the equilibrium solidification model from FA to F. As a result, the mass fraction of ferrite phase in ingot increased gradually;
- (3)
- Owing to increase of the ferrite phase mass fraction and decrease of the equiaxed grain size, the YS and UTS at 350 °C of ingot increased gradually, but tensile elongation changed little.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pryds, N.H.; Huang, X. The effect of cooling rate on the microstructures formed during solidification of ferritic steel. Metall. Mater. Trans. A 2000, 31, 3155–3166. [Google Scholar] [CrossRef]
- Xu, P.G.; Yin, F.; Nagai, K. Solidification cooling rate and as-cast textures of low-carbon steel strips. Mater. Sci. Eng. A 2006, 441, 157–166. [Google Scholar] [CrossRef]
- Simmons, J.W. Overview: High-nitrogen alloying of stainless steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Xiang, H.; He, F. Effects of N content on microstructure and properties of 29Cr casting super duplex stainless steel. Acta Metall. Sin. 2010, 46, 304–310. [Google Scholar] [CrossRef]
- Yang, S.H.; Lee, Z.H. A method for predicting nitrogen gas pores in nitrogen alloying stainless steels. Mater. Sci. Eng. A 2006, 417, 307–314. [Google Scholar] [CrossRef]
- Zhu, H.C.; Jiang, Z.H.; Li, H.B.; Feng, H.; Zhang, S.C.; Liu, G.H.; Li, G.P. Effects of nitrogen segregation and solubility on the formation of nitrogen gas pores in 21.5Cr-1.5Ni duplex stainless steel. Metall. Mater. Trans. B 2017, 48, 2493–2503. [Google Scholar] [CrossRef]
