Preparation and Mechanical Properties of ZK61-Y Magnesium Alloy Wheel Hub via Liquid Forging—Isothermal Forging Process
Abstract
1. Introduction
2. Material and Experiment Procedure
2.1. Materials
2.2. Liquid Forging Procedure
2.3. Isothermal Forging Procedure
2.4. Post Heat Treatment
2.5. Properties Test
3. Results and Discussion
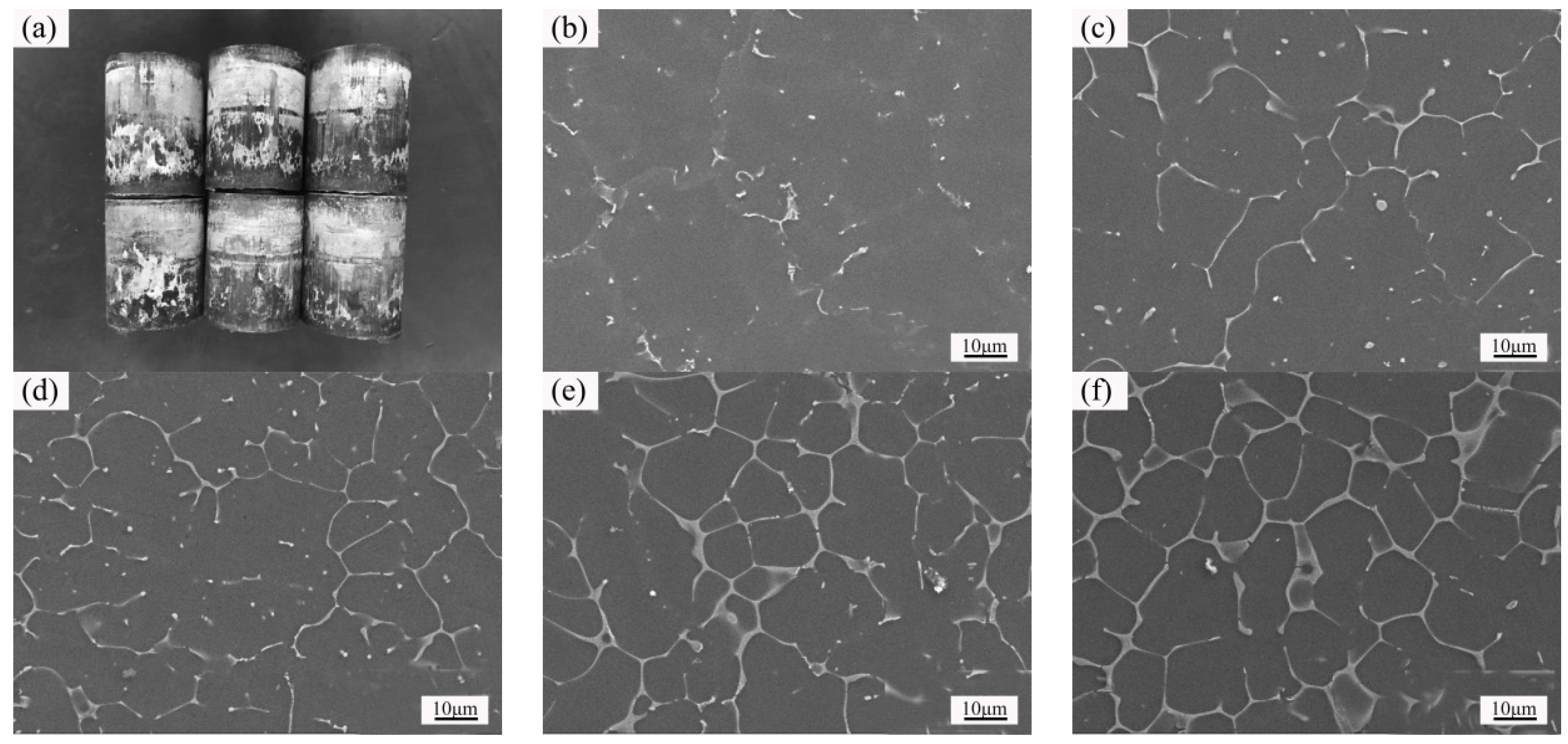
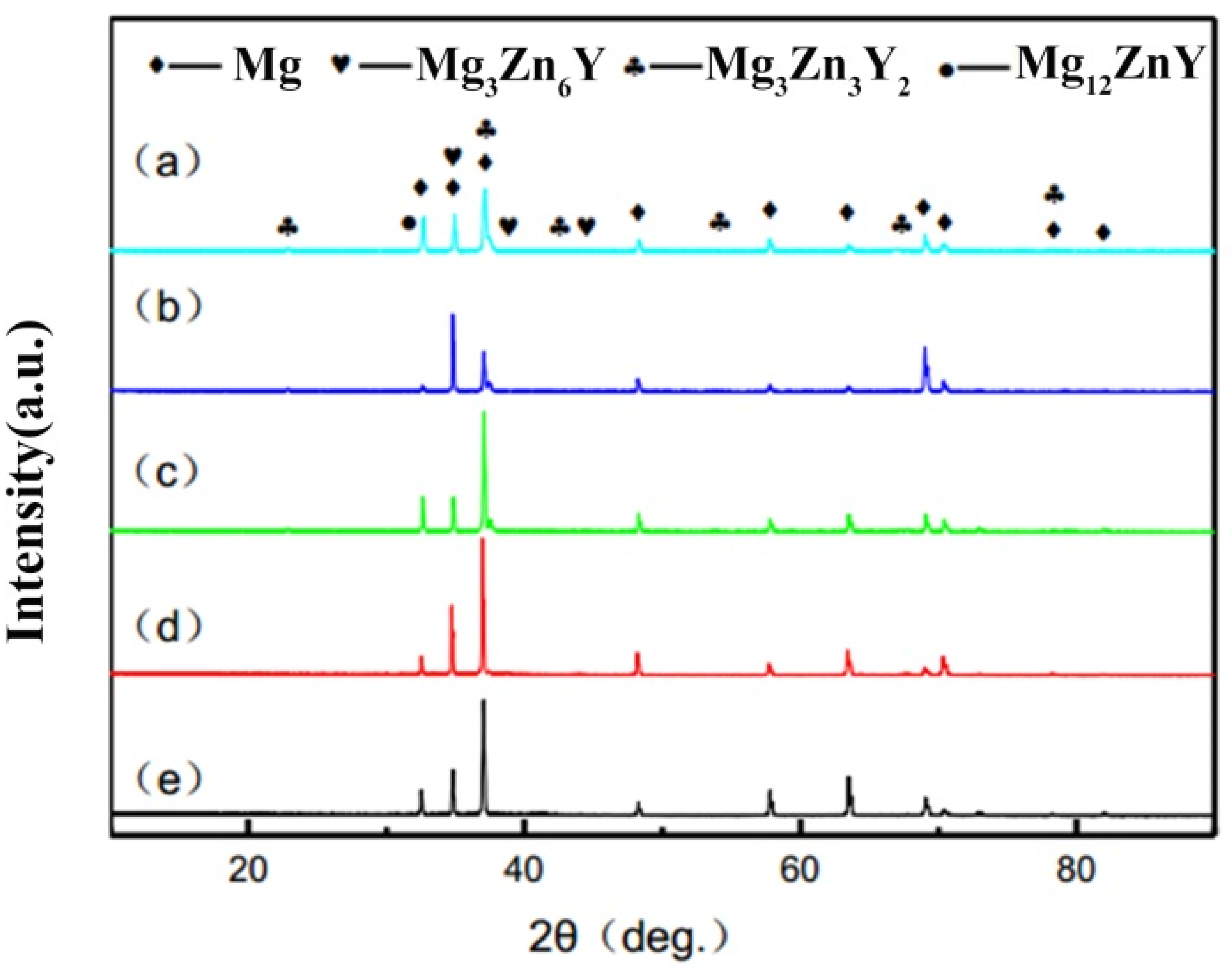
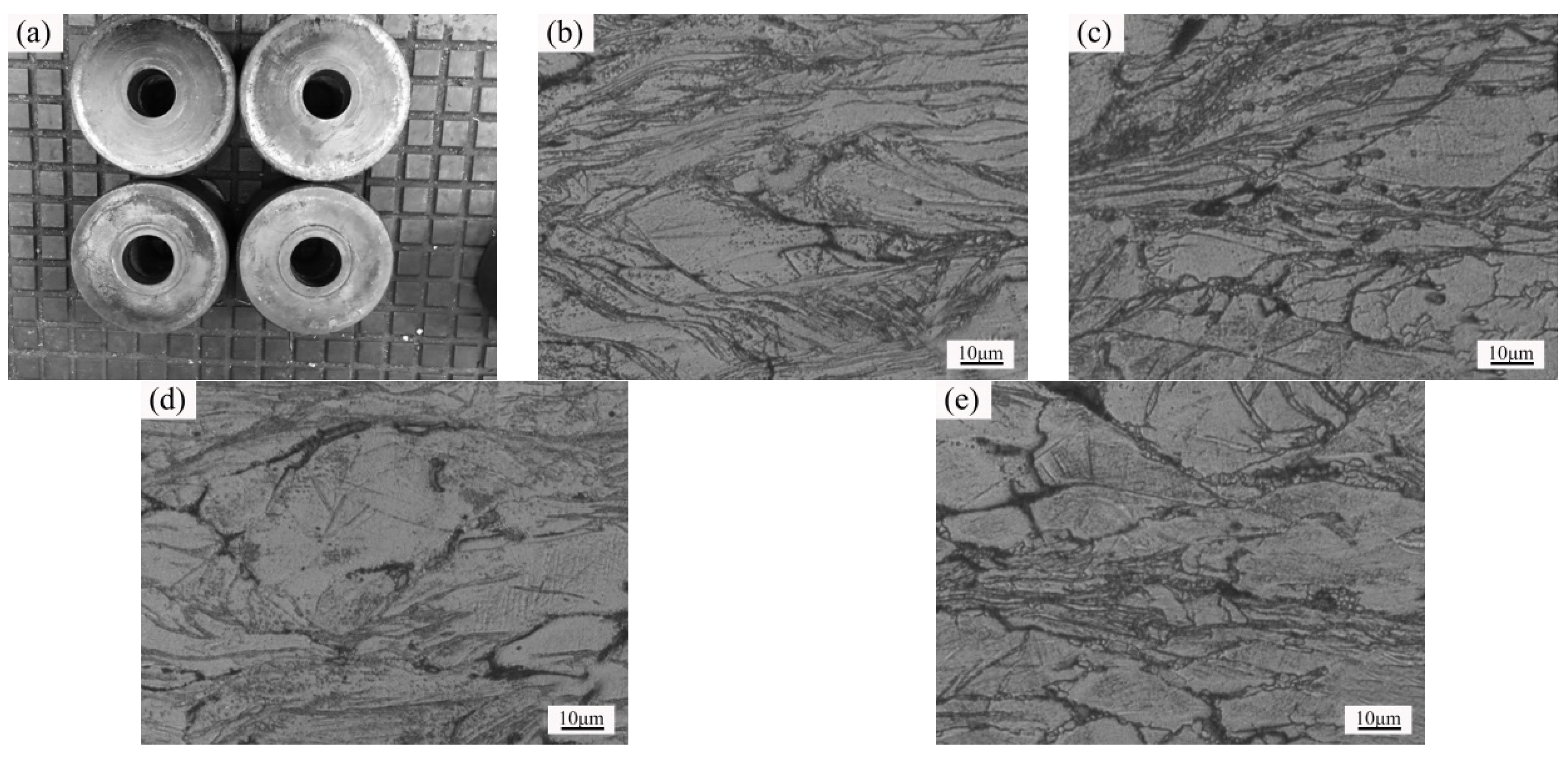
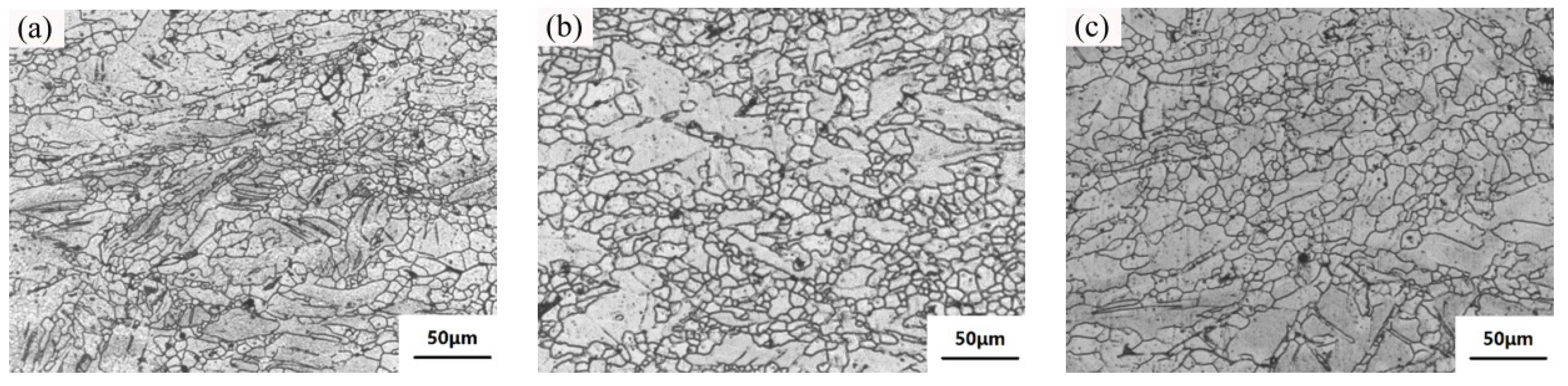
3.1. Microstructure and Properties of the Liquid Forging Blanks with Different Y-Element Contents
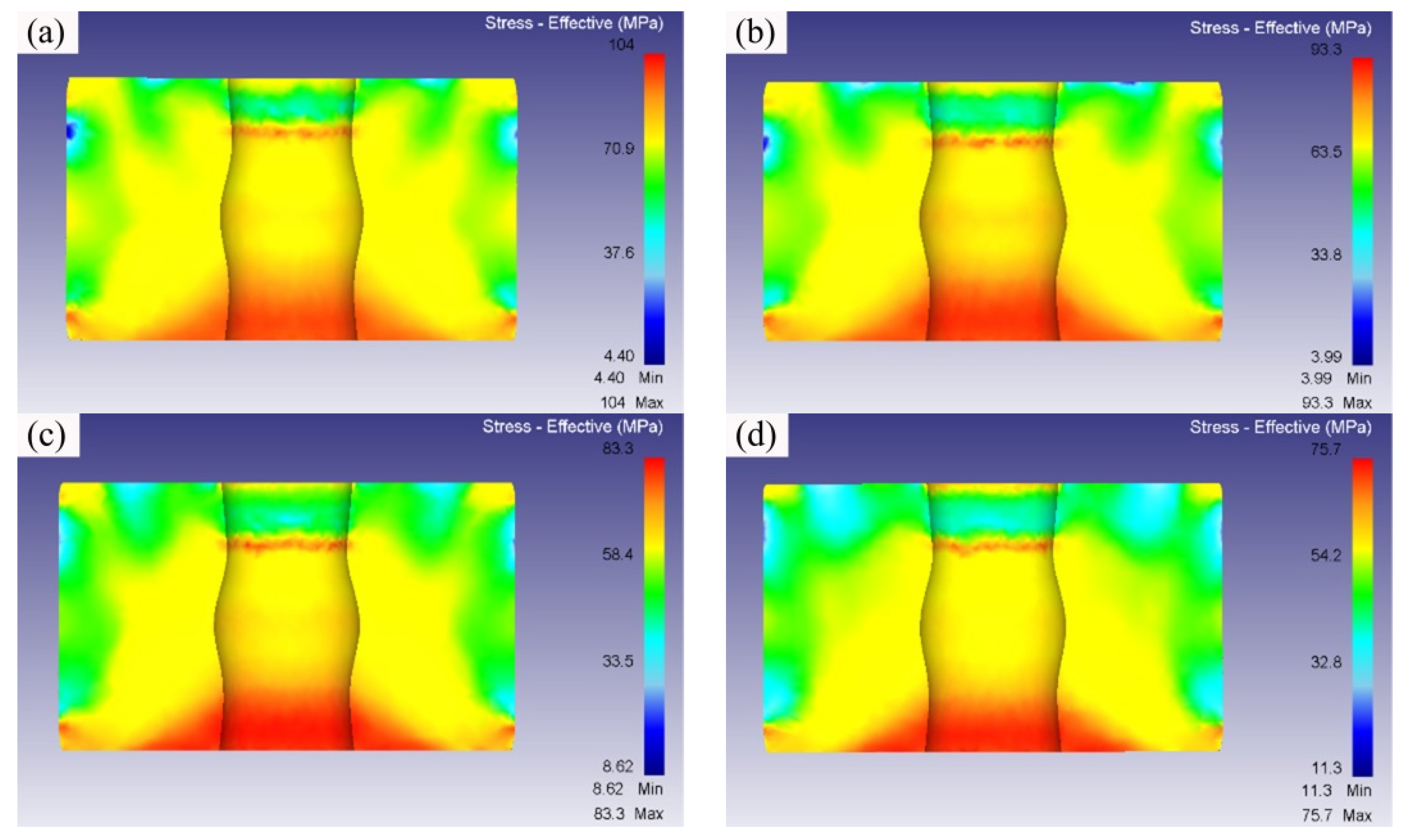
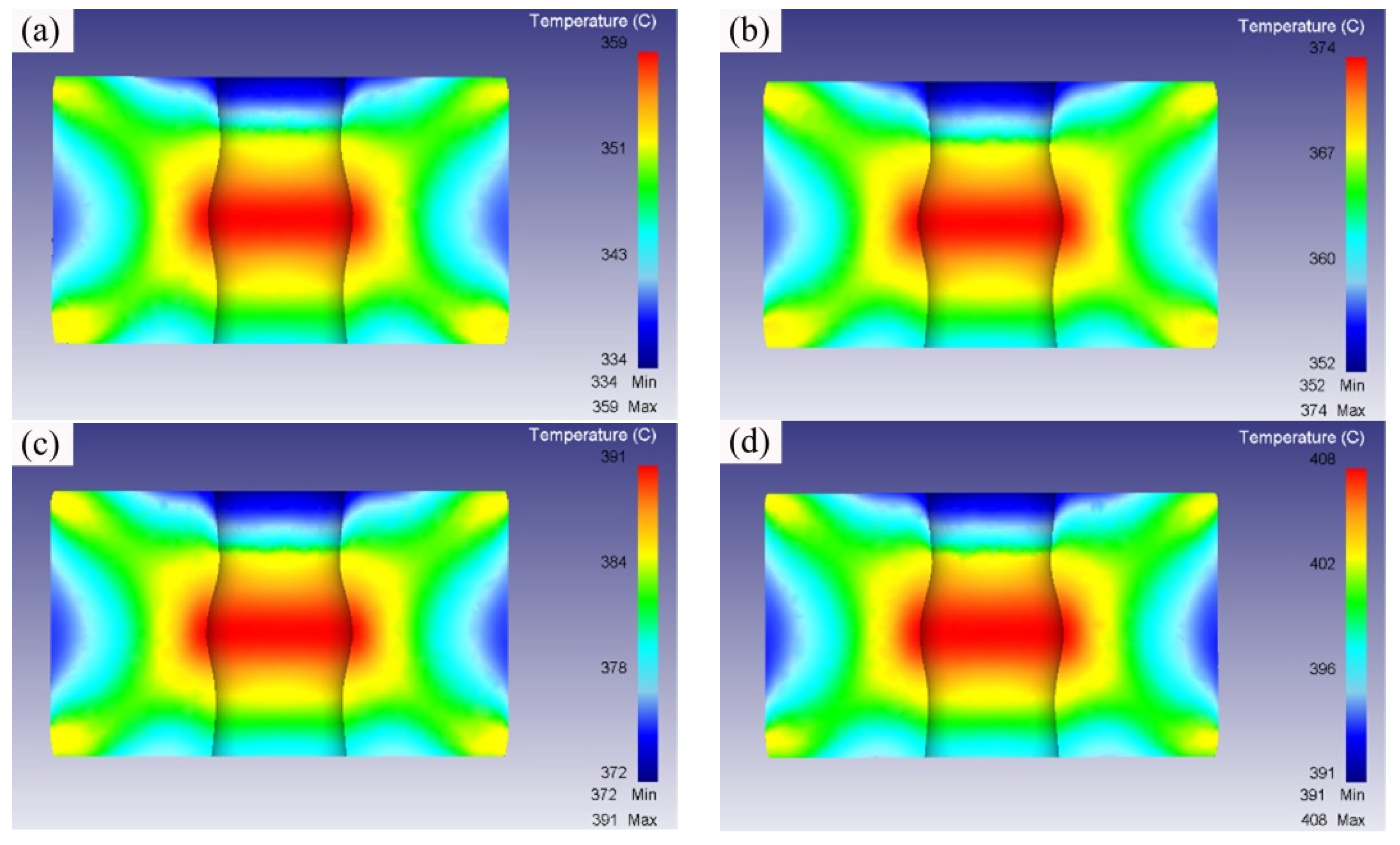
3.2. Simulation Analysis of the Isothermal Forging Procedure
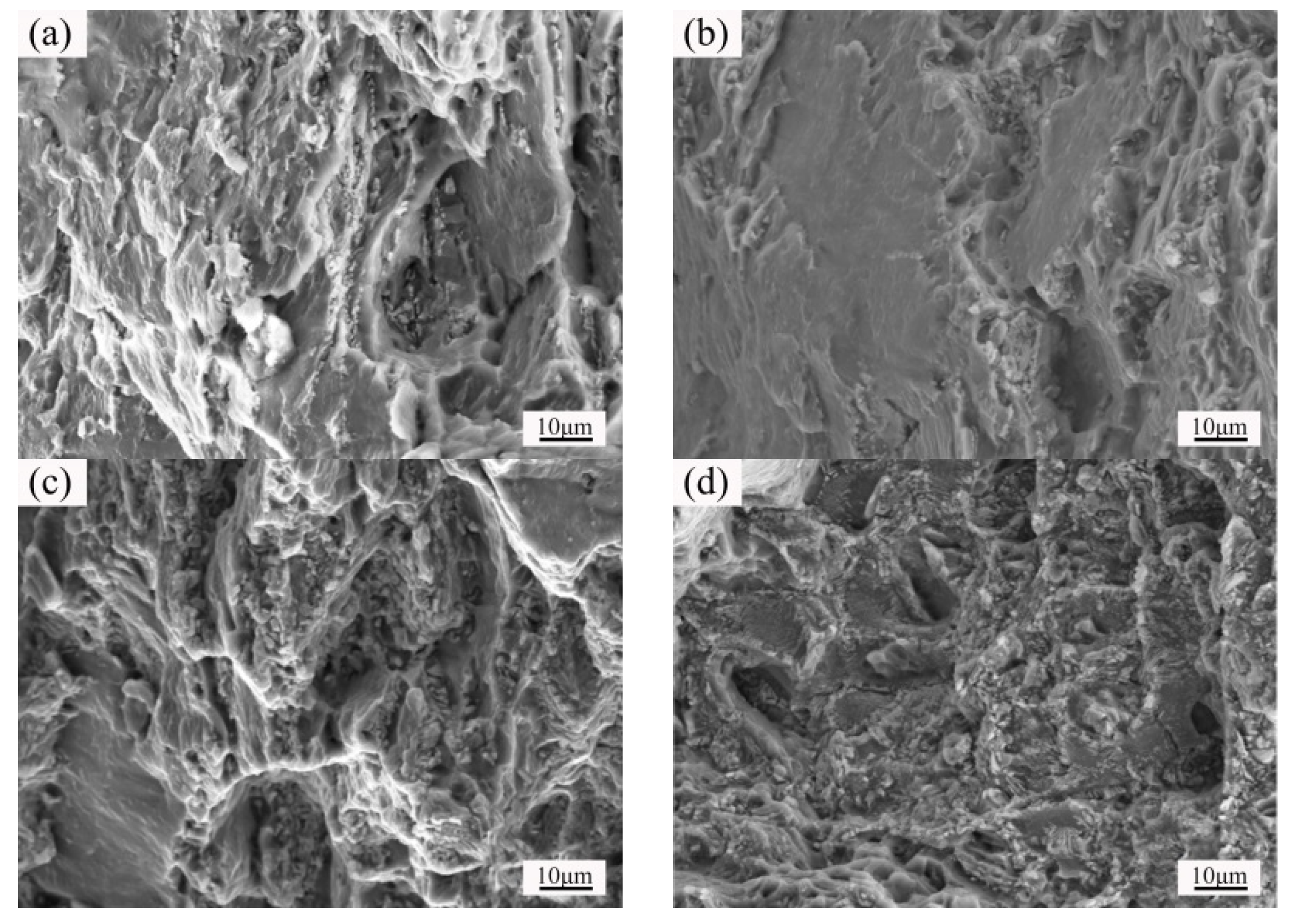
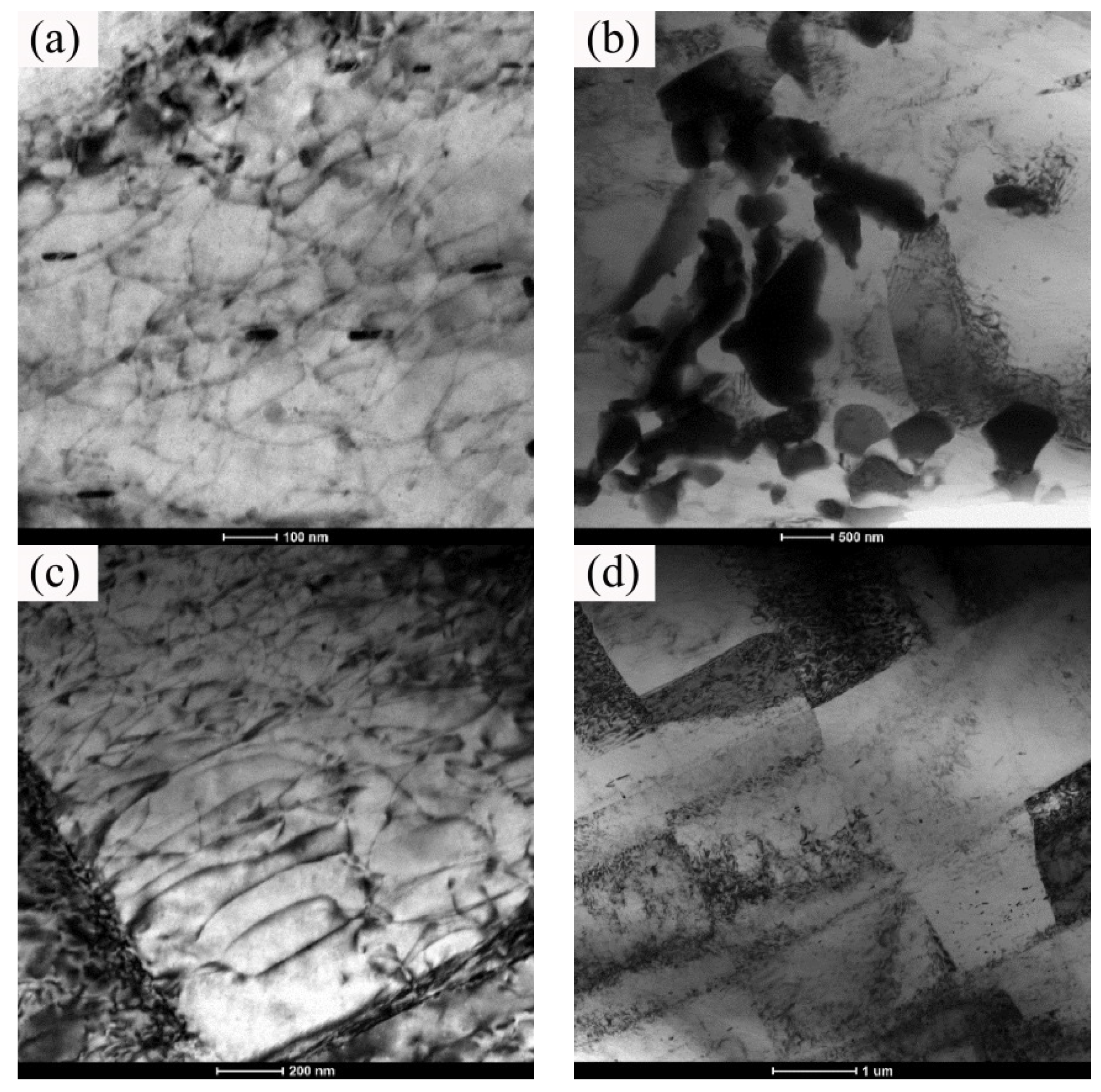
3.3. Microstructure and Properties of the Isothermal Forging Parts at Different Temperatures
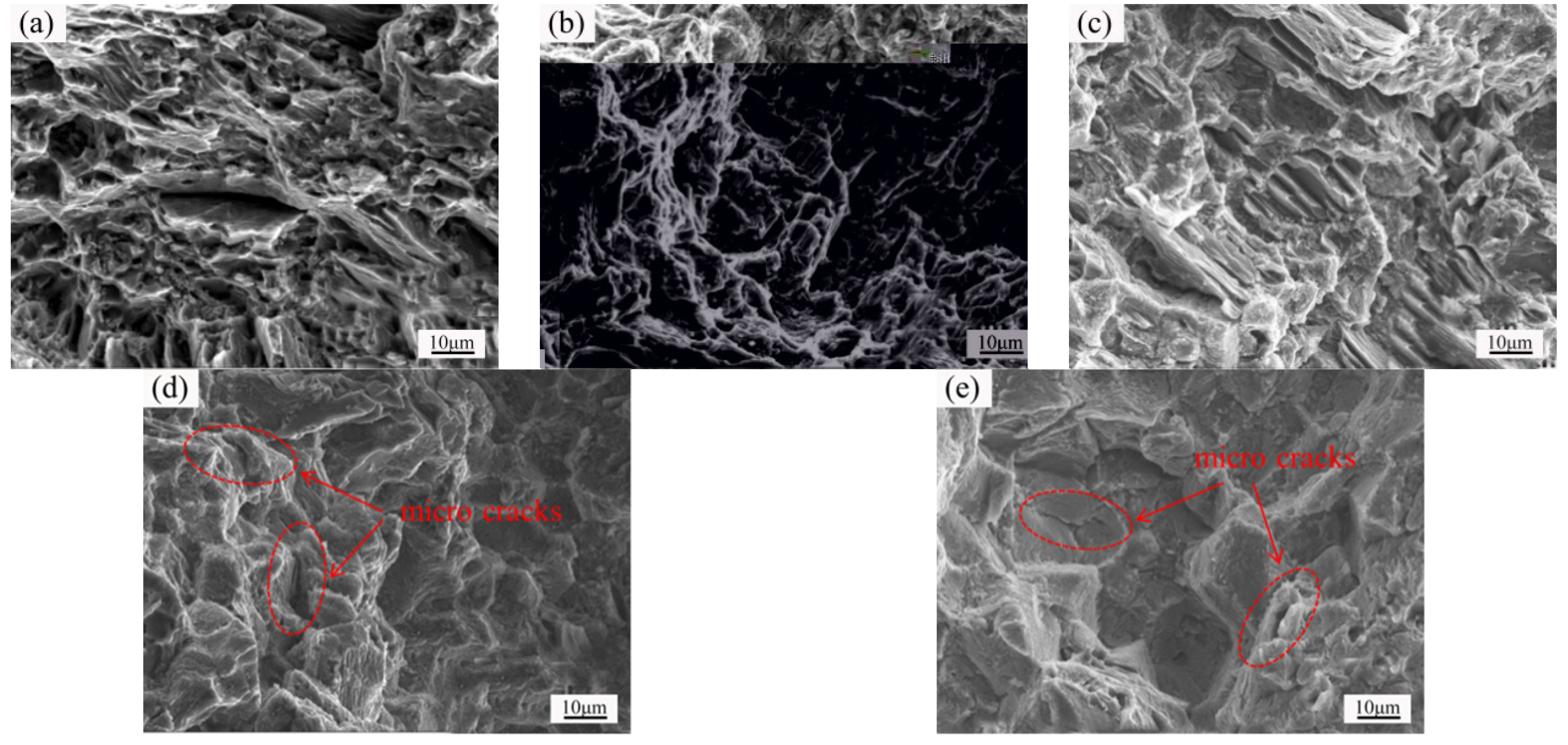
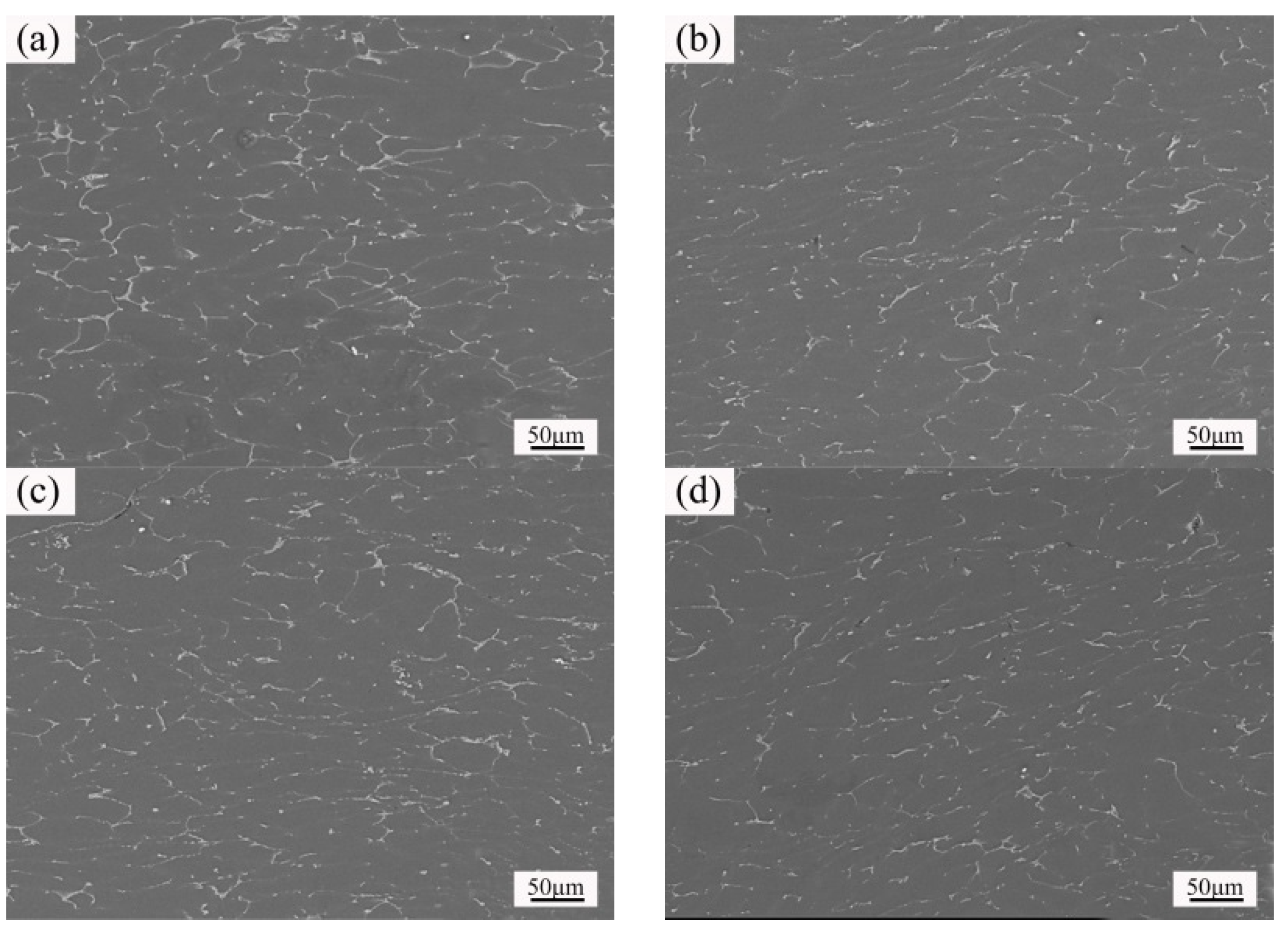
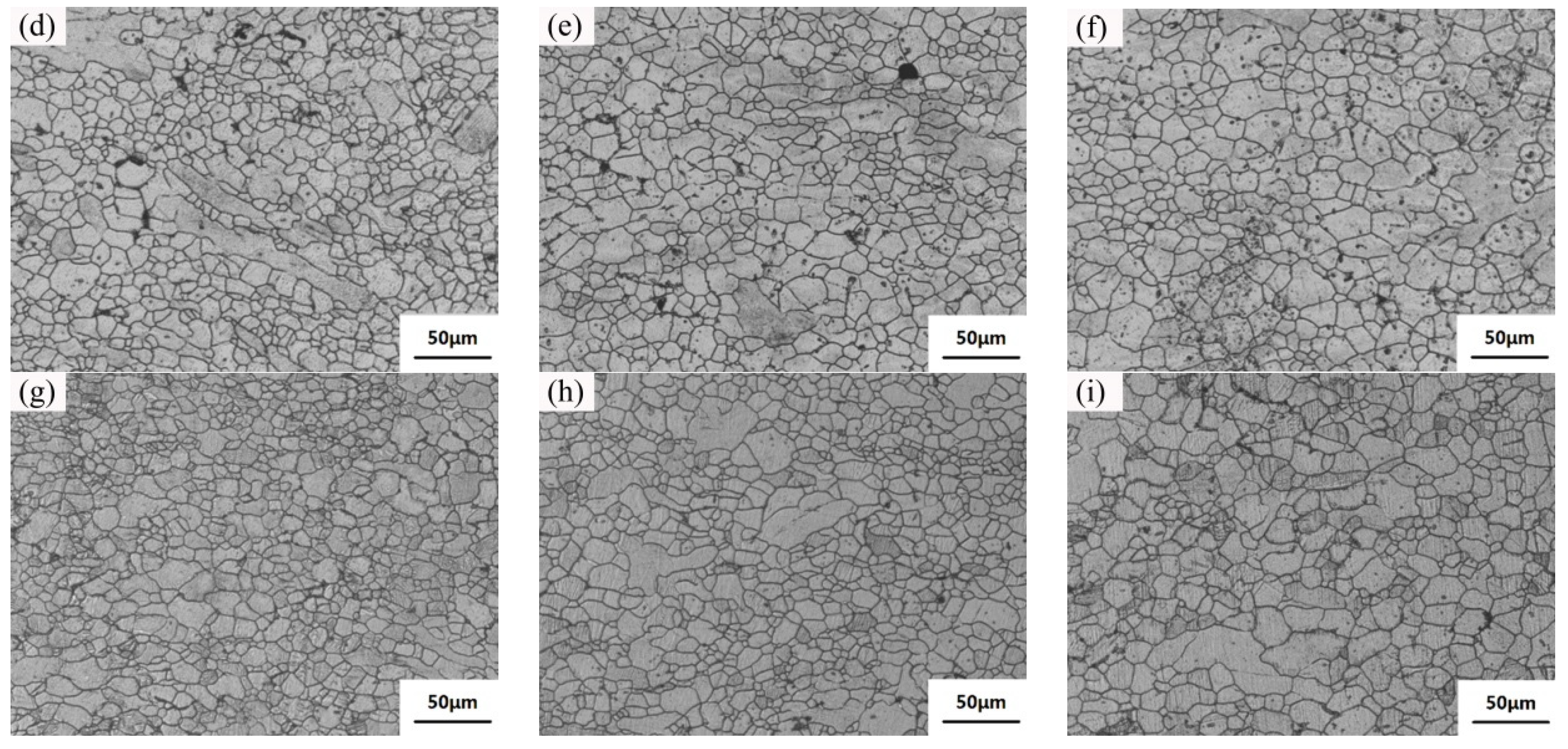
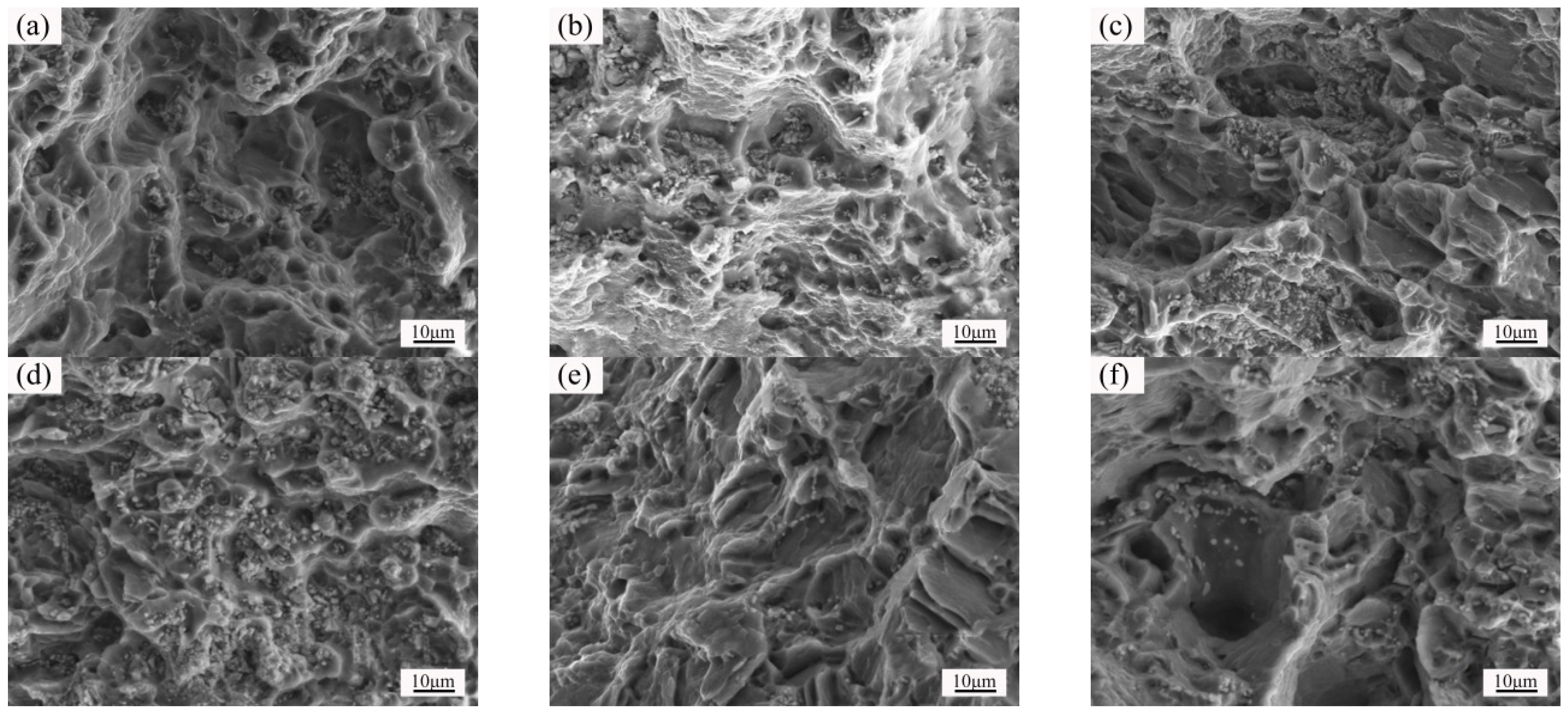
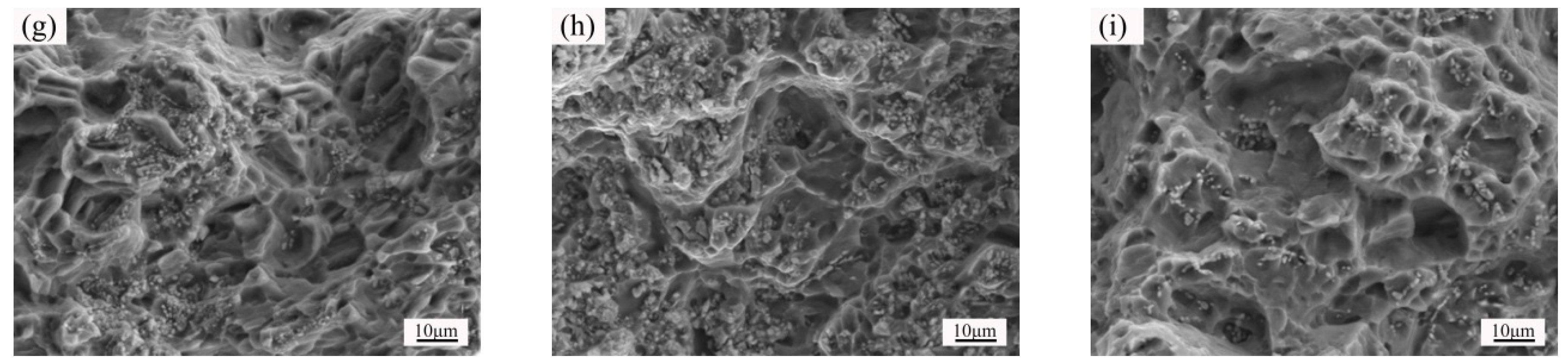
3.4. Microstructure and Properties of the Isothermal Forging Parts after Post Heat Treatments
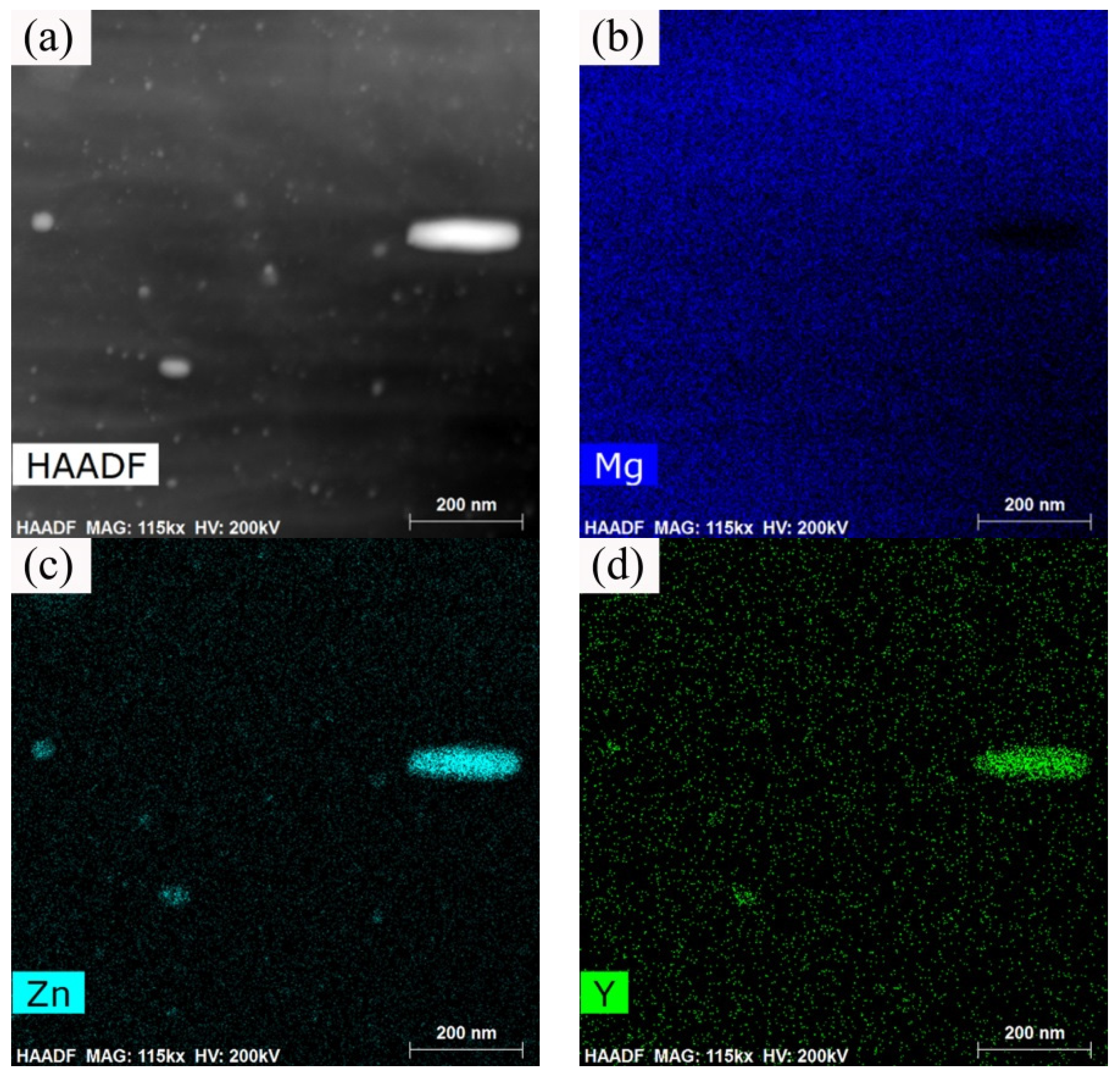
3.5. Strengthening Mechanism of the Isothermal Forging Process
4. Conclusions
- The undercooling of liquid alloy increased due to the application of pressure during the liquid forging process, which refined the grains and prevented the dendrites formation. The Mg-1YL displayed the best properties with the elongation of 16.0%, the yield stress of 104 MPa, and the ultimate tensile strength of 232 MPa. The constitutional undercooling increased as the Y-element increased, which would facilitate dendrites growth. The formation order of the second phase was I-phase (Mg3Zn6Y) → W-phase (Mg3Zn3Y2) → Z-phase (Mg12ZnY) with the increasing of the Y-element content. Meanwhile, the I-phase and Z-phase formed in the liquid forging process were beneficial to the grain refinement.
