The Relationship between Nil-Strength Temperature, Zero Strength Temperature and Solidus Temperature of Carbon Steels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Investigated Steels
2.2. Determination of Nil-Strength Temperature, Zero Strength Temperature and Solidus Temperature
3. Results and Discussion
3.1. Evaluation of the Nil-Strength Temperature and Zero Strength Temperature
3.2. Evaluation of Solidus Temperature
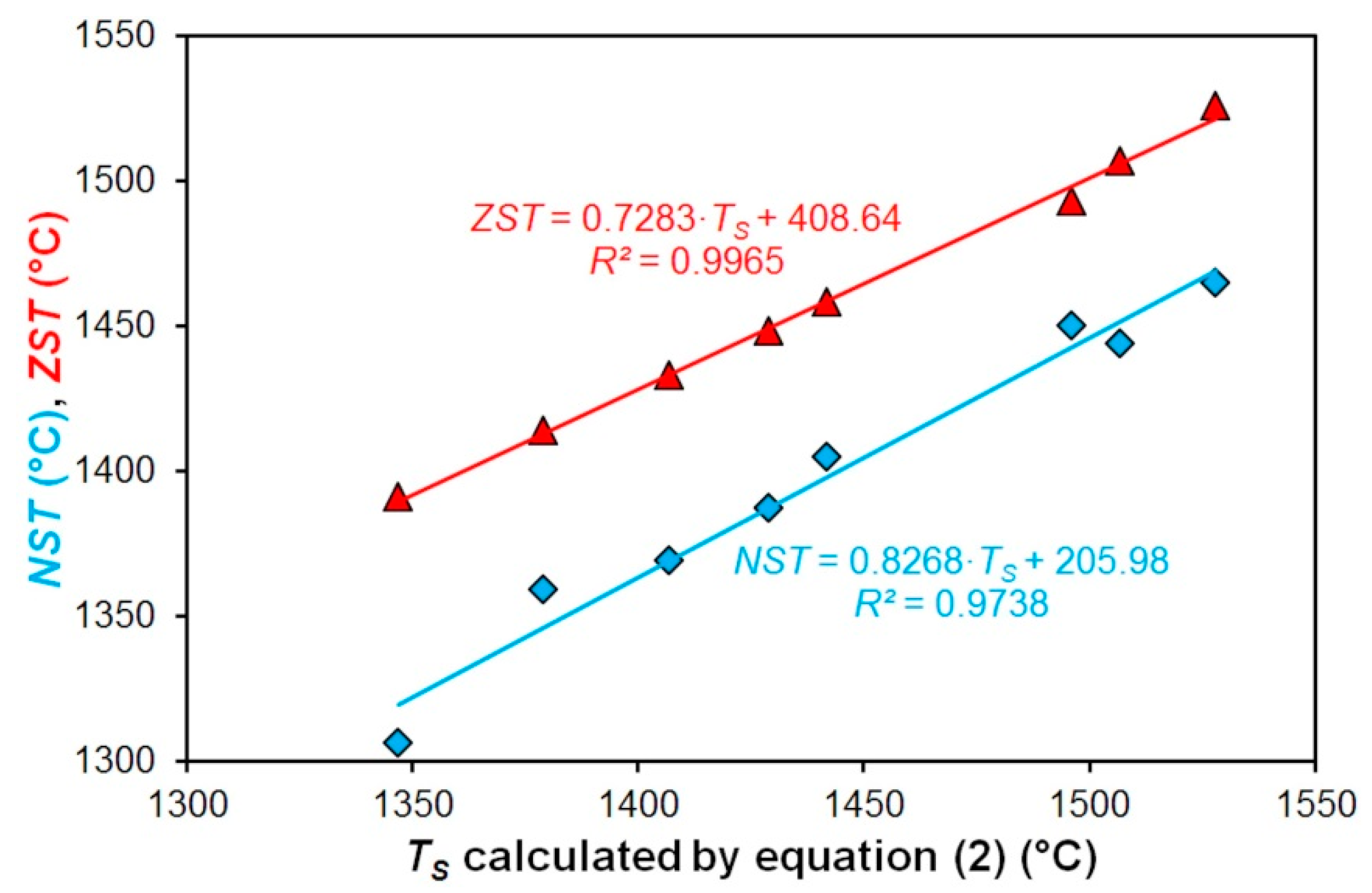
3.3. The Dependence of the Nil-Strength Temperature and the Zero Strength Temperature on the Solidus Temperature of Investigated Steels
4. Conclusions
- It is clear from the obtained results that the nil-strength temperature determined during heating is not the same value as the zero strength temperature determined during the solidification of steels during their casting.
- The linear dependence of experimentally measured nil-strength temperature on the calculated zero strength temperature of the investigated steels was determined.
- The mathematical relations which described with high accuracy a linear dependence of the nil-strength and zero strength temperatures of the investigated steels on the solidus temperature were determined. Equations (9) and (10), together with Equation (2), enable a relatively easy and accurate prediction of the nil-strength and zero strength temperatures of carbon steels (during their heating or solidification) in dependence on their chemical composition.
