Abstract
Studying the dissolution of chalcocite allows to understand the behavior of the most abundant secondary sulfide ore in copper deposits, while digenite (Cu1.8S) and other intermediate sulfides (Cu2−xS) are often associated with chalcocite. The most common mechanism of dissolution is by two stages, and chloride ions benefit the kinetics of dissolution. In this study, a pure chalcocite mineral (99.9% according to XRD (X-Ray Diffraction) analysis) is leached in chloride media using NaCl and wastewater as the sources of chloride. Magnetic leaching tests are performed at 65, 75, and 95 °C, using a particle size between −150 and + 106 μm. Chloride concentration and leaching time are the main variables. A substantial dissolution of chalcocite was obtained with 0.5 M H2SO4, 100 g/L of chloride and a leaching time of 3 h. The apparent activation energy (Ea) derived from the slopes of the Arrhenius plots was 36 kJ/mol. The XRD analysis proves the presence of elemental sulfur (S0) as the main component in the leaching residue. No significant differences in copper extraction were detected when using 100 g/L of chloride ion or wastewater (39 g/L).
1. Introduction
Chilean copper production in 2018 was 5.83 million metric tons—the highest in the world [1]. Currently, Chilean copper mining continues to deepen its research to sustainably improve the efficiency of the processes and increase the copper production in a viable way. The challenges vary and include energy issues, water scarcity, relationship with local communities, and changes in mineralogy as porphyritic minerals are located at greater depths. In this case, copper sulfide may appear, which is refractory to the conventional leaching conditions [2,3].
One of the problems facing the mining industry in Chile is the lack of water. In 2017, it was used 13.26 m3/s of water from continental origin, 3.16 m3/s of seawater, and 38.07 m3/s of recirculated water [4]. In the future there will be an increase in the seawater use, and Cochilco predicts an increase of up to 10.82 m3/s by 2029, where some companies are building their own desalination plants [5].
Many researches address the use of seawater in mining, and promising results have been gotten in both hydrometallurgical and concentration processes [6,7,8]. However, many companies are desalinating seawater through reversal osmosis plants, due to the operational complexities caused by ions like calcium, magnesium, phosphate, carbonates, and chloride. These species may delay the kinetics of copper dissolution (except chloride), precipitate as undesired and unstable waste, and they may consume the sulfuric acid, incrementing the operational costs. In copper concentration stages, ions like magnesium and calcium affect the performance of both froth flotation and tailing management [9,10].
Seawater desalination plants generate wastewater with high chloride content that is discharged into the sea; however, the high chloride content may be of interest in the hydrometallurgical processes to treat copper sulfide. The presence of chloride works as a catalyst in the kinetics of copper dissolution, and help to obtain stable residues that do not produce acid drainage (AMD, acid mine/metalliferous drainage). Besides, it generates elemental sulfur as a residue and not a sulfate. Finally, cupric and cuprous ions stabilize as chloride complexes.
The first stage of leaching of chalcocite is much faster than the second stage. This is controlled by the diffusion of the oxidant on the ore surface at low values of activation energy (4–25 kJ mol−1) [11]. The second stage is much slower but this can be accelerated according to the temperature [12,13], indicating that the process is controlled by chemical and/or electrochemical reaction [14]. The authors of reference [13] indicated that the covellite dissolution rate is mostly independent of chloride and HCl concentration in the range of 0.2 to 2.5 M and 0.1 to 1 M, reporting activation energy values of 71.5 kJ mol−1. This means that the process is controlled by chemical or electrochemical reaction on the surface of the ore.
Several researches address the leaching of copper sulfides in chlorinated media under varied conditions of temperature and/or pressure [13,15,16,17]. Table 1 details the experiments of each investigation, specifying the operating conditions and the results provided. The common factor is the addition of chloride, since when no chloride is added, the dissolution decreases, and there is a tendency to passivation. Chloride ions promote the formation of long sulfide crystals, which allow the penetration of the reagent through the passivating layer [18].

Table 1.
Comparison of previous investigations of chalcocite with the use of Cl− and T used.
