Effect of TiO2-Nanoparticles on Ni Electrodeposition on Copper Wire
Abstract
1. Introduction
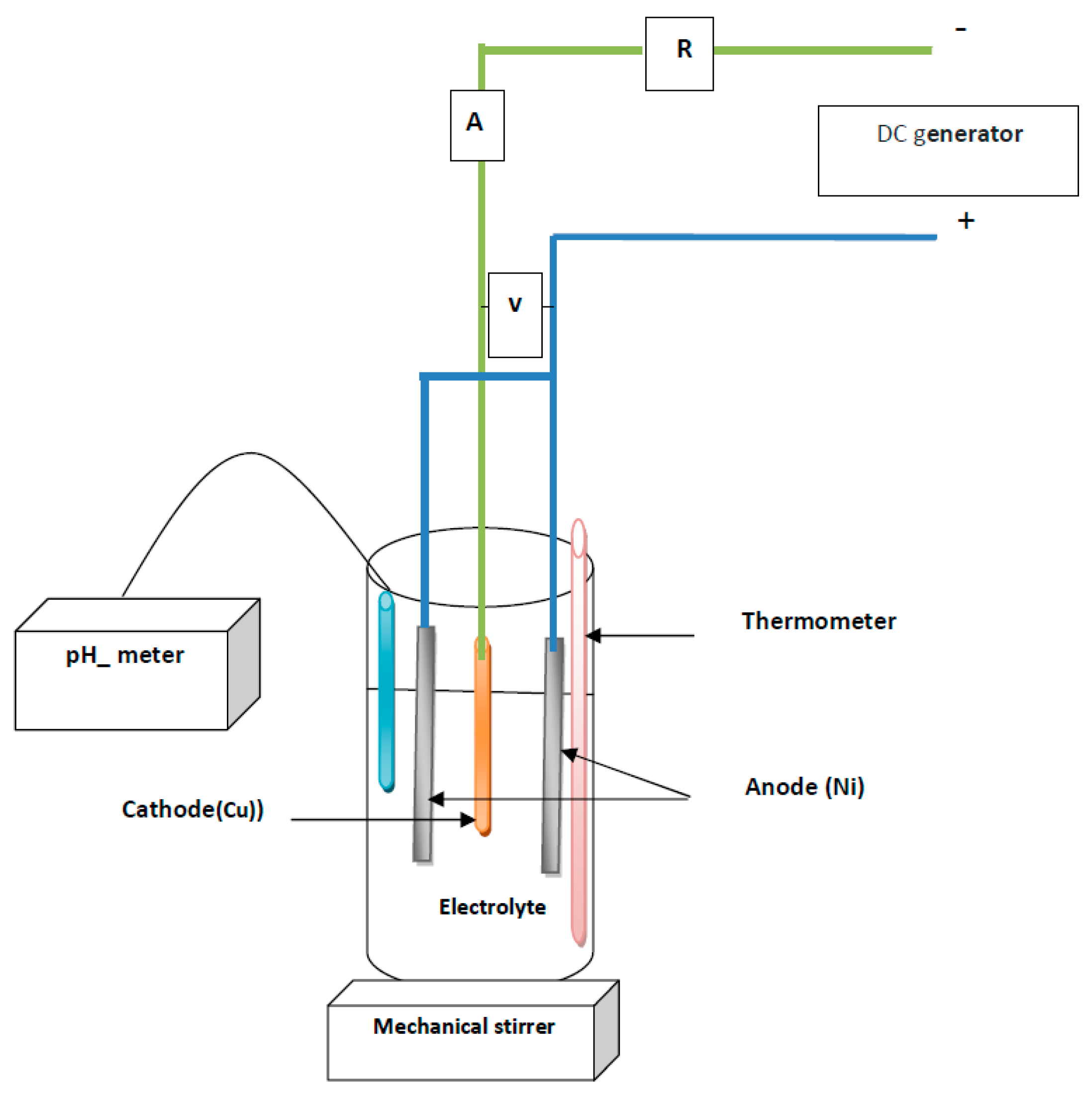
2. Materials and Methods
2.1. Electrodeposition of Ni–TiO2 Coatings
2.2. Morphological and Structural Characterization