- The obtained results can be applied to optimization processes for the welding, casting or forming of non-alloy carbon steels.
Author Contributions
Funding
Conflicts of Interest
References
- Gryc, K.; Smetana, B.; Žaludová, M.; Michalek, K.; Klus, P.; Tkadlečková, M.; Socha, L.; Dobrovská, J.; Machovčák, P.; Válek, L.; et al. Determination of the Solidus and Liquidus Temperatures of the Real-steel Grades with Dynamic Thermal-analysis Methods. Mater. Technol. 2013, 47, 569–575. [Google Scholar]
- Zhuang, C.L.; Liu, J.H.; Bernhard, C.; Presoly, P. Analysis of Solidification of High Manganese Steels Using Improved Differential Thermal Analysis Method. J. Iron Steel Res. Int. 2015, 22, 709–714. [Google Scholar] [CrossRef]
- Štětina, J. Dynamický model teplotního pole plynule odlévané bramy. Ph.D. Thesis, VSB-TU Ostrava, Ostrava, Czech Republic, 2007. (In Czech). [Google Scholar]
- Thomas, B.G.; Samarasekera, I.V.; Brimacombe, J.K. Mathematical Model of the Thermal Processing of Steel Ingots 1. Heat Flow Model. Metall. Trans. B 1987, 18, 119–130. [Google Scholar] [CrossRef]
- Kuzsella, L.; Lukács, J.; Szücs, K. Nil-strength Temperature and Hot Tensile Tests on S960QL High-strength Low-alloy Steel. Prod. Process. Syst. 2013, 6, 67–78. [Google Scholar]
- Mandziej, S.T. Physical Simulation of Metallurgical Processes. Mater. Technol. 2010, 44, 105–119. [Google Scholar]
- Gryc, K.; Smetana, B.; Žaludová, M.; Klus, P.; Michálek, K.; Tkadlečková, M.; Dobrovská, J.; Socha, L.; Chmiel, B. High-temperature Thermal Analysis of Specific Steel Grades. In Proceedings of the 21st International Conference on Metallurgy and Materials Metal 2012, Brno, Czech Republic, 23–25 May 2012; pp. 103–108. [Google Scholar]
- Piekarski, B. The influence of Nb, Ti, and Si additions on the liquidus and solidus temperatures and primary microstructure refinement in 0.3C-30Ni-18Cr cast steel. Mater. Charact. 2010, 61, 899–906. [Google Scholar] [CrossRef]
- Khosravifard, A.; Hematiyan, M.R.; Wrobel, L.C. Simultaneous control of solidus and liquidus lines in alloy solidification. Eng. Anal. Bound. Elem. 2013, 34, 211–224. [Google Scholar] [CrossRef]