- Fruehan, R.J. Activities in liquid Fe–Al–O and Fe–Ti–O alloys. Metall. Trans. 1970, 1, 3403–3410. [Google Scholar] [CrossRef]
- Seok, S.H.; Miki, T.; Hino, M. Equilibrium between Ti and O in molten Fe–Ni, Fe–Cr and Fe–Cr–Ni alloys equilibrated with Ti3O5 solid solution. ISIJ Int. 2011, 51, 566–572. [Google Scholar] [CrossRef]
- Cha, W.Y.; Miki, T.; Sasaki, Y.; Hino, M. Temperature dependence of Ti deoxidation equilibria of liquid iron in coexistence with Ti3O5 and Ti2O3. ISIJ Int. 2008, 48, 729–738. [Google Scholar] [CrossRef]
- Pak, J.J.; Jo, J.O.; Kim, S.I.; Kim, W.Y.; Chung, T.I.; Seo, S.M.; Kim, D.S. Thermodynamics of titanium and oxygen dissolved in liquid iron equilibrated with titanium oxides. ISIJ Int. 2007, 47, 16–24. [Google Scholar] [CrossRef]
- Cha, W.Y.; Nagasaka, T.; Miki, T.; Sasaki, Y.; Hino, M. Equilibrium between titanium and oxygen in liquid Fe–Ti alloy coexisted with titanium oxides at 1873 K. ISIJ Int. 2006, 46, 996–1005. [Google Scholar] [CrossRef]
- Seo, K.; Kim, Y.M.; Kim, H.J.; Lee, C. Characterization of inclusions formed in Ti-containing steel weld metals. ISIJ Int. 2015, 55, 1730–1738. [Google Scholar] [CrossRef]
- Ozturk, B.; Matway, R.; Fruehan, R.J. Thermodynamics of inclusion formation in Fe-Cr-Ti-N alloys. Metall. Mater. Trans. B 1995, 26, 563–567. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.; Yang, Y.; Bai, X.; Barati, M.; Mclean, A. Formation of inclusions in Ti-stabilized 17Cr austenitic stainless steel. Metall. Mater. Trans. B 2016, 47, 3274–3284. [Google Scholar] [CrossRef]
