Abstract
The present study aims to reveal the mechanism of element vaporization of Ti-6Al-4V alloy during selective laser melting (SLM). The equations of Redlich–Kister and the thermodynamics principles were employed to calculate the vaporization thermodynamics, which contributes to the obtaining the vaporization kinetic based on the Chapman-Enskog theory and the diffusion model. According to the achieved vaporization model, the elements with the most prominent tendency and flux to vaporize were distinguished. Moreover, the effect of the process parameters on the vaporization of Al and Ti is experimentally investigated using inductively coupled plasma optical emission spectrometer (ICP) technology. The analyzed results of the chemical composition of the powders and builds show a great agreement with the kinetic results calculated by the vaporization model. Notably, the element vaporization can be curbed by regulating the laser energy input.
1. Introduction
Selective laser melting (SLM) is an advanced additive manufacturing (AM) technique in which the components are fabricated layer-by-layer by selectively melting powders with a high-energy laser beam in an inert gas atmosphere [1,2,3]. This technology is characterized by high forming precision and can freely fabricate extremely complex components, such as molds with internal flow channels and various lightweight structures [4,5]. So far, SLM has been successfully used in the manufacturing of stainless steel, aluminum alloy, nickel-based alloy, titanium alloy, etc. [6].
Since titanium alloys have the advantages of high strength-to-weight ratio, favorable biocompatibility, excellent corrosion resistance, and high-temperature mechanical properties, it is widely used in aerospace and medical fields. In titanium alloys, the application of Ti-6Al-4V alloy, which is a kind of α+β two-phase titanium alloy consisting of about 6 wt.% Al and 4 wt.% V [7,8], exceeds 50%.
To date, SLM of Ti-6Al-4V alloy has been widely used in the aerospace industry and medical treatment. However, the energy source of SLM is a high-energy laser, inducing a high-temperature molten pool. As a result, the vaporization of elements is difficult to avoid, which disturbs the stability of the molten pool and alters the composition of raw materials, consequently affecting the mechanical properties of the builds [9,10]. Hence, it is of great importance to explore the mechanism of vaporization during SLM of Ti-6Al-4V parts.
The vaporization of elements for the conventional forming methods of titanium alloy is well documented. Electron beam cooling bed melting [11] and vacuum induction shell melting [12] are two major methods for fabricating titanium components in conventional industry. These forming processes are conducted under vacuum to prevent oxidation due to the high activity and natural oxidation of melted titanium alloy. Therefore, the vaporization loss of elements is difficult to avoid in such conditions. Powell et al. conducted careful work establishing a mathematical model to predict the vaporization of titanium and titanium alloy in an electron-beam furnace [13]. Akhonin et al. studied the element vaporization during vacuum electron beam melting (EBM) of Ti-6Al-4V alloy, and established a kinetic vaporization model that accords with the experimental data. However, those studies failed to consider the diffusion of vaporization elements from the melt pool to the liquid/gas interface [14]. Semiatin et al. analyzed and calculated the vaporization loss of Al in the vacuum electron beam melting of Ti-6Al-4V alloy. They found that the vaporization flux was controlled by both the interfacial reaction and the mass transferred in the liquid [15]. Wei et al. studied the vaporization of Mg and Zn in SLM process of Mg-Zn-Zr alloy. The results illustrated that the total content of Mg and Zn decreased after the building process [16]. Recently, Yao et al. studied the vaporization behavior of Ti-Mo alloy during EBM, with the results showing that the melting power and the melting time were two critical factors in controlling the vaporization [17].
The vaporization kinetic models used in the previous reports were mainly based on the Langmuir model, which is widely applied in the vacuum processing environment [18]. Nevertheless, during the SLM process, the processing environment is filled with protective gas to construct a non-vacuum inert gas protection atmosphere. As is well documented, there is a critical pressure for element vaporization under a non-vacuum environment [19]. When the ambient pressure is less than the critical pressure, the vaporization process is mainly controlled by interfacial reaction, which can be descripted by the Langmuir model. Conversely, as the ambient pressure is higher than the critical pressure, the vaporization process will be mainly controlled by the diffusion of volatile elements in the inert gas, which can be described by the diffusion model. The value of critical pressure is considered to be approximately equal to the saturated vapor pressure of the volatile element [20].