- Fu, J.W.; Qiu, W.X.; Nie, Q.Q.; Wu, Y.C. Precipitation of TiN during solidification of AISI 439 stainless steel. J. Alloys Compd. 2017, 699, 938–946. [Google Scholar] [CrossRef]
- Sawicki, S.; Dyja, H.; Kawalek, A.; Knapinksi, M.; Kwapisz, M.; Laber, K. High-Temperature Characteristics of 20MnB4 and 30MnB4 Micro-Addition Cold Upsetting Steels and C45 and C70 High-Carbon-Steels. Metalurgija 2016, 55, 643–646. [Google Scholar]
- Böllinghaus, T.; Herold, H. Hot Cracking Phenomena in Welds, 1st ed.; Springer: Berlin/Heidelberg, Deutschland, 2005; p. 394. [Google Scholar]
- Lukács, J.; Kuzsella, L.; Koncsik, Z.; Gáspár, M.; Meilinger, Á. Role of the Physical Simulation for the Estimation of the Weldability of High Strength Steels and Aluminum Alloys. Mater. Sci. Forum 2014, 812, 149–154. [Google Scholar] [CrossRef]
- Santillana, B.; Boom, R.; Eskin, D.; Mizukami, H.; Hanao, M.; Kawamoto, M. High-Temperature Mechanical Behavior and Fracture Analysis of a Low-Carbon Steel Related to Cracking. Metall. Mater. Trans. A 2012, 43A, 5048–5057. [Google Scholar] [CrossRef]
- Lyczkowska, K.; Adamiec, J.; Jachym, R.; Kwiecinski, K. Properties of the Inconel 713 Alloy Within the High Temperature Brittleness Range. Arch. Foundry Eng. 2017, 17, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Eskin, D.G.; Suyitno; Katgeman, L. Mechanical properties in the semi-solid state and hot tearing of aluminium alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Andersson, J.; Sjöberg, G.; Chaturvedi, M. Hot Ductility Study of Haynes 282 Superalloy. In Proceedings of the 7th International Symposium on Superalloy 718 and Derivatives, Pittsburgh, PA, USA, 10–13 October 2010. [Google Scholar] [CrossRef]
- Smetana, B.; Zla, S.; Kawuloková, M.; Gryc, K.; Strouhalová, M.; Kalup, A.; Tkadlečková, M.; Dobrovská, J.; Michalek, K.; Jonšta, P.; et al. Temperatures of Solidus and Liquidus of Tool Steel. In Proceedings of the 25th Anniversary International Conference on Metallurgy and Materials Metal 2016, Brno, Czech Republic, 25–27 May 2016; pp. 91–96. [Google Scholar]
