Are Large Particles of Iron Detrimental to Properties of Powder Metallurgy Steels?
Abstract
1. Introduction
- Compression is a single-stage process carried out at room temperature. Specifically, neither hot nor warm isostatic compaction is employed.
- It is not intended to fabricate a ferrous material with a small fraction of non-interconnected pores; post-sintering density around 7.0 g/cm3 approximately corresponding to the fraction of pores equal to 0.1 is acceptable.
- After sintering, a final part is solutionized and quenched with an intent to produce a fully martensitic structure, which is then held at temperature, which is sufficient to relieve internal stresses, but not high enough to trigger a decomposition of martensite into ferrite and carbides.
- A characteristic size of the final part (such as the thickness of a plate) is less than an ideal diameter calculated for quenching to fully martensitic microstructure for the given overall composition.
- Iron with insignificant percentages of inevitable impurities instead of deliberately pre-alloyed iron powders is used in the process.
- Metallic alloying additions are brought into a blend as tiny particles of either individual elements or master alloys. These particles are much smaller in comparison with Fe particles.
- Carbon is brought into a mixture as a submicron graphite powder. A duration and temperature of sintering are such that a uniform carbon concentration is guaranteed.
2. Literature Review
3. Experimental Procedure
3.1. Choice of the Chemical Composition
3.2. Characteristics of Used Powders
3.3. Compressibility Tests
3.4. Preparation of Samples