- The numerical simulation results indicated that the effective stress decreased and the temperature increased as the forming temperatures were elevated during the isothermal forging process. The forming part forged at 380 °C displayed the outstanding properties. The elongation, yield strength, and ultimate tensile strength were 18.5%, 150 MPa and 315 MPa, respectively. The DRX, second-phase hardening, and work hardening were the reasons for the properties improvement after the isothermal forging process.
- The elongation increased and the strength decreased after heat treatments for the forming parts. This can be ascribed to the recrystallization, the weakening of pinning effect of second phases, and the elimination of working hardening. Meanwhile, the second phase transformation (I-phase → W-phase → Mg2Y + MgZn2), dissolution, and decomposition can be detected, as well. The 520 °C—1 h sample possessed the superior mechanical properties, in which the elongation was 25.5%, the yield stress was 125 MPa, and the ultimate tensile strength was 282 MPa.
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.J.; Kim, S.H.; Lee, J.U.; Choi, J.O.; Kim, H.S.; Kim, Y.M.; Kim, Y.; Park, S.H. Effects of cold pre-forging on microstructure and tensile properties of extruded AZ80 alloy. Mater. Sci. Eng. A 2017, 708, 405–410. [Google Scholar] [CrossRef]
- Pan, H.C.; Ren, Y.P.; Fu, H.; Zhao, H.; Wang, L.Q.; Meng, X.Y.; Qin, G.W. Recent developments in rare-earth free wrought magnesium alloys having high strength: A review. J. Alloy Compd. 2016, 663, 321–331. [Google Scholar] [CrossRef]