In this work, we study the leaching of pure chalcocite in chloride media from wastewater with the application of high temperatures to analyze its dissolution kinetics and calculate the activation energy of the leaching process.
2. Materials and Methods
2.1. Chalcocite
The chalcocite mineral was collected from Atómica Mine, located in the city of Antofagasta, Chile. The material was crushed in a porcelain mortar to reach a size range between −150 and +106 μm, and then it was chemically analyzed by atomic emission spectrometry via induction-coupled plasma (ICP-AES), developed in the Applied Geochemistry Laboratory of the Department of Geological Sciences of the Universidad Católica del Norte. Table 2 shows the chemical composition of the samples.

Table 2.
Chemical analysis of the chalcocite ore.
The sample was analyzed mineralogically using a Bruker brand X-ray diffractometer, automatic and computerized model D8 (Bruker, Billerica, MA, USA). In Figure 1, it is seen that the chalcocite mineral has a purity of 99.9%.
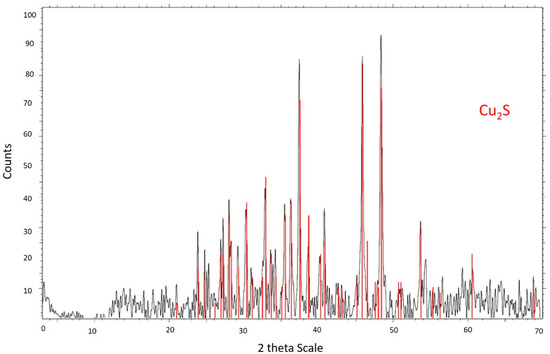
Figure 1.
X-ray diffractogram for the chalcocite mineral.
2.2. Reagents and Leaching Tests
The sulfuric acid used for the leaching tests is grade P.A., Merck brand, purity 95–97%, it also works with wastewater from the “Aguas Antofagasta” Desalination Plant. Table 3 shows its chemical composition:

Table 3.
Chemical analysis of waste water.
Leaching tests were carried out in a 50 mL glass reactor with a 0.01 S/L ratio of leaching solution. A total of 200 mg of chalcocite ore in a size range between −147 and +104 μm remained dispersed with the use of a 5-position magnetic stirrer (IKA ROS, CEP 13087-534, Campinas, Brasil) at a rotation rate of 600 rpm. The temperature was controlled with an oil-heated circulator (Julabo, St. Louis, MO, USA). The tests were conducted at 65, 75, 85 and 95, °C, with 0.5 M of H2SO4, 100 and 39 g/L of chloride ion concentration; acid concentration and S/L ratio parameters were based in a previous research [19]. Furthermore, the assays were performed in duplicate, measurements (or analyses) were carried on 5 mL aliquot and diluted to a range of using atomic absorption spectrometry with a coefficient of variation ≤ 5% and a relative error between 5% to 10%. Measurements of pH and oxidation-reduction potential (ORP) of leach solutions were made using a pH-ORP meter (HANNA HI-4222, St. Louis, MO, USA). The solution ORP was measured in a combination ORP electrode cell composed of a platinum working electrode and a saturated Ag/AgCl reference electrode.
2.3. Experimental Design
The effect of temperature on the dissolution of pure chalcocite with the addition of sodium chloride and waste water was performed. The tests were carried out at four temperatures (65, 75, 85, and 95 °C) using NaCl (100 g/L Cl−) and wastewater (39 g/L Cl−). 0.5 M H2SO4 was used in all leaching tests. Copper extraction was measured as a function of time (3–180 min) for each temperature. Finally, each point (as an independent test) was part of the curve that gives the final leaching trend.
An Arrhenius plot was made for the 2 groups (with NaCl and waste water) depending on the unreacted core model. Theoretically, chalcocite leaching was governed by diffusional control, so the necessary activation energy, under the conditions studied, was calculated.
XRD analysis was performed on two samples at different leaching times, showing the formation of the species. The samples are analyzed at 6 and 180 min, from the test at 95 °C using 100 g/L of Cl–.