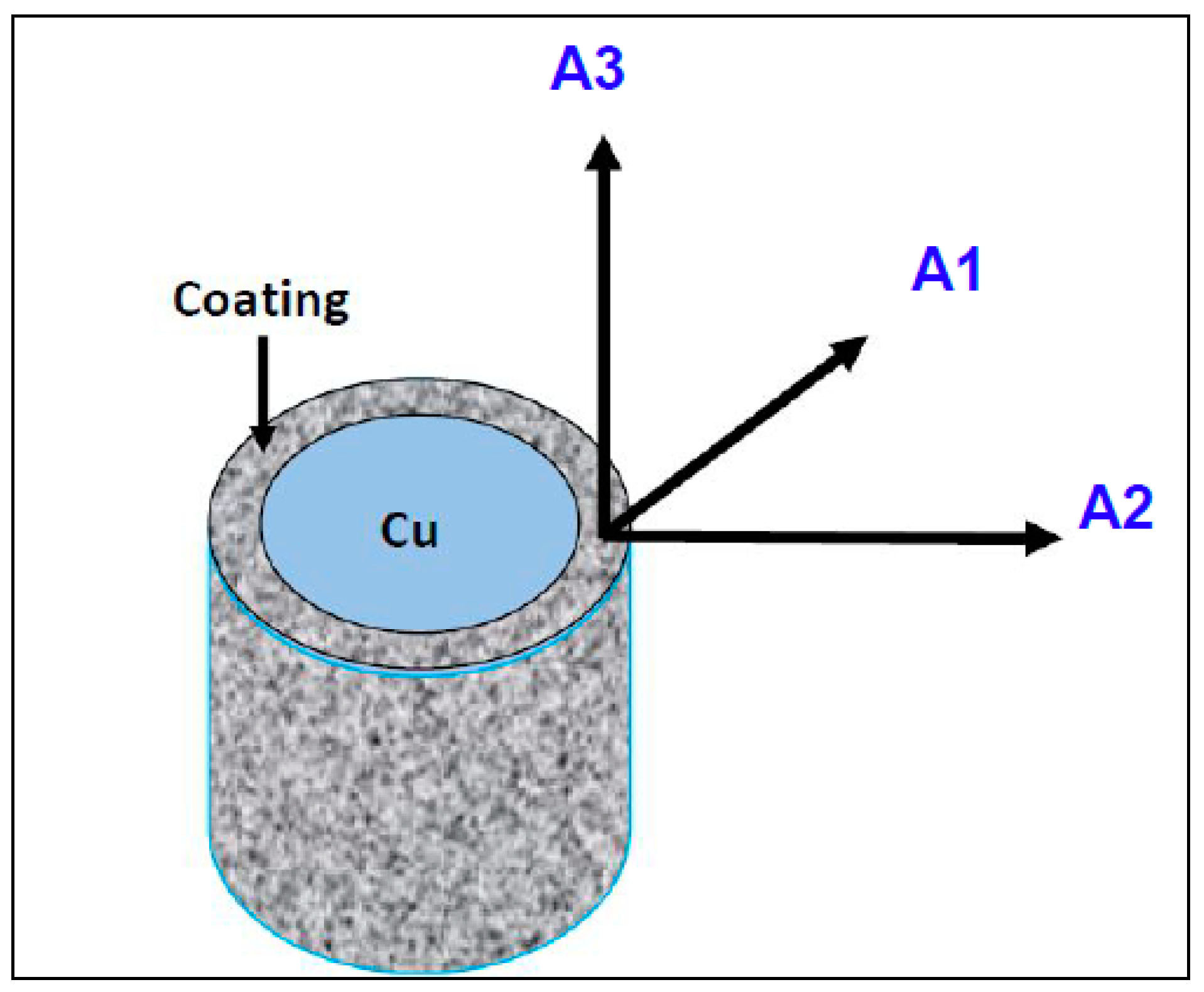
2.3. Electron Backscatter Diffraction (EBSD) Technique
3. Results
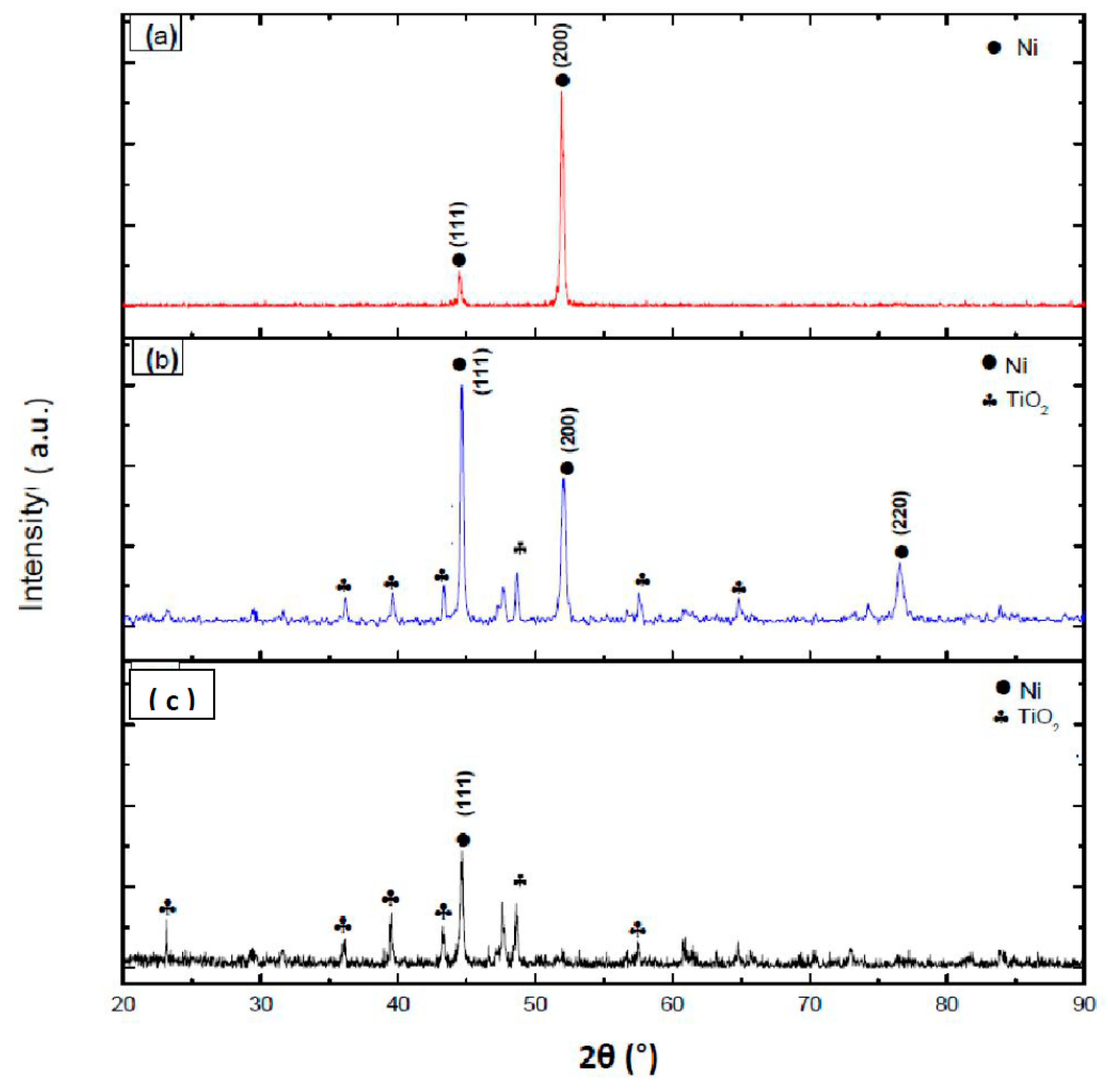
3.1. X-ray Diffraction Technique
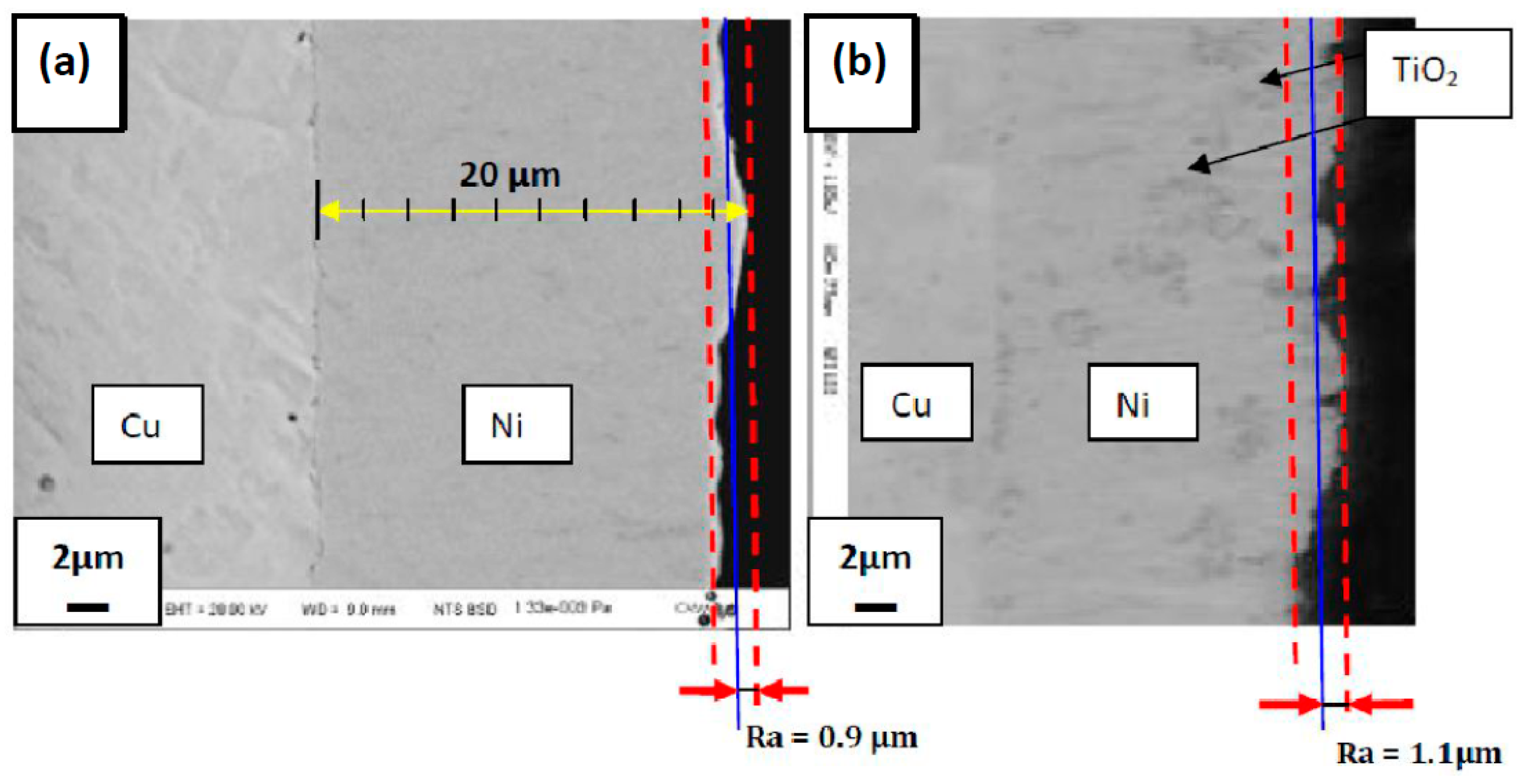
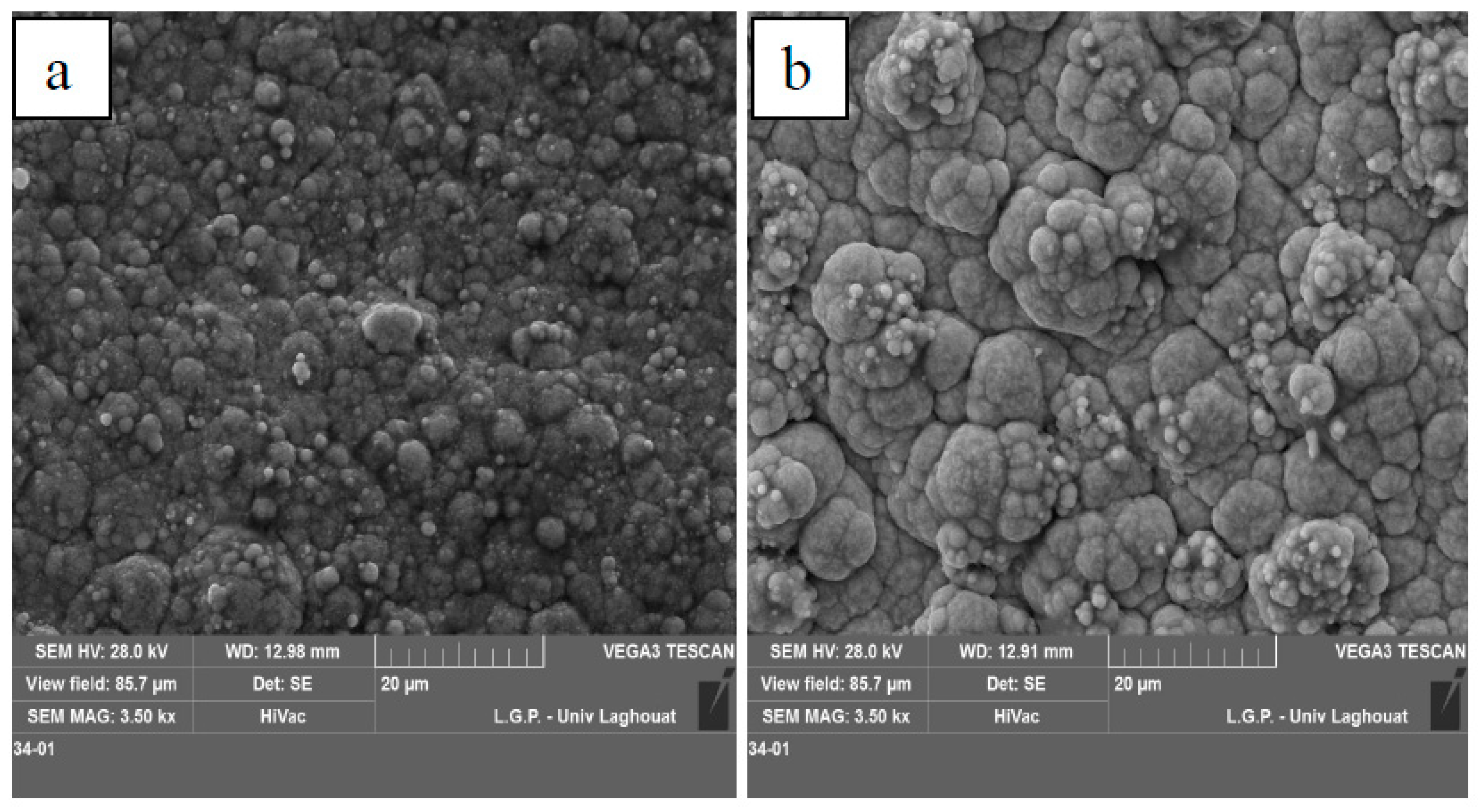