To the authors’ knowledge, the model adopted to depict the vaporization behavior during SLM process has rarely been reported. In this paper, a model of vaporization thermodynamics and kinetics was developed to analyze the vaporization behavior of Al and Ti elements in Ti-6Al-4V during SLM process. Meanwhile, both the orthogonal experiment and single experiment were carried out to explore the effect of processing parameters on the vaporization loss of Al and Ti. This work aims to provide a reliable foundation for optimizing the SLM process parameters and guaranteeing the composition of Ti-6Al-4V builds. On the other hand, the proposed methodology is also promising for characterizing the vaporization behavior of other alloys such as aluminum alloy and magnesium alloy during the SLM process.
2. Experiment and Mathematical Model
2.1. Experiments
The as-used SLM system is a Concept Laser M2, containing a fiber laser with the wavelength of 1060~1100 nm. This system has a maximum laser power of 400 W, and the laser spot diameter is 50 μm. During the building process, the working chamber is filled with inert argon gas, and the pressure is fixed as a constant of 0.1 MPa to ensure the content of oxygen less than 0.5%.
The building plate used in the experiment is forged Ti-6Al-4V alloy, and the powder used for printing is the spherical powder of Ti-6Al-4V prepared by plasma-rotating electrode process. The chemical compositions of this powder are shown in Table 1.

Table 1.
Composition of Ti-6Al-4V alloy powder (wt.%), part based on the literature [21].
The orthogonal experiment with three factors and five levels was used to evaluate the vaporization of alloying elements. The characteristics of the orthogonal experiment are shown in Table 2. Layer thicknesses of 30 μm, 45 μm, and 60 μm were selected to research the effect of the layer thickness on the element vaporization. Seventy-five different sets of parameters were used to fabricate samples in the orthogonal experiment. Two identical samples were built using each kind of parameter to ensure the reliability, so there were 150 samples in total. The size of each sample is Φ 8 × 10 mm.

Table 2.
Process parameters of orthogonal experiment.
At the same time, a single-factor experiment was designed to explore the effect of the scanning speed on the element vaporization. The experimental parameters are shown in Table 3. To reduce the error induced by the experiment, three identical samples were formed under each set of parameters.

Table 3.
Single-factor experiment of scanning speed.
It is difficult to detect the mass fraction of the pure elements in the alloy. One method was a non-destructive method, which used energy dispersive spectroscopy (EDS) or X-ray diffraction (XRD) to measure the mass fraction from bottom to top on the sample surfaces [22]. The other was a destructive method using the inductively coupled plasma optical emission spectrometer (ICP) to measure the mass fraction by dissolving a part of the samples [6]. The latter was able to provide better accuracy and was adopted to measure the composition of the fabricated samples in this work. However, direct measurement of the mass fraction of the main alloying element Ti caused a large error by ICP. Thereby, the mass fraction of Ti was obtained based on the measurements of Al and V in the alloy. The instrument model was a PerkinElmer-pe 8300.
2.2. Vaporization Thermodynamic
The thermodynamic calculation includes the activity coefficient (the ratio of activity (effective concentration) to concentration in a mixture of chemical substances) and the saturated vapor pressure of the elements in the alloy. The elements with high saturated vapor pressure present a significant tendency to vaporize. Meanwhile, the activity coefficient is essential for obtaining the saturated vapor pressure.
Compared to several different calculation methods for the activity coefficient, it can be found that the excess free energy of the ternary system is obtained by the partial molar excess free energy of the three binary systems. The partial molar excess free energy of each element in the alloy is obtained based on the excess free energy of the ternary system as the molar fraction of the elements in the alloy is certain. Finally, the relationship between the partial molar excess energy and the activity coefficient is used to obtain the activity coefficients of the three elements in a ternary alloy [23].
The calculation of the activity coefficient of each element i in the alloy is given as:
where is the activity coefficient of element i in the alloy, is the partial molar excess free energy of element i, R is the gas constant, and T is absolute temperature.
For ternary alloy, the partial molar excess free energy can be obtained from the excess energy of the ternary system. i, j and k represent three different elements in ternary alloy, respectively. The partial molar free energy of element i, j and k is given by:
where and represent the mole fraction of element i and j in the alloy, respectively.
The excess free energy of the ternary system can be approximately calculated by the excess free energy of three binary systems.
The Redlich-Kister equation [24] is applied to obtain the excess free energy of three binary systems.
For the ternary titanium alloy system of Ti-Al-V, the relevant parameter values in Equation (6) are shown in Table 4.

