Role of Ag+ in the Bioleaching of Arsenopyrite by Acidithiobacillus ferrooxidans
Abstract
1. Introduction
2. Thermodynamic Calculations
3. Experimental
3.1. Minerals, Strain and Media
3.2. Bioleaching Experiment
3.3. Analytical Methods
4. Results and Discussion
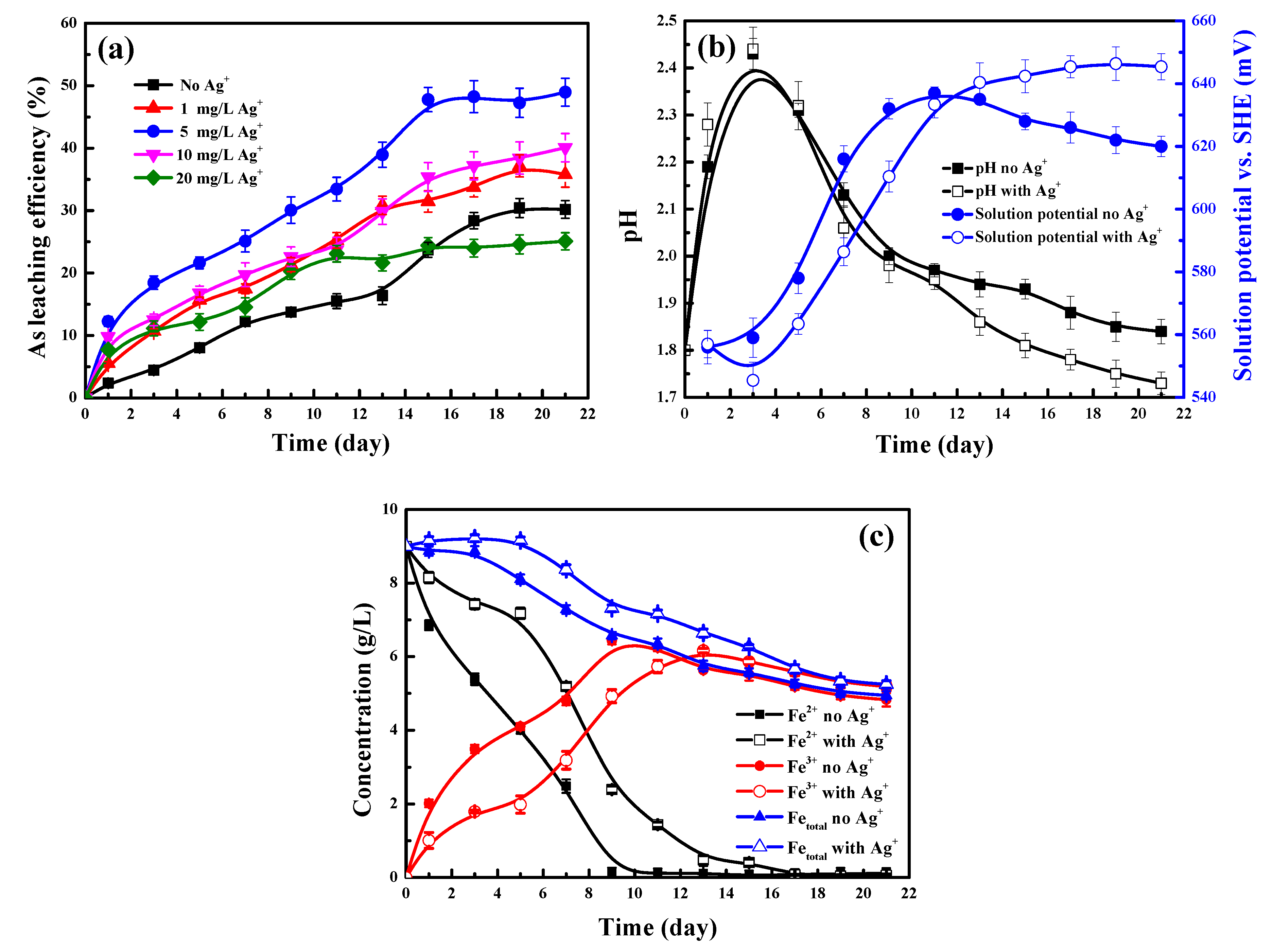
4.1. Effect of Ag+ Addition on Arsenopyrite Bioleaching
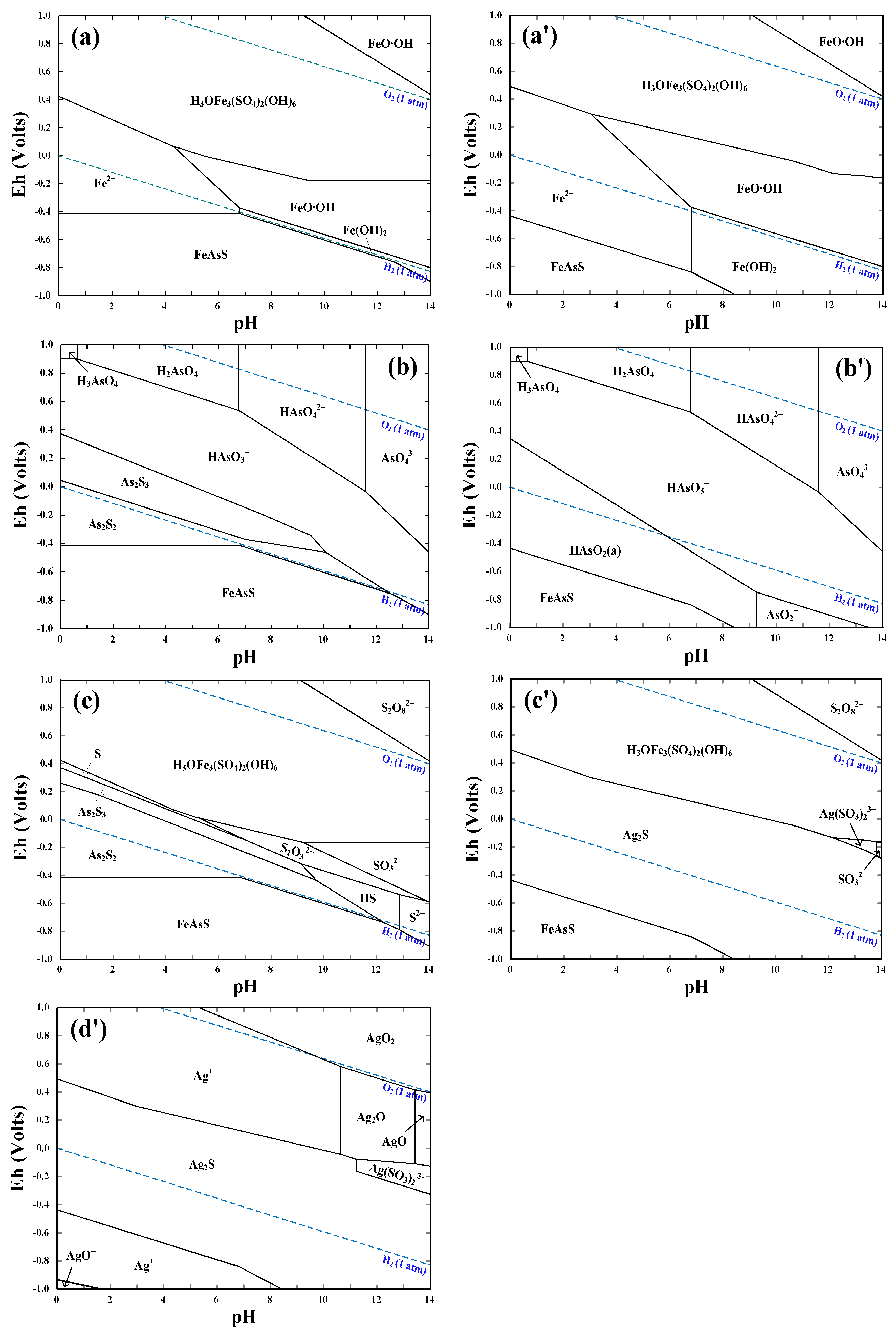
4.2. Thermodynamics of Arsenopyrite Dissolution in the Presence of Ag+
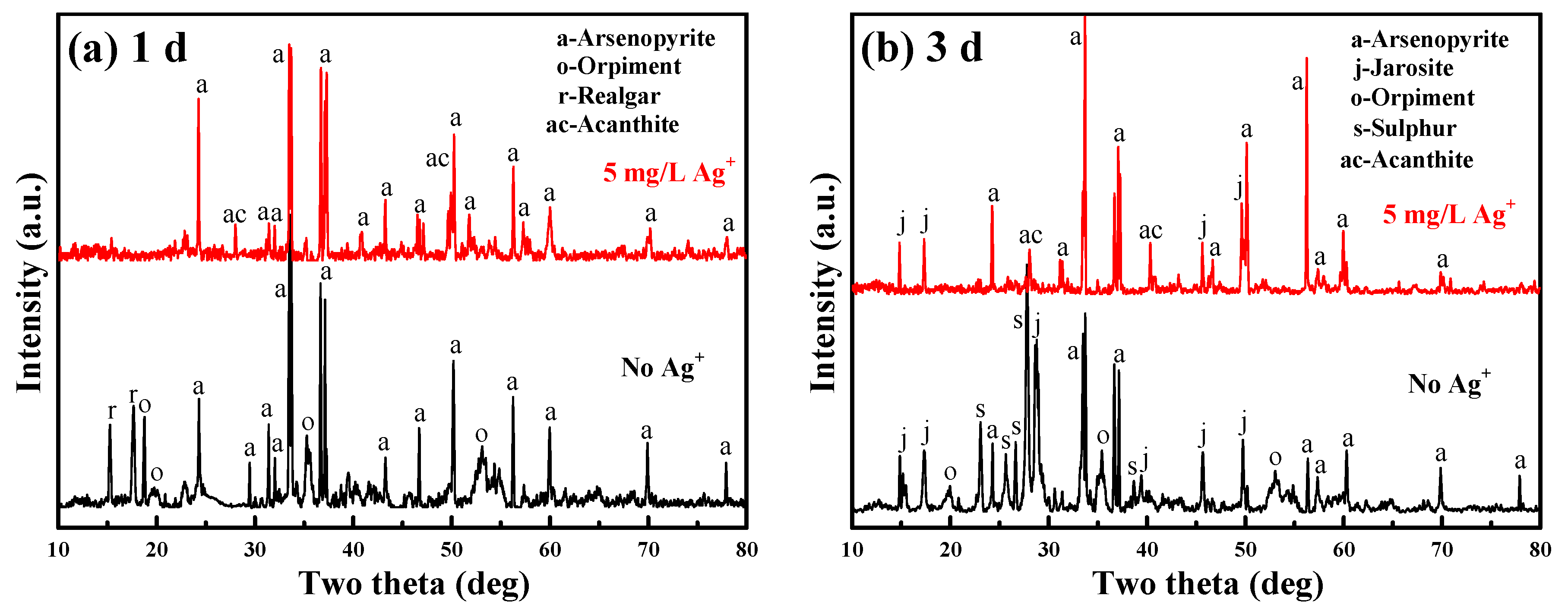
4.3. Mineralogical Phase Transformation
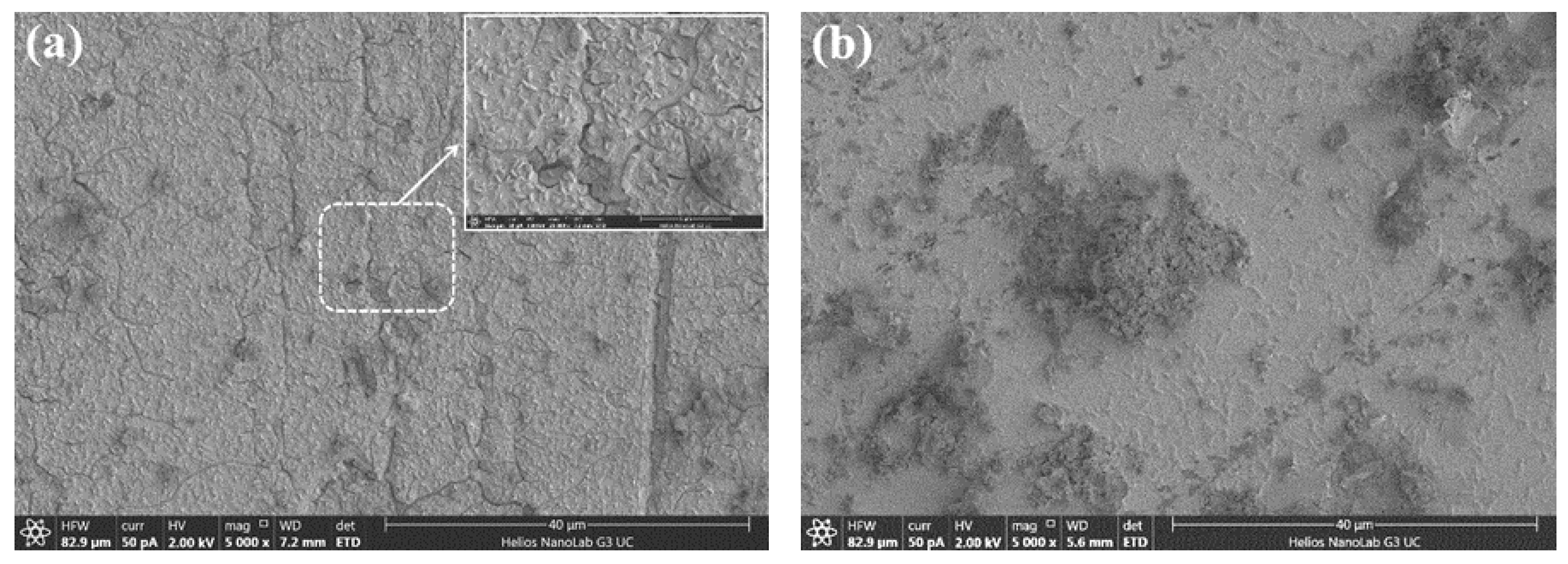
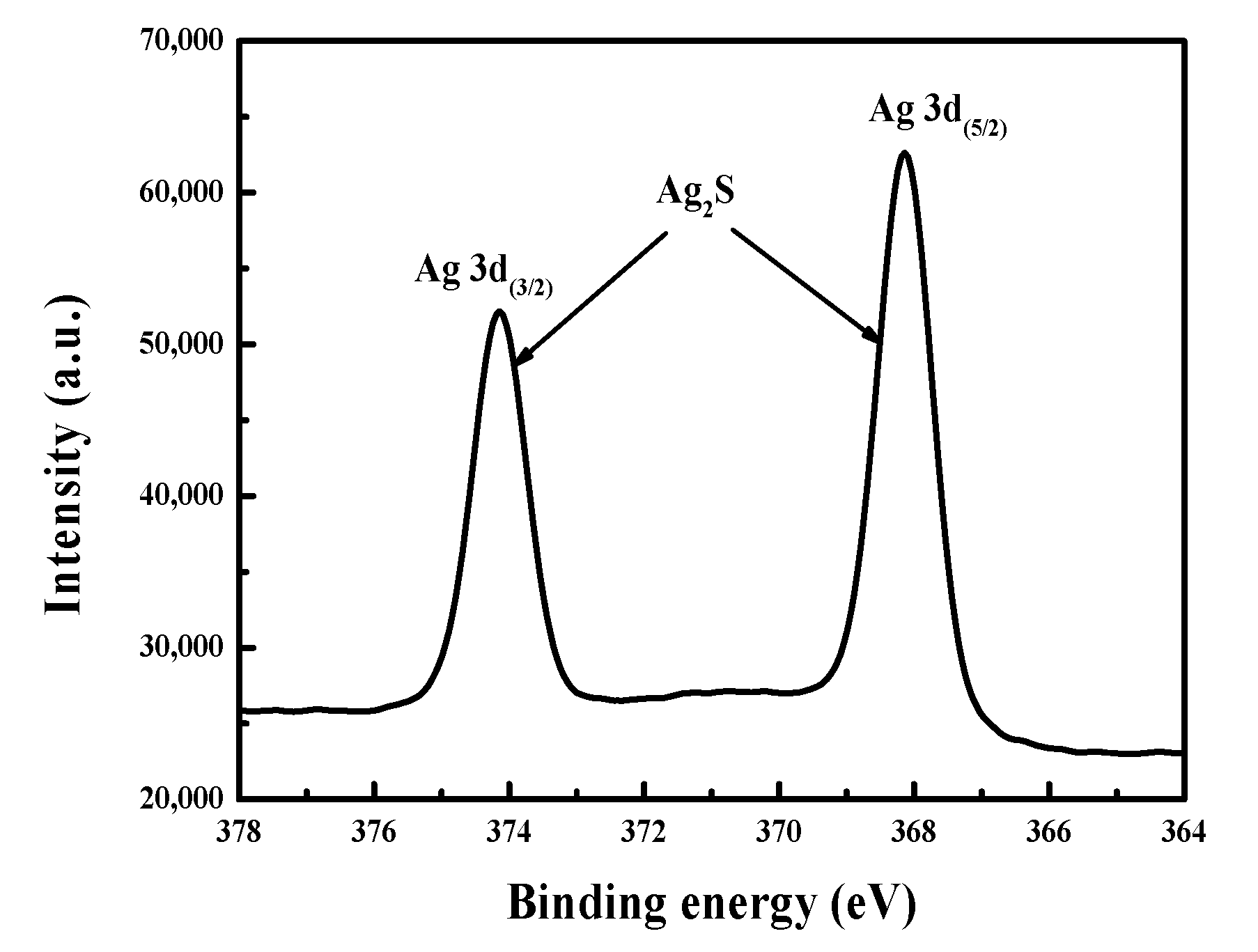
4.4. Morphology and Surface Composition Changes
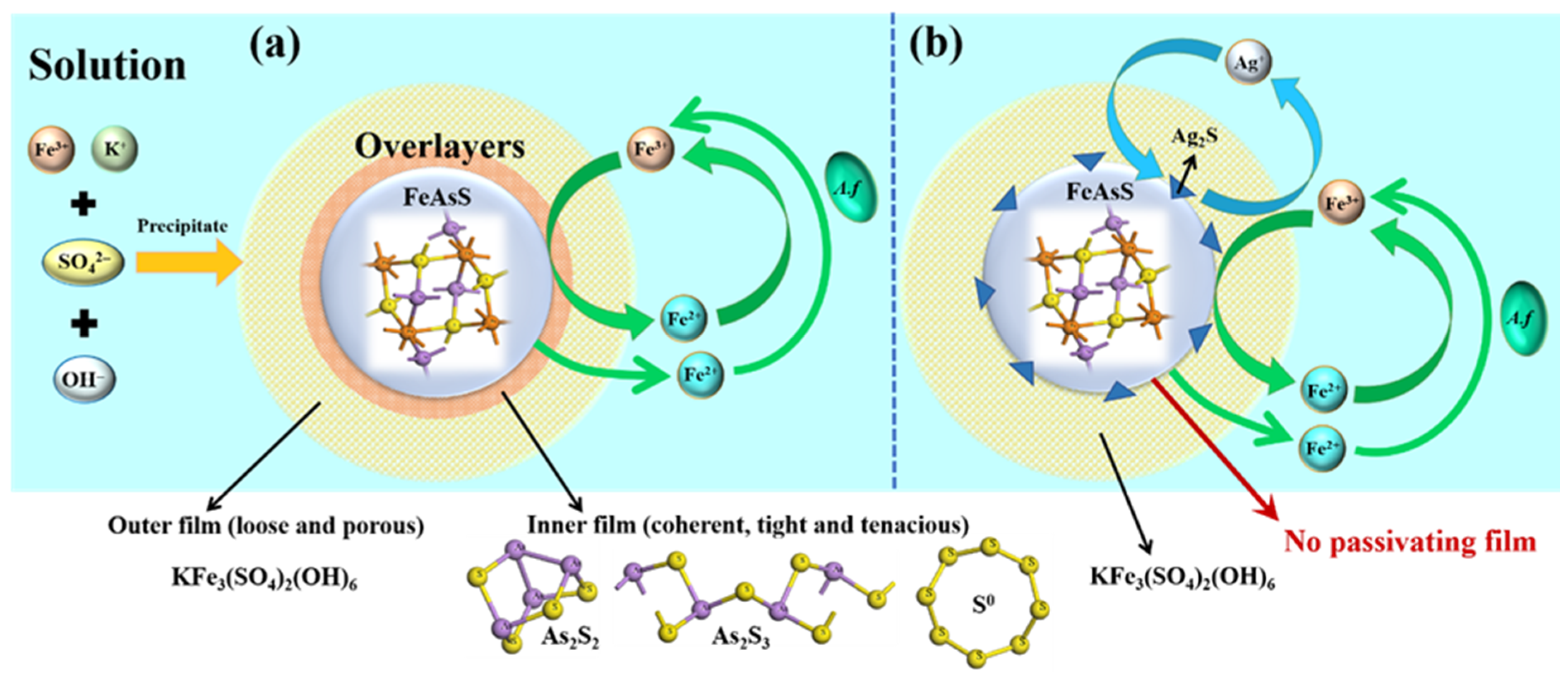
4.5. Possible Mechanisms for the Ag+ Catalysed Bioleaching of Arsenopyrite
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | ΔGf°298 | Species | ΔGf°298 | Species | ΔGf°298 | Species | ΔGf°298 |
| /(kJ/mol) | /(kJ/mol) | /(kJ/mol) | /(kJ/mol) | ||||
| FeAsS | −49.7616 | As2S2 | −68.5508 | S | 0 | AgO | 14.50 |
| FeAsO4 | −772.727 | As2S3 | −91.4907 | S2− | 86.00982 | AgO2 | −10.996 |
| Fe3(AsO4)2 | −1766.73 | As2O3 | −576.899 | S22− | 79.76167 | Ag2O | −11.180 |
| Fe(OH)2 | −492.158 | As2O4 | −701.161 | SO32− | −486.755 | Ag2O2 | 27.429 |