- Kawuloková, M.; Zlá, S.; Dobrovská, J.; Smetana, B.; Kalup, A.; Strouhalová, M.; Vontorová, J.; Válek, L.; Rosypalová, S.; Francová, H. Phase Transformations Temperatures of Real Steel Grade. In Proceedings of the 24th International Conference on Metallurgy and Materials Metal 2015, Brno, Czech Republic, 3–5 June 2015; pp. 636–641. [Google Scholar]
- Kawulok, R.; Schindler, I.; Kawulok, P.; Rusz, S.; Opěla, P.; Solowski, Z.; Čmiel, K.M. Effect of Deformation on the Continuous Cooling Transformation CCT Diagram of Steel 32CrB4. Metalurgija 2015, 54, 473–476. [Google Scholar]
- Kawuloková, M.; Smetana, B.; Zlá, S.; Kalup, A.; Mazancová, E.; Váňová, P.; Kawulok, P.; Dobrovská, J.; Rosypalová, S. Study of Equilibrium and Nonequilibrium Phase Transformations Temperatures of Steel by Thermal Analysis Methods. J. Therm. Anal. Calorim. 2017, 127, 423–429. [Google Scholar] [CrossRef]
- Saunders, N.; Guo, Z.; Li, X.; Miodownik, A.P.; Schille, J.P. Using JMatPro to Model Materials Properties and Behavior. JOM 2003, 55, 60–65. [Google Scholar] [CrossRef]
- Andersson, J.O.; Helander, T.; Hoglund, L.H.; Shi, P.F.; Sundman, B. THERMO-CALC & DICTRA, computational tools for materials science. CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Diederichs, R.; Bleck, W. Modelling of manganese sulphide formation during solidification. Part I: Description of MnS formation parameters. Steel Res. Int. 2006, 77, 202–209. [Google Scholar] [CrossRef]
- Takeuchi, E.; Brimacombe, J.K. Effect of Oscillation-Mark Formation on the Surface Quality of Continuously Cast Steel Slabs. Metall. Trans. B 1985, 16, 605–625. [Google Scholar] [CrossRef]
- Peitsch, P.R.; Danón, C.A. Comparative Study of 9% Cr Martensitic-ferritic Steels Using Differential Scanning Calorimetry. Procedia Mater. Sci. 2015, 9, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Fabrichnaya, O.; Ullrich, C.; Wendler, M.; Savinykh, G.; Rafaja, D. High-temperature phase transformations in strongly metastable austenitic-martensitic CrMnNi-N-C cast steels. J. Alloys Compd. 2016, 686, 511–521. [Google Scholar] [CrossRef]