Table 4.
Relevant parameters in the Redlich-Kister equation.
The function between the activity coefficient and temperature is shown in Figure 1a. It can be observed that as the temperature increases, the activity coefficients of Ti and V consistently decrease, while the activity coefficient of Al increases distinctly. The activity coefficient of the three elements would approach 1 with the increase of temperature, which is consistent with the classical thermo-dynamical theory. When the temperature is below 2900 K, the activity coefficient of Al is smaller than that of Ti and V. However, as the temperature is higher than 2950 K, the activity coefficient of Al is more than 1, indicating that the activity coefficient has deviated from the normal range.
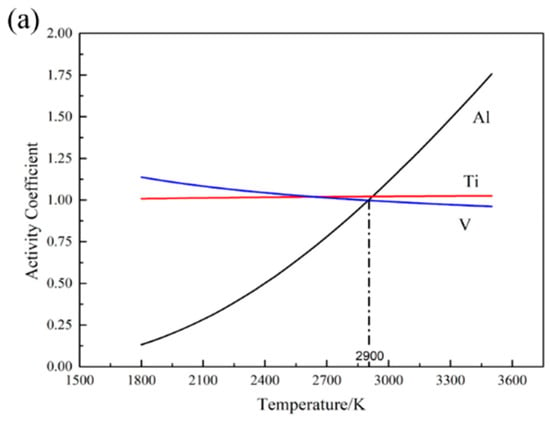
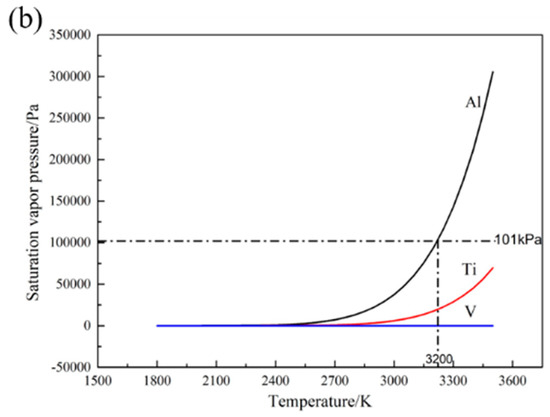
Figure 1.
Thermodynamic calculation results of element vaporization: (a) the activity coefficient; (b) the saturated vapor pressure.
The vaporization behavior of the elements is closely related to the saturated vapor pressure. The higher the saturated vapor pressure is, the larger the quantity of vaporization loss becomes. Therefore, the calculation of saturated vapor pressure is crucial for determining the most sensitive element for vaporization in Ti-6Al-4V alloy [25].
Element-saturated vapor pressure in Ti-6Al-4V alloy is:
where is the saturated vapor pressure of element i in alloy, and is saturated vapor pressure of pure metal i.
The saturated vapor pressure of pure metal is the function of the temperature, which can be expressed as:
The saturated vapor pressure of pure metal is attained when the gas phase is equilibrium [26].
where A, B, C, and D represent the vapor pressure constants of alloying elements. The vapor pressure constants of three main elements in Ti-6Al-4V alloy are shown in Table 5.

Table 5.
Vapor pressure constant of main elements in Ti-6Al-4V alloy.
According to the calculation of the activity coefficient and the saturated vapor pressure, the function between the saturated vapor pressure and the temperature of three elements in Ti-6Al-4V alloy is obtained. The calculated saturated vapor pressure versus temperature curve is shown in Figure 1b. It can be seen that Al element shows the maximum saturated vapor pressure in three elements, indicating that Al has more tendency to vaporize. It should be noted that the saturated vapor pressures of these three elements in Ti-6Al-4V are less than 101 KPa (close the ambient pressure of SLM chamber) as the temperature is lower than 3200 K. It means that the ambient pressure is higher than the critical pressure as the temperature of molten pool is below 3200 K, and the element vaporization is mainly controlled by the diffusion of volatile element in SLM chamber. When the temperature of molten pool exceeds 3200 K, the element vaporization is mainly controlled by interfacial reaction which can be expressed by Langmuir model. However, the boiling temperature of Ti-6Al-4V is about 3133 K, which illustrates elements diffusion plays a primary role in the vaporization process. Therefore, it is necessary to build a diffusion model to character the element vaporization during SLM process.
