Statistical Study for Leaching of Covellite in a Chloride Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Covellite
2.2. Reagent and Leaching Tests
2.3. Experimental Design
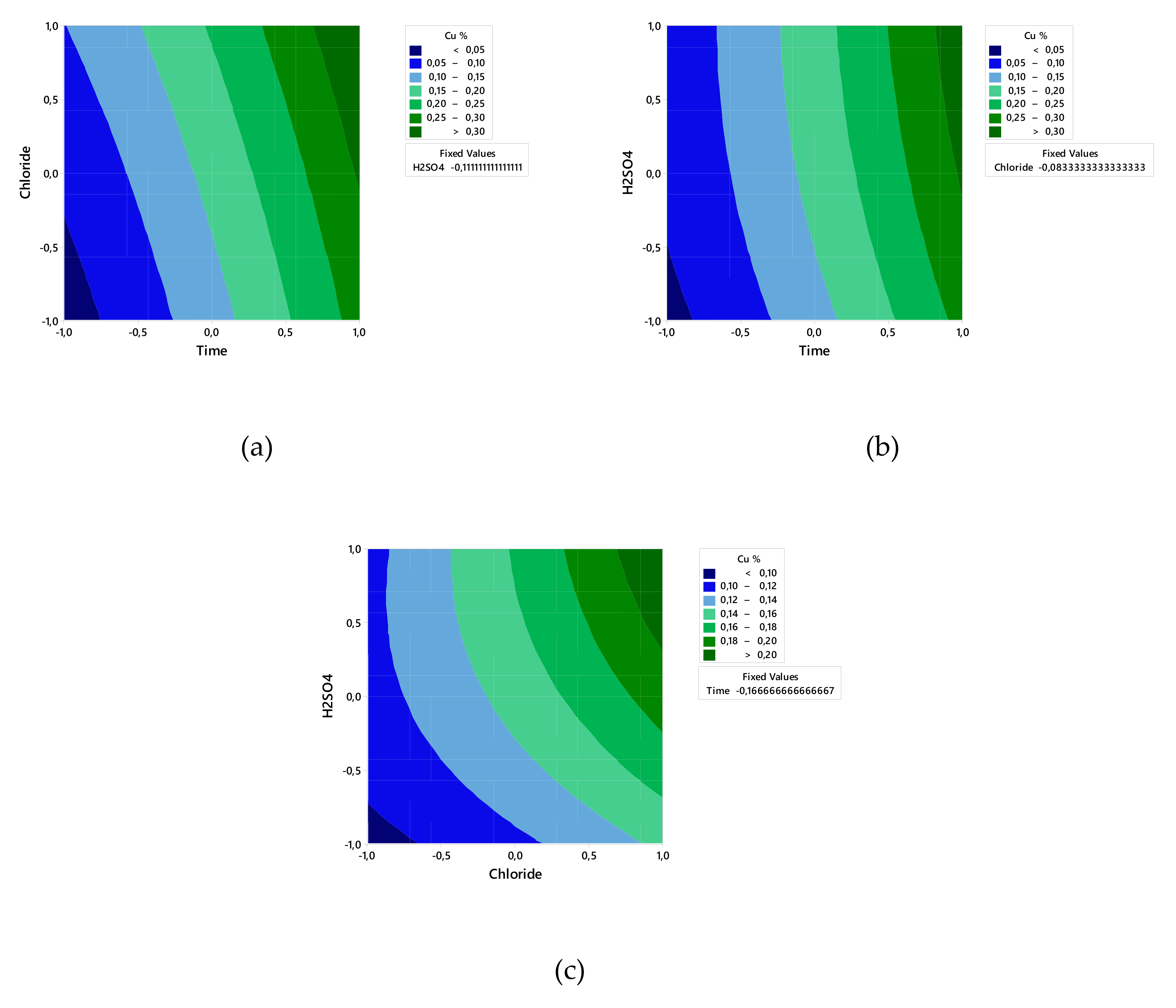