- Meng, S.; Yu, H.; Zhou, J.; Han, H.; Li, Y.; Dong, L.; Nan, X.; Li, Z.; Shin, K.S.; Zhao, W. Enhancing the Mechanical Properties of AZ80 Alloy by Combining Extrusion and Three Pass Calibre Rolling. Metals 2020, 10, 249. [Google Scholar] [CrossRef]
- Meng, X.R.; Wu, R.Z.; Zhang, M.L.; Wu, L.B.; Cui, C.L. Microstructures and properties of superlight Mg-Li-Al-Zn wrought alloys. J. Alloy Compd. 2009, 486, 722–725. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Liu, X.; Wang, Z.K.; Le, Q.C.; Hu, W.Y.; Bao, L.; Cui, J.Z. Effects of phase composition and content on the microstructures and mechanical properties of high strength Mg-Y-Zn-Zr alloys. Mater. Des. 2015, 88, 915–923. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Sun, J.P.; Wang, C.; Bai, J.; Ma, A.B.; Chen, X.H. Microstructure and Mechanical Properties of Mg–RE–TM Cast Alloys Containing Long Period Stacking Ordered Phases: A Review. Acta Metall. Sin. (Engl. Lett.) 2018, 32, 269–285. [Google Scholar] [CrossRef]
- Liu, K.; Meng, J.A. Microstructures and mechanical properties of the extruded Mg-4Y-2Gd-xZn-0.4Zr alloys. J. Alloy Compd. 2011, 509, 3299–3305. [Google Scholar] [CrossRef]
- Wu, C.M.L.; Yu, D.Q.; Law, C.M.T.; Wang, L. Properties of lead-free solder alloys with rare earth element additions. Mater. Sci. Eng. R 2004, 44, 1–44. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Morton, A.J.; Nie, J.F. The 18R and 14H long-period stacking ordered structures in Mg-Y-Zn alloys. Acta Mater. 2010, 58, 2936–2947. [Google Scholar] [CrossRef]
- Li, B.; Teng, B.G.; Xu, W.C. Hot Deformation Characterization of Homogenized Mg-Gd-Y-Zn-Zr Alloy during Isothermal Compression. JOM 2019, 71, 4059–4070. [Google Scholar] [CrossRef]