2.3. Vaporization Kinetics
For the element vaporization dynamic behavior in the induction skull melting process, Guo et al. [27] suggested an applicable model that divides the vaporization behavior into six stages. Nevertheless, there are three differences between the SLM process and the induction skull melting process:
- 1)
- The surrounding of the molten pool is full of inert gas with a pressure close to atmospheric pressure during SLM process;
- 2)
- During the SLM process, the inert gas will be continuously filled to purify the forming chamber, since on the upside of the molten pool, a gas stratosphere will be generated during the building process;
- 3)
- The molten pool size of SLM is minimal, and the existent time of molten pool is extremely short.
Accordingly, the element vaporization behavior during SLM should be divided into the following five stages:
- 1)
- The volatile element i in the alloy diffuses to the liquid/gas interface in the molten pool;
- 2)
- Interfacial reaction of element i occurs at liquid/gas interface, which makes element i convert from liquid state to gas state;
- 3)
- The vapor of element i diffuses to the gas stratosphere, which is created by continuously filled inert gas;
- 4)
- The vapor of element i is brought to the air-vent by constantly inert airflow. The air-vent is set in the SLM machine to exhaust the gas, which is generated during SLM working;
- 5)
- The vapor of element i transforms into a stable solid at the air-vent.
Based on the analysis of the vaporization thermodynamics, the third stage, which can be expressed by the diffusion model, is the primary controlling stage for element vaporization during SLM process.
An element diffusion model accessible for SLM process was proposed by Geiger et al. [28], which can be used to calculate the quantity of the element vaporization loss when the atmosphere is filled with static inert gas. This model is shown in Figure 2a. The vaporization flux of element i can be expressed as:
where is the actual vaporization flux of element i on the surface of the molten pool, is diffusion coefficient of element i in inert gas, and is the molar concentration of volatile elements on the melt surface, and is the molar concentration of the volatile elements in the gas stratosphere. Since the flow velocity of the inert gas in the stratosphere is swift and can carry off the volatile elements quickly during the SLM process, can be further assumed to zero.
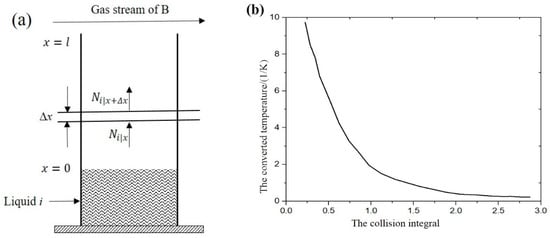
Figure 2.
Element vaporization kinetics calculation [28]: (a) element vaporization in static gas atmosphere; (b) the functional between collision integral and converted temperature.
The diffusion coefficient of element i in inert gas B can be obtained by using the Chapman–Enskog model [29].
where is the diffusion coefficient of element i in inert gas B, is the pressure in the working chamber, is the average atomic collision radius, is the collision integral, which can be obtained by the converted temperature . The relationship between the two variables is shown in Figure 2b. is the molar mass of element i, and is the molar mass of the inert gas B.
The converted temperature can be obtained by the equation:
where is the boiling point of the element i, and is the boiling point of the inert gas B.
The characterization of the vaporization flux and the temperature of the molten pool is shown in Figure 3a. The vaporization fluxes of Al and Ti are exponentially increased with the increment of temperature. It should be noted that the boiling point of Ti-6Al-4V alloy is 3133 K. Therefore, the rapid vaporization loss hardly happens. The ratio of the saturation vapor pressure and the vaporization flux of Al and Ti is shown in Figure 3b. The vaporization flux ratio of Al and Ti decreased compared to the saturation vapor pressure ratio (as shown in Figure 3b), which demonstrates the gap between Al and Ti decreases due to the maximum mole fraction of Ti in Ti-6Al-4V alloy. However, the element of Al also has the biggest vaporization flux. Therefore, the vaporization loss of Al is more than Ti according to the vaporization kinetics.
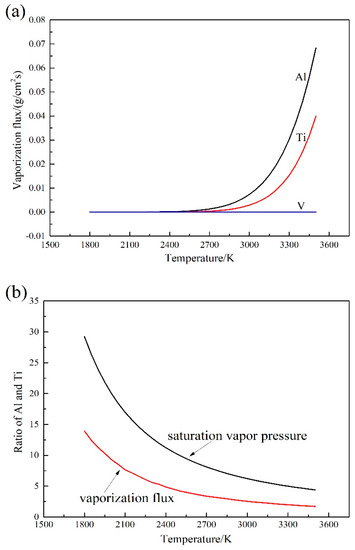
Figure 3.