3. Results
3.1. Effect of Temperature in Copper Extraction
Figure 2 shows the strong effect of the temperature in the leaching of a pure chalcocite. In a time close to 15 min, copper extraction close to 40% is achieved (except for the test performed at 65 °C), which presumes the first stage of leaching ruled by the fast reaction of the transformation of chalcocite to covellite, in which low activation energy is required [13]. When covellite is formed, it needs more energy (about 72 kJ approximately) to achieve its dissolution, and to later become a copper polysulfide (CuS2) that requires more demanding conditions to reach its complete dissolution [20].
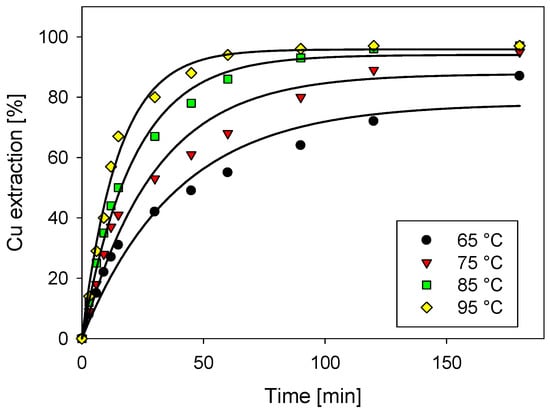
Figure 2.
Cu extraction as a function of temperature with 0.5 M H2SO4 and 100 g/L of Cl.
The kinetics of dissolution increase as a function of temperature, reaching a copper extraction of 95% after 90 min and 97% after 3 h, considering a temperature of 95 °C, 0.5 M of H2SO4 and 100 g/L of chloride. Under similar conditions, other researchers [17] carried out leaching tests of white metal (chalcocite and djurleite) with high temperatures but without the addition of chloride, obtaining copper extractions of the order of 55% using a temperature of 105 °C for 5 h, which shows the importance of including chloride ions in the chalcocite dissolution [21,22].
The behavior of copper extraction by using wastewater does not change the trend. Wastewater has been previously evaluated for the dissolution of copper sulfides minerals [19,23,24] showing favorable outcomes, even better in some cases compared to seawater. In Figure 3, it is appreciated that the presence of chloride (39 g/L) would be enough to achieve high copper extraction and that other wastewater ions would not impact the chalcocite dissolution.
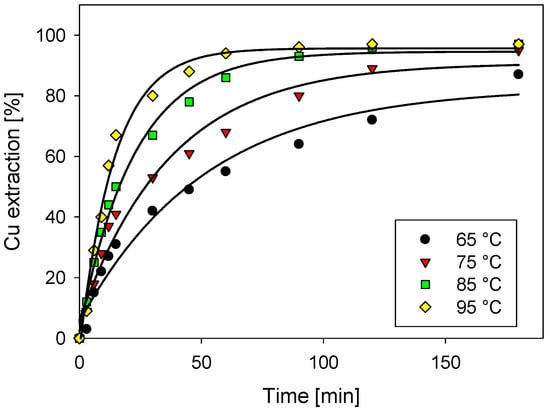
Figure 3.
Cu extraction as a function of temperature with 0.5 M H2SO4 and waste water.
According to several authors [11,15,22], chalcocite leaching is governed by the unreacted core model and is controlled by the oxidative diffusion of the surface layer. This reaction is used to describe the kinetics of chalcocite leaching, so the equation used is:
and:
Kt = α
α: fraction of dissolved copper.
t: leaching time.
K: reaction rate constant.
The Arrhenius plot in Figure 4 is obtained by the slopes of each extraction curve as a function of temperature in the linear zones (approximately up to 12 min), which finally provides the activation energy of 36 kJ/mol. A similar value was obtained in other investigations with comparable conditions [15], that obtained an activation energy of 33.5 kJ/mol with the addition of 0.5 M of H2SO4 and 0.5 M of NaCl. Nevertheless, it was higher than that previously obtained with chalcocite and white metal using low temperatures (10–30 °C) and molecular chlorine, obtaining activation energies of 23.6 and 22.4 kJ/mol, respectively [25]. The diffusion-controlled processes are not greatly affected by temperature, and the chemically controlled processes depends strongly on temperature.