- Kishida, K.; Nagai, K.; Matsumoto, A.; Yasuhara, A.; Inui, H. Crystal structures of highly-ordered long-period stacking-ordered phases with 18R, 14H and 10H-type stacking sequences in the Mg-Zn-Y system. Acta Mater. 2015, 99, 228–239. [Google Scholar] [CrossRef]
- Takagi, K.; Yamasaki, M.; Mine, Y.; Takashima, K. Temperature dependence of prismatic slip in a single-crystalline long-period stacking ordered Mg–Zn–Y alloy. Scr. Mater. 2020, 178, 498–502. [Google Scholar] [CrossRef]
- Mehrabi-Mehdiabadi, M.; Mahmudi, R. Effects of yttrium addition on microstructural stability and elevated temperature mechanical properties of a cast Mg-Zn alloy. J. Alloy Compd. 2020, 820, 153083. [Google Scholar] [CrossRef]
- Wu, J.; Ikeda, K.; Shi, Q.; Chiu, Y.L. Kink boundaries and their role in dynamic recrystallisation of a Mg-Zn-Y alloy. Mater. Charact. 2019, 148, 233–242. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, X.S.; Yuan, B.G.; Shu, D.Y.; Zhao, Z.D.; Han, J.C. Hot workfability behavior of as-cast Mg-Zn-Y-Zr alloy. Mater. Sci. Eng. A 2014, 593, 38–47. [Google Scholar] [CrossRef]
- Luo, Z.P.; Sui, H.X.; Zhang, S.Q. On the stable Mg-Zn-Y quasicrystals. Metall. Mater. Trans. A 1996, 27, 1779–1784. [Google Scholar] [CrossRef]
- Bae, D.H.; Lee, M.H.; Kim, K.T.; Kim, W.T.; Kim, D.H. Application of quasicrystalline particles as a strengthening phase in Mg-Zn-Y alloys. J. Alloy Compd. 2002, 342, 445–450. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Zeng, Z.R.; Wang, B.J.; Sheng, L.Y.; Chen, X.B.; Han, E.H. Effect of volume fraction of LPSO phases on corrosion and mechanical properties of Mg-Zn-Y alloys. Mater. Des. 2017, 121, 430–441. [Google Scholar] [CrossRef]