- Hechu, K.; Slater, C.; Santillana, B.; Clark, S.; Sridhar, S. A novel approach for interpreting the solidification behaviour of peritectic steels by combining CSLM and DSC. Mater. Charact. 2017, 133, 25–32. [Google Scholar] [CrossRef]
- Gumienny, G. Carbidic Bainitic and Ausferritic Ductile Cast Iron. Arch. Metall. Mater. 2013, 58, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Rapiejko, C.; Pisarek, B.; Czekaj, E.; Pacyniak, T. Analysis of AM60 and AZ91 alloy crystallisation in ceramic moulds by thermal derivative analysis (TDA). Arch. Metall. Mater. 2014, 59, 1449–1455. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, T.; Takeshita, K.; Suzuki, R.O. Mathematical analysis of the solidification behavior of multi-component alloy steel based on the heat- and solute-transfer equations in the liquid–solid zone. Int. J. Heat Mass Tran. 2019, 130, 797–812. [Google Scholar] [CrossRef]
- Won, Y.M.; Thomas, B.G. Simple Model of Microsegregation during Solidification of Steels. Metall. Mater. Trans. A 2001, 32A, 1755–1767. [Google Scholar] [CrossRef]
- Kawulok, P.; Schindler, I.; Kawulok, R.; Rusz, S.; Opěla, P.; Kliber, J.; Kawuloková, M.; Solowski, Z.; Čmiel, K.M. Plastometric Study of Hot Formability of Hypereutectoid C–Mn–Cr–V Steel. Metalurgija 2016, 55, 365–368. [Google Scholar]
- Mittinen, J. Solidification Analysis Package for Steels—User’s Manual of DOS Version, 1st ed.; University of Technology: Helsinky, Finland, 1999. [Google Scholar]
- Miettinen, J.; Louhenkilpi, S. Calculation of thermophysical properties of carbon and low-alloyed steels for modeling of solidification processes. Metall. Mater. Trans. B 1994, 25, 909–916. [Google Scholar] [CrossRef]
- Miettinen, J.; Louhenkilpi, S.; Kytonen, H.; Laine, J. IDS: Thermodynamic-kinetic-empirical tool for modelling of solidification, microstructure and material properties. Math. Comput. Simulat. 2010, 80, 1536–1550. [Google Scholar] [CrossRef]