Kinetic calculation results of element vaporization: (a) relationship between vaporization fluxes of different elements and temperature; (b) ratio of saturation vapor pressure and vaporization flux of Al and Ti.
3. Experimental Verification and Discussion
3.1. The Orthogonal Experiment
In this work, the orthogonal experimental method is used to investigate the effects of the laser power, the scanning speed, and the hatch distance on the vaporization of Al and Ti elements under different layer thicknesses during the SLM process. Meanwhile, the effect of the layer thickness on the vaporization behavior is also investigated, as shown in Figure 4.
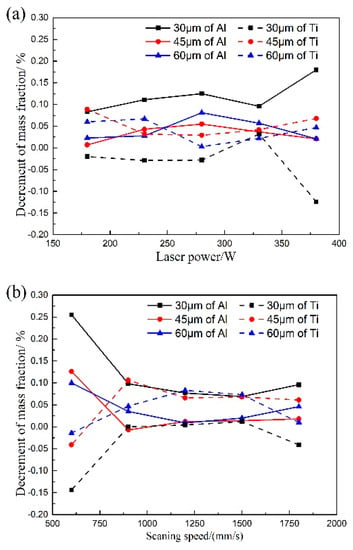
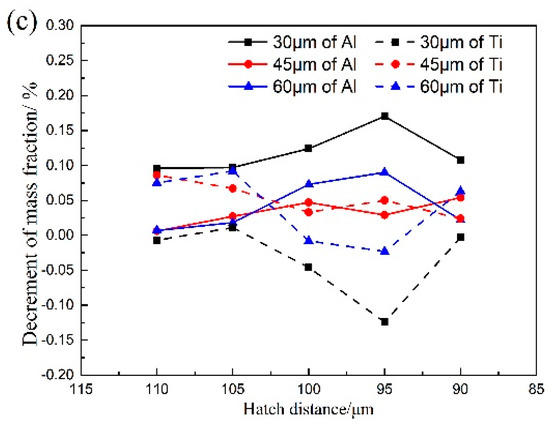
Figure 4.
Effect of process parameters on the element vaporization of Al and Ti in alloy: (a) laser power; (b) scanning speed; (c) hatch distance.
Figure 4a presents the effect of the laser power on the vaporization loss in different layer thicknesses. With the increase of the laser power, the decrement of the mass fraction of Al and Ti fails to change distinctly. Therefore, it can be inferred that the laser power has little influence on the vaporization loss. In contrast, the vaporization loss of Al and Ti is significantly different under the three different layer thicknesses. When the layer thickness decreases from 60 μm to 30 μm, the vaporization loss of Al picks up, while the vaporization loss of Ti drops. The decrement of the mass fraction (increment of vaporization loss) of Al, due to the increment of the vaporization loss, will induce a relative increment of Ti in mass fraction.
It can be seen from Figure 4b that the vaporization loss of Al in the SLMed samples exhibits a noticeable change with the increase of the scanning speed. Notably, as the scanning speed increases from 600 mm/s to 900 mm/s, the vaporization loss of Al significantly declines, while the vaporization loss of Ti sharply increases. It can be deduced that if the scanning speed was less than 600 mm/s, there would be an apparent vaporization loss of Al. It can be seen from Figure 4c that with the decrease of the hatch distance, the vaporization loss of Al smoothly increases but the vaporization loss of Ti decreases.
The effect of the layer thickness on the vaporization loss is legible in Figure 4. When the layer thicknesses of 45 μm and 60 μm are chosen, there is no noticeable vaporization loss of Al and Ti. However, adopting a layer thickness of 30 μm entails a pronounced vaporization loss of Al and Ti. During SLM building of the multi-layer samples, the top region of each layer would be partially remolten when the adjacent layer is deposited. The remolten depth increases with the decrement of the layer thickness, which allows a longer time to vaporize for the molten pool. As a result, the vaporization loss of Al and Ti increases at the layer thickness of 30 μm. As for the layer thickness between 45 μm and 60 μm, the increment of remolten depth will be small compared to the layer thickness between 30 μm and 45 μm. Thus, the vaporization loss between 45 μm and 60 μm is not apparent.