3.5. Characterization of Samples
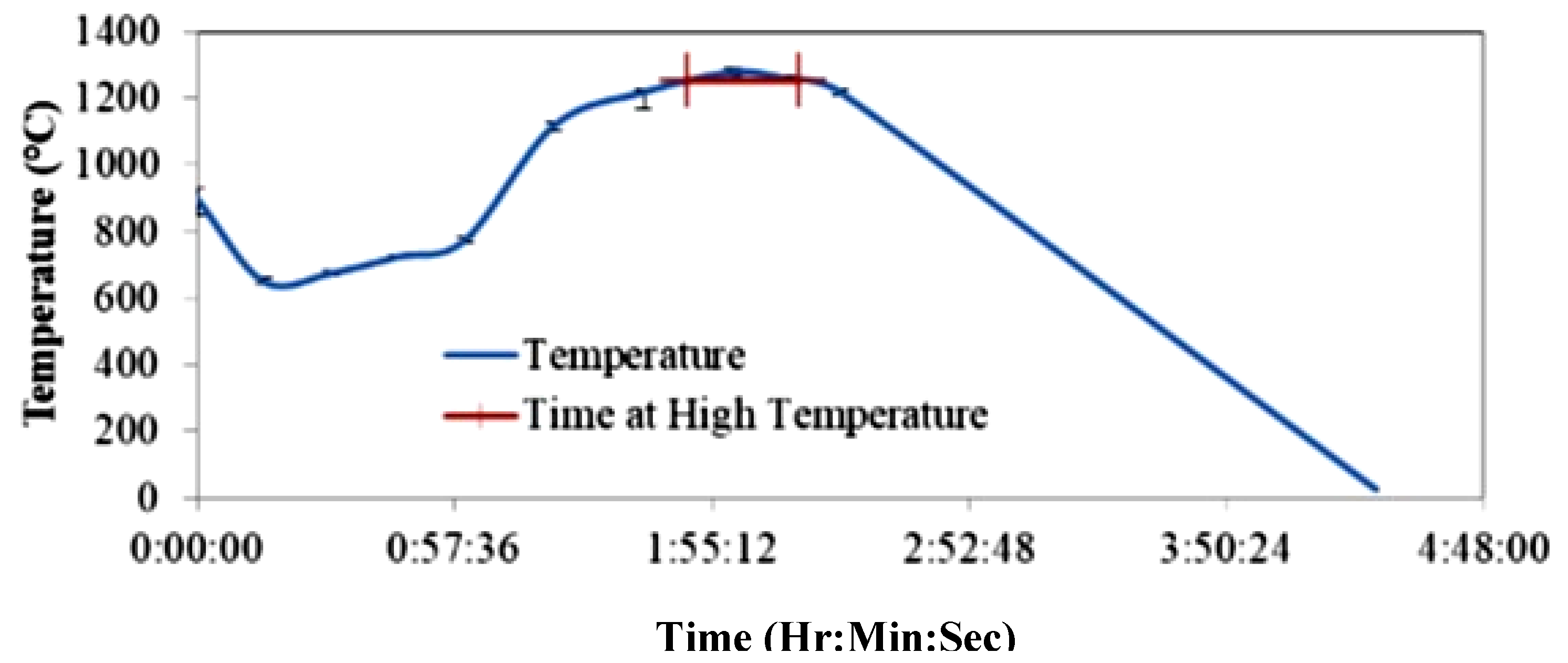
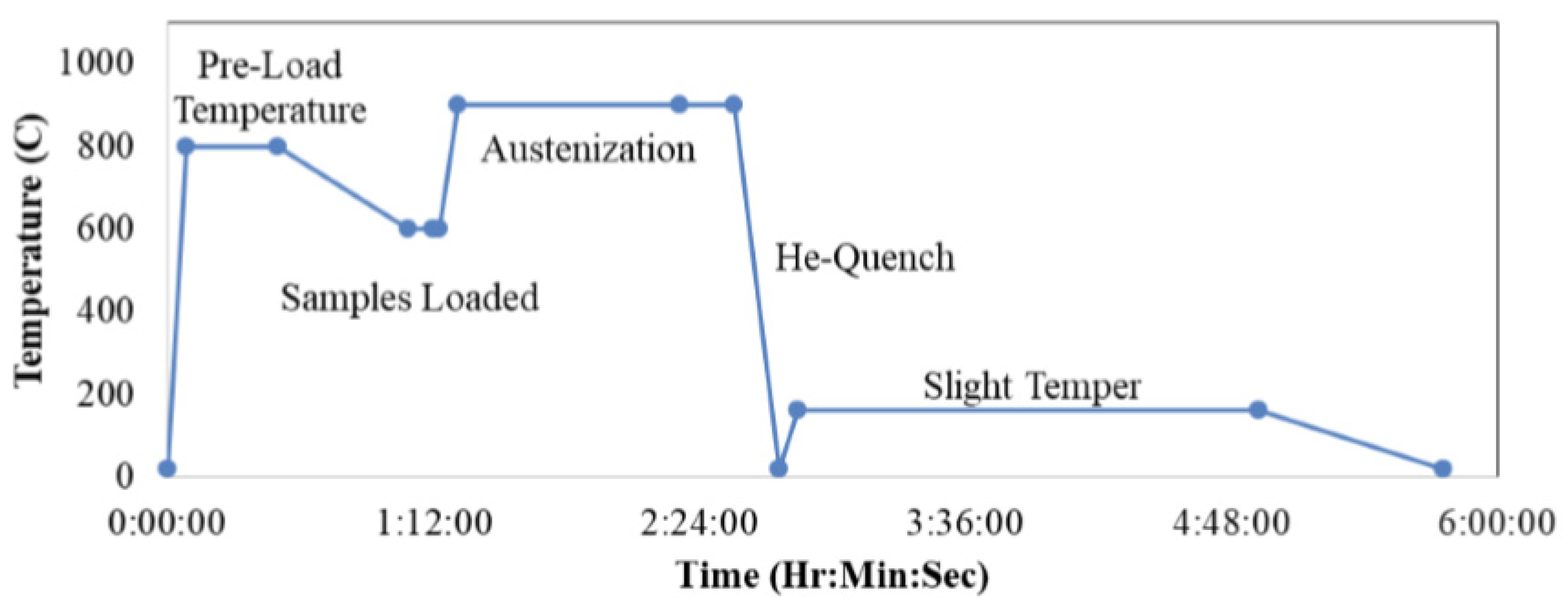
3.6. Sequence of the Experimental Work
4. Results and Discussion
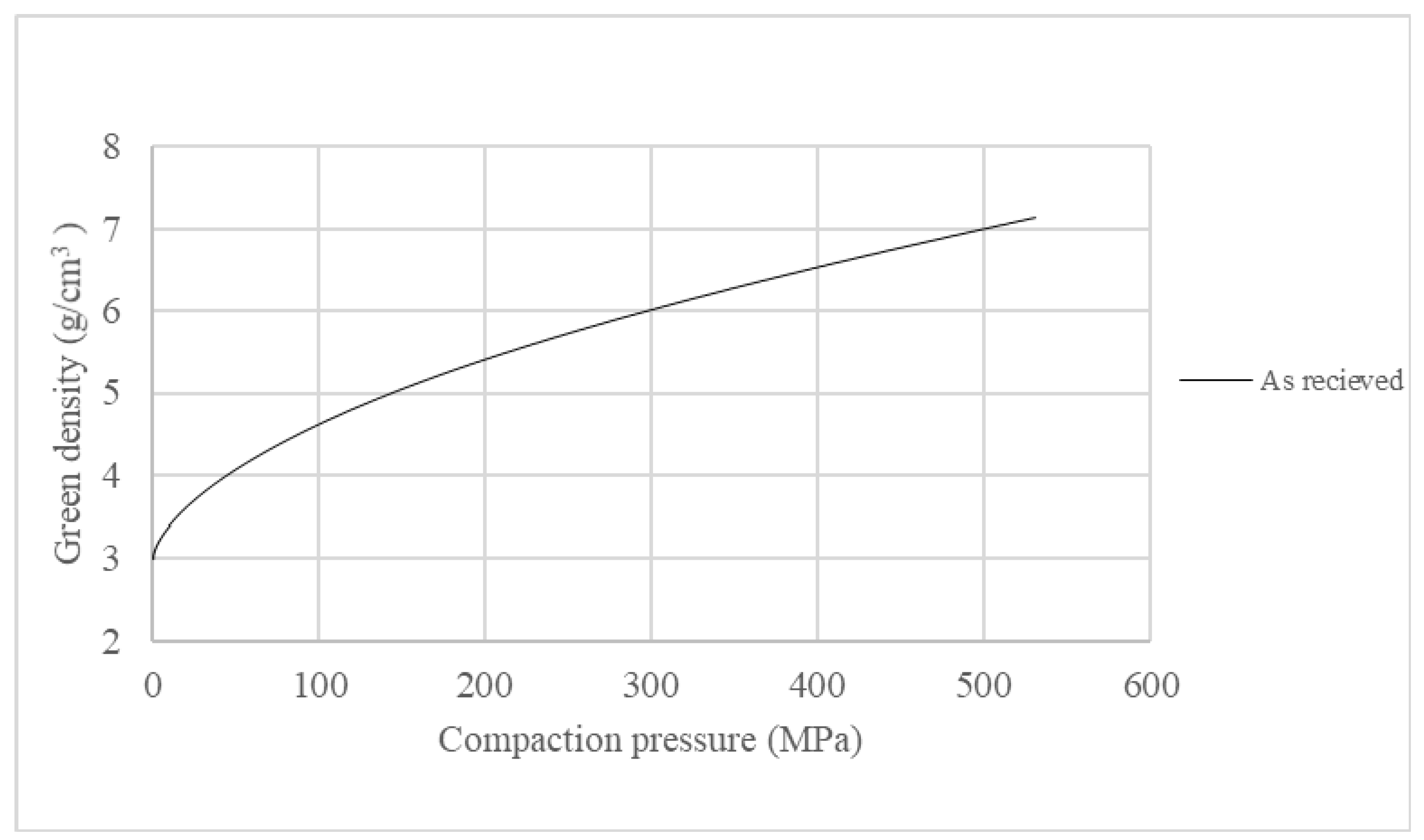
4.1. Compressibility of Pure Iron Powder
4.2. Properties of Metal Powders
4.2.1. Size Dependent Properties of Pure Iron Powder
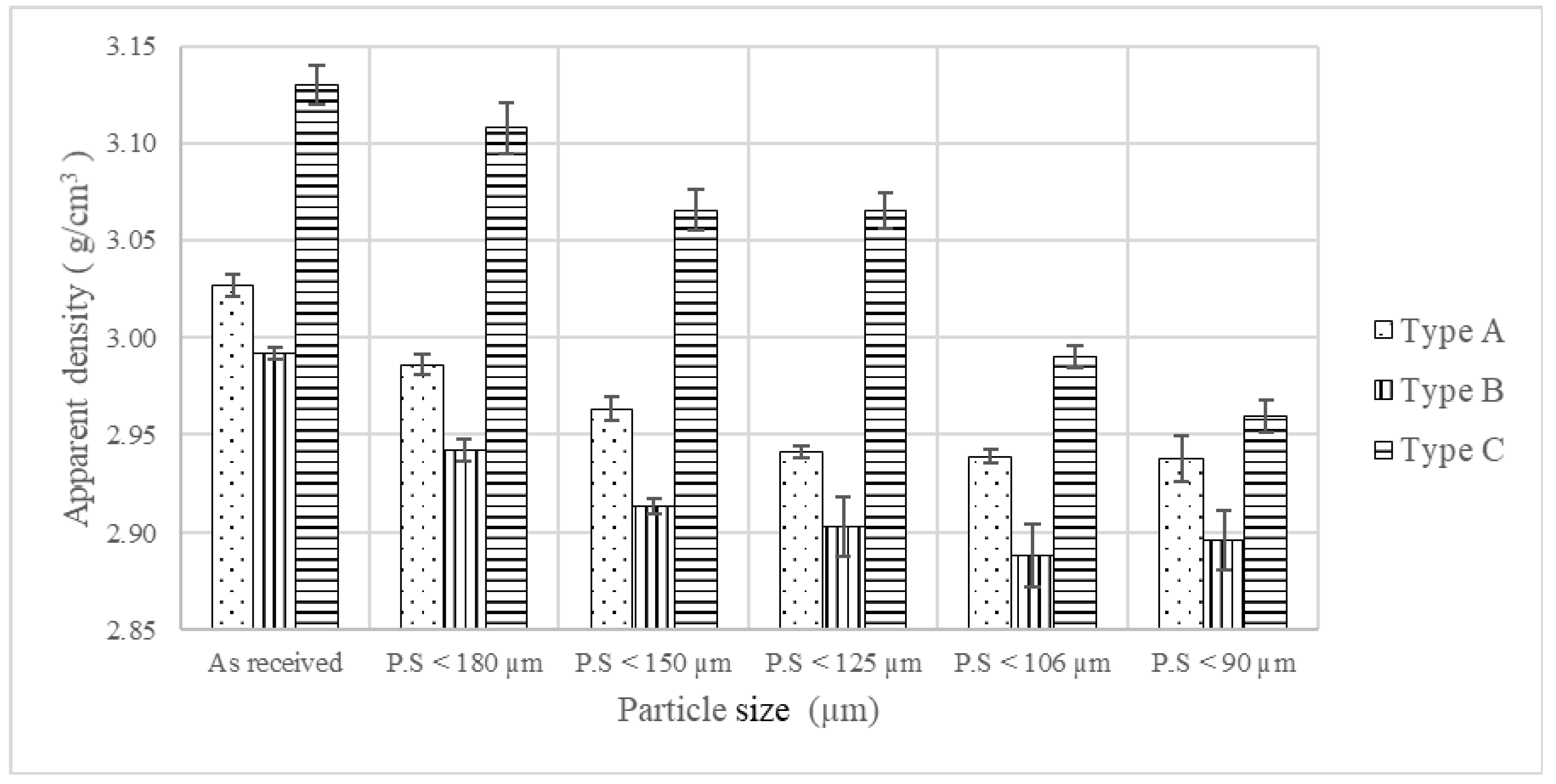
Apparent Density