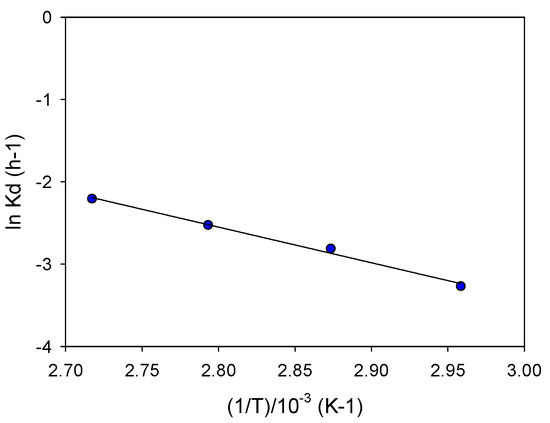
Figure 4.
Arrhenius plot.
3.2. Residue Analysis
It was segmented into two tests performed comparing to the initial sample without leaching to know the conditions in which it started. In Figure 1, it is observed that the non-leached mineral is in a pure state of chalcocite, evidencing the non-presence of other species. Then in Figure 5, it is observed that its transformation to covellite (68.0% wt) and elemental sulfur S0 (15.8% wt) together with small intermediate rests of chalcocite and copper chloride CuCl, occurred in a time of 6 minutes of leaching, in which 29% copper was extracted. It is appreciated that despite that short time course, most of the initial chalcocite was transformed into covellite, and it also formed elemental sulfur as a stable residue. Copper chloride can be reoxidized in solution in the form of CuCl+ [16], so it is not a major issue in the residue.
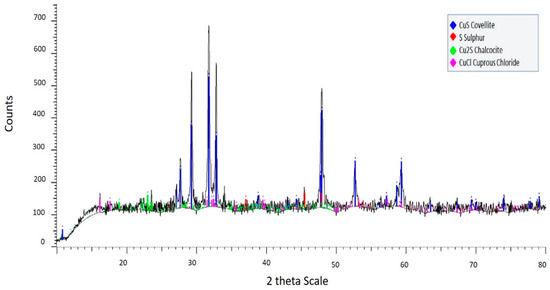
Figure 5.
X-ray diffractogram for solid residue after leaching for 6 min at 95 °C using 100 g/L chloride and 0.5 M H2SO4.
Figure 6 indicates that there is no chalcocite in the residue but it appears a remaining of covellite (14.6% wt) and copper chloride CuCl (5.2% wt), together with the elemental sulfur S0 (80.2% wt). Chalcocite was converted to a covellite-like product which then reacted to liberate the remaining copper and form each sulfur [26]. The remaining covellite was not leached because the diffusion barrier became more complicated [11].
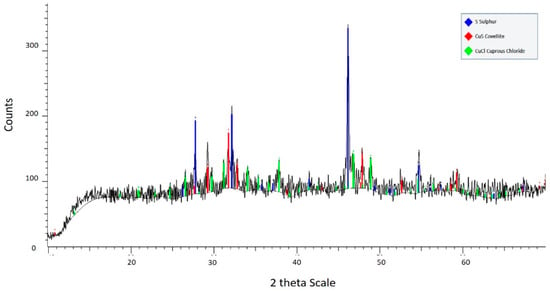
Figure 6.
X-ray diffractogram for solid residue after leaching for 3 h at 95 °C using 100 g/L of chloride and 0.5 M of H2SO4.
4. Conclusions
The present study shows the experimental results of dissolving copper from a chalcocite mineral in chloride media. The findings of this research were:
With the application of temperature (65–95 °C) in leaching with 0.5 M H2SO4, 100 g/L of chloride, and a leaching time of up to 3 h, an activation energy of 36 kJ/mol was obtained. It is stated that the dissolution of this mineral is controlled by oxidative diffusion on the surface according to the model of the unreacted core.