By comparing the vaporization loss of Al and Ti at the layer thickness of 30 μm, it can be concluded that Al has more significant vaporization flux than Ti, which is in line with the results obtained from the kinetic calculations. Because of the limitation to acquire the molten pool geometry and temperature distributions, which are critical in calculating the vaporization fluxes and vaporization loss of elements, the proposed model in this work was qualitatively validated by experimental results only.
The vaporization of Al is greater than that of Ti, so the vaporization of Al should be paid more attention, and is valuable for performing further research. The salience analysis of the laser power, scanning speed and hatch distance on the vaporization of element Al are calculated. The analytical results are shown in Table 6, Table 7 and Table 8, respectively. Among these three process parameters, it can be found that the scanning speed has the most significant effect on the vaporization of Al. Basically, the single-factor experiment on the scanning speed is performed to further study its effect on the element vaporization.

Table 6.
Significant level of each factor under a layer thickness of 30 µm.

Table 7.
Significant level of each factor under a layer thickness of 45 µm.

Table 8.
Significant level of each factor under a layer thickness of 60 µm.
3.2. The Single-Factor Experiment
The resulting effect of the single variable of the scanning speed on the vaporization of Al element is shown in Figure 5. With the increase of the scanning speed, the mass fraction of Al gradually picks up, which demonstrates the vaporization loss of Al gradually drops. When the scanning speed increases from 250 mm/s to 500 mm/s, the decrement of the vaporization loss is remarkable.
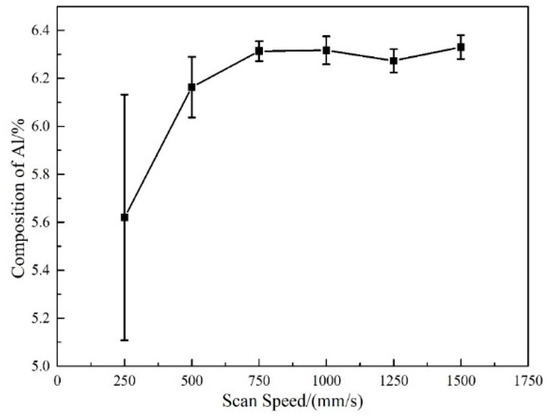
Figure 5.
Effect of scanning speed on the mass fraction of Al.
In this paper, the laser energy density is measured by the body energy density [1]:
where is the laser body energy density, is the laser power, is the laser scanning speed, is the hatch distance, and is the layer thickness. The body energy density dramatically decreases from 440 J/mm3 to 220 J/mm3 as the scanning speed increases from 250 mm/s to 500 mm/s. The decrement of the body energy density would result in a decline of both the molten pool temperature and the existent time. Thereby, the vaporization loss of Al would also decrease. For that reason, the pronounced adjustment of the body energy density should be responsible for the significant change of the vaporization loss as the scanning speed is elevated from 600 mm/s to 900 mm/s in the orthogonal experiment. However, when the scanning speed exceeds 750 mm/s (the body energy density is less than 146.7 J/mm3), the mass fraction of Al barely changes, which indicates the vaporization loss of Al hardly appears.
3.3. Prediction of Vaporization
All of the SLM parameters in the orthogonal experiment can be transformed into the body energy density. Setting the mass fraction of Al in the SLMed samples as Y-axis and the laser body energy density as X-axis, a simple functional relationship between the two variables could be established, which is shown in Figure 6. The formula could be expressed as:
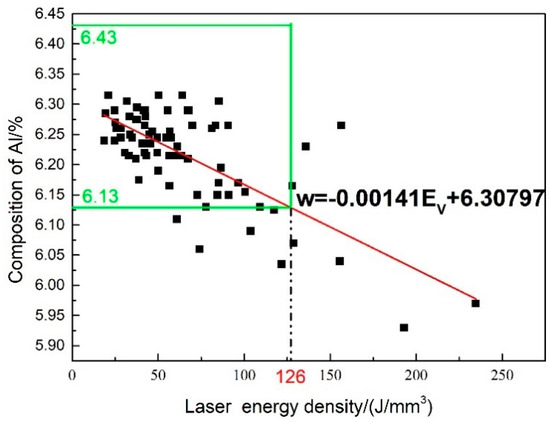
Figure 6.
Relationship between body energy density and mass fraction of Al in formed components.