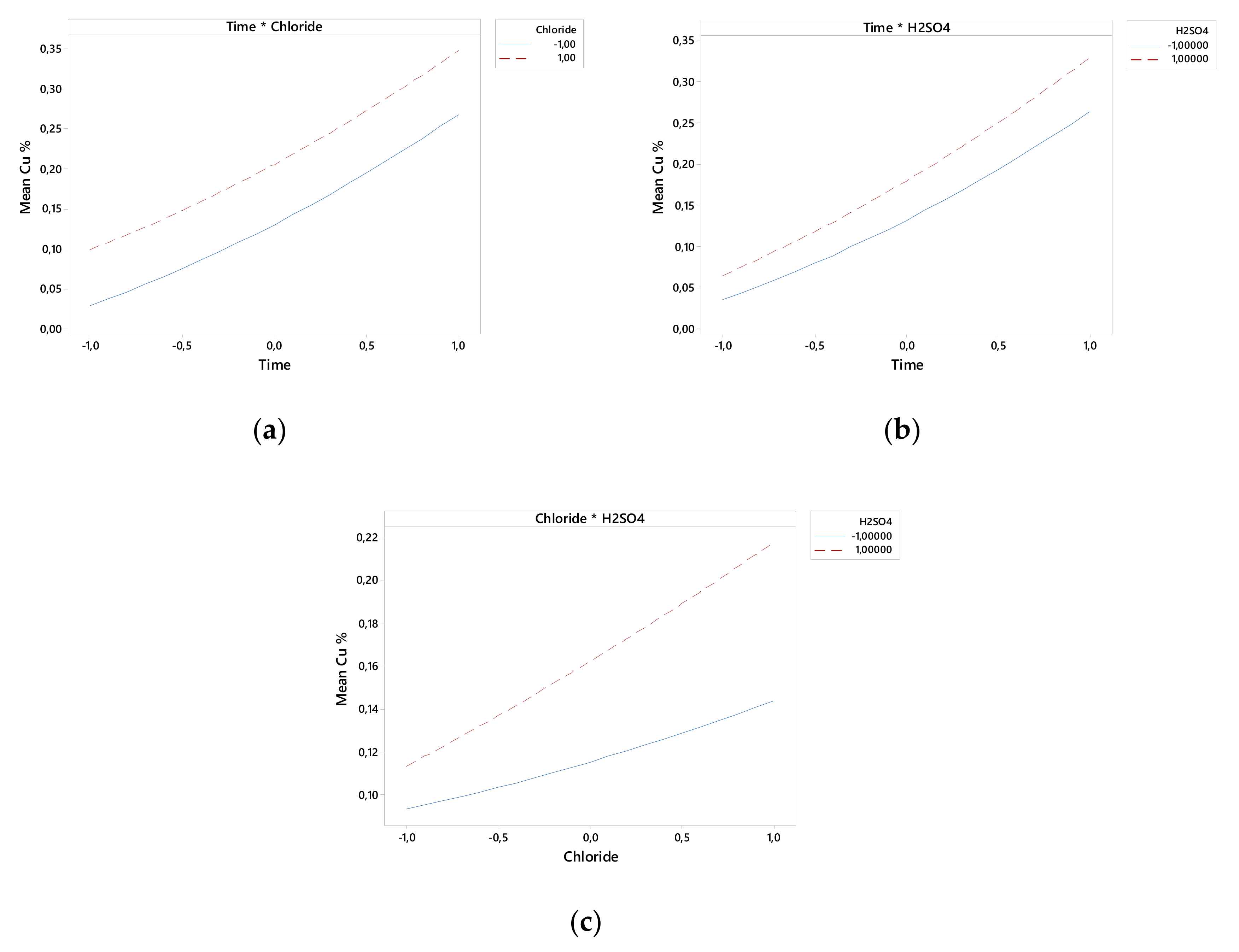
3. Results
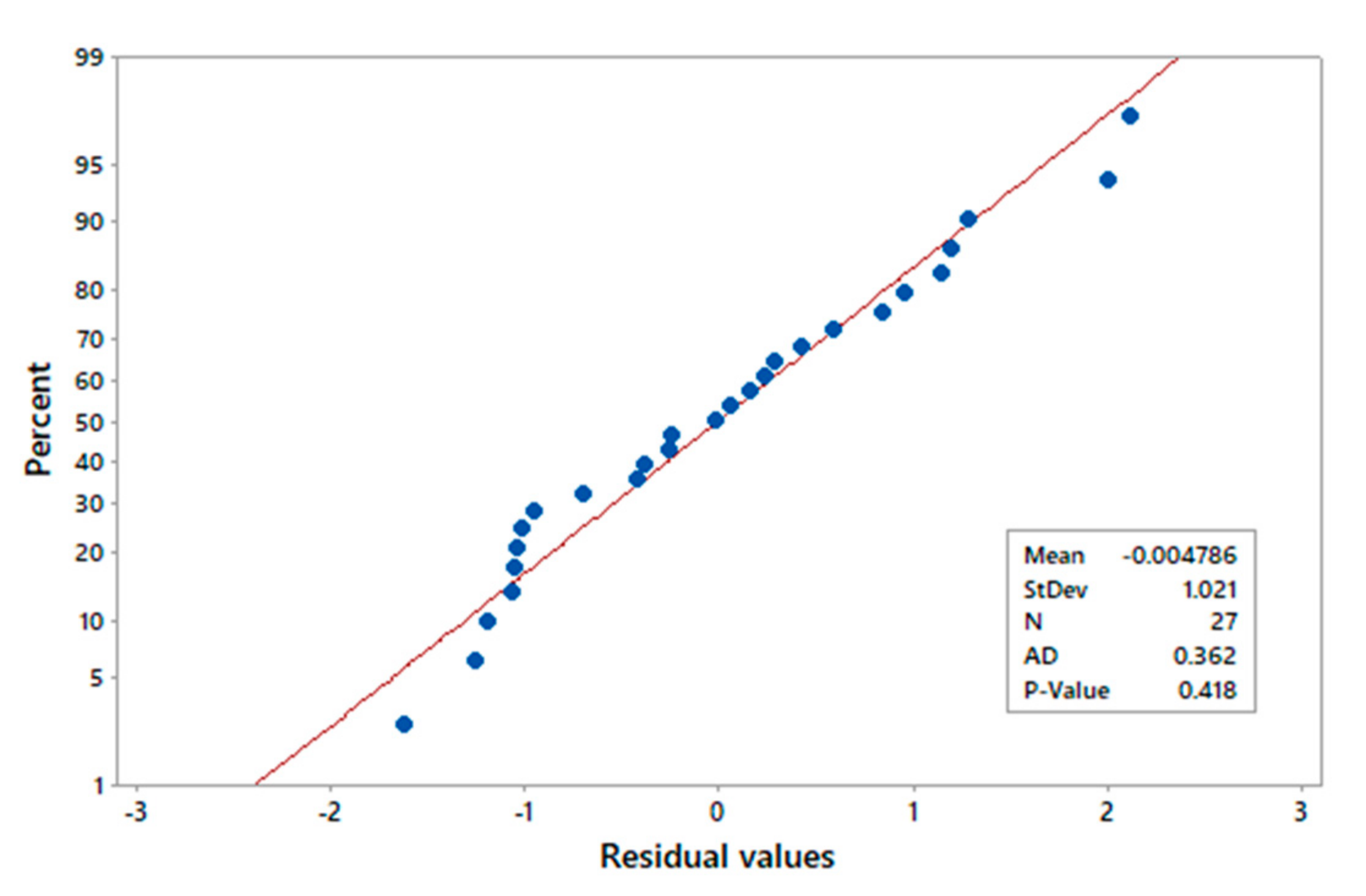
3.1. ANOVA
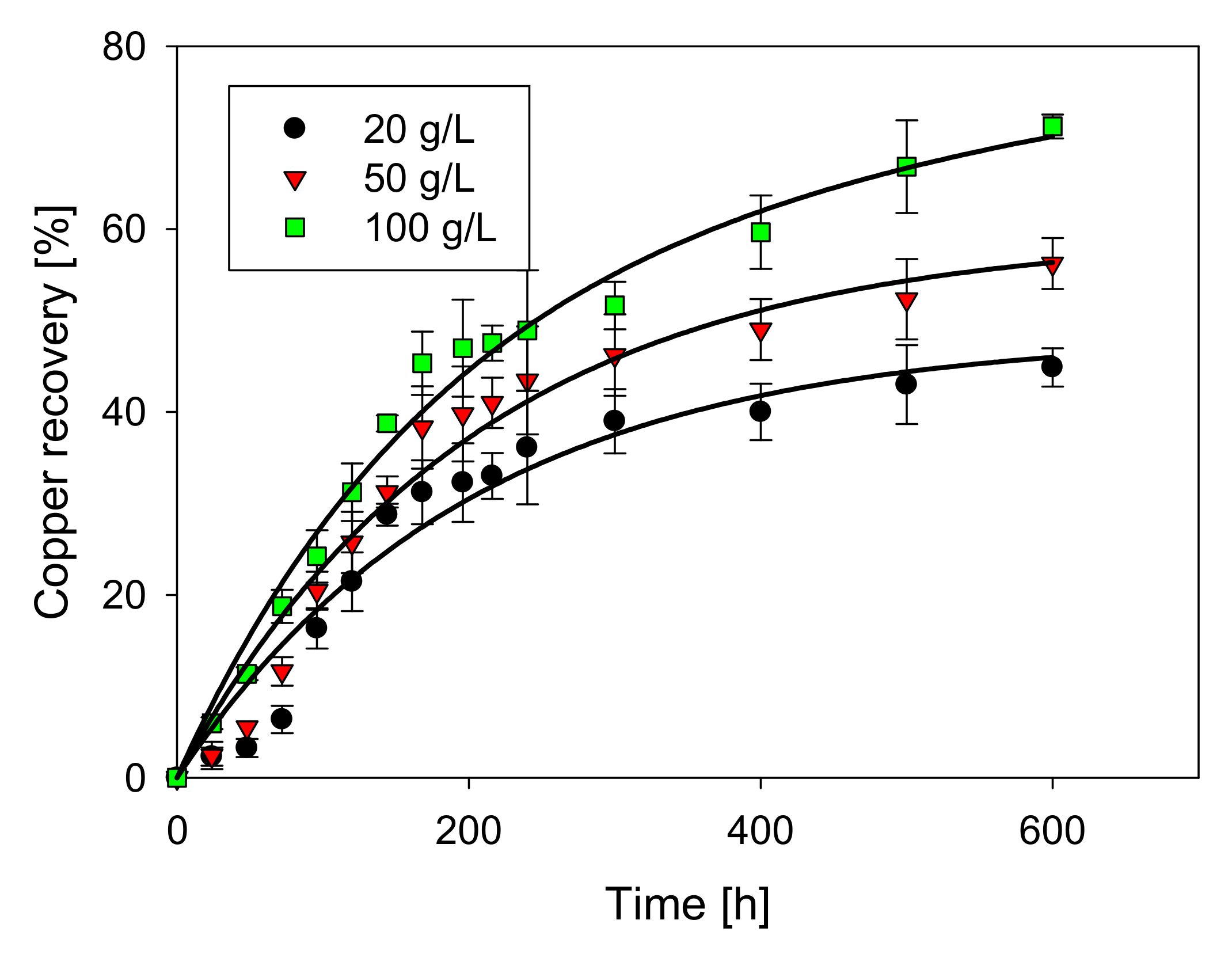
3.2. Effect of Chloride Concentration
4. Conclusions
- The linear variables of time and chloride concentration have the greatest influence on the model.
- Under ambient conditions of pressure and temperature, H2SO4 concentration–time and chloride concentration–time have significant effects on copper extraction kinetics from covellite.
- The ANOVA analysis indicates that the quadratic model adequately represents copper extraction, which was validated by the R2 value (0.9945).