- Xu, C.; Nakata, T.; Qiao, X.G.; Zheng, M.Y.; Wu, K.; Kamado, S. Effect of LPSO and SFs on microstructure evolution and mechanical properties of Mg-Gd-Y-Zn-Zr alloy. Sci. Rep. 2017, 7, 40846. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhao, D.S.; Ye, L.; Zhuang, Y.L.; Gao, S.B.; Wang, J.B. Effect of Er contents on the microstructure of long period stacking ordered phase and the corresponding mechanical properties in Mg-Dy-Er-Zn alloys. Mater. Sci. Eng. A 2018, 718, 461–467. [Google Scholar] [CrossRef]
- Kim, W.J.; Hwang, B.G.; Lee, M.J.; Park, Y.B. Effect of speed-ratio on microstructure, and mechanical properties of Mg-3Al-1Zn alloy, in differential speed rolling. J. Alloys Compd. 2011, 509, 8510–8517. [Google Scholar] [CrossRef]
- Li, B.; Teng, B.G.; Luo, D.G. Effects of Passes on Microstructure Evolution and Mechanical Properties of Mg-Gd-Y-Zn-Zr Alloy during Multidirectional Forging. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 1009–1018. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wang, C.; Zha, M.; Wang, H.Y.; Yang, Z.Z.; Jiang, Q.C. Microstructure and tensile properties of rolled Mg-4Al-2Sn-1Zn alloy with pre-rolling deformation. Mater. Sci. Eng. A 2018, 719, 132–139. [Google Scholar] [CrossRef]
- Inao, D.; Mori, A.; Tanaka, S.; Hokamoto, K. Explosive Welding of Thin Aluminum Plate onto Magnesium Alloy Plate Using a Gelatin Layer as a Pressure-Transmitting Medium. Metals 2020, 10, 106. [Google Scholar] [CrossRef]
- Minárik, P.; Veselý, J.; Král, R.; Bohlen, J.; Kubásek, J.; Janeček, M.; Stráská, J. Exceptional mechanical properties of ultra-fine grain Mg-4Y-3RE alloy processed by ECAP. Mater. Sci. Eng. A 2017, 708, 193–198. [Google Scholar] [CrossRef]
- Verma, R.; Jayaganthan, R.; Nath, S.K.; Srinivasan, A. Effect of multiaxial forging followed by hot rolling on non-basal planes and its influence on tensile and fracture toughness behaviour of Mg–4Zn–4Gd alloy. Mater. Sci. Eng. A 2020, 774, 138890. [Google Scholar] [CrossRef]