- Fujimura, H.; Tsuge, S.; Komizo, Y.; Nishizawa, T. Effect of oxide composition on solidification structure of Ti added ferritic stainless steel. Tetsu-to-Hagané 2001, 87, 707–712. [Google Scholar] [CrossRef]
- Fukumoto, S.; Kimura, K.; Takahashi, A. Formation of Fine Macrostructure in Ferritic Stainless Steel. Tetsu-to-Hagané 2012, 98, 351–357. [Google Scholar] [CrossRef][Green Version]
- Suito, H.; Ohta, H.; Morioka, S. Refinement of solidification microstructure and austenite grain by fine inclusion particles. ISIJ Int. 2006, 46, 840–846. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Park, J.H. Effect of inclusions on the solidification structures of ferritic stainless steel: Computational and experimental study of inclusion evolution. Calphad 2011, 35, 455–462. [Google Scholar] [CrossRef]
- Nakajima, K.; Hasegawa, H.; Khumkoa, S.; Mizoguchi, S. Effect of a catalyst on heterogeneous nucleation in pure and Fe-Ni alloys. Metall. Mater. Trans. B 2003, 34, 539–547. [Google Scholar] [CrossRef]
- Nakajima, K.; Hasegawa, H.; Khumkoa, S.; Hayashi, M. Effect of catalyst on heterogeneous nucleation in Fe–Ni–Cr alloys. ISIJ Int. 2006, 46, 801–806. [Google Scholar] [CrossRef]
- Hansen, N. Hall–Petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Ulvan, E.; Koursaris, A. The effect of grain size on the bulk formability and tensile properties of austenitic stainless steel types 304 and 316. Metall. Trans. A 1988, 19, 2287–2298. [Google Scholar] [CrossRef]