- The highest copper extraction rate at ambient temperature of 71.2% was obtained with a low sulfuric acid concentration (0.5 M), high level of chloride (100 g/L), and extended leaching time (600 h).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, C.; Hurlbut, C.S. Manual de Mineralogía; Reverté: Madrid, Spain, 1996; ISBN 8429146067. [Google Scholar]
- Lundström, M.; Liipo, J.; Taskinen, P.; Aromaa, J. Copper precipitation during leaching of various copper sulfide concentrates with cupric chloride in acidic solutions. Hydrometallurgy 2016, 166, 136–142. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Honores, S.; Padilla, R. Leaching kinetics of digenite concentrate in oxygenated chloride media at ambient pressure. Metall. Mater. Trans. B 1998, 29, 961–969. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Oxford, UK, 2011; ISBN 9780080967899. [Google Scholar]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A. Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: A review. J. Clean. Prod. 2016, 131, 795–809. [Google Scholar] [CrossRef]
- Turan, M.D.; Sari, Z.A.; Miller, J.D. Leaching of blended copper slag in microwave oven. Trans. Nonferrous Met. Soc. China 2017, 27, 1404–1410. [Google Scholar] [CrossRef]
- Kelm, U.; Avendaño, M.; Balladares, E.; Helle, S.; Karlsson, T.; Pincheira, M. The use of water-extractable Cu, Mo, Zn, As, Pb concentrations and automated mineral analysis of flue dust particles as tools for impact studies in topsoils exposed to past emissions of a Cu-smelter. Chemie der Erde 2014, 74, 365–373. [Google Scholar] [CrossRef]
- Afif, C.; Chélala, C.; Borbon, A.; Abboud, M.; Adjizian-Gérard, J.; Farah, W.; Jambert, C.; Zaarour, R.; Saliba, N.B.; Perros, P.E.; et al. SO2 in Beirut: Air quality implication and effects of local emissions and long-range transport. Air Qual. Atmos. Heal. 2008, 1, 167–178. [Google Scholar] [CrossRef]
- Dijksira, R.; Senyard, B.; Shah, U.; Lee, H. Economical abatement of high-strength SO2 off-gas from a smelter. J. South. African Inst. Min. Metall. 2017, 117, 1003–1007. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Kostov, A.; Tasić, V.; Milosević, N. Influence of pyrometallurgical copper production on the environment. J. Hazard. Mater. 2009, 164, 892–899. [Google Scholar] [CrossRef]
- Sánchez de la Campa, A.M.; de la Rosa, J.D.; Sánchez-Rodas, D.; Oliveira, V.; Alastuey, A.; Querol, X.; Gómez Ariza, J.L. Arsenic speciation study of PM2.5 in an urban area near a copper smelter. Atmos. Environ. 2008, 42, 6487–6495. [Google Scholar] [CrossRef]
- Serbula, S.M.; Milosavljevic, J.S.; Radojevic, A.A.; Kalinovic, J.V.; Kalinovic, T.S. Extreme air pollution with contaminants originating from the mining—Metallurgical processes. Sci. Total Environ. 2017, 586, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Balladares, E.; Jerez, O.; Parada, F.; Baltierra, L.; Hernández, C.; Araneda, E.; Parra, V. Neutralization and co-precipitation of heavy metals by lime addition to effluent from acid plant in a copper smelter. Miner. Eng. 2018, 122, 122–129. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2018; ISBN 9789241565585. [Google Scholar]
- Baba, A.A.; Balogun, A.F.; Olaoluwa, D.T.; Bale, R.B.; Adekola, F.A.; Alabi, A.G.F. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 2017, 34, 1133–1140. [Google Scholar] [CrossRef]
- González, C.; Parra, R.; Klenovcanova, A.; Imris, I.; Sánchez, M. Reduction of Chilean copper slags: A case of waste management project. Scand. J. Metall. 2005, 34, 143–149. [Google Scholar] [CrossRef]