- Li, N.; Xing, S.M.; Bao, P.W. Microstructure and Mechanical Properties of Nodular Cast Iron Produced by Melted Metal Die Forging Process. J. Iron Steel Res. Int. 2013, 20, 58–62. [Google Scholar] [CrossRef]
- Wang, D.Y.; Du, L.J.; Liu, Y.W.; Qi, Y.S.; Du, Z.M. Effects of variable-cavity liquid forging on microstructure and mechanical properties of Mg-Zn-Y-Zr alloy. Mater. Charact. 2019, 151, 96–102. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, L.; Chen, G.; Li, K.; Li, C.; Du, Z. Preparation and Properties of Special Vehicle Cover via a Novel Squeeze Casting Quantitative Feeding System of Molten Metal. Metals 2020, 10, 266. [Google Scholar] [CrossRef]
- Chen, G.; Chang, X.; Zhang, J.; Jin, Y.; Sun, C.; Chen, Q.; Zhao, Z. Microstructures and Mechanical Properties of In-Situ Al3Ti/2024 Aluminum Matrix Composites Fabricated by Ultrasonic Treatment and Subsequent Squeeze Casting. Met. Mater. Int. 2019. [Google Scholar] [CrossRef]
- Fang, X.G.; Lu, S.L.; Zhao, L.; Wang, J.; Liu, L.F.; Wu, S.S. Microstructure and mechanical properties of a novel Mg-RE-Zn-Y alloy fabricated by rheo-squeeze casting. Mater. Des. 2016, 94, 353–359. [Google Scholar] [CrossRef]
- Wang, K.; Kopp, R.; Hirt, G. Investigation on forming defects during thixo-forging of aluminum alloy AlSi7Mg. Adv. Eng. Mater. 2006, 8, 724–730. [Google Scholar] [CrossRef]
- Zheng, C.K.; Zhang, W.W.; Zhang, D.T.; Li, Y.Y. Low cycle fatigue behavior of T4-treated Al-Zn-Mg-Cu alloys prepared by squeeze casting and gravity die casting. T. Nonferr. Metal. Soc. 2015, 25, 3505–3514. [Google Scholar] [CrossRef]
- Selin, M. Comparing Three Equations Used for Modeling the Tensile Flow Behavior of Compacted Graphite Cast Irons at Elevated Temperatures. Metall. Mater. Trans. A 2010, 41, 2805–2815. [Google Scholar] [CrossRef]