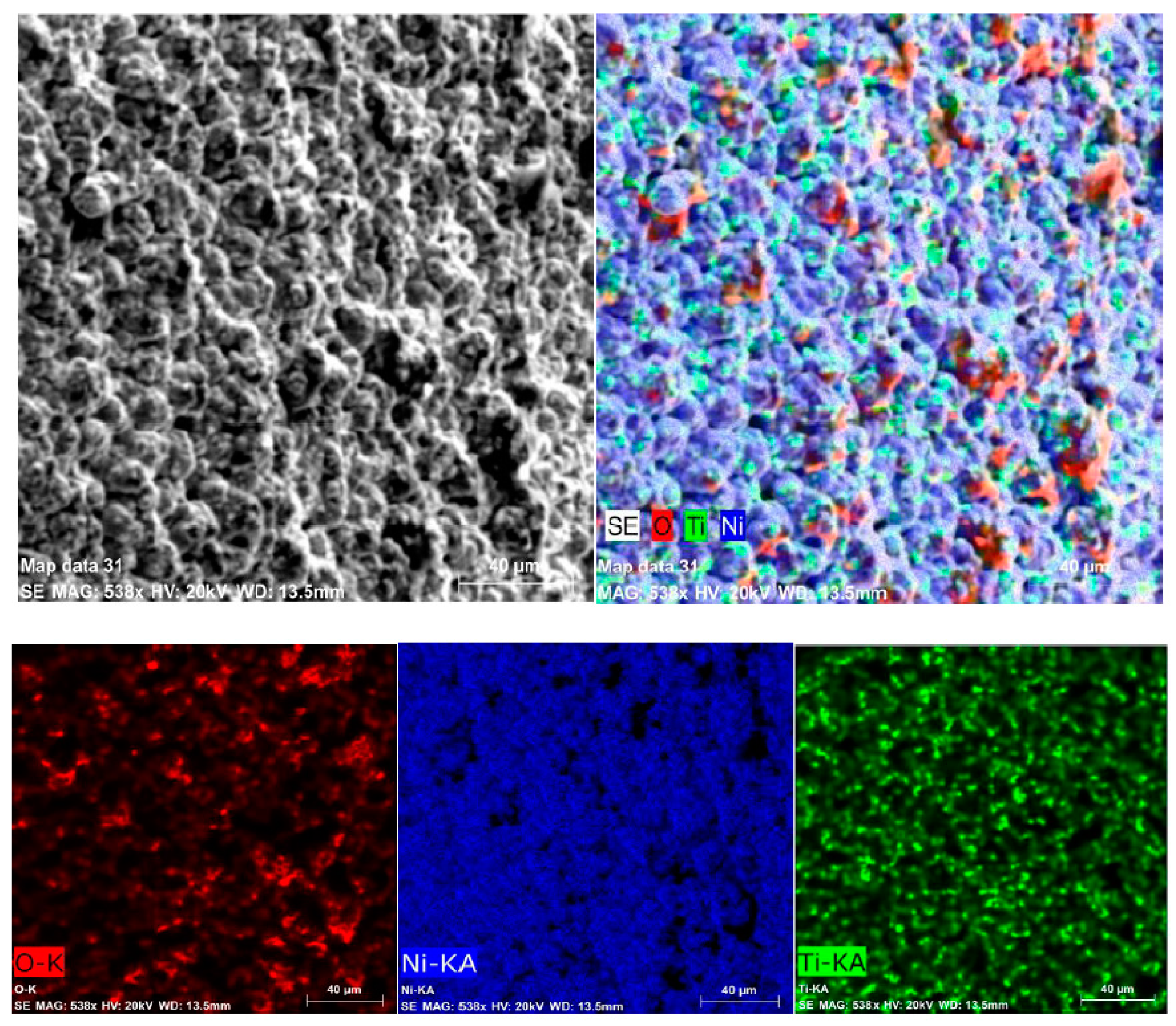
3.2. SEM Observations
3.2.1. Cross Section Observation
3.2.2. Surface Observation
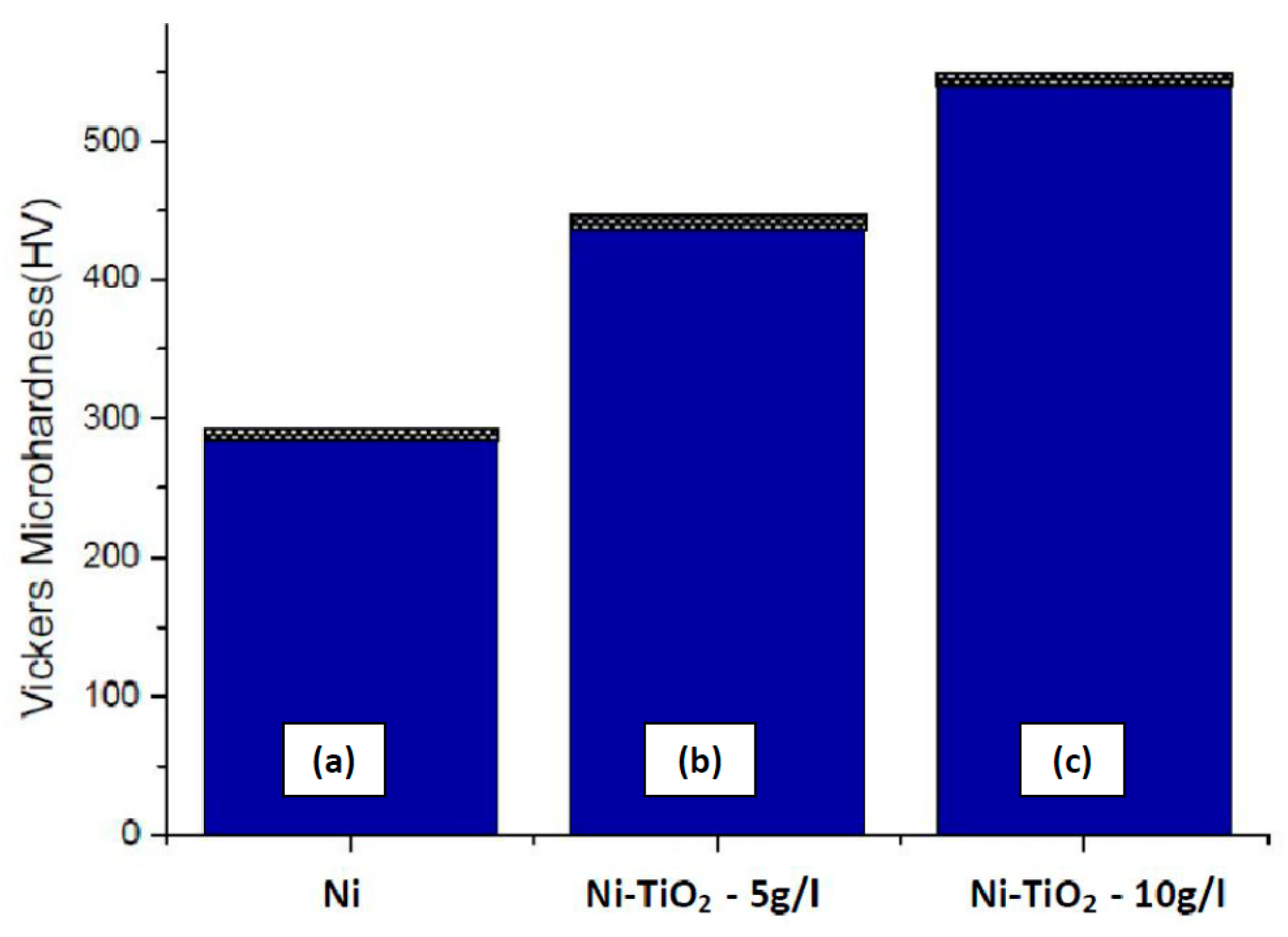
3.3. Microhardness Measurements
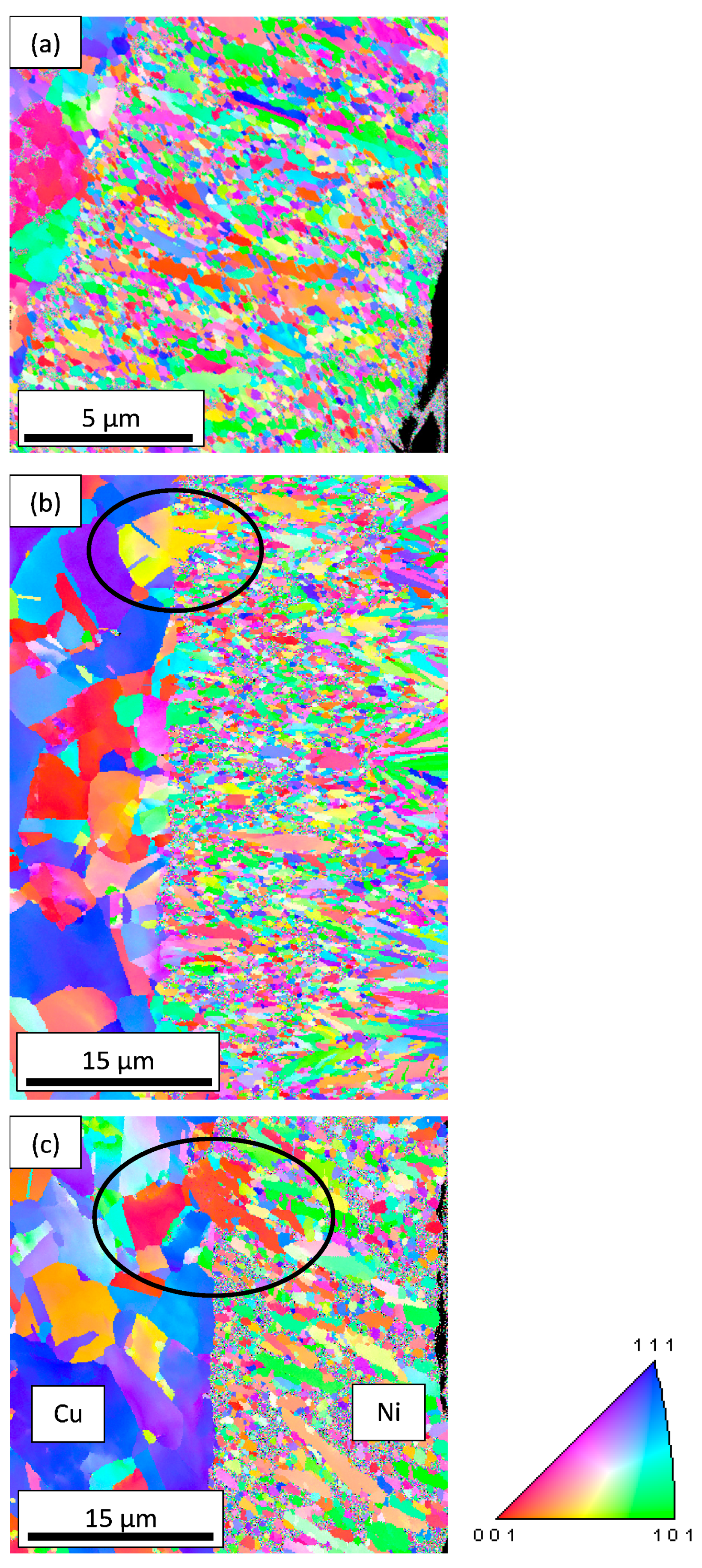
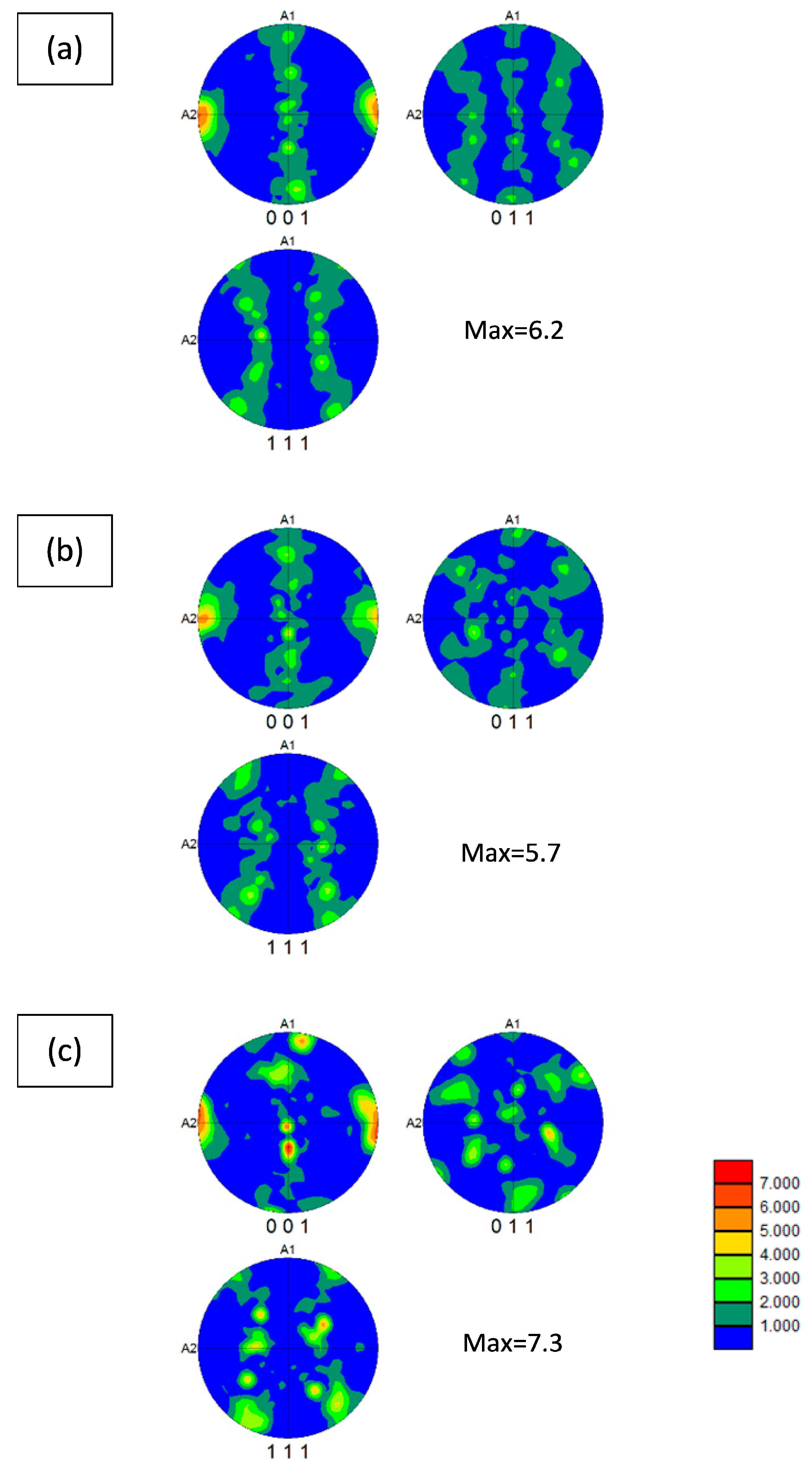
3.4. EBSD Analysis
4. Discussions
5. Conclusions
- TiO2 nanoparticles can be successfully co-deposited with Ni matrix from a Watt bath.
- TiO2 nanoparticles are homogeneously distributed into the Ni matrix.
- From the X-ray results, Ni grains have preferred orientation with the addition of TiO2 nanoparticles.
- TiO2 nanoparticles cause a rougher surface.
- Some limited number of large grains was formed with the incorporation of TiO2 in the nickel layer
- At the maximum amount of TiO2 added (10 g/L), the agglomerated particles propagated perpendicularly to the cathode surface from big size agglomerations to particles of smaller size to the external surface.
- The enhancement of microhardness of Ni-TiO2 composite coating can be attributed to the dispersion hardening effect of reinforced TiO2 particles.