- Behjati, P.; Kermanpur, A.; Najafizadeh, A.; Baghbadorani, H.S.; Karjalainen, L.P.; Jung, J.G.; Lee, Y.K. Effect of nitrogen content on grain refinement and mechanical properties of a reversion-treated Ni-free 18Cr-12Mn austenitic stainless steel. Metall. Mater. Trans. A 2014, 45, 6317–6328. [Google Scholar] [CrossRef]
- Di Schino, A.; Barteri, M.; Kenny, J.M. Effects of grain size on the properties of a low nickel austenitic stainless steel. J. Mater. Sci. 2003, 38, 4725–4733. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Z.H.; Zhang, Z.R.; Yang, Y. Effect of grain size on mechanical properties of nickel-free high nitrogen austenitic stainless steel. J. Iron Steel Res. Int. 2009, 16, 58–61. [Google Scholar] [CrossRef]
- Jang, Y.; Son, J.; Kim, S.; Lee, J.; Choi, B. Effect of different Cr contents on tensile and corrosion behaviors of 0.13 pct N-containing CD4MCU cast duplex stainless steels. Metall. Mater. Trans. A 2004, 35, 3431–3438. [Google Scholar] [CrossRef]
- Jang, Y.H.; Kim, S.S.; Lee, J.H. Effect of different Mn contents on tensile and corrosion behavior of CD4MCU cast duplex stainless steels. Metall. Mater. Trans. A 2005, 396, 302–310. [Google Scholar] [CrossRef]