- Lü, C.; Wang, Y.; Qian, P.; Liu, Y.; Fu, G.; Ding, J.; Ye, S.; Chen, Y. Separation of chalcopyrite and pyrite from a copper tailing by ammonium humate. Chinese J. Chem. Eng. 2018, 26, 1814–1821. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Tepavitcharova, S.; Todorov, T.; Dassenakis, M.; Paraskevopoulou, V.; Petrov, M. Chemical speciation in mining affected waters: The case study of Asarel-Medet mine. Environ. Monit. Assess. 2009, 159, 353–366. [Google Scholar] [CrossRef]
- Reilly, I.G.; Scott, D.S. The leaching of cupric sulfide in ammonia. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 60–67. [Google Scholar] [CrossRef]
- Fisher, W.W. Comparison of chalcocite dissolution in the sulfate, perchlorate, nitrate, chloride, ammonia, and cyanide systems. Miner. Eng. 1994, 7, 99–103. [Google Scholar] [CrossRef]
- Vračar, R.Ž.; Vučković, N.; Kamberović, Ž. Leaching of copper(I) sulphide by sulphuric acid solution with addition of sodium nitrate. Hydrometallurgy 2003, 70, 143–151. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching covellite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef]
- Nicol, M.; Basson, P. The anodic behaviour of covellite in chloride solutions. Hydrometallurgy 2017, 172, 60–68. [Google Scholar] [CrossRef]
- Donati, E.; Pogliani, C.; Boiardi, J.L. Anaerobic leaching of covellite by Thiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 1997, 47, 636–639. [Google Scholar] [CrossRef]
- Monteiro, F.V.; Garcia, O.; Tuovinen, O. Oxidative dissolution of covellite by Thiobacillus ferrooxidans. Process Metall. 1999, 9, 283–290. [Google Scholar]
- Falco, L.; Pogliani, C.; Curutchet, G.; Donati, E. A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 2003, 71, 31–36. [Google Scholar] [CrossRef]
- Lee, J.; Acar, S.; Doerr, D.L.; Brierley, J.A. Comparative bioleaching and mineralogy of composited sulfide ores containing enargite, covellite and chalcocite by mesophilic and thermophilic microorganisms. Hydrometallurgy 2011, 105, 213–221. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Tan, Q.; Jia, Y.; Sun, H. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Abarzúa, E.; Padilla, R. Oxygen pressure leaching of white metal. Hydrometallurgy 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Senanayake, G. A review of chloride assisted copper sulfide leaching by oxygenated sulfuric acid and mechanistic considerations. Hydrometallurgy 2009, 98, 21–32. [Google Scholar] [CrossRef]
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity-Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Montes, K.S.; Padilla, R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Padilla, R.; Jerez, O.; Ruiz, M.C. Hydrometallurgy Kinetics of the pressure leaching of enargite in FeSO4–H2SO4–O2 media. Hydrometallurgy 2015, 158, 49–55. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Gallardo, E.; Padilla, R. Copper extraction from white metal by pressure leaching in H2SO4-FeSO4-O2. Hydrometallurgy 2009, 100, 50–55. [Google Scholar] [CrossRef]
- Aguirre, C.L.; Toro, N.; Carvajal, N.; Watling, H.; Aguirre, C. Leaching of chalcopyrite (CuFeS2) with an imidazolium-based ionic liquid in the presence of chloride. Miner. Eng. 2016, 99, 60–66. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljic, D. Response Surface Methodology. In Design and Analysis of Experiments; Springer Nature: Cham, Switzerland, 2017; pp. 565–614. [Google Scholar]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.; Sepúlveda, R. Initial investigation into the leaching of manganese from nodules at room temperature with the use of sulfuric acid and the addition of foundry slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Gálvez, E.; Cánovas, M.; Trigueros, E.; Castillo, J.; Hernández, P.C. Optimization of parameters for the dissolution of mn from manganese nodules with the use of tailings in an acid medium. Minerals 2019, 9, 387. [Google Scholar] [CrossRef]