- EBSD results indicate a <100> // A2 fiber texture for all samples with or without TiO2-nanoparticles. This crystallographic direction corresponds to the Ni grain growth one.
Author Contributions
Funding
Conflicts of Interest
References
- Erb, U. Electrodeposited nanocrystals: Synthesis, properties and industrial applications. Nanostruct. Mater. 1995, 6, 533–538. [Google Scholar] [CrossRef]
- Cheung, C.; Djuanda, F.; Erb, U.; Palumbo, G. Electrodeposition of nanocrystalline Ni-Fe alloys. Nanostruct. Mater. 1995, 5, 513–523. [Google Scholar] [CrossRef]
- Wu, G.; Li, N.; Zhou, D.; Mitsuo, K. Electrodeposited Co–Ni–Al2O3 composite coatings. Surf. Coat. Technol. 2004, 176, 157–164. [Google Scholar] [CrossRef]
- Clark, D.; Wood, D.; Erb, U. Industrial applications of electrodeposited nanocrystals. Nanostruct. Mater. 1997, 9, 755–758. [Google Scholar] [CrossRef]
- Rastogi, M.C. Surface and Interfacial Science, Applications to Engineering and Technology, 1st ed.; Narosa Publishing House: New Delhi, India, 2003. [Google Scholar]
- Gul, H.; Kilic, F.; Aslan, S.; Alp, A.; Akbulut, H. Characteristics of electro-co-deposited Ni–Al2O3 nano-particle reinforced metal matrixcomposite (MMC) coatings. Wear 2009, 267, 976–990. [Google Scholar]
- Du, L.; Xu, B.; Dong, S.; Yang, H.; Tu, W. Study of tribological characteristics and wear mechanism of nano-particle strengthened nickel-based composite coatings under abrasive contaminant lubrication. Wear 2004, 257, 1058–1063. [Google Scholar] [CrossRef]
- Zimmerman, A.F.; Palumbo, G.; Aust, K.T.; Erb, U. Mechanical properties of nickel silicon carbide nanocomposites. Mater. Sci. Eng. A 2002, 328, 137–146. [Google Scholar] [CrossRef]
- Baghery, P.; Farzam, M.; Mousavi, A.B.; Hosseini, M. Ni–TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf. Coat. Technol. 2010, 204, 3804–3810. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, C.S.; Xiao, H.N.; Cheng, F.Q.; Zhang, G.; Yi, G.J. Corrosion behavior of carbon nanotubes–Ni compositecoating. Surf. Coat. Technol. 2005, 191, 351–356. [Google Scholar]
- Stroumbouli, M.; Gyftou, P.; Pavlatou, E.A.; Spyrellis, N. Codeposition of ultrafine WC particles in Ni matrix composite electrocoatings. Surf. Coat. Technol. 2005, 195, 325–332. [Google Scholar] [CrossRef]
- Aruna, S.T.; William, V.K.; Rajam, K.S. Ni-based electrodeposited composite coating exhibiting improved microhardness, corrosion and wear resistance properties. J. Alloys Com. 2009, 468, 546–552. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Baraniak, M.; Pernak, J.; Lota, G. Nickel coatings electrodeposited from Watts type baths containing quaternary ammonium sulphate Salts. Int. J. Electrochem. Sci. 2017, 12, 3350–3360. [Google Scholar] [CrossRef]
- Tilak, B.V.; Gendron, A.S.; Mosoiu, M.A. Borate buffer equilibria in nickel refining electrolytes. J. Appl. Electrochem. 1977, 7, 495–500. [Google Scholar] [CrossRef]
- Cui, C.Q.; Lee, J.Y. Nickel deposition from unbuffered neutral chloride solutions in the presence of oxygen. Electrochim. Acta 1995, 40, 1653–1662. [Google Scholar] [CrossRef]
- Tian, B.R.; Cheng, Y.F. Electrolytic deposition of Ni–Co–Al2O3 composite coating on pipe steel for corrosion/erosion resistance in oil sand slurry. Electrochim. Acta 2007, 53, 511–517. [Google Scholar] [CrossRef]
- Gvozden, T.; Sladjana, M.; Dragana, Z.; Aleksandar, D.M.; Milica P., M.K. Characterization of the Ni–Mo catalyst formed in situ during hydrogen generation from alkaline water electrolysis. Int. J. Hydrogen Energy. 2011, 36, 11588–11595. [Google Scholar]
- Vereecken, P.M.; Shao, I.; Searson, P.C. Particle Codeposition in Nanocomposite Films. J. Electrochem. Soc. 2002, 147, 2572. [Google Scholar] [CrossRef]
- Ben Temam, H.; Chala, A.; Rahmane, S. Microhardness and corrosion behavior of Ni–SiC electrodeposited coatings in presence of organic additives. Surf. Coat. Technol. 2011, 205, S161–S164. [Google Scholar] [CrossRef]
- Indyka, P.; Beltowska-Lehman, E.; Tarkowski, L.; Bigos, A.; García-Lecina, E. Structure characterization of nanocrystalline Ni–W alloys obtained by electrodeposition. J. Alloys Compd. 2014, 590, 75–79. [Google Scholar] [CrossRef]
- Calleja, P.; Esteve, J.; Cojocaru, P.; Magagnin, L.; Vallés, E.; Gómez, E. Developing plating baths for the production of reflective Ni–Cu films. Electrochim. Acta 2012, 62, 381–389. [Google Scholar] [CrossRef]
- Jovic, B.M.; La cnjevac, U.C.; Krstaji, N.V.; Jović, V.D. Ni–Sn coatings as cathodes for hydrogen evolution in alkaline solutions. Electrochim. Acta 2013, 114, 813. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Halim, J.; El-Raghy, S.; Nabil, M.; Waheed, A. Surface morphology and electrochemical characterization of electrodeposited Ni–Mo nanocomposites as cathodes for hydrogen evolution. J. Alloys Compd. 2012, 530, 85–90. [Google Scholar] [CrossRef]