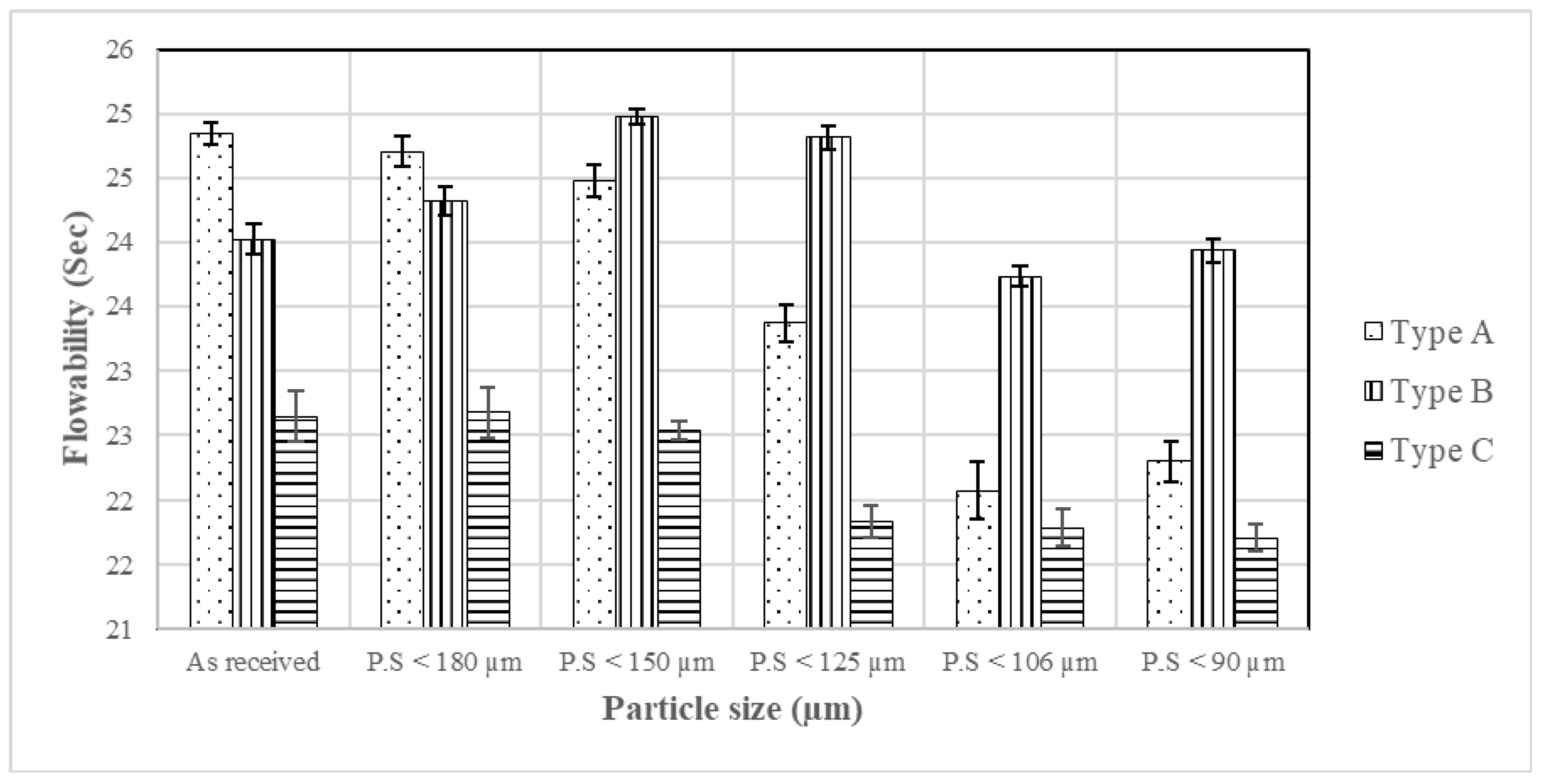
Flowability
Compressibility
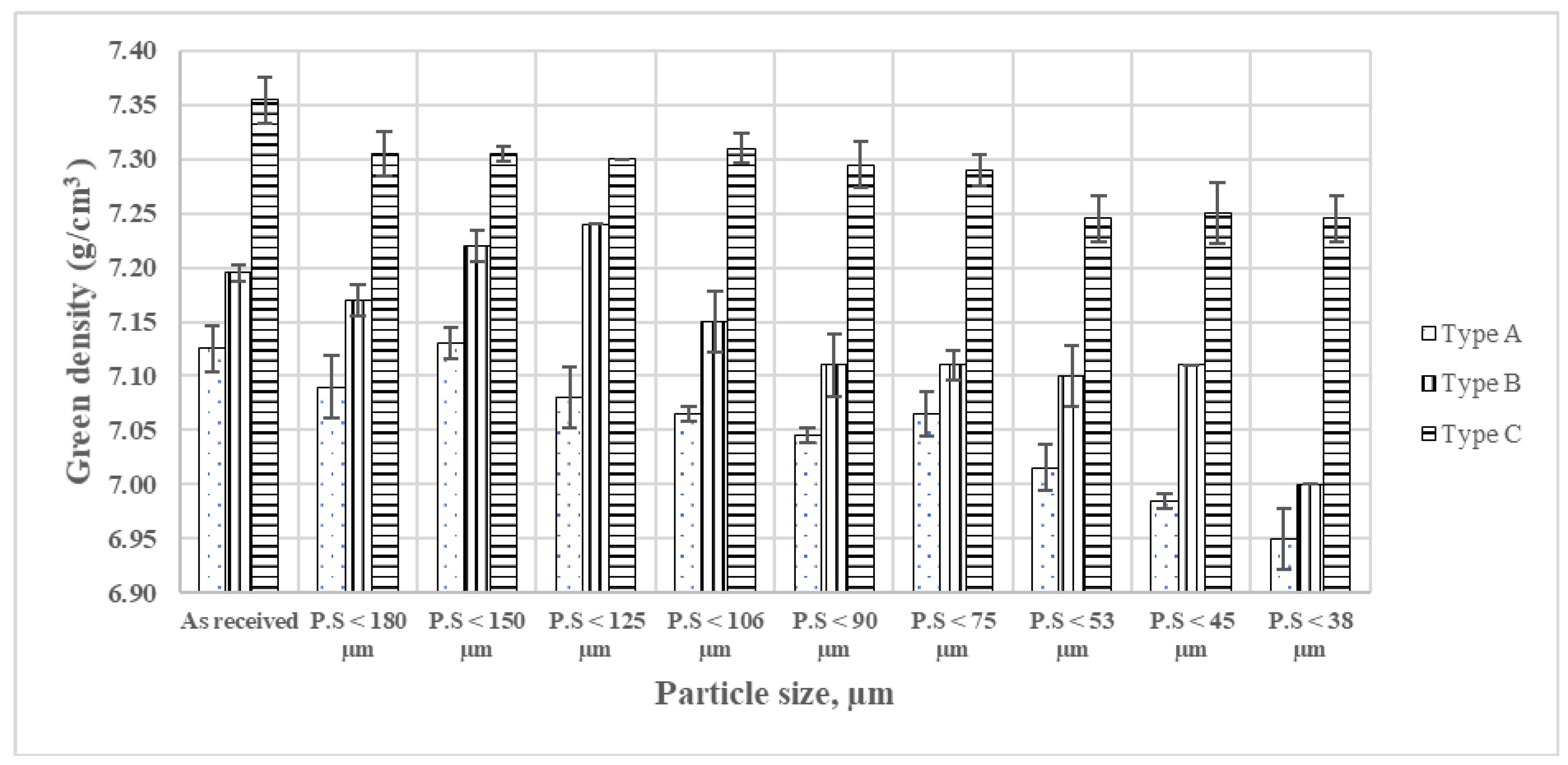
4.2.2. Size Dependent Properties of Blends
Constant-Load-Green Body Densities
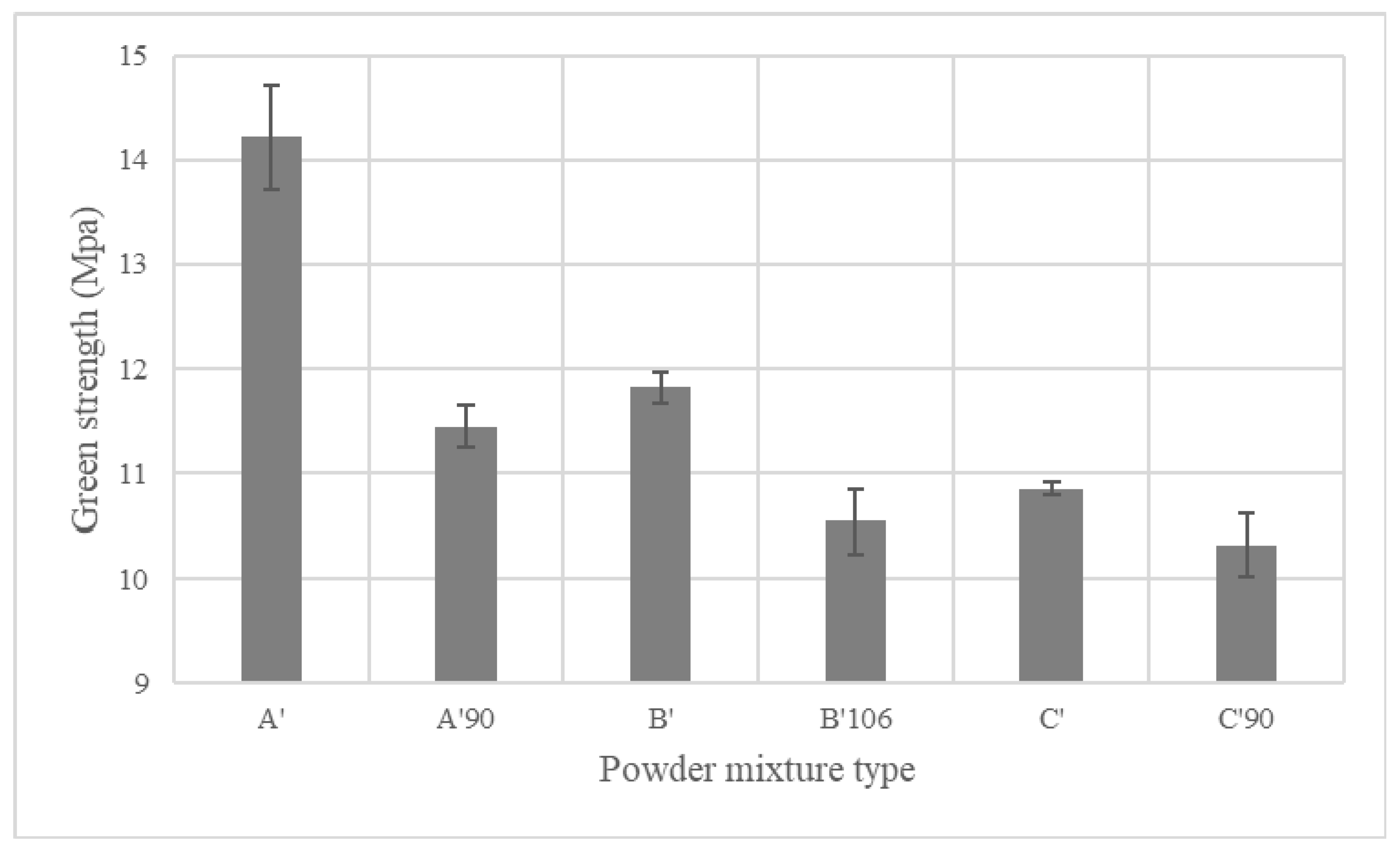
4.3. Green Body Strength
4.4. Sintered PMS Alloy Properties
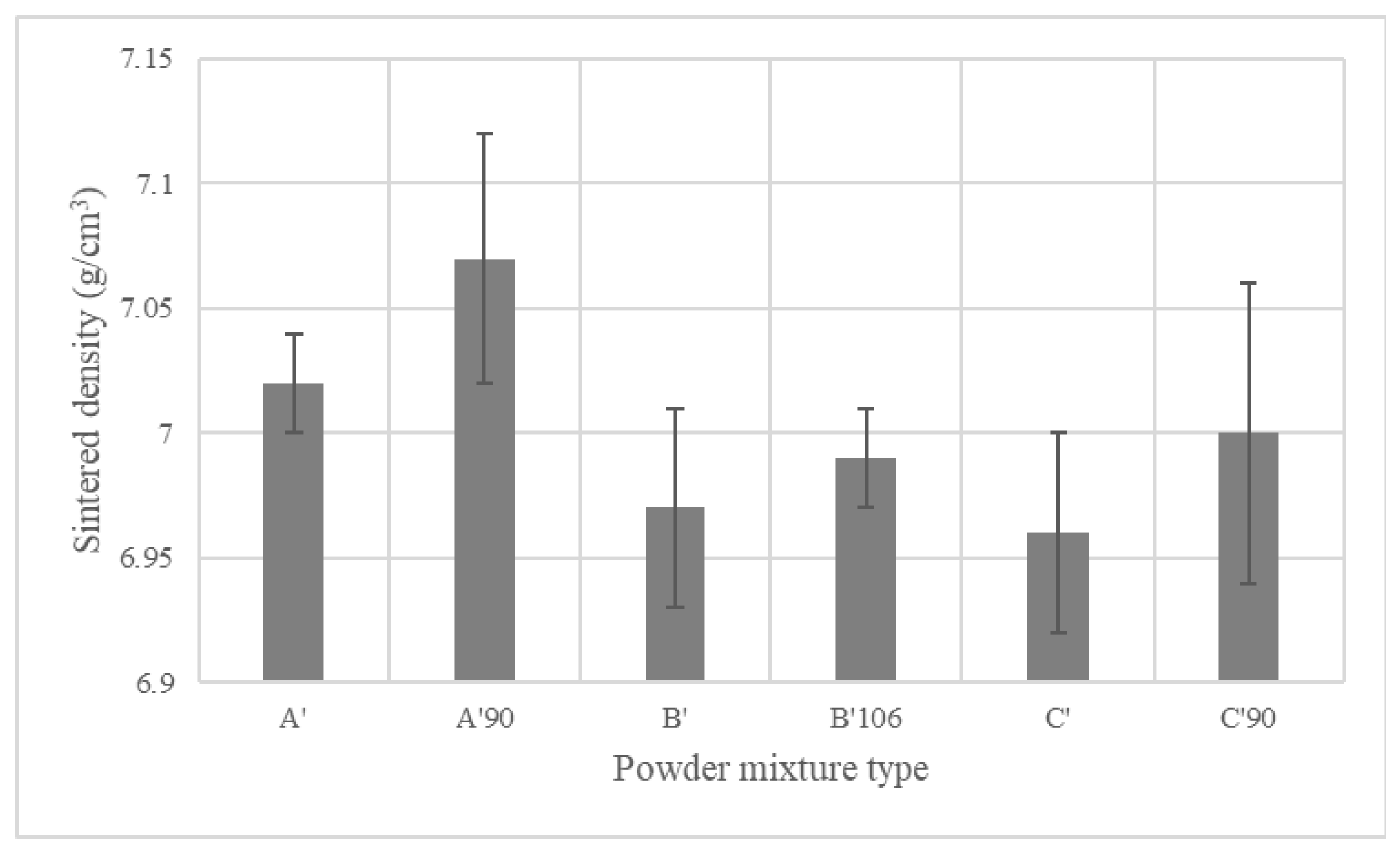
4.4.1. Sintered Density
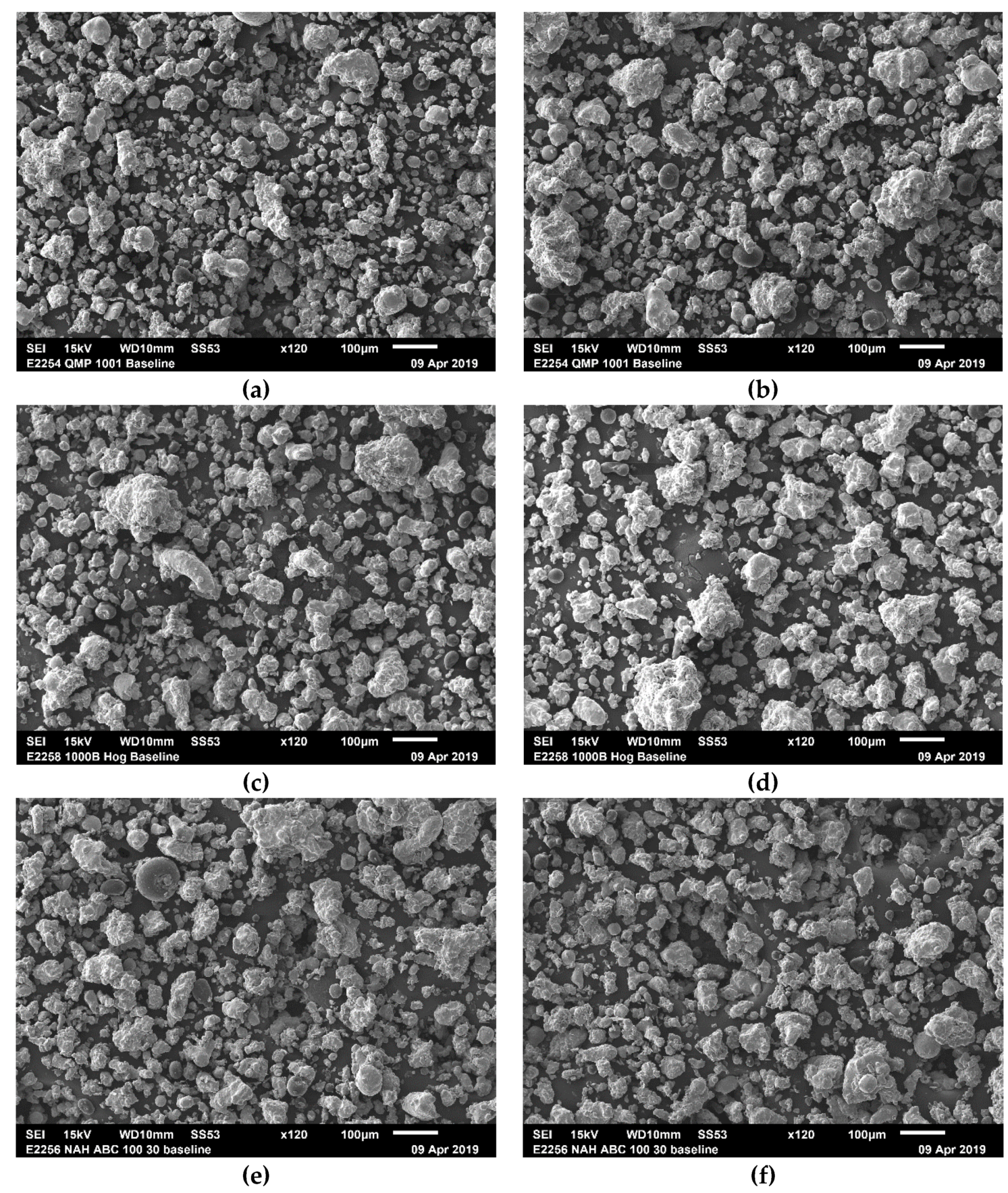
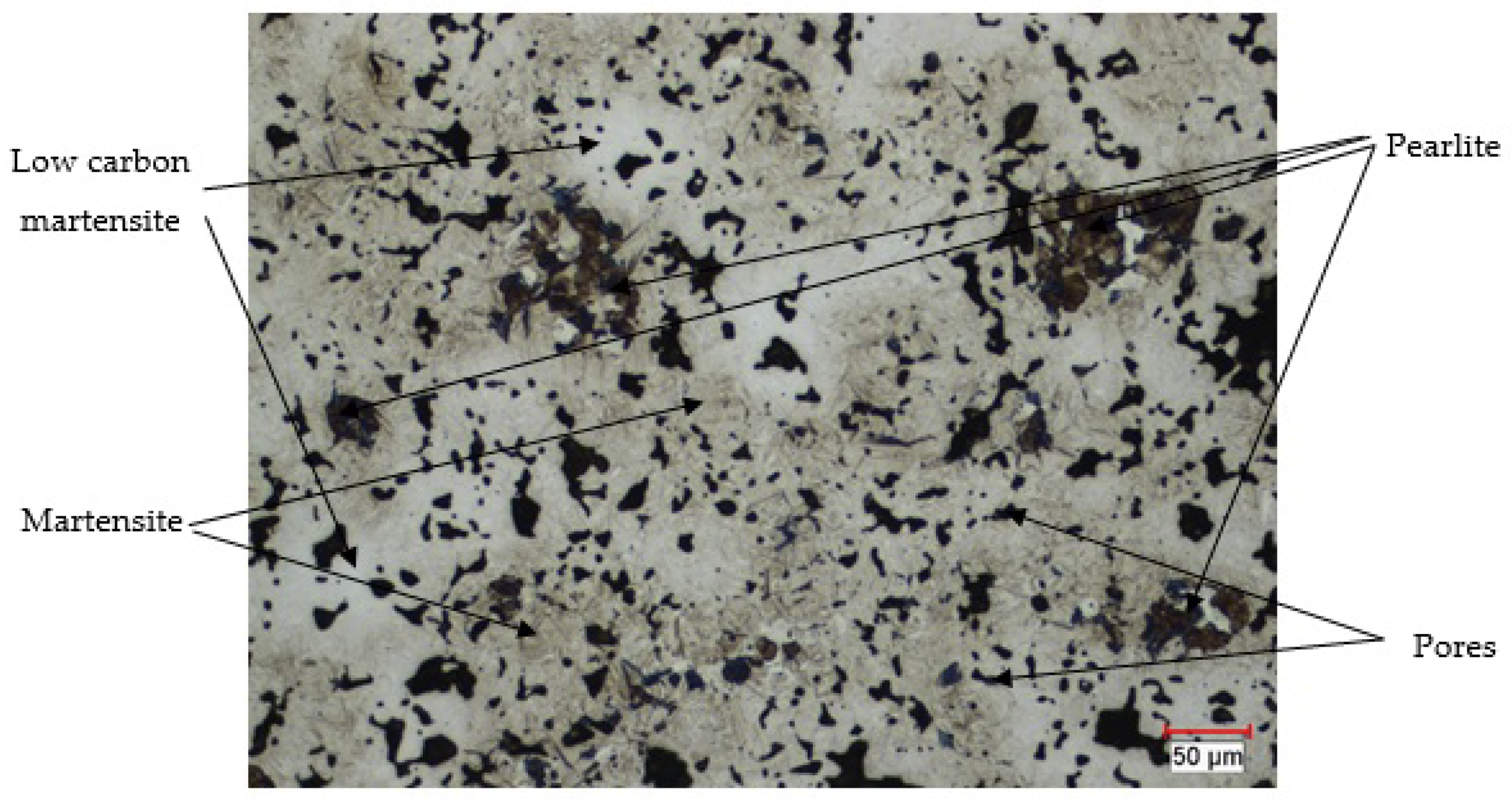