| Fe(OH)3 | −705.885 | As2O5 | −782.437 | S2O32− | −518.87 | Ag2O3 | 121.329 |
| FeO·OH | −489.439 | As4O6 | −1152.42 | S2O42− | −600.825 | Ag2S | −40.401 |
| Fe3+ | −17.1907 | AsO2– | −349.991 | S2O52− | −791.217 | Ag2SO3 | −411.615 |
| Fe2+ | −91.5644 | AsO43– | −648.477 | S2O62− | −969.453 | Ag2+ | 268.686 |
| FeOH2+ | −242.064 | As(OH)4– | −824.457 | S2O72− | −795.432 | Ag+ | 77.148 |
| FeOH+ | −275.615 | HAsO3− | −606.638 | S2O82− | −1115.35 | Ag(HS)2− | 0.247 |
| Fe(OH)2+ | −452.391 | HAsO42− | −714.732 | HS2− | 11.51053 | AgO− | −22.762 |
| Fe2(OH)24+ | −467.733 | H2AsO3− | −587.149 | HSO3− | −527.84 | Ag(OH)2− | −260.214 |
| H3OFe3(SO4)2(OH)6 b | −3230.36 | H2AsO4− | −753.399 | HS− | 12.44438 | AgS− | 59.968 |
| H3AsO3 (a) | −638.142 | HS2O3− | −532.363 | Ag(SO3)− | −441.572 | ||
| H2O | −237.177 | H3AsO4 (a) | −764.001 | H2S (a) | −27.656 | Ag(SO3)23− | −946.744 |
| HAsO2 (a) | −402.951 | Ag(SO3)35− | −1434.875 | ||||
| Ag(S2O3)− | −490.262 | ||||||
| Ag(S2O3)23− | −1023.386 | ||||||
| Ag(S2O3)35− | −2241.344 |
References
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Márquez, M.A.; Ospina, J.D.; Morales, A.L. New insights about the bacterial oxidation of arsenopyrite: A mineralogical scope. Miner. Eng. 2012, 39, 248–254. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of pyrite on thiosulfate leaching of gold and the role of ammonium alcohol polyvinyl phosphate (AAPP). Metals 2017, 7, 278. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Romanchenko, A.S.; Asanov, I.P. Oxidation of arsenopyrite and deposition of gold on the oxidized surfaces: A scanning probe microscopy, tunneling spectroscopy and XPS study. Geochim. Cosmochim. Acta 2006, 70, 4874–4888. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Muravyov, M. Thermodynamic and XRD analysis of arsenopyrite biooxidation and enhancement of oxidation efficiency of gold-bearing concentrates. Int. J. Miner. Process. 2014, 133, 112–118. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Improving gold recovery from a refractory ore via Na2SO4 assisted roasting and alkaline Na2S leaching. Hydrometallurgy 2019, 185, 133–141. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Simultaneous removal of S and As from a refractory gold ore in a single stage O2-enriched roasting process. Metall. Mater. Trans. B 2019, 50, 1588–1596. [Google Scholar] [CrossRef]
- Miller, P.; Brown, A.R.G. Bacterial Oxidation of Refractory Gold Concentrates. In Gold Ore Processing: Project Development and Operations; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 359–372. [Google Scholar]
- Craw, D.; Falconer, D.; Youngson, J.H. Environmental arsenopyrite stability and dissolution: Theory, experiment, and field observations. Chem. Geol. 2003, 199, 71–82. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Licheri, C.; Atzei, D.; Loi, G.; Elsener, B.; Rossi, G.; Rossi, A. Arsenopyrite and pyrite bioleaching: Evidence from XPS, XRD and ICP techniques. Anal. Bioanal. Chem. 2011, 401, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Henao, D.M.O.; Godoy, M.A.M. Jarosite pseudomorph formation from arsenopyrite oxidation using Acidithiobacillus ferrooxidans. Hydrometallurgy 2010, 104, 162–168. [Google Scholar] [CrossRef]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Yin, H.; Yang, Y.; Xu, B.; Jiang, T.; He, Y. The catalytic effect of copper ion in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans in 9K culture medium. J. Clean. Prod. 2020, 256, 120391. [Google Scholar] [CrossRef]
- Abdollahi, H.; Shafaei, S.Z.; Noaparast, M.; Manafi, Z.; Niemelä, S.I.; Tuovinen, O.H. Mesophilic and thermophilic bioleaching of copper from a chalcopyrite-containing molybdenite concentrate. Int. J. Miner. Process. 2014, 128, 25–32. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Xin, Y.; Gao, K.; Yang, J.; Liu, T.; Zhang, L.; Wang, W. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus. Bioresour. Technol. 2013, 129, 456–462. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the Galvanox™ process. Hydrometallurgy 2012, 113–114, 122–130. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Hu, M.; Zhang, E.; Qin, W.; Qiu, G. Cooperative bioleaching of chalcopyrite and silver-bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis. Miner. Eng. 2015, 81, 29–39. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, G.; Cao, J.; Li, Y.; Fang, Z.; Yang, C. Catalytic effect of Ag+ and Cu2+ on leaching realgar (As2S2). Hydrometallurgy 2011, 106, 99–103. [Google Scholar] [CrossRef]
- Zhang, G.; Chao, X.; Guo, P.; Cao, J.; Yang, C. Catalytic effect of Ag+ on arsenic bioleaching from orpiment (As2S3) in batch tests with Acidithiobacillus ferrooxidans and Sulfobacillus sibiricus. J. Hazard. Mater. 2015, 283, 117–122. [Google Scholar] [CrossRef]
- Fang, F.; Zhong, H.; Jiang, F.; Zhan, X. Catalytic effect of silver on bioleaching of arsenopyrite. Int. J. Chem. Eng. Appl. 2014, 5, 474–478. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Li, Q.; Zhang, Y.; Yang, Y.; Xu, B.; Jiang, T. Formation process of the passivating products from arsenopyrite bioleaching by Acidithiobacillus ferrooxidans in 9K culture medium. Metals 2019, 9, 1320. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Electrochemical behaviour of the dissolution and passivation of arsenopyrite in 9K culture medium. Appl. Surf. Sci. 2020, 508, 145269. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows: Chemical reaction and equilibrium software with extensive thermochemical database. In User’s Guide, Version 6.0.; Outokumpu Research Oy: Pori, Finland, 2006. [Google Scholar]
- Lázaro, I.; Cruz, R.; González, I.; Monroy, M. Electrochemical oxidation of arsenopyrite in acidic media. Int. J. Miner. Process. 1997, 50, 63–75. [Google Scholar] [CrossRef]
- Cruz, R.; Lázaro, I.; Rodríguez, J.M.; Monroy, M.; González, I. Surface characterization of arsenopyrite in acidic medium by triangular scan voltammetry on carbon paste electrodes. Hydrometallurgy 1997, 46, 303–319. [Google Scholar] [CrossRef]
- Liu, J.Y.; Tao, X.X.; Cai, P. Study of formation of jarosite mediated by thiobacillus ferrooxidans in 9K medium. Procedia Earth Planet. Sci. 2009, 1, 706–712. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Synthesis and properties of ammoniojarosites prepared with iron-oxidizing acidophilic microorganisms at 22–65 °C. Geochim. Cosmochim. Acta 2007, 71, 155–164. [Google Scholar] [CrossRef]