- Sanches, L.S.; Domingues, S.H.; Marino, C.E.; Mascaro, H.M. Characterisation of electrochemically deposited Ni–Mo alloy coatings. Electrochem. Commun. 2004, 6, 543–548. [Google Scholar] [CrossRef]
- Spanou, S.; Pavlatou, E.A.; Spyrellis, N. Ni/nano-TiO2 Composite Electrodeposits:Textural and Structural Modifications. Electrochim. Acta 2008, 54, 2547–2555. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, P.A.I. Tribological and Corrosion Performance of Electrodeposited Nickel Composite Coatings, Chapter 10, Electrodeposition of Composite Materials, 1st ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Duan, G.; Cai, W.; Luo, Y.I. Transferable ordered Ni hollow sphere arrays induced by electrodeposition on colloidal monolayer. Phys. Chem. B 2006, 110, 14–7184. [Google Scholar] [CrossRef]
- Yılmaz, G.; Hapcı, G.; Orhan, G. Properties of Ni/Nano-TiO2 composite coatings prepared by direct and pulse current electroplating. JMEPEG 2015, 24, 709–720. [Google Scholar] [CrossRef]
- Benea, L.; and Danaila, E. Nucleation and growth mechanism of Ni/TiO2 nanoparticles electro-codeposition. J. Electrochem. Soc. 2016, 163, D655–D662. [Google Scholar] [CrossRef]
- Kolonits, T.; Czigány, Z.; László Péter, L. Influence of Bath Additives on the Thermal Stability of the Nanostructure and Hardness of Ni Films Processed by Electrodeposition. Coatings 2019, 9, 644. [Google Scholar] [CrossRef]
- Amblard, J.; Froment, M. New interpretation of texture formation in nickel electrodeposits. Faraday Symp. Chem. Soc. 1977, 12, 136–144. [Google Scholar]
- Bhushan, B. Surface roughness analysis and measurement techniques. In Modern Tribology Handbook; CRC Press LLC: New York, NY, USA, 2001. [Google Scholar]
- Gyawali, G.; Tripathi, K.; Joshi, B.; Lee, S.W. Mechanical and tribological properties of Ni-W-TiB2 composite coatings. J. Alloys Compd. 2017, 721, 757–763. [Google Scholar] [CrossRef]
- Mohajeri, S.; Dolati, A.; Ghorbani, M. The influence of pulse plating parameters on the electrocodeposition of Ni-TiO2 nanocomposite single layer and multilayer structures on copper substrates. Surf. Coat. Technol. 2015, 262, 173–183. [Google Scholar] [CrossRef]
- Lajevardi, S.A.; Shahrabi, T. Effects of pulse electrodeposition parameters on the properties of Ni–TiO2 nanocomposite coatings. Appl. Surf. Sci. 2010, 256, 6775–6781. [Google Scholar] [CrossRef]
- Lampke, T.; Wielage, B.; Dietrich, D.; Leopold, A. Details of crystalline growth in co-deposited electroplated nickel films with hard (nano) particles. Appl. Surf. Sci. 2006, 253, 2399–2408. [Google Scholar] [CrossRef]
- Thiemig, T.; Bund, A. Characterization of electrodeposited Ni–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Lampe, T.; Leopold, A.; Dietrich, D.; Alisch, G.; Wielage, B. Correlation between structure and corrosion behaviour of nickel dispersion coatings ceramic particles of different sizes. Surf. Coat. Technol. 2006, 201, 3510–3517. [Google Scholar] [CrossRef]
- Goranova, D.; Avdeev, G.; Rashkov, R. Electrodeposition and characterization of Ni–Cu alloys. Surf. Coat. Technol. 2014, 240, 204–210. [Google Scholar] [CrossRef]
- Cardinal, M.F.; Castro, P.A.; Baxi, J.; Liang, H.; Williams, F.J. Characterization and frictional behavior of nanostructured Ni–W–MoS2 composite coatings. Surf. Coat. Technol. 2009, 204, 85–90. [Google Scholar] [CrossRef]
- Stankovic, V.; Gojo, M.; Grekulovic, V.; Pajkić, N.; Cigula, T. Surface quality of the Ni-TiO2 composite coatings produced by electroplating. J. Min. Metall. Sect. B Metal. 2017, 53, 341–348. [Google Scholar] [CrossRef]
- Magdy, A.M.; Ibrahim Kooli, F.; Saleh, N. Electrodeposition and characterization of nickel-TiN microcomposite coatings. Int. J. Electrochem. Sci. 2013, 8, 12308–12320. [Google Scholar]
- Erler, F.; Jakob, C.; Romanus, H.; Spiess, L.; BWielage, L.; Lampke, T.; Steinhäuser, S. Interface behavior in nickel composite coatings with nano-particles of oxidic ceramic. Electrochim. Acta. 2003, 48, 3063. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, S.; Boumerzoug, Z.; Helbert, A.L.; Brisset, F.; Baudin, T. Effect of TiO2-Nanoparticles on Ni Electrodeposition on Copper Wire. Metals 2020, 10, 406. https://doi.org/10.3390/met10030406
Saad S, Boumerzoug Z, Helbert AL, Brisset F, Baudin T. Effect of TiO2-Nanoparticles on Ni Electrodeposition on Copper Wire. Metals. 2020; 10(3):406. https://doi.org/10.3390/met10030406
Chicago/Turabian StyleSaad, Samiha, Zakaria Boumerzoug, Anne Laure Helbert, François Brisset, and Thierry Baudin. 2020. "Effect of TiO2-Nanoparticles on Ni Electrodeposition on Copper Wire" Metals 10, no. 3: 406. https://doi.org/10.3390/met10030406
APA StyleSaad, S., Boumerzoug, Z., Helbert, A. L., Brisset, F., & Baudin, T. (2020). Effect of TiO2-Nanoparticles on Ni Electrodeposition on Copper Wire. Metals, 10(3), 406. https://doi.org/10.3390/met10030406