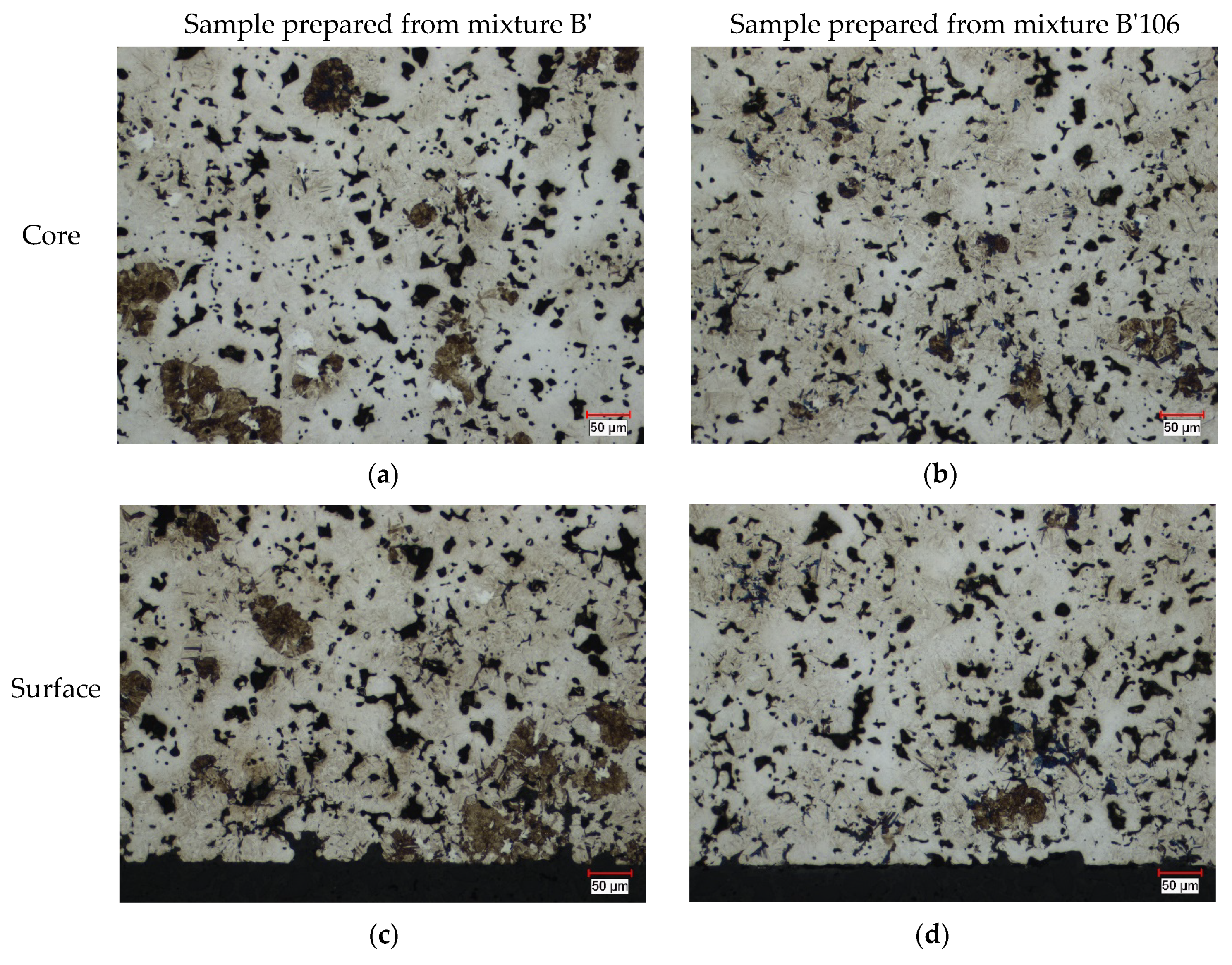
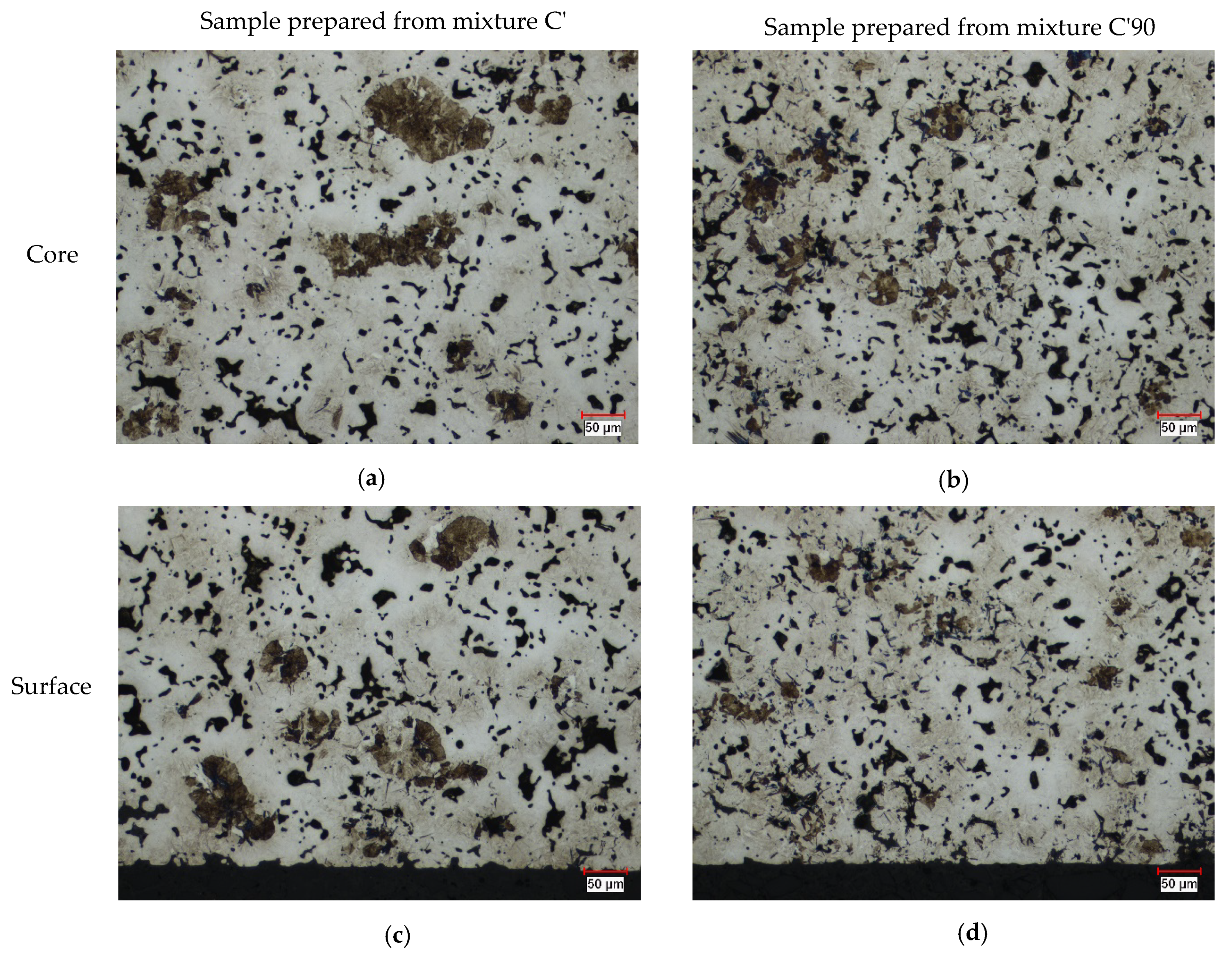
4.4.2. Optical Microscopy
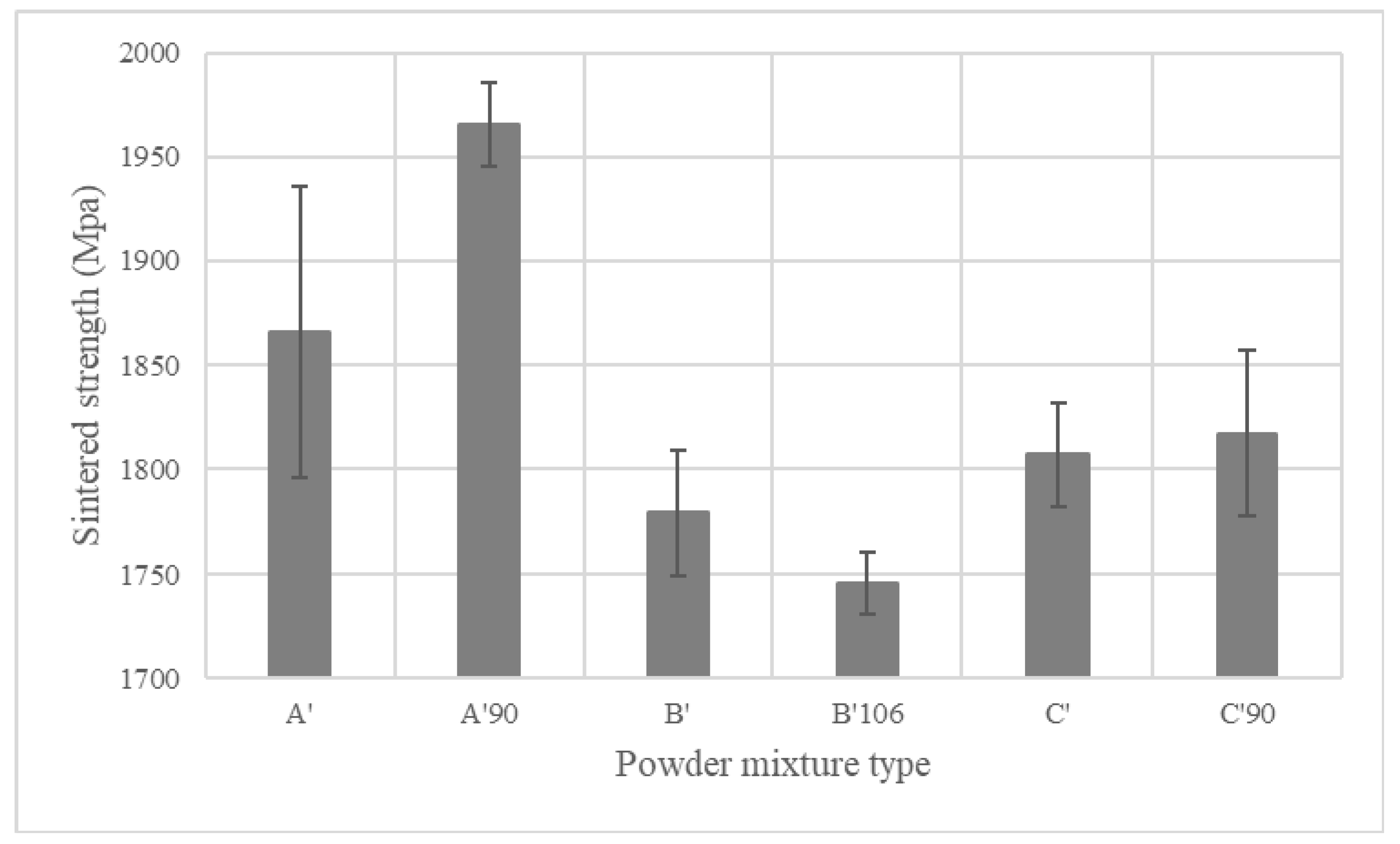
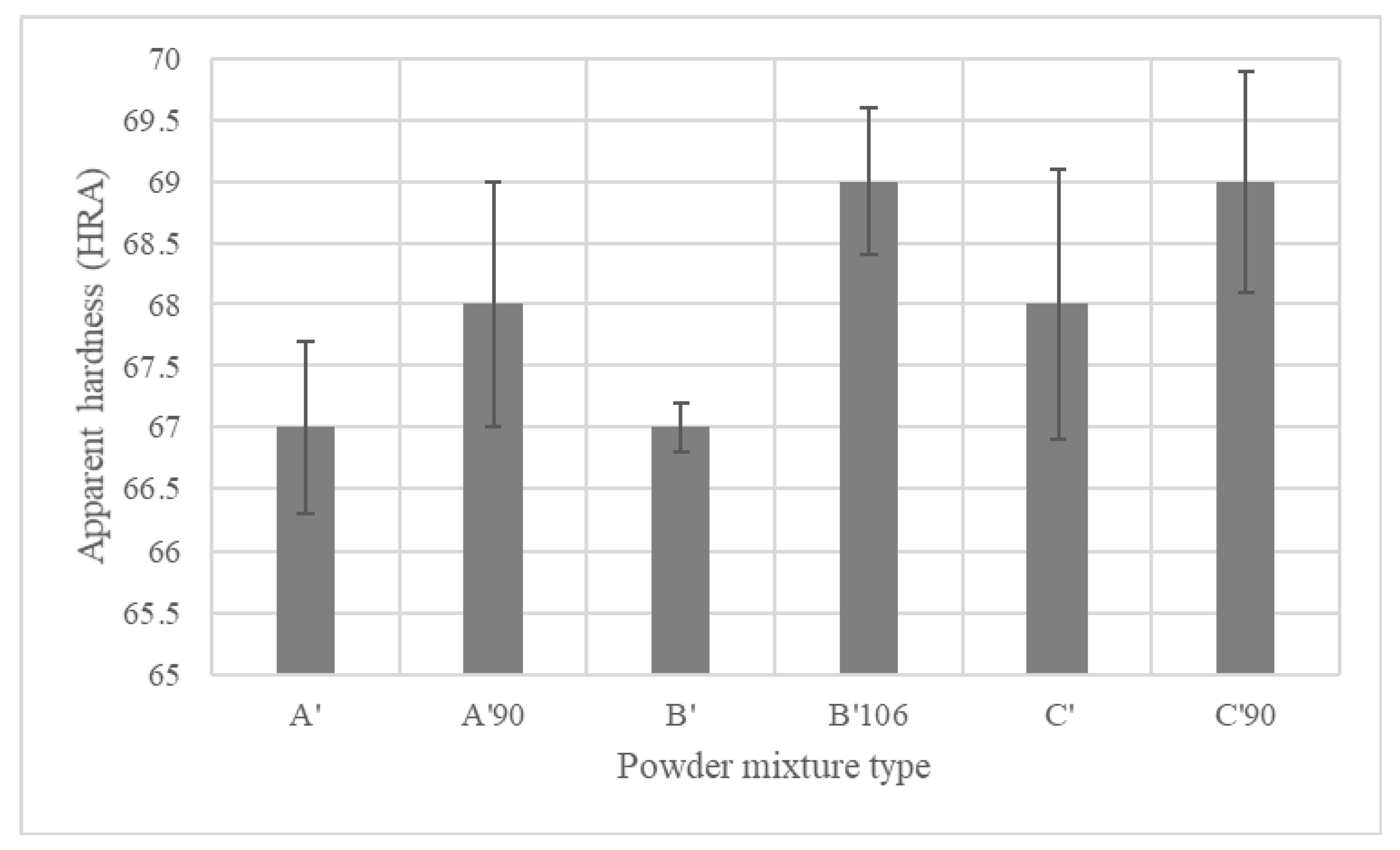
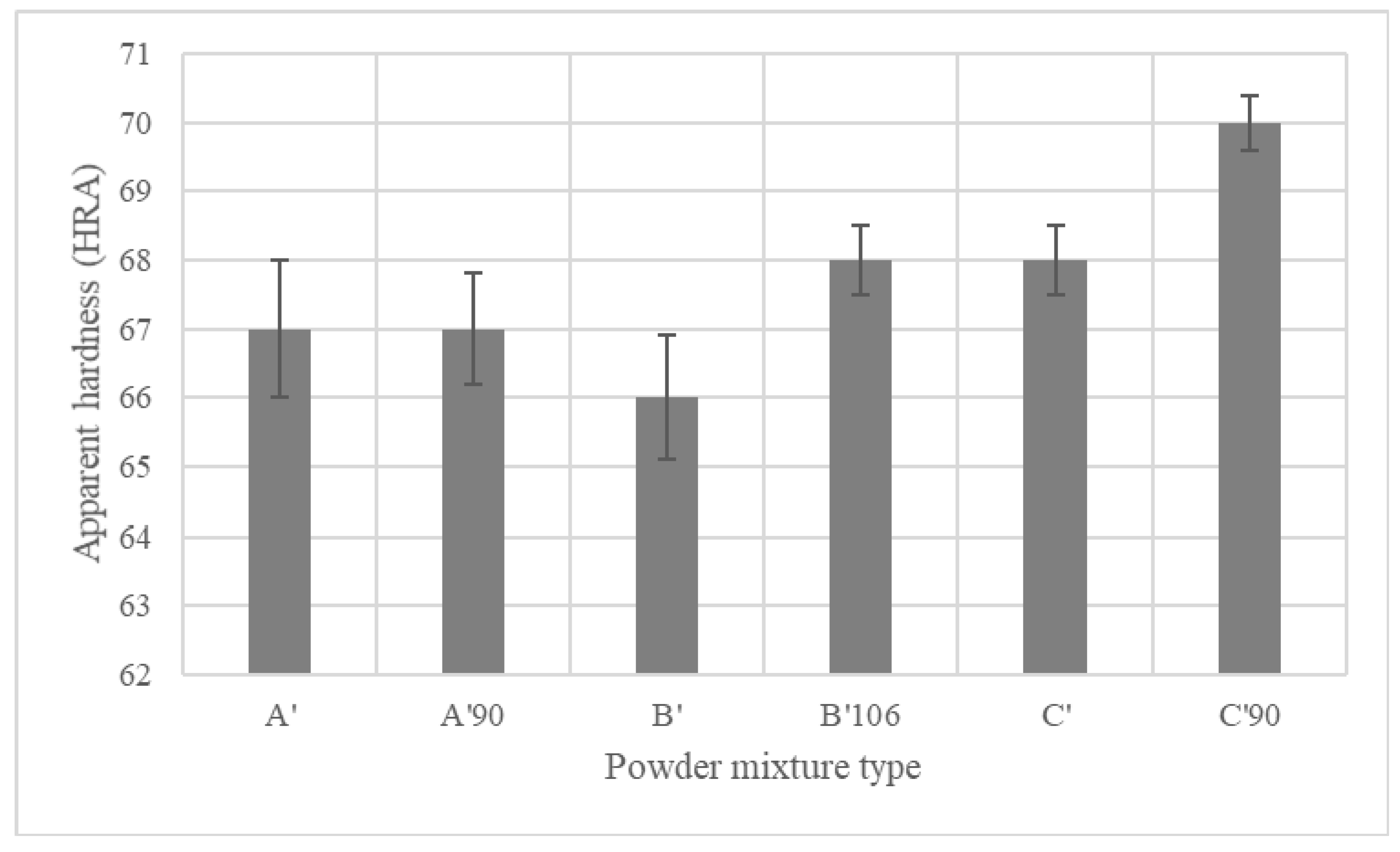
4.4.3. Mechanical Properties of Sintered PMS
5. Conclusions
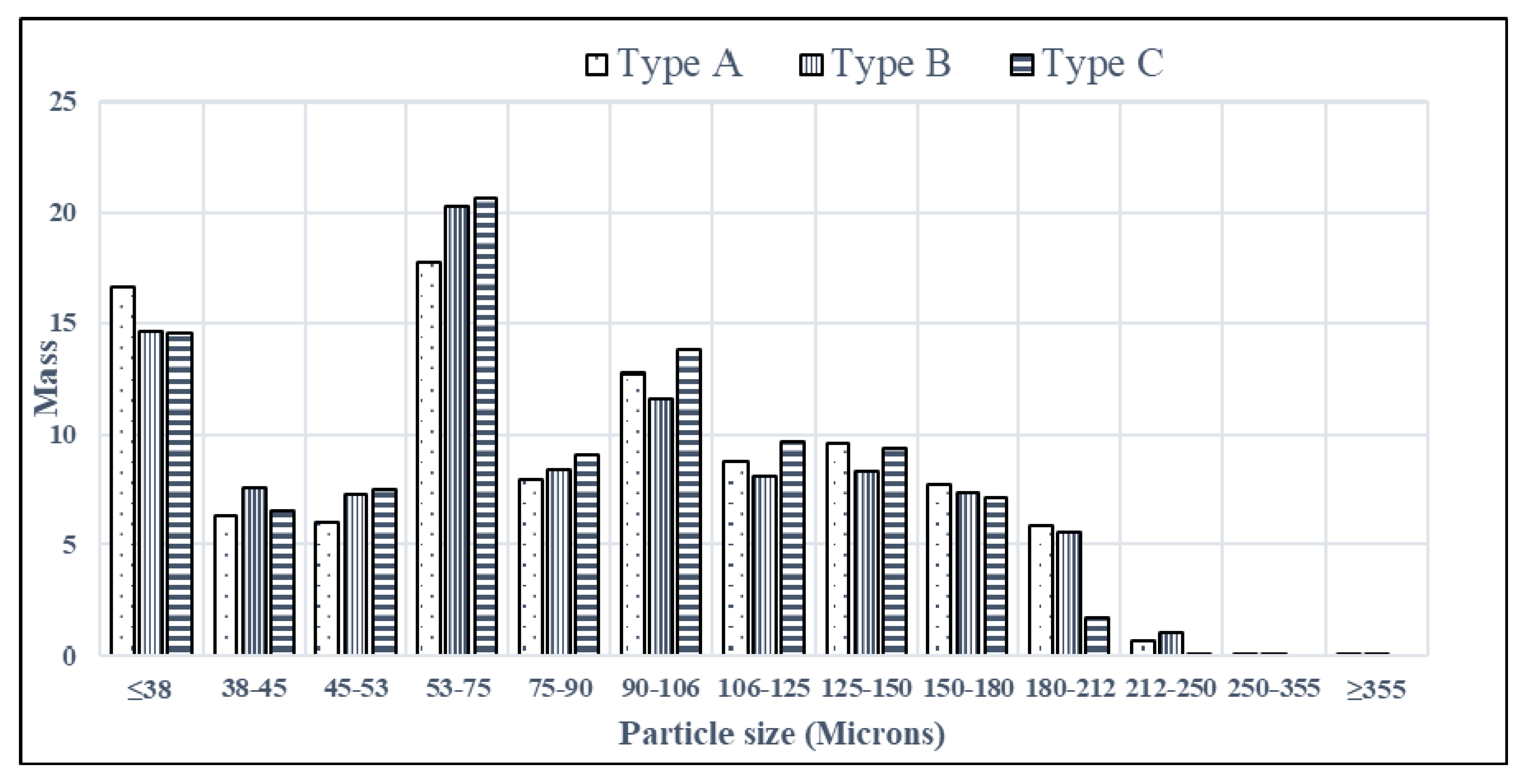
- A histogram showing a size distribution was constructed.