- Qorbani, M.; Gholami, M.; Moradlou, O.; Naseri, N.; Moshfegh, A.Z. Optimal Ag2S nanoparticle incorporated TiO2 nanotube array for visible water splitting. RSC Adv. 2014, 4, 7838–7844. [Google Scholar]
- Ristova, M.; Ristov, M. XPS profile analysis on CdS thin film modified with Ag by an ion exchange. Appl. Surf. Sci. 2001, 181, 68–77. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Puigdomenech, I. Make equilibrium diagrams using sophistacated algorithms (MEDUSA). In Inorganic Chemistry; Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]
| Oxidative Dissolution of Arsenopyrite | ∆Gr0/(kJ/mol) a | No. |
|---|---|---|
| 3FeAsS + 2H2O + 7Fe3+ = As2S2 + S2– + 10Fe2+ + AsO2– + 4H+ | −504.203 | 1 |
| 4FeAsS + 4H2O + 12Fe3+ = As2S3 + S2– + 16Fe2+ + 2AsO2– + 8H+ | −816.451 | 2 |
| 2FeAsS + 6H2O + 10Fe3+ = S0 + 2HAsO3– + S2– + 12Fe2+ + 10H+ | −531.546 | 3 |
| 3FeAsS + 2H2O + 2Ag+ + 7Fe3+ = Ag2S + As2S2 + 10Fe2+ + AsO2– + 4H+ | −784.910 | 4 |
| 4FeAsS + 4H2O + 2Ag+ + 12Fe3+ = Ag2S + As2S3 + 16Fe2+ + 2AsO2– + 8H+ | −1097.156 | 5 |
| 2FeAsS + 6H2O + 2Ag+ + 10Fe3+ = Ag2S + S0 + 2HAsO3– + 12Fe2+ + 10H+ | −812.255 | 6 |
| Oxidation of Passivating Products from Arsenopyrite Leaching | ||
| 2As2S2 + 15H2O + 16Fe3+ = 4HAsO3– + 2S2– + HS2O3– + 16Fe2+ + 25H+ | −282.116 | 7 |