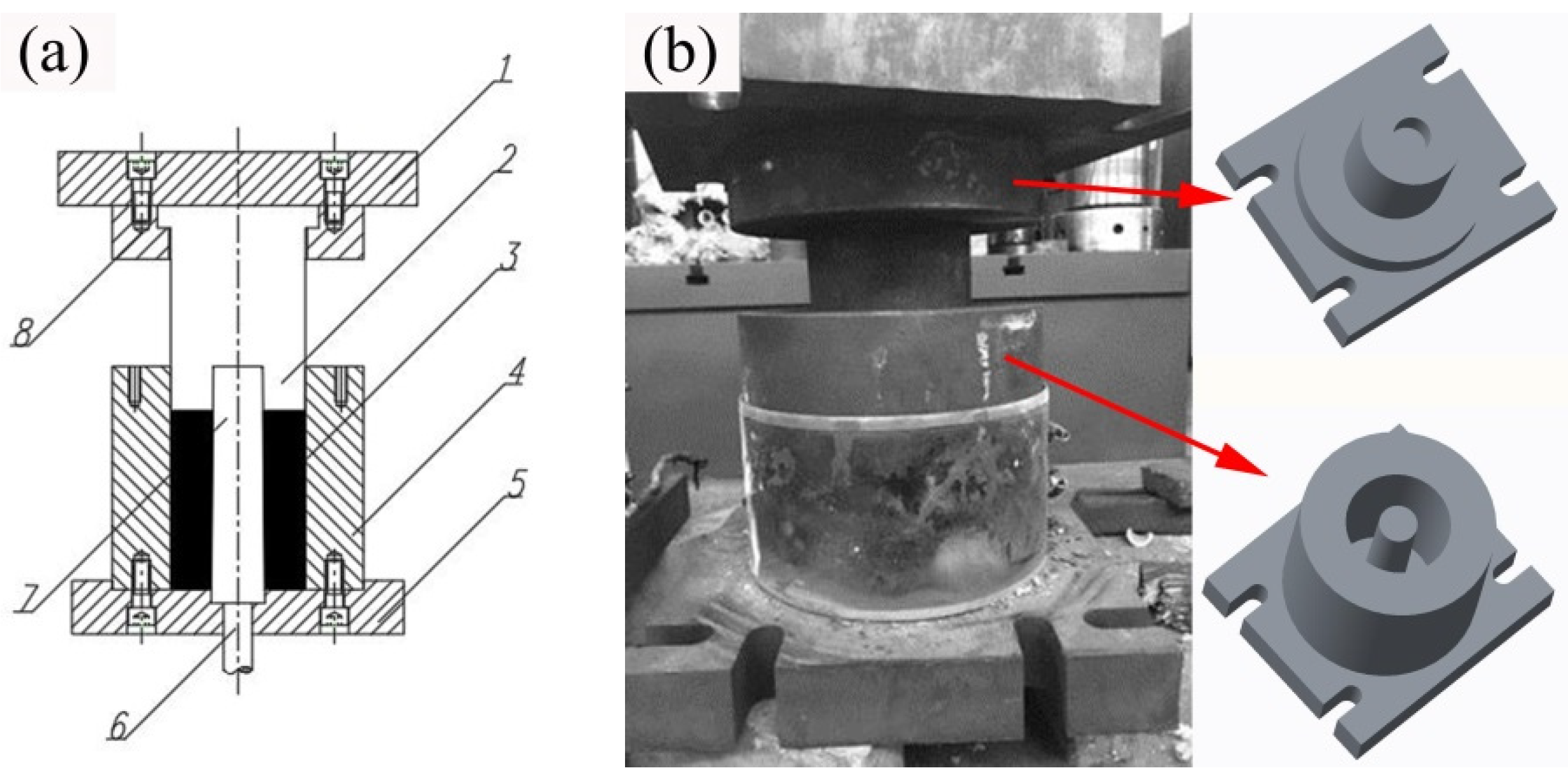
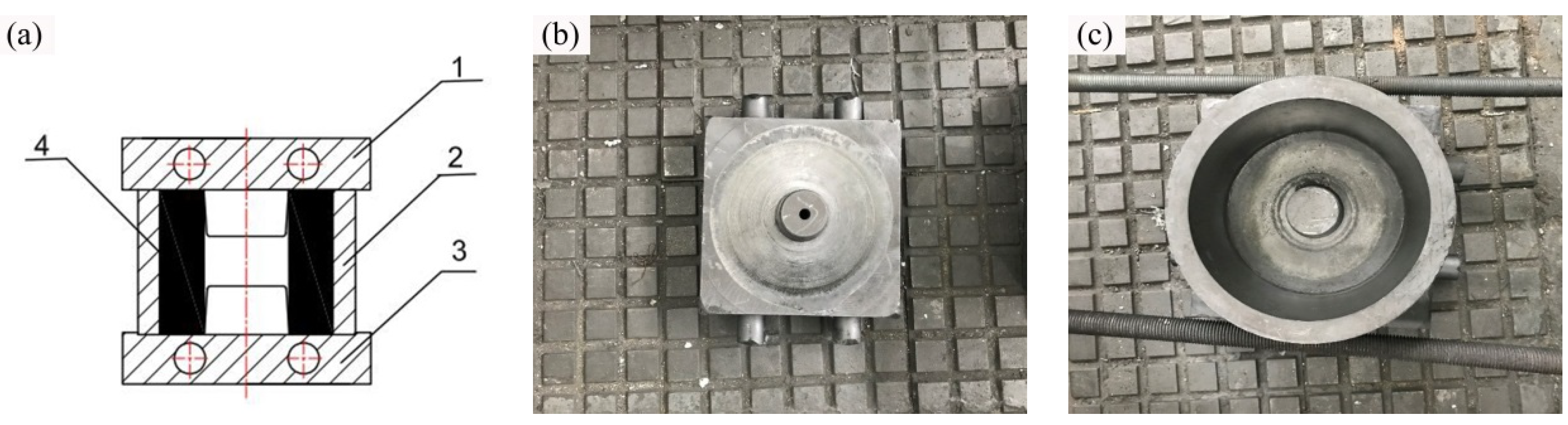
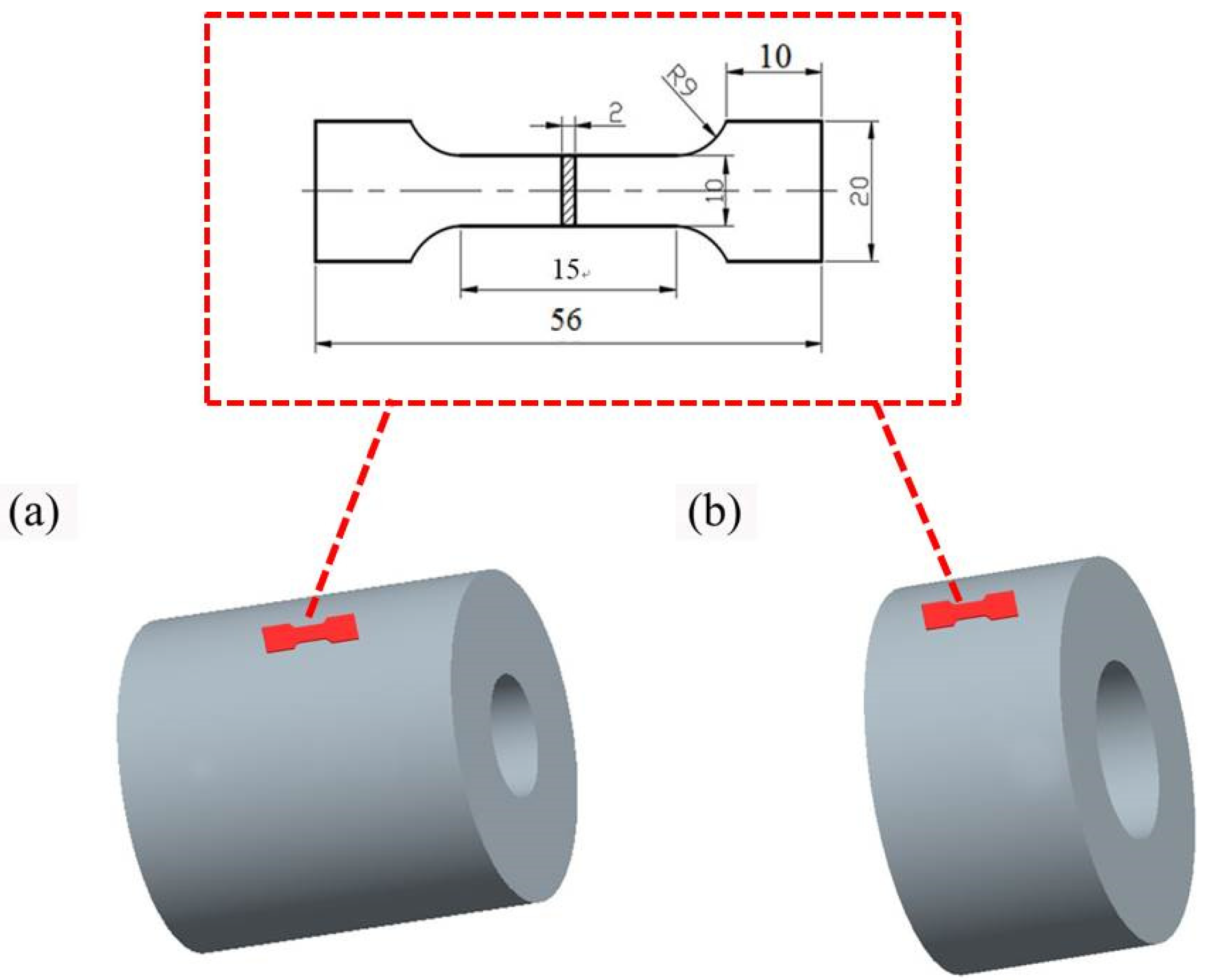
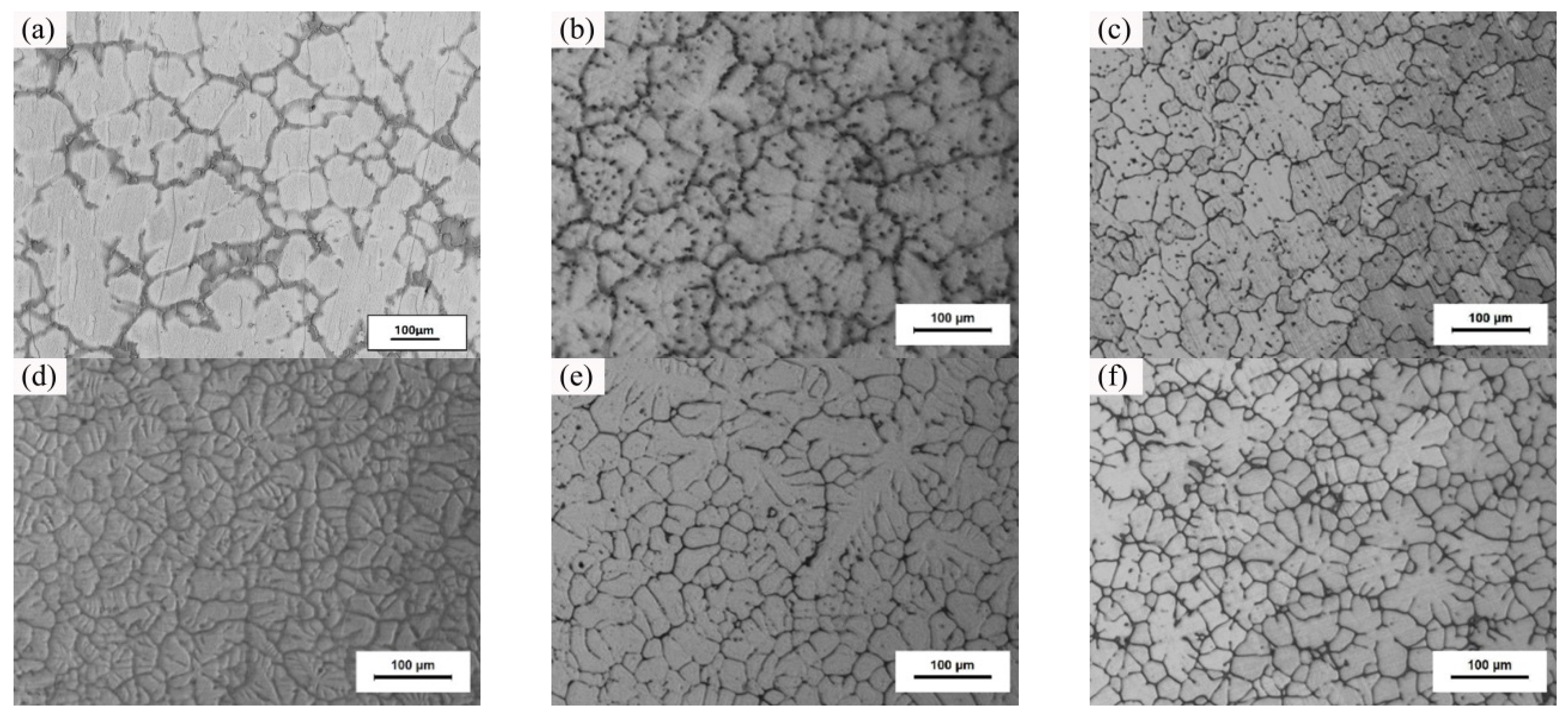
| Element | Zn | Zr | Mn | Fe | Al | S | Si | Mg |
|---|---|---|---|---|---|---|---|---|
| Content | 6.06 | 0.67 | 0.02 | 0.04 | 0.04 | <0.01 | <0.01 | Bal. |
| Element | Mg | Y | Si | Others |
|---|---|---|---|---|
| Content | 70.66 | 28.97 | 0.25 | Bal. |
| No. | Y-element Content (wt %) | Procedure |
|---|---|---|
| Mg-0YG | 0 | Gravity Casting |
| Mg-0YL | 0 | Liquid Forging |
| Mg-1YL | 1 | Liquid Forging |
| Mg-2YL | 2 | Liquid Forging |
| Mg-3YL | 3 | Liquid Forging |
| Mg-4YL | 4 | Liquid Forging |
| Treatment | Temperature/°C | Dwell Time/h |
|---|---|---|
| Solution | 440 | 1, 2, 3 |
| Solution | 480 | 1, 2, 3 |
| Solution | 520 | 1, 2, 3 |
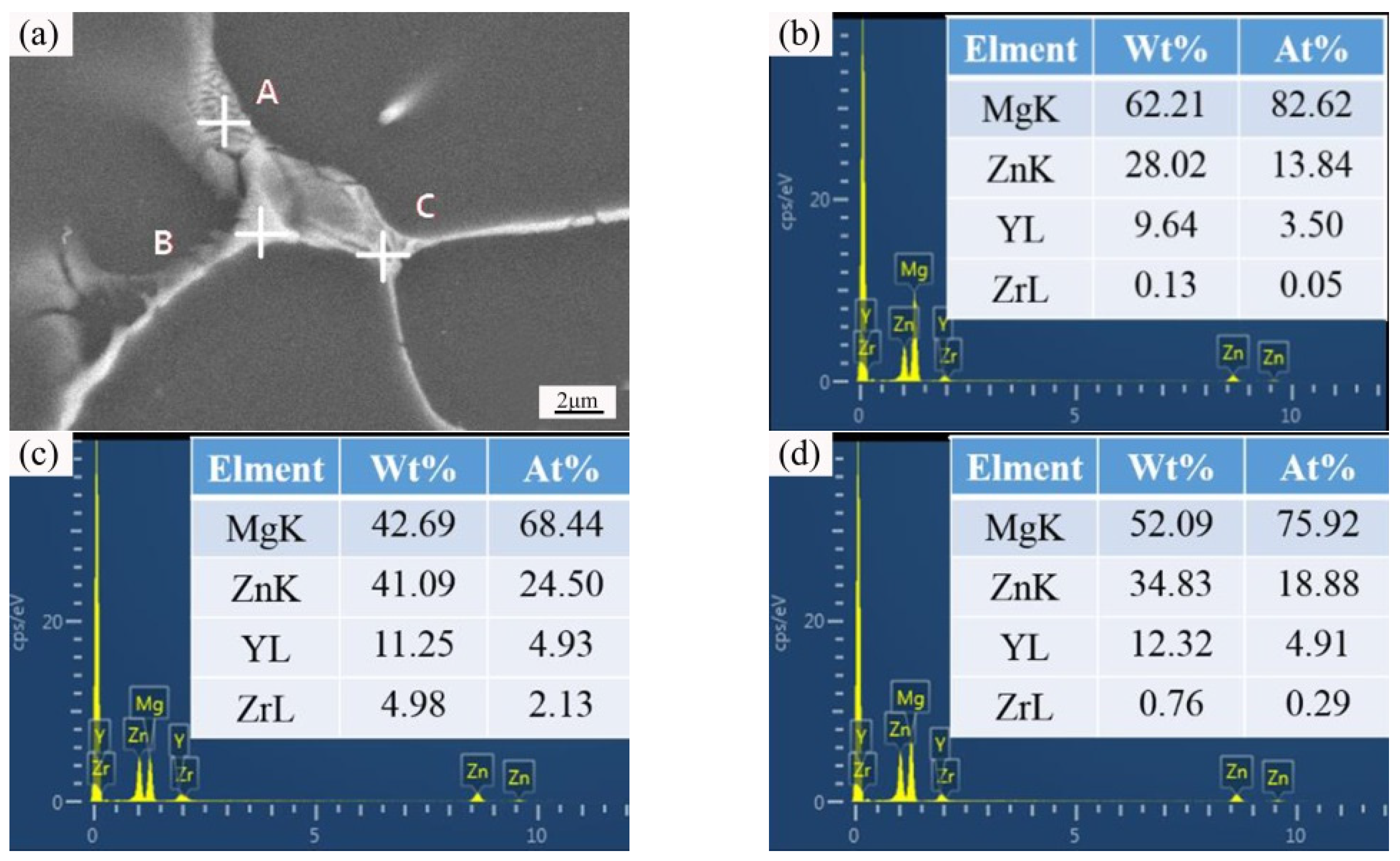
| Position | Mg | Zn | Y | Zr |
|---|---|---|---|---|
| Spot A | 82.62 | 13.84 | 3.50 | 0.05 |
| Spot B | 68.44 | 24.50 | 4.93 | 2.13 |
| Spot C | 75.92 | 18.88 | 4.91 | 0.29 |
| Y-Element Content (wt %) | Elongation (δ/%) | Yield Stress (σs/MPa) | Ultimate Tensile Strength (σb/MPa) |
|---|---|---|---|
| 0 | 9.5 | 103 | 220 |
| 1 | 16.0 | 104 | 232 |
| 2 | 12.5 | 95 | 217 |
| 3 | 11.5 | 97 | 228 |
| 4 | 11.0 | 125 | 231 |
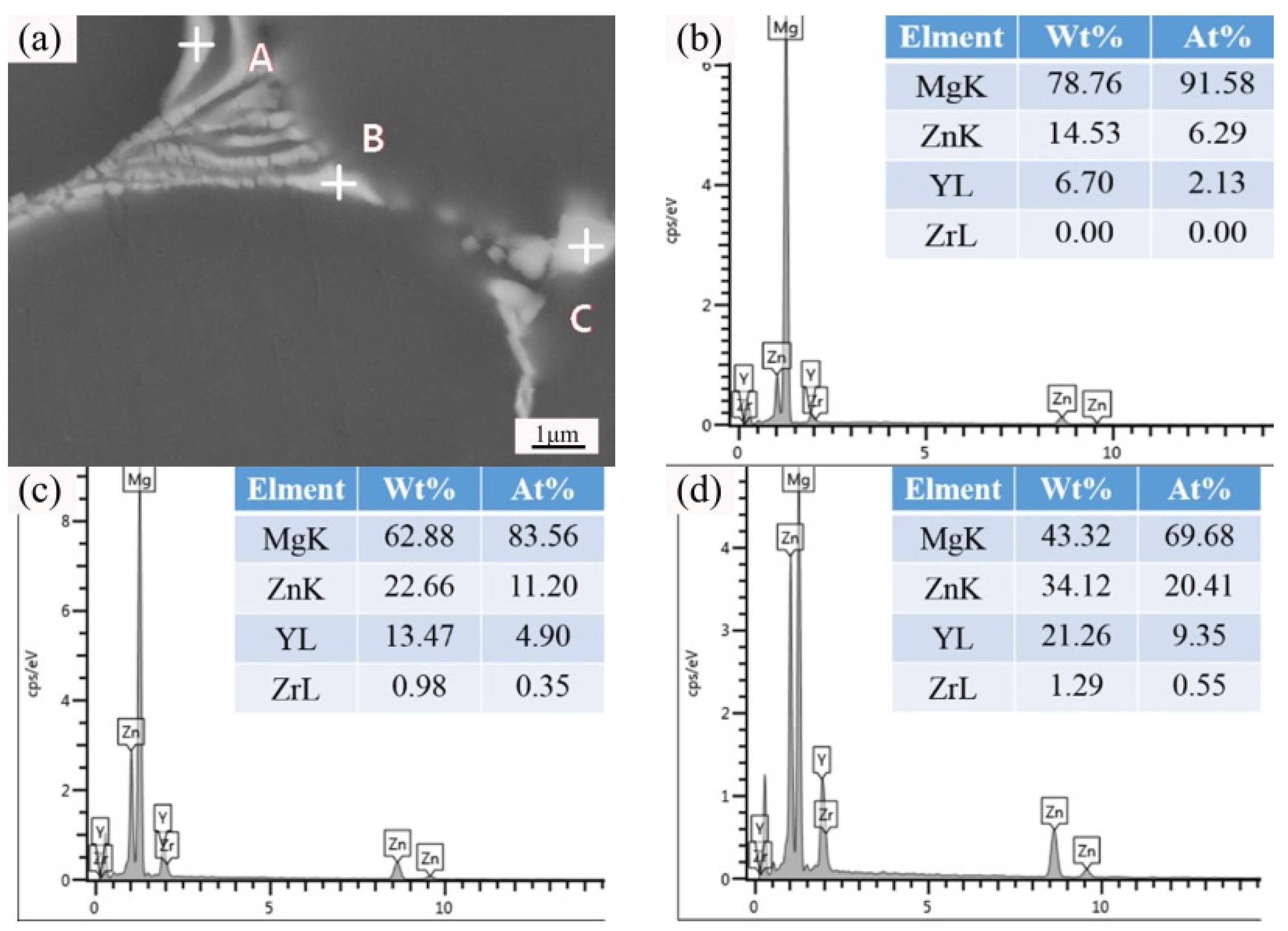
| Position | Mg | Zn | Y | Zr |
|---|---|---|---|---|
| Spot A | 91.58 | 6.29 | 2.13 | 0 |
| Spot B | 83.56 | 11.20 | 4.90 | 0.35 |
| Spot C | 69.68 | 20.41 | 9.35 | 0.55 |
| Temperature (°C) | Elongation (δ/%) | Yield Stress (σs/MPa) | Ultimate Tensile Strength (σb/MPa) |
|---|---|---|---|
| 320 | 8.5 | 142 | 283 |
| 340 | 10.0 | 142 | 308 |
| 360 | 10.5 | 147 | 300 |