- Apparent density, flowability and compressibility were measured.
- Particles whose sizes exceeded 180 μm were sieved away and the properties of the remaining powder were examined. Then particles with sizes greater than 150 μm were eliminated and the characteristics of the remaining powder were quantified.
- Iterations continued until either flowability or compressibility became compromised by the described incremental removal of coarse particles. In other words, a threshold telling one when to stop taking away large particles was ascertained. According to experimental observations, the threshold was located within the 90–106 μm interval.
- Iron powder from which particles with sizes exceeding the threshold were eliminated were then used for making a blend containing, in addition to Fe, Cr, Mo, Ni, graphite and a lubricant. Another blend with an almost identical chemical composition was made of with-received (unsieved) Fe powder.
- Both blends were compacted, sintered, austenitized and quenched. Then their microstructures and mechanical properties were compared.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poquillon, D.; Lemaitre, J.; Baco-Carles, V.; Tailhades, P.; Lacaze, J. Cold compaction of iron powders-relations between powder morphology and mechanical properties Part I: Powder preparation and compaction. Powder Technol. 2002, 126, 65–74. [Google Scholar] [CrossRef]
- Sanchez, F.; Bolarin, A.M.; Molera, P.; Mendoza, J.E.; Ocampo, M. Relationship between particle size and manufacturing processing and sintered characteristics of iron powders. Revista Lationoamericana de Metalurgia y Materials 2003, 23, 35–40. [Google Scholar]
- Shima, S.; Saleh, M.A.E. The effect of particle characteristics on compaction behviour of powders-Experiments. In Proceedings of the Powder Metallurgy World Congress, Kyoto, Japan, 12–15 July 1993; Part 1. pp. 331–334. [Google Scholar]
- Tikhonov, G.F.; Sorokin, V.K. Theory, production technology and properties of powders and fibers-Effect of particle size distribution on technological properties of stainless steel powder. Poroshkovaya Metallurgiya 1971, 10, 1–4. [Google Scholar]
- Peterson, J.E.; Small, W.M. Physical behavior of water atomized iron powders-Effects of relative humidity and particle size. Int. J. Powder Metall. 1993, 29, 121–130. [Google Scholar]
- Peterson, J.E.; Small, W.M. Physical behavior of water atomized iron powders-Particle size distribution and apparent density. Int. J. Powder Metall. 1993, 29, 131–137. [Google Scholar]
- Moon, L.H.; Kim, K.H. Relationship between compacting pressure, green density and green strength of copper powder compacts. Int. J. Powder Metall. 1984, 27, 80–84. [Google Scholar] [CrossRef]
- Tallon, P.G. An Experimental Investigation of the Hardenability, Tensile and Fracture Properties of Powdered Metal Steels. Master’s Thesis, McMaster University, Hamilton, ON, Canada, 26 April 2018. [Google Scholar]
- Suh, S.; Patel, S.; Nash, P. A study of compressibility, green and sintered strength of iron powders. In Proceedings of the 1991 Powder Metallurgy Conference and Exhibition, Chicago, IL, USA, 9–12 June 1991; Volume 5, pp. 151–160. [Google Scholar]
- Zhornyak, A.F.; Oliker, V.E. Effect of the particle size distribution of atomized iron powder and mixtures of atomized and reduced iron powders on some properties of materials produced from them by pressing and sintering. Poroshkovaya Metallurgiya 1978, 17, 558–561. [Google Scholar] [CrossRef]
- Oikonomou, C.; Hryha, E.; Nyborg, L.; Ahlin, Å. Effect of powder properties on the compressibility of water atomized iron and low alloyed steel grades. In Proceedings of the Euro PM, Gothenburg, Sweden, 15–18 September 2013. [Google Scholar]
- Sanchez, F.; Bolarin, A.M.; Coreno, J.; Mendoza, J.E.; Bas, J.A. Effect of compaction process sequence on axial density distribution of green compacts. Int. J. Powder Metall. 2001, 44, 351–354. [Google Scholar] [CrossRef]
- Sorokin, V.K. Compressibility of stainless-steel powder. Poroshkovaya Metallurgiya 1968, 10, 22–26. [Google Scholar]
- Fischmeister, H.F.; Arzt, E.; ’Olsson, L.R. Particle deformation and sliding during compaction of spherical powders: A study by quantitative metallography. Powder Metall. 1978, 21, 179–181. [Google Scholar] [CrossRef]
- Fischmeister, H.F.; Arzt, E. Densification of powders by particle deformation. Powder Metall. 1983, 26, 82–88. [Google Scholar] [CrossRef]
- Kiparisov, S.S.; Panov, V.S.; Smirnova, M.M.; Kots, Y.F. Properties of steel powders of various particle size and the structure of the steel in the sintered condition. Poroshkovaya Metallurgiya 1981, 6, 9–15. [Google Scholar] [CrossRef]
- Lund, J.A. Origins of green strength in Iron P/M compacts. Int. J. Powder Metall. 1982, 18, 117–127. [Google Scholar]
- Jackson, J.G.; Ehrgott, J.Q.; Rohani, B. Loading rate effects on compressibility of sands. J. Geotech. Eng. Div. 1980, 106, 839–852. [Google Scholar]
- Barr, A.D.; Clarke, S.D.; Petkovski, M.; Tyas, A.; Rigby, S.E.; Warren, J.; Kerr, S. Effects of strain rate and moisture content on the behaviour of sand under one-dimensional compression. Exp. Mech. 2016, 56, 1625–1639. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy Science; MPIF: Princeton, NJ, USA, 1994. [Google Scholar]
- Danninger, H.; Spoljaric, D.; Weiss, B. Microstructural features limiting the performance of P/M steels. Int. J. Powder Metall. 1997, 33, 43–53. [Google Scholar]
- Beiss, P.; Dalgic, M. Structure property relationships in porous sintered steels. Mater. Chem. Phys. 2001, 67, 37–42. [Google Scholar] [CrossRef]


| Element | C | Ni | Cr | Mo | Fe |
|---|---|---|---|---|---|
| Mass % | 0.5 | 0.5 | 0.8 | 0.2 | balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, A.; Habibnejad-Korayem, M.; Malakhov, D.V. Are Large Particles of Iron Detrimental to Properties of Powder Metallurgy Steels? Metals 2020, 10, 431. https://doi.org/10.3390/met10040431
Abdallah A, Habibnejad-Korayem M, Malakhov DV. Are Large Particles of Iron Detrimental to Properties of Powder Metallurgy Steels? Metals. 2020; 10(4):431. https://doi.org/10.3390/met10040431
Chicago/Turabian StyleAbdallah, Ahmed, Mahdi Habibnejad-Korayem, and Dmitri V. Malakhov. 2020. "Are Large Particles of Iron Detrimental to Properties of Powder Metallurgy Steels?" Metals 10, no. 4: 431. https://doi.org/10.3390/met10040431
APA StyleAbdallah, A., Habibnejad-Korayem, M., & Malakhov, D. V. (2020). Are Large Particles of Iron Detrimental to Properties of Powder Metallurgy Steels? Metals, 10(4), 431. https://doi.org/10.3390/met10040431