- Adamiec, J.; Kalka, M. Brittleness temperature range of Fe-Al alloy. JAMME 2006, 18, 43–46. [Google Scholar]
- Žídek, M. Metallurgical Formability of Steels at hot and cold Conditions, 1st ed.; Aleko: Praha, Czech Republic, 1995. (In Czech) [Google Scholar]
- Suzuki, H.G.; Nishimura, S.; Nakamura, Y. Improvement of hot ductility of continuously cast carbon steels. Trans. ISIJ 1984, 24, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Schmidtmann, E.; Rakoski, F. Einfluß des Kohlenstoffgehaltes von 0,015 bis 1 % und der Gefügestruktur auf das Hochtemperaturfestigkeits- und -zähigkeitsverhalten von Baustählen nach der Erstarrung aus der Schmelze (Influence of sulphur and manganese and of the cooling conditions on the structure and toughness properties of structural steels after solidification from the melt). Arch. Eisenhüttenwes. 1983, 54, 357–362. (In German) [Google Scholar]
- Shin, G.; Kajitani, T.; Suzuki, T.; Umeda, T. Mechanical properties of carbon steels during solidification. Tetsu-to-Hagane 1992, 78, 587–593. [Google Scholar] [CrossRef] [Green Version]
- You, D.; Bernhard, C.H.; Wieser, G.; Michelic, S. Microsegregation model with local equilibrium partition coefficients during solidification of steels. Steel Res. Int. 2016, 87, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Flesh, R.; Bleck, W. Crack susceptibility of medium and high alloyed tool steels under continuous casting conditions. Steel Res. 1998, 69, 292–299. [Google Scholar] [CrossRef]
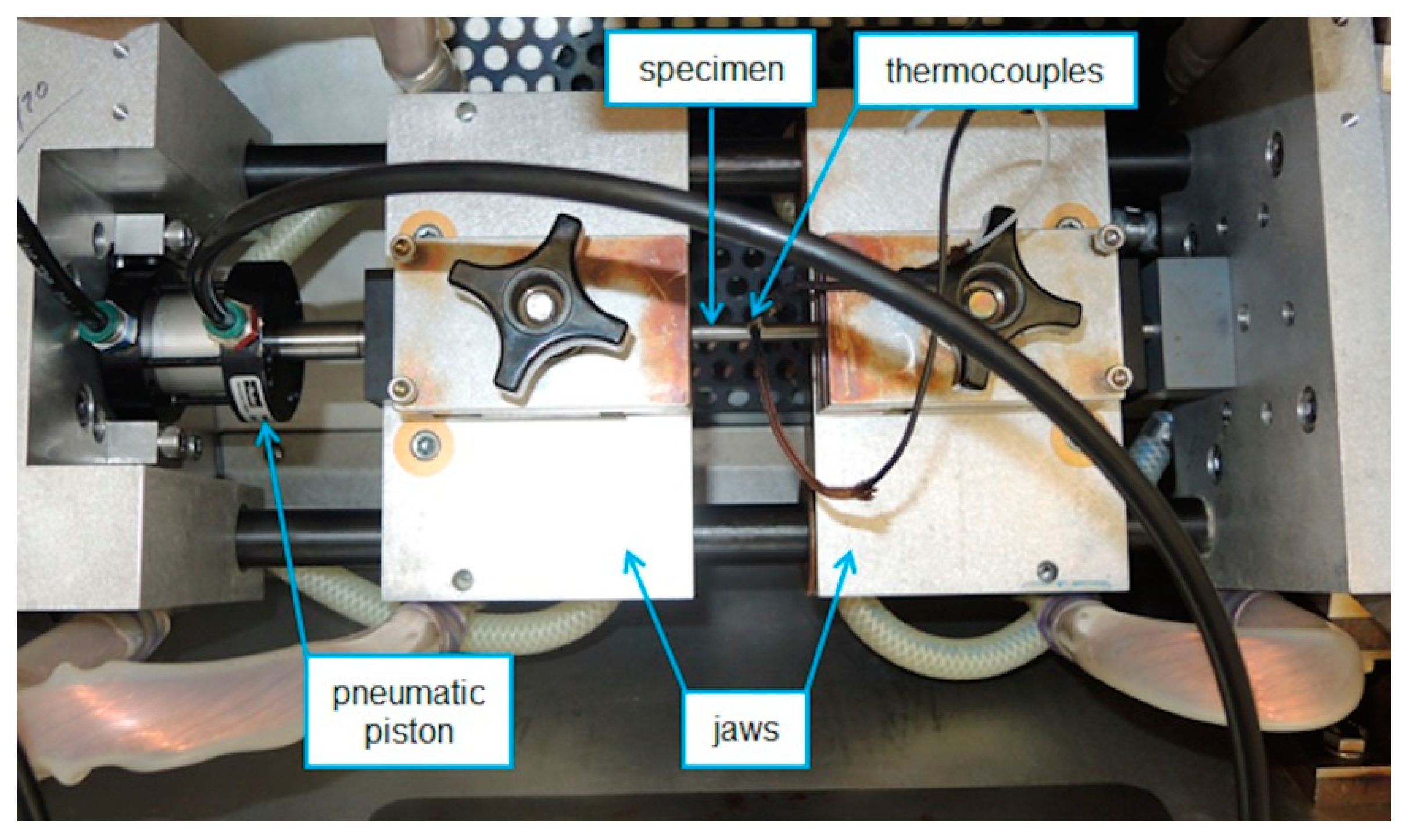

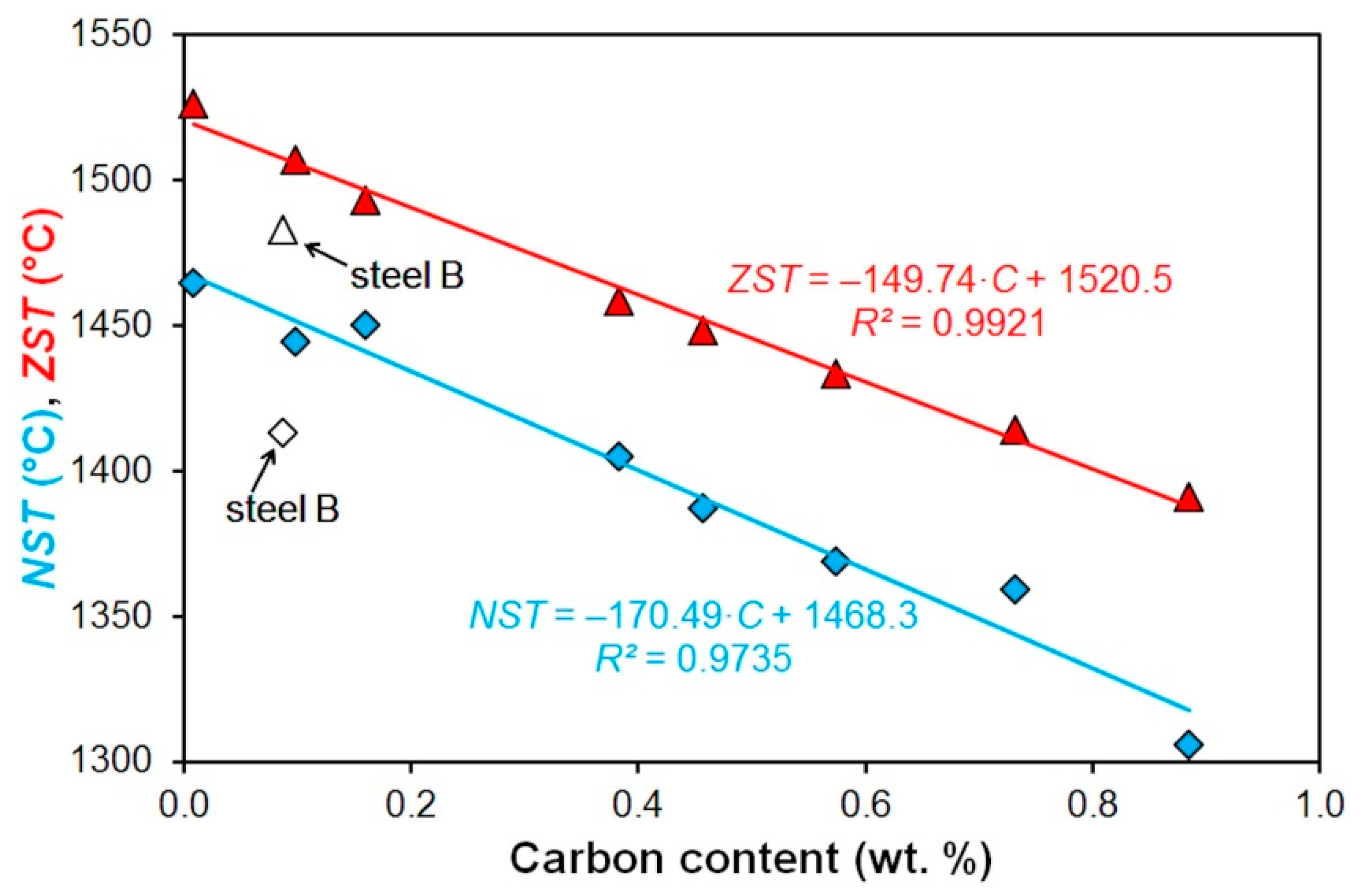
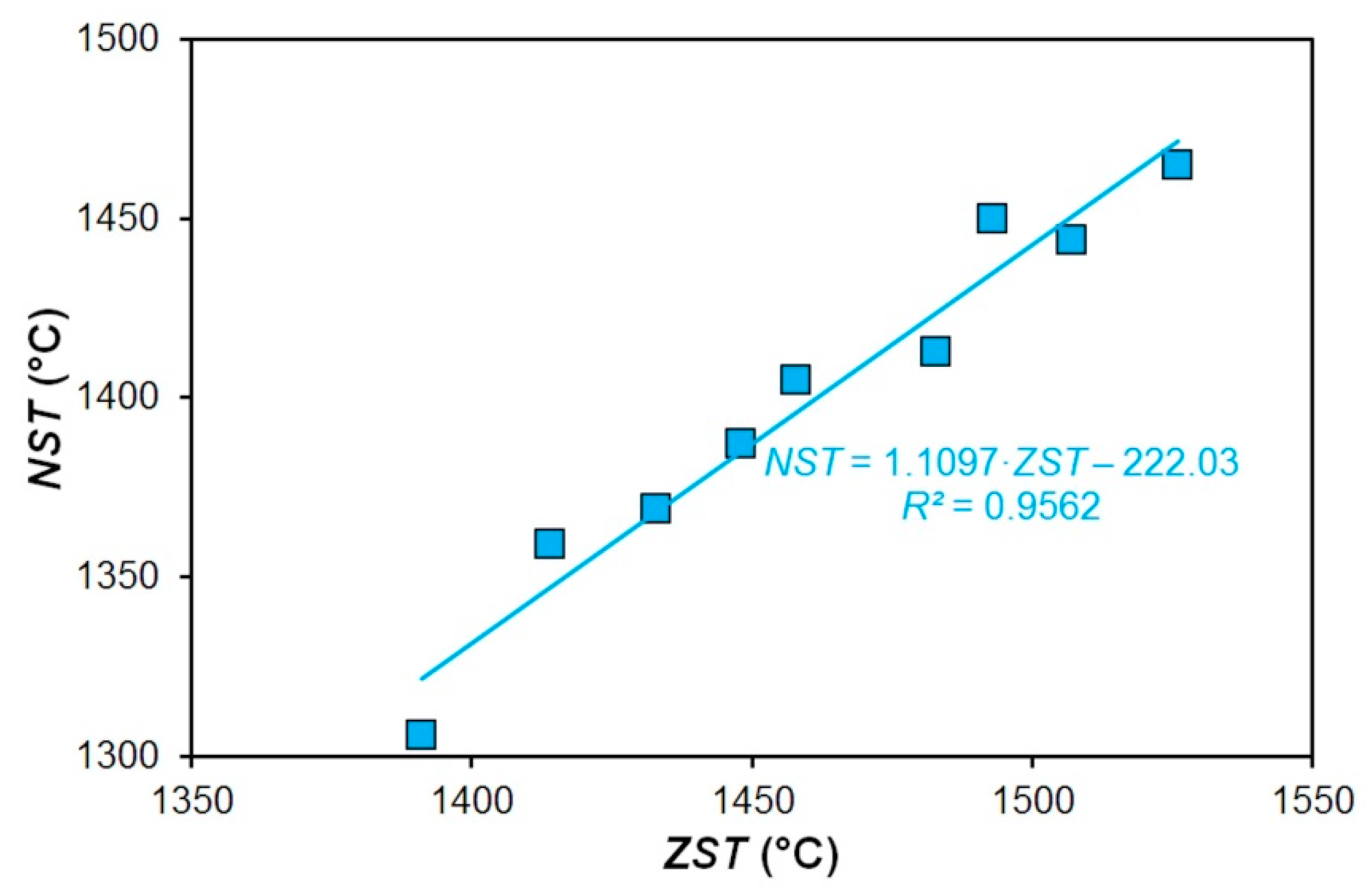
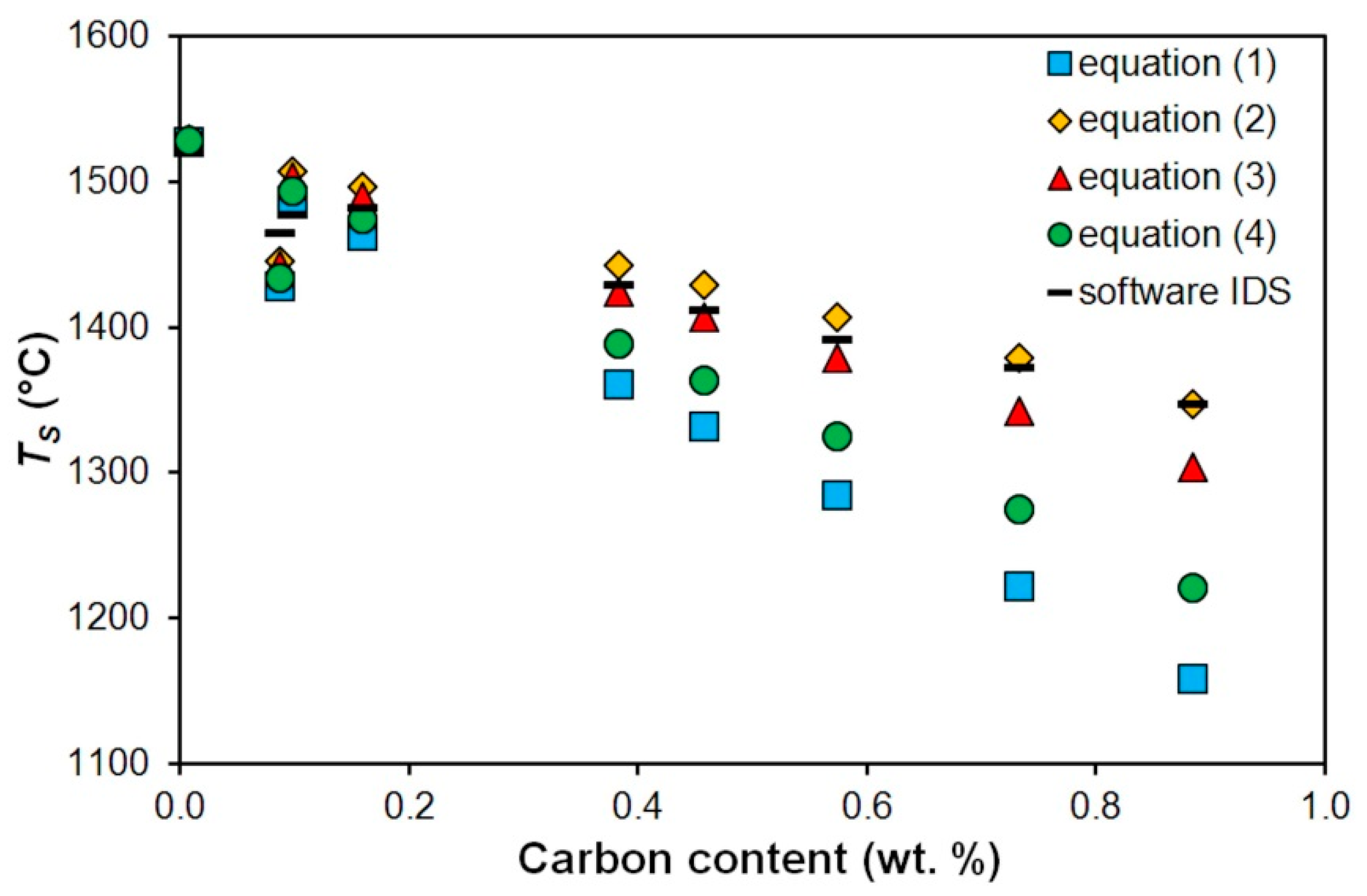

| Steel | C | Mn | Si | P | S | Cr | Al | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|
| A | 0.008 | 0.14 | 0.020 | 0.008 | 0.017 | 0.04 | 0.003 | 0.01 | 0.01 |
| B | 0.087 | 1.13 | 0.030 | 0.055 | 0.311 | 0.03 | 0.003 | 0.03 | 0.03 |
| C | 0.098 | 0.38 | 0.065 | 0.009 | 0.020 | 0.05 | 0.036 | 0.02 | 0.06 |
| D | 0.160 | 0.37 | 0.055 | 0.017 | 0.006 | 0.06 | 0.035 | 0.03 | 0.04 |