| As2S3 + 9H2O + 10Fe3+ = 2HAsO3– + S2– + HS2O3– + 10Fe2+ + 15H+ | −177.281 | 8 |
| 2S0 + 3H2O + 2Fe3+ = HS2O3– + S2– + 2Fe2+ + 5H+ | −116.431 | 9 |
| 2As2S2 + 15H2O + 4Ag+ + 16Fe3+ = 2Ag2S + 4HAsO3– + HS2O3‒ + 16Fe2+ + 25H+ | −843.533 | 10 |
| As2S3 + 9H2O + 2Ag+ + 10Fe3+ = Ag2S + 2HAsO3– + HS2O3‒ + 10Fe2+ + 15H+ | −457.990 | 11 |
| 3S0 + 3H2O + 2Ag+ + 2Fe3+ = Ag2S + HS2O3‒ + 2Fe2+ + 5H+ | −164.276 | 12 |
| Oxidation of Ag2S | ||
| 8Fe3+ + 2Ag2S + 3H2O = 8Fe2+ + 4Ag+ + HS2O3– + 5H+ | −26.421 | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Q.; Liu, X. Role of Ag+ in the Bioleaching of Arsenopyrite by Acidithiobacillus ferrooxidans. Metals 2020, 10, 403. https://doi.org/10.3390/met10030403
Zhang Y, Li Q, Liu X. Role of Ag+ in the Bioleaching of Arsenopyrite by Acidithiobacillus ferrooxidans. Metals. 2020; 10(3):403. https://doi.org/10.3390/met10030403
Chicago/Turabian StyleZhang, Yan, Qian Li, and Xiaoliang Liu. 2020. "Role of Ag+ in the Bioleaching of Arsenopyrite by Acidithiobacillus ferrooxidans" Metals 10, no. 3: 403. https://doi.org/10.3390/met10030403
APA StyleZhang, Y., Li, Q., & Liu, X. (2020). Role of Ag+ in the Bioleaching of Arsenopyrite by Acidithiobacillus ferrooxidans. Metals, 10(3), 403. https://doi.org/10.3390/met10030403