According to the ASTM B381-13 standard specification for titanium and titanium alloy forgings [30], once the mass fraction change of Al element is more than 0.15%, it is considered to be unacceptable. Therefore, 0.15% is termed as the critical value to determine whether or not the notable vaporization loss generates. The green box depicts a domain where the variation range of Al in the SLMed samples is less than 0.15% compared to the powder. When the Al in Ti-6Al-4V alloy shows an evident vaporization loss (more than 0.15%), the body energy density is higher than 126 J/mm3 from the fitting curve. In addition, the excellent mechanical properties of SLM Ti-6Al-4V alloy are achieved in the literature [3,31,32,33] as the laser body energy density is less than 126 J/mm3. Therefore, it can be deduced that in the regular SLM building process of Ti-6Al-4V, the element vaporization loss barely happens.
4. Conclusions
In the present investigation, a diffusion model is proposed to describe the element vaporization behavior of Ti-6Al-4V during SLM process. The influence of the processing parameters on the vaporization loss of element Al is discussed in detail. The critical conclusions are summarized as follows:
- (1)
- In Ti-6Al-4V alloy, Al element has the maximum saturated vapor pressure, followed by Ti element, and the saturated vapor pressure of V element is almost zero, which indicates that Al element has the potential to vaporize in the building process.
- (2)
- During the SLM process of Ti-6Al-4V alloy, the element vaporization is mainly controlled by the diffusion of the metal vapor in an inert atmosphere. The diffusion model is used to calculate the kinetics of the element vaporization flux. The result indicates that Al has the most significant vaporization flux and vaporization loss.
- (3)
- Orthogonal experiment and single factor experiment demonstrate that the scanning speed pronouncedly affects the element vaporization loss. The quantity of the vaporization loss of Al is larger than Ti, supporting the result that the vaporization flux of Al is higher than Ti. This is in line with the vaporization kinetic prediction by the diffusion model.
- (4)
- The quantity of the vaporization loss picks up as the laser body energy density increases. When the laser energy density exceeds 126 J/mm3 in Ti-6Al-4V SLM process, the temperature of the molten pool is significantly elevated to contribute to a remarkable vaporization loss (>0.15%) of Al.
Author Contributions
Conceptualization, J.C., X.L. (Xin Lin) and G.Z.; methodology, W.H.; data curation, M.Z. and G.Z.; writing—original draft preparation, G.Z.; writing—review and editing, M.Z. and X.L. (Xufei Lu); supervision, J.C. and Z.Y.; project administration, Z.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Program on Key Basic Research Project (973 Program) of China (Grant No. 613281).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Tong, J.; Bowen, C.R.; Persson, J.; Plummer, A.R. Mechanical properties of titanium-based Ti-6Al-4V alloys manufactured by powder bed additive manufacture. Mater. Sci. Technol. 2017, 33, 138–148. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.; Humbeeck, J.V. Heat treatment of Ti-6Al-4V produced by Selective Laser Melting: Microstructure and mechanical properties. J. Alloy. Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Ott, R.T. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B 2018, 143, 172–196. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Zhang, W. Additive manufacturing of metallic components–process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Peters, M.; Leyens, C. Titanium and Titanium Alloys, 1st ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 1–35. [Google Scholar]
- Zhang, Z.; Wang, Q.J.; Mo, W. Metallography and Heat Treatment of Titanium, 4th ed.; Metallurgical Industry Press: Beijing, China, 2009; pp. 247–302. [Google Scholar]
- Dias, D.; Santos, O.S.; Alves, W.; Lima, M.S.F.; Silva, M.M.D. Fiber Laser Surface Melting of a NiTi Superelastic Alloy: Influence on Structural and Mechanical Properties. Metals 2019, 9, 1268. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Feng, Y.; Luo, B. 3D Multiphysical Modelling of Fluid Dynamics and Mass Transfer in Laser Welding of Dissimilar Materials. Metals 2018, 8, 443. [Google Scholar] [CrossRef]