- Son, J.; Kim, S.; Lee, J.; Choi, B. Effect of N addition on tensile and corrosion behaviors of CD4MCU cast duplex stainless steels. Metall. Mater. Trans. A 2003, 34, 1617–1625. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, G. Formation Mechanism and Nucleation Effect of Ti2O3–TiN Complex Nucleus at Solidification Front of 18Cr Ferritic Stainless Steel. Metall. Mater. Trans. B 2019, 50, 1351–1364. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, G. Effect of Nb on the as-cast structure and compactness degree of ferritic stainless steel dual stabilized by Ti and Nb. ISIJ Int. 2018, 58, 2298–2307. [Google Scholar] [CrossRef]
- Villafuerte, J.C.; Pardo, E.; Kerr, H.W. The effect of alloy composition and welding conditions on columnar-equiaxed transitions in ferritic stainless steel gas-tungsten arc welds. Metall. Trans. A 1990, 21, 2009–2016. [Google Scholar] [CrossRef]
- Liang, J.P.; Gao, Y.L.; Li, R.X.; Zhai, Q.J. Phase evolution of AISI 321 stainless steel during directional solidification. Ironmak. Steelmak. 2009, 36, 603–609. [Google Scholar] [CrossRef]
- Knezevic, V.R.; Cvijovic, Z.M.; Mihajlovic, D.V. Effect of rapid cooling on the solidification mode and microstructure morphology of austenitic-ferritic stainless steels. J. Serb. Chem. Soc. 1995, 60, 525–534. [Google Scholar]



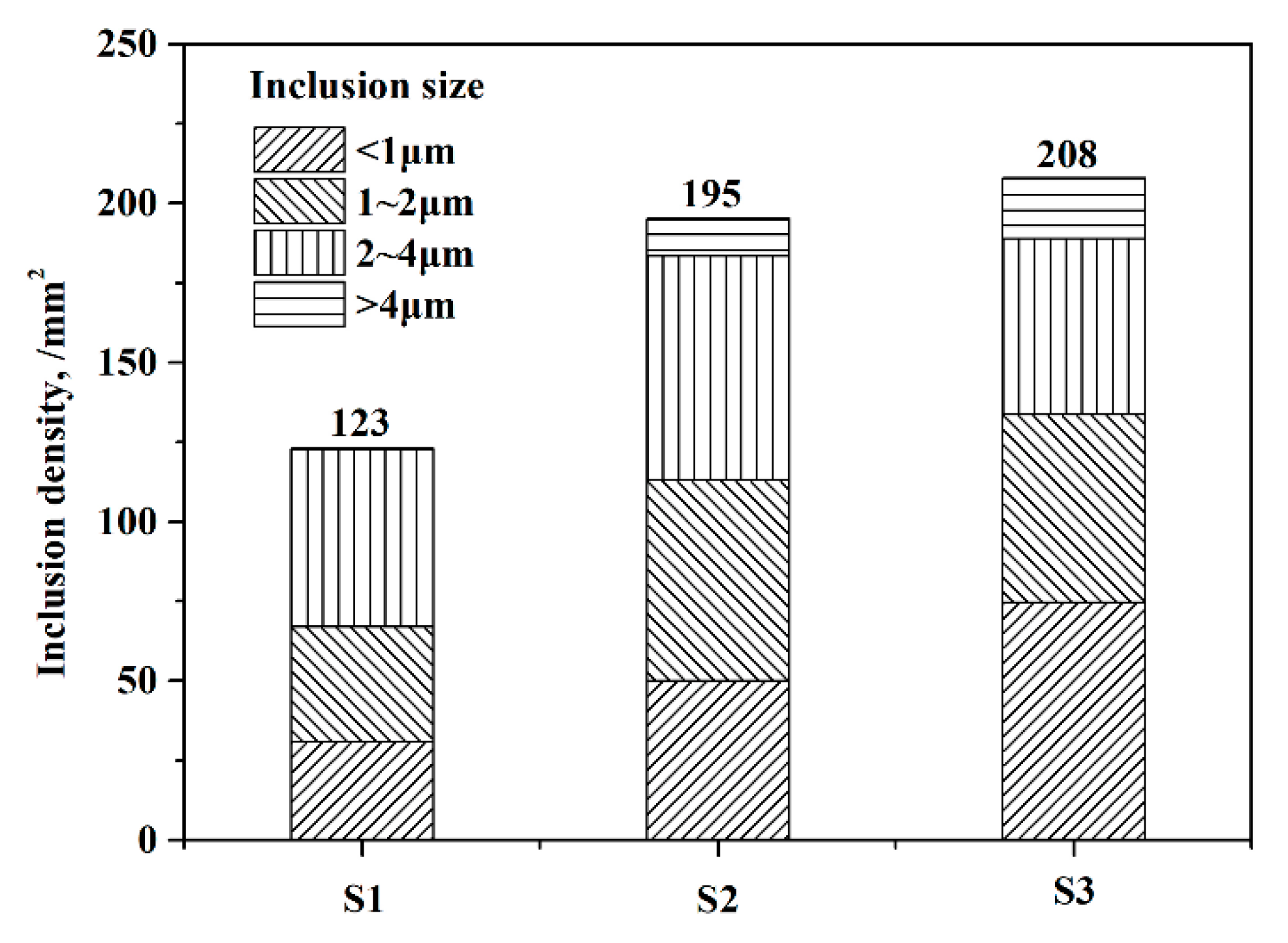
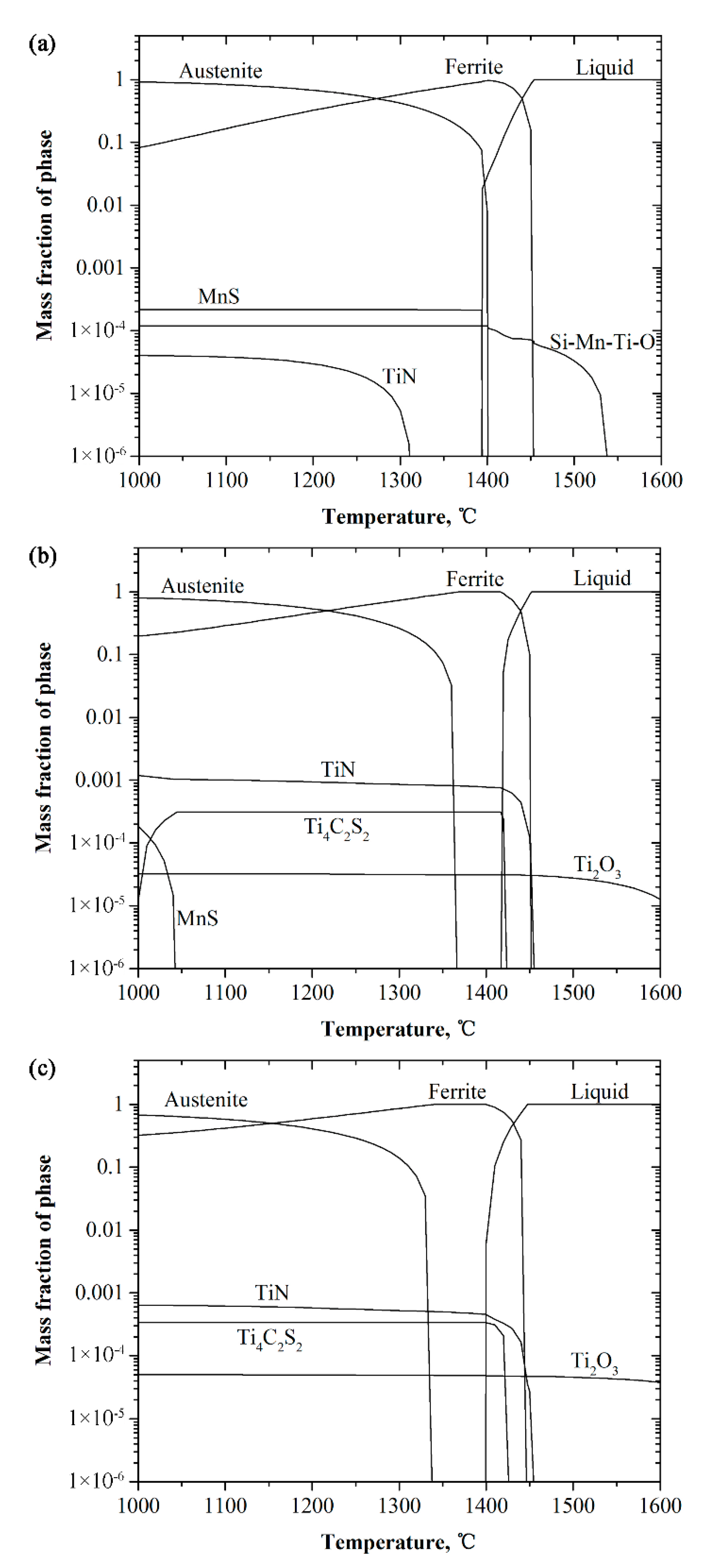
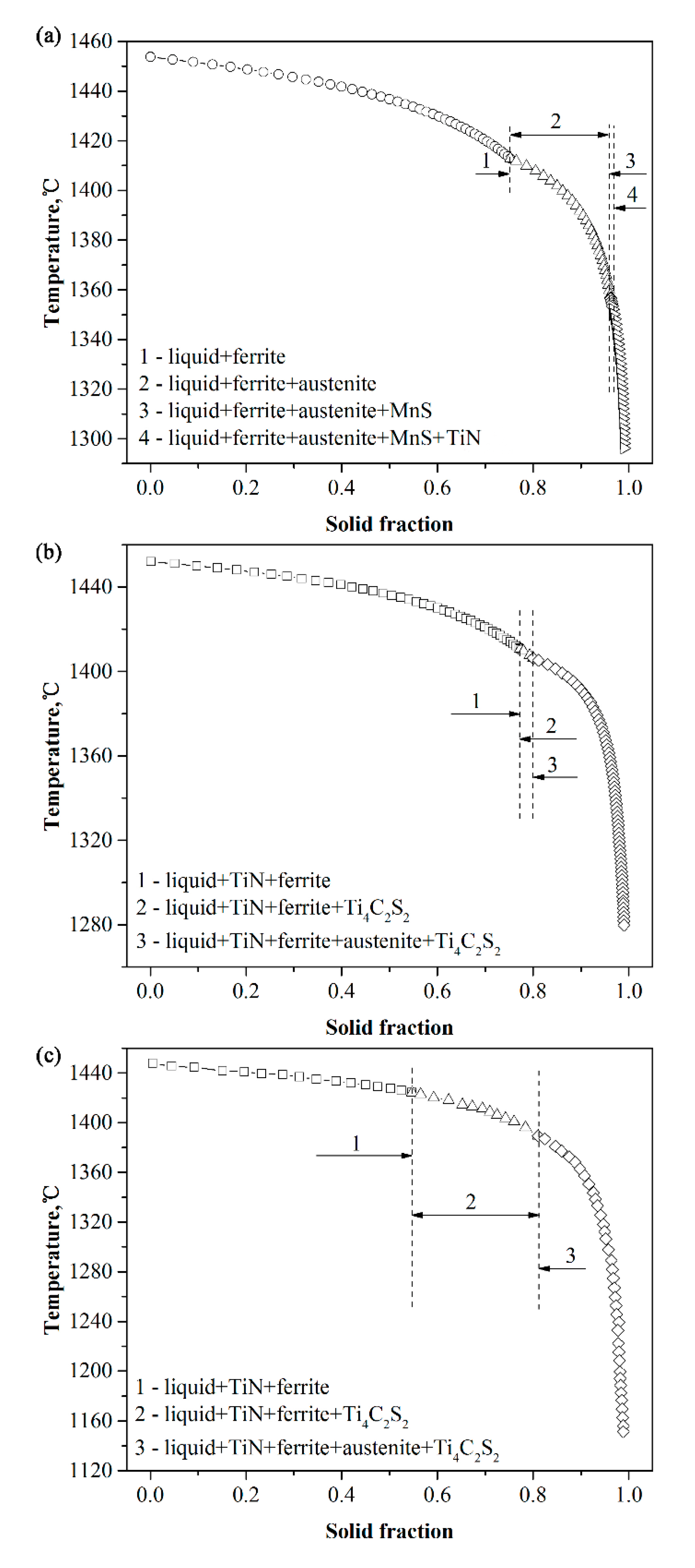

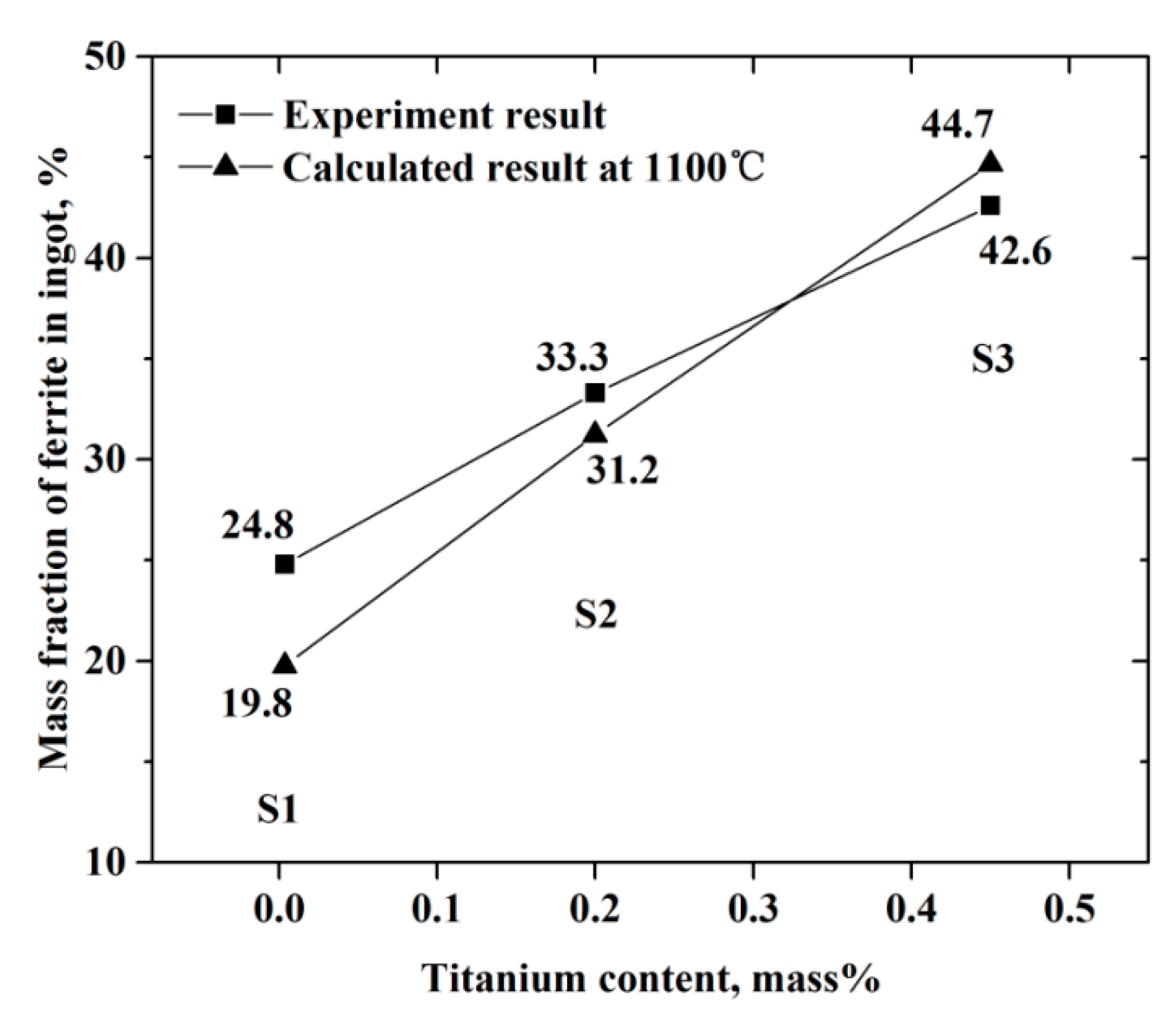
| Ingot | C | Si | Mn | S | Cr | Ni | Mo | Ti | N | O |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.0170 | 1.60 | 1.32 | 0.0080 | 20.11 | 8.55 | 0.4 | 0.0036 | 0.0190 | 0.0056 |