- Saldaña, M.; Toro, N.; Castillo, J.; Hernández, P.; Trigueros, E.; Navarra, A. Development of an analytical model for the extraction of manganese from marine nodules. Metals 2019, 9, 903. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]

| Research Title | Dissolution Agents | Parameters Evaluated | Ref. | Maximum Cu Extraction (%) | Type of Covellite |
|---|---|---|---|---|---|
| The kinetics of leaching covellite in acidic oxygenated sulfate—chloride solutions | HCl, HNO3, NaCl, H2SO4 | Temperature, oxygen partial pressure, particle size, stirring speed, and sulfuric acid concentration | [23] | 85% | Synthetic covellite |
| The kinetics of dissolution of synthetic covellite, chalcocite, and digenite in dilute chloride solutions at ambient temperatures | HCl, Cu2+ and Fe3+ | Potential effect, chloride concentration, acid concentration, temperature, dissolved oxygen, and pyrite effect | [24] | >90% | Synthetic covellite |
| Element | Cu | S | Ca | O | H |
|---|---|---|---|---|---|
| Mass (%) | 56.14 | 31.08 | 3.66 | 8.76 | 0.36 |
| Experimental Variable | Low | Medium | High |
|---|---|---|---|
| Time (h) | 48 | 72 | 144 |
| Chloride Concentration (g/L) | 20 | 50 | 100 |
| H2SO4 Concentration (M) | 0.5 | 1 | 2 |
| Codifications | −1 | 0 | 1 |
| Exp. No. | Time (h) | Cl (g/L) | H2SO4 (M) | Cu Extraction Rate (%) |
|---|---|---|---|---|
| 1 | 48 | 20 | 0.5 | 2.50 |
| 2 | 48 | 50 | 0.5 | 3.50 |
| 3 | 48 | 100 | 0.5 | 6.00 |
| 4 | 48 | 20 | 1 | 3.00 |
| 5 | 48 | 50 | 1 | 3.63 |
| 6 | 48 | 100 | 1 | 9.13 |
| 7 | 48 | 20 | 2 | 3.25 |
| 8 | 48 | 50 | 2 | 5.50 |
| 9 | 48 | 100 | 2 | 11.38 |
| 10 | 72 | 20 | 0.5 | 5.13 |
| 11 | 72 | 50 | 0.5 | 8.75 |
| 12 | 72 | 100 | 0.5 | 11.25 |
| 13 | 72 | 20 | 1 | 5.88 |
| 14 | 72 | 50 | 1 | 9.25 |
| 15 | 72 | 100 | 1 | 13.88 |
| 16 | 72 | 20 | 2 | 6.38 |
| 17 | 72 | 50 | 2 | 11.63 |
| 18 | 72 | 100 | 2 | 18.75 |
| 19 | 144 | 20 | 0.5 | 24.63 |
| 20 | 144 | 50 | 0.5 | 24.88 |
| 21 | 144 | 100 | 0.5 | 28.75 |
| 22 | 144 | 20 | 1 | 26.25 |
| 23 | 144 | 50 | 1 | 29.75 |
| 24 | 144 | 100 | 1 | 35.00 |
| 25 | 144 | 20 | 2 | 28.75 |
| 26 | 144 | 50 | 2 | 31.25 |
| 27 | 144 | 100 | 2 | 38.75 |
| Source | F-Value | p-Value |
|---|---|---|
| Regression | 371.42 | 0.000 |
| Time | 2624.36 | 0.000 |
| Cl | 257.04 | 0.000 |
| H2SO4 | 105.5 | 0.000 |
| Time × Time | 9.7 | 0.006 |
| Cl × Cl | 0.56 | 0.466 |
| H2SO4 × H2SO4 | 3.39 | 0.083 |
| Time × Cl | 0.81 | 0.379 |
| Time × H2SO4 | 11.22 | 0.004 |
| Cl × H2SO4 | 22.6 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, K.; Toro, N.; Saldaña, M.; Salinas-Rodríguez, E.; Robles, P.; Torres, D.; Jeldres, R.I. Statistical Study for Leaching of Covellite in a Chloride Media. Metals 2020, 10, 477. https://doi.org/10.3390/met10040477
Pérez K, Toro N, Saldaña M, Salinas-Rodríguez E, Robles P, Torres D, Jeldres RI. Statistical Study for Leaching of Covellite in a Chloride Media. Metals. 2020; 10(4):477. https://doi.org/10.3390/met10040477
Chicago/Turabian StylePérez, Kevin, Norman Toro, Manuel Saldaña, Eleazar Salinas-Rodríguez, Pedro Robles, David Torres, and Ricardo I. Jeldres. 2020. "Statistical Study for Leaching of Covellite in a Chloride Media" Metals 10, no. 4: 477. https://doi.org/10.3390/met10040477
APA StylePérez, K., Toro, N., Saldaña, M., Salinas-Rodríguez, E., Robles, P., Torres, D., & Jeldres, R. I. (2020). Statistical Study for Leaching of Covellite in a Chloride Media. Metals, 10(4), 477. https://doi.org/10.3390/met10040477