| 380 | 18.5 | 150 | 315 |
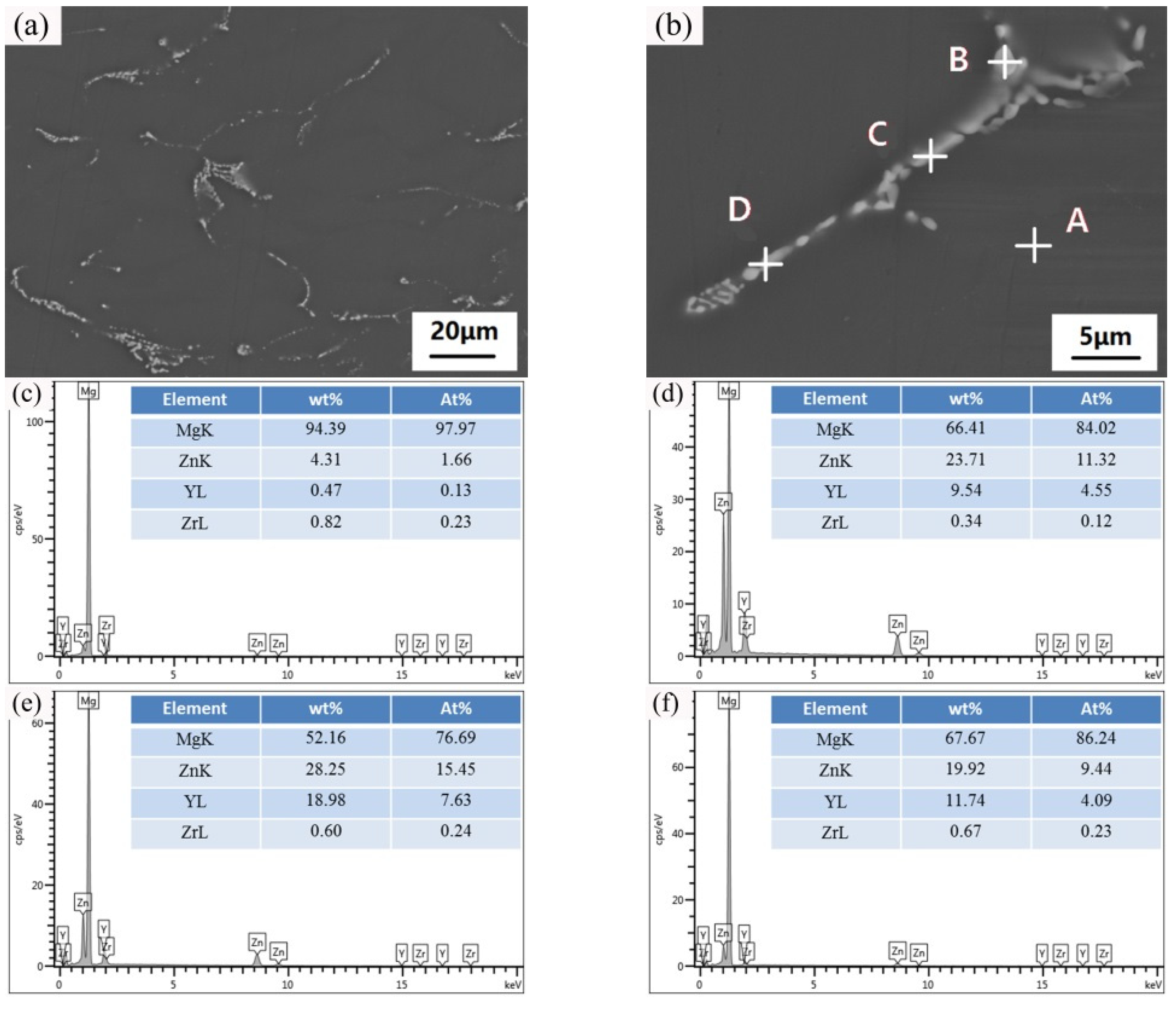
| Position | Mg | Zn | Y | Zr |
|---|---|---|---|---|
| Spot A | 97.97 | 1.66 | 0.13 | 0.23 |
| Spot B | 84.02 | 11.32 | 4.55 | 0.12 |
| Spot C | 76.69 | 15.45 | 7.63 | 0.24 |
| Spot D | 86.24 | 9.44 | 4.09 | 0.23 |
| Samples | Elongation (δ/%) | Yield Stress (σs/MPa) | Ultimate Tensile Strength (σb/MPa) |
|---|---|---|---|
| 440 °C—1 h | 19.5 | 113 | 282 |
| 440 °C—2 h | 19.0 | 116 | 269 |
| 440 °C—3 h | 18.5 | 114 | 274 |
| 480 °C—1 h | 19.0 | 121 | 273 |
| 480 °C—2 h | 18.5 | 123 | 268 |
| 480 °C—3 h | 25.0 | 122 | 280 |
| 520 °C—1 h | 25.5 | 125 | 282 |
| 520 °C—2 h | 25.0 | 126 | 273 |
| 520 °C—3 h | 23.5 | 123 | 280 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, H.; Chen, L.; Zhang, H.; Chen, G.; Chen, L.; Du, Z. Preparation and Mechanical Properties of ZK61-Y Magnesium Alloy Wheel Hub via Liquid Forging—Isothermal Forging Process. Metals 2020, 10, 385. https://doi.org/10.3390/met10030385
Qi Y, Wang H, Chen L, Zhang H, Chen G, Chen L, Du Z. Preparation and Mechanical Properties of ZK61-Y Magnesium Alloy Wheel Hub via Liquid Forging—Isothermal Forging Process. Metals. 2020; 10(3):385. https://doi.org/10.3390/met10030385
Chicago/Turabian StyleQi, Yushi, Heng Wang, Lili Chen, Hongming Zhang, Gang Chen, Lihua Chen, and Zhiming Du. 2020. "Preparation and Mechanical Properties of ZK61-Y Magnesium Alloy Wheel Hub via Liquid Forging—Isothermal Forging Process" Metals 10, no. 3: 385. https://doi.org/10.3390/met10030385
APA StyleQi, Y., Wang, H., Chen, L., Zhang, H., Chen, G., Chen, L., & Du, Z. (2020). Preparation and Mechanical Properties of ZK61-Y Magnesium Alloy Wheel Hub via Liquid Forging—Isothermal Forging Process. Metals, 10(3), 385. https://doi.org/10.3390/met10030385