| S2 | 0.0076 | 1.59 | 1.38 | 0.0071 | 20.10 | 8.46 | 0.4 | 0.20 | 0.0170 | 0.0012 |
| S3 | 0.0074 | 1.65 | 1.37 | 0.0077 | 20.15 | 8.58 | 0.4 | 0.45 | 0.0081 | 0.0017 |
| Ingot | Ti Contents (mass%) | YS (MPa) | UTS (MPa) | Tensile Elongation (%) |
|---|---|---|---|---|
| S1 | 0.0036 | 207.41 | 452.31 | 31.20 |
| S2 | 0.20 | 229.11 | 471.24 | 30.92 |
| S3 | 0.45 | 238.89 | 494.87 | 31.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Cheng, G.; Hou, Y. Effect of Titanium Addition on As-Cast Structure and High-Temperature Tensile Property of 20Cr-8Ni Stainless Steel for Heavy Castings. Metals 2020, 10, 529. https://doi.org/10.3390/met10040529
Wang Q, Cheng G, Hou Y. Effect of Titanium Addition on As-Cast Structure and High-Temperature Tensile Property of 20Cr-8Ni Stainless Steel for Heavy Castings. Metals. 2020; 10(4):529. https://doi.org/10.3390/met10040529
Chicago/Turabian StyleWang, Qiming, Guoguang Cheng, and Yuyang Hou. 2020. "Effect of Titanium Addition on As-Cast Structure and High-Temperature Tensile Property of 20Cr-8Ni Stainless Steel for Heavy Castings" Metals 10, no. 4: 529. https://doi.org/10.3390/met10040529
APA StyleWang, Q., Cheng, G., & Hou, Y. (2020). Effect of Titanium Addition on As-Cast Structure and High-Temperature Tensile Property of 20Cr-8Ni Stainless Steel for Heavy Castings. Metals, 10(4), 529. https://doi.org/10.3390/met10040529