| E | 0.384 | 0.75 | 0.246 | 0.018 | 0.028 | 0.17 | 0.026 | 0.02 | 0.03 |
| F | 0.458 | 0.71 | 0.291 | 0.013 | 0.023 | 0.27 | 0.021 | 0.03 | 0.04 |
| G | 0.574 | 0.72 | 0.251 | 0.019 | 0.016 | 0.07 | 0.025 | 0.03 | 0.04 |
| H | 0.733 | 0.53 | 0.220 | 0.009 | 0.013 | 0.03 | 0.002 | 0.01 | 0.01 |
| I | 0.885 | 0.67 | 0.195 | 0.011 | 0.013 | 0.04 | 0.003 | 0.02 | 0.03 |
| Steel | NST (°C) | Standard Deviation (°C) | ZST (°C) | ΔT (°C) |
|---|---|---|---|---|
| A | 1465 | 33.3 | 1526 | 61 |
| B | 1413 | 3.8 | 1483 | 70 |
| C | 1444 | 5.2 | 1507 | 63 |
| D | 1450 | 5.4 | 1493 | 43 |
| E | 1405 | 4.1 | 1458 | 53 |
| F | 1387 | 6.5 | 1448 | 61 |
| G | 1369 | 2.3 | 1433 | 64 |
| H | 1359 | 4.9 | 1414 | 55 |
| I | 1306 | 9.2 | 1391 | 85 |
| Steel | TS (°C) | ||||
|---|---|---|---|---|---|
| Equation (1) | Equation (2) | Equation (3) | Equation (4) | Software IDS | |
| A | 1527 | 1528 | 1529 | 1528 | - |
| B | 1428 | 1445 | 1442 | 1434 | 1464 |
| C | 1487 | 1507 | 1503 | 1494 | 1477 |
| D | 1463 | 1496 | 1489 | 1474 | 1482 |
| E | 1361 | 1442 | 1424 | 1388 | 1429 |
| F | 1331 | 1429 | 1406 | 1364 | 1411 |
| G | 1284 | 1407 | 1378 | 1325 | 1391 |
| H | 1222 | 1379 | 1342 | 1274 | 1372 |
| I | 1157 | 1347 | 1303 | 1221 | 1347 |
| Steel | NST − TS(2) (°C) | NST − TS(3) (°C) | ZST − TS(2) (°C) | ZST − TS(3) (°C) |
|---|---|---|---|---|
| A | −63 | −64 | −2 | −3 |
| B | −32 | −29 | 38 | 41 |
| C | −63 | −59 | 0 | 4 |
| D | −46 | −39 | −3 | 4 |
| E | −37 | −19 | 16 | 34 |
| F | −42 | −19 | 19 | 42 |
| G | −38 | −9 | 26 | 55 |
| H | −20 | 17 | 35 | 72 |
| I | −41 | 3 | 44 | 88 |
| Standard deviation (°C) | 13.0 | 25.3 | 16.9 | 29.8 |
| Steel | NST(exp.) (°C) | NST(6) (°C) | NST(9) (°C) | NST(exp.) − NST(6) (%) | NST(exp.) − NST(9) (%) |
|---|---|---|---|---|---|
| A | 1465 | 1467 | 1469 | −0.1 | −0.3 |
| B | 1413 | 1453 | 1401 | −2.8 | 0.8 |
| C | 1444 | 1452 | 1452 | −0.6 | −0.6 |
| D | 1450 | 1441 | 1443 | 0.6 | 0.5 |
| E | 1405 | 1403 | 1398 | 0.1 | 0.5 |
| F | 1387 | 1390 | 1387 | −0.2 | 0 |