- Wood, J.R. Producing Ti-6Al-4V plate from single-melt EBCHM ingot. JOM 2002, 54, 56–58. [Google Scholar] [CrossRef]
- Hong, D. Microstructure and properties of Ti-6Al-4V alloy with cold-wall induction melting preparation. Mater. Sci. Technol. 1999, s1, 12–16. [Google Scholar]
- Powell, A.C.; Pal, U.B.; Den Avyle, J.A.; Damkroger, B.; Szekely, J. Analysis of multicomponent evaporation in electron beam melting and refining of titanium alloys. Metall. Mater. Trans. B 1997, 28, 1227–1239. [Google Scholar] [CrossRef]
- Akhonin, S.V.; Trigub, N.P.; Zamkov, V.N.; Semiatin, S.L. Mathematical modeling of aluminum volatilization during electron-beam cold-hearth melting of Ti-6Al-4V ingots. Metall. Mater. Trans. B-Proc. Metall. Mater. Proc. Sci. 2003, 34, 447–454. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Ivanchenko, V.G.; Ivasishin, O.M. Diffusion models for evaporation losses during electron-beam melting of alpha/beta-titanium alloys. Metall. Mater. Trans. B 2004, 35, 235–245. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of the selective laser melted Mg-Zn-Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Yao, K.; Min, X.H.; Shi, S.; Tan, Y. Volatilization Behavior of β-Type Ti-Mo Alloy Manufactured by Electron Beam Melting. Metals 2018, 8, 206. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Guo, J.J.; Liu, G.Z. Melt Quality Control in Non-Ferrous Alloy Vacuum Melting Process, 2nd ed.; Harbin Institute of Technology Press: Harbin, China, 2005; pp. 71–86. [Google Scholar]
- Qiu, K.Q.; Duan, W.J.; Chen, Q.Y. Rate of metal evaporation under vacuum condition. Nonferrous Met. 2002, 54, 48–52. [Google Scholar]
- White, D.C. Vaporization and vaporizers. BJA Br. J. Anaesth. 1985, 57, 658–671. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Chen, J.; Tan, H.; Lin, X.; Huang, W. Evolution of plastic deformation and its effect on mechanical properties of laser additive repaired Ti64ELI titanium alloy. Opt. Laser Technol. 2017, 92, 36–43. [Google Scholar]
- Maamoun, A.H.; Elbestawi, M.; Dosbaeva, G.K.; Veldhuis, S.C. Thermal post-processing of AlSi10Mg parts produced by Selective Laser Melting using recycled powder. Addit. Manuf. 2018, 21, 234–247. [Google Scholar] [CrossRef]
- Ivanchenko, V.G.; Ivasishin, O.M.; Semiatin, S.L. Evaluation of evaluation losses during electron-beam melting of Ti-Al-V alloys. Metall. Mater. Trans. B 2003, 34, 911–915. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic Representation of Thermodynamic Properties and the Classification of Solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Lei, L.; Lanlan, Y.; Wenguang, L.; Xiaonan, M.; Guanjun, Y.; Rongrong, N.; Chen, W. Research on evaporation mechanism of elements in TC4 alloys during Electron Beam Cold hearth melting. Rare Met. Mater. Eng. 2011, 40, 625–629. [Google Scholar]
- Lópezarias, T. Comment on Clausius-Clapeyron equation and saturation vapour pressure: Simple theory reconciled with practice. Eur. J. Phys. 2012, 33, L11–L12. [Google Scholar] [CrossRef]
- Guo, J.; Jia, J.; Liu, Y.; Su, Y.; Ding, H. Volatilization behavior of aluminum during the cold crucible induction skull melting of titanium aluminum alloys. Metall. Mater. Trans. B 2000, 31, 837–844. [Google Scholar] [CrossRef]
- Geiger, G.H.; Poirier, D.R. Transport Phenomena in Metallurgy; Addison-Wesley Publishing Company: Boston, MA, USA, 1973; pp. 515–542. [Google Scholar]
- Present, R.D. Chapman-Enskog Method in Chemical Kinetics. J. Chem. Phys. 2003, 48, 4875–4877. [Google Scholar] [CrossRef]
- ASTM B381-13. Standard Specification for Titanium and Titanium Alloy Forgings; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Wycisk, E.; Siddique, S.; Herzog, D.; Walther, F.; Emmelmann, C. Fatigue performance of laser additive manufactured Ti-6Al-4V in very high cycle fatigue (VHCF) regime up to 109 cycles. Front. Mater. 2015, 2, 72. [Google Scholar] [CrossRef]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Qian, M. Additive manufacturing of strong and ductile Ti-6Al-4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Qiu, C.; Adkins, N.J.; Attallah, M.M. Microstructure and tensile properties of selectively laser-melted and of HIPed laser-melted Ti-6Al-4V. Mater. Sci. Eng. A 2013, 578, 230–239. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).