| G | 1369 | 1370 | 1369 | −0.1 | 0 |
| H | 1359 | 1343 | 1346 | 1.2 | 1.0 |
| I | 1306 | 1317 | 1320 | −0.8 | −1.1 |
| Standard deviation (°C) | 1.1 | 0.6 | |||
| Correlation coefficient | 0.9554 | 0.9836 | |||
| Steel | ZST(IDS) (°C) | ZST(7) (°C) | ZST(10) (°C) | ZST(IDS) − ZST(7) (%) | ZST(IDS) − ZST(10) (%) |
|---|---|---|---|---|---|
| A | 1526 | 1519 | 1521 | 0.5 | 0.3 |
| B | 1483 | 1507 | 1461 | −1.6 | 1.5 |
| C | 1507 | 1506 | 1506 | 0.1 | 0.1 |
| D | 1493 | 1497 | 1498 | −0.3 | −0.3 |
| E | 1458 | 1463 | 1459 | −0.3 | −0.1 |
| F | 1448 | 1452 | 1449 | −0.3 | −0.1 |
| G | 1433 | 1435 | 1433 | −0.1 | 0 |
| H | 1414 | 1411 | 1413 | 0.2 | 0.1 |
| I | 1391 | 1388 | 1390 | 0.2 | 0.1 |
| Standard deviation (°C) | 0.6 | 0.5 | |||
| Correlation coefficient | 0.9817 | 0.9848 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawulok, P.; Schindler, I.; Smetana, B.; Moravec, J.; Mertová, A.; Drozdová, Ľ.; Kawulok, R.; Opěla, P.; Rusz, S. The Relationship between Nil-Strength Temperature, Zero Strength Temperature and Solidus Temperature of Carbon Steels. Metals 2020, 10, 399. https://doi.org/10.3390/met10030399
Kawulok P, Schindler I, Smetana B, Moravec J, Mertová A, Drozdová Ľ, Kawulok R, Opěla P, Rusz S. The Relationship between Nil-Strength Temperature, Zero Strength Temperature and Solidus Temperature of Carbon Steels. Metals. 2020; 10(3):399. https://doi.org/10.3390/met10030399
Chicago/Turabian StyleKawulok, Petr, Ivo Schindler, Bedřich Smetana, Ján Moravec, Andrea Mertová, Ľubomíra Drozdová, Rostislav Kawulok, Petr Opěla, and Stanislav Rusz. 2020. "The Relationship between Nil-Strength Temperature, Zero Strength Temperature and Solidus Temperature of Carbon Steels" Metals 10, no. 3: 399. https://doi.org/10.3390/met10030399
APA StyleKawulok, P., Schindler, I., Smetana, B., Moravec, J., Mertová, A., Drozdová, Ľ., Kawulok, R., Opěla, P., & Rusz, S. (2020). The Relationship between Nil-Strength Temperature, Zero Strength Temperature and Solidus Temperature of Carbon Steels. Metals, 10(3), 399. https://doi.org/10.3390/met10030399