Tests conducted at 0.5 M H2SO4, temperatures between 65–95 °C using 100 g/L chloride ion versus tests performed in wastewater (39 g/L Cl−) show that there was no significant effect on copper extraction neither in the trend of the curves.
The XRD analysis shows the formation of a stable and non-polluting residue such as elemental sulfur (S0) and a small quantity of unreacted covellite. This residue was obtained in a leaching time of 3 h at 95 °C under conditions of 0.5 mol/L H2SO4 and 100 g/L Cl−.
Author Contributions
K.P., N.T., R.I.J., P.R. contributed in project administration, investigation and wrote paper, V.Q. contributed in the data curation, E.S.-R. and J.H.-Á. contributed in validation and supervision and S.N. performed the experiments, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
R.I.J. thanks Centro CRHIAM Project ANID/Fondap/15130015.
Acknowledgments
The authors are grateful for the contribution of the Scientific Equipment Unit—MAINI—of the Universidad Católica del Norte for facilitating the chemical analysis of the solutions. Pedro Robles thanks the Pontificia Universidad Católica de Valparaíso for the support provided.
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- Pérez, K.; Toro, N.; Campos, E.; González, J.; Jeldres, R.I.; Nazer, A.; Rodriguez, M.H. Extraction of Mn from Black Copper Using Iron Oxides from Tailings and Fe2+ as Reducing Agents in Acid Medium. Metals 2019, 9, 1112. [Google Scholar] [CrossRef]
- Beiza, L.; Quezada, V.; Melo, E.; Valenzuela, G. Electrochemical behaviour of chalcopyrite in chloride solutions. Metals 2019, 9, 67. [Google Scholar] [CrossRef]
- Velásquez Yévenes, L. The Kinetics of the Dissolution of Chalcopyrite in Chloride Media. Ph.D. Thesis, Murdoch University, Perth, Australia, March 2009. [Google Scholar]
- Consumo De Agua En La Mineria Del Cobre. Available online: http://www.cochilco.cl/descargas/estudios/informes/agua/CONSUMO-DE-AGUA-EN-LA-MINERIA-DEL-COBRE-2011.pdf (accessed on 11 December 2019).
- Proyección De Consumo De Agua En La Minería Del Cobre 2018–2029. Available online: https://www.cochilco.cl/Mercado de Metales/Proyección de la producción esperada de cobre 2018–2029 Vfinal.pdf (accessed on 11 December 2019).
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Torres, C.M.; Ghorbani, Y. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy 2015, 157, 325–332. [Google Scholar] [CrossRef]
- Watling, H.R.; Shiers, D.W.; Li, J.; Chapman, N.M.; Douglas, G.B. Effect of water quality on the leaching of a low-grade copper sulfide ore. Miner. Eng. 2014, 58, 39–51. [Google Scholar] [CrossRef]
- Torres, C.M.; Taboada, M.E.; Graber, T.A.; Herreros, O.O.; Ghorbani, Y.; Watling, H.R. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y. The effect of saline water on copper activation of pyrite in chalcopyrite flotation. Miner. Eng. 2019, 131, 336–341. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Forbes, L.; Cisternas, L.A. Effect of Seawater on Sulfide Ore Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2016, 37, 369–384. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Tan, Q.; Jia, Y.; Sun, H. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D. Principles, mechanisms and dynamics of chalcocite heap bioleaching. Microb. Process. Met. Sulfides 2007, 193–218. [Google Scholar]
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef]
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity-Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl-H2SO4-O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Abarzúa, E.; Padilla, R. Oxygen pressure leaching of white metal. Hydrometallurgy 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Toro, N.; Pérez, K.; Saldaña, M.; Jeldres, R.I.; Jeldres, M.; Cánovas, M. Dissolution of pure chalcopyrite with manganese nodules and waste water. J. Mater. Res. Technol. 2019, 9, 798–805. [Google Scholar] [CrossRef]
- Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water. Metals 2019, 9, 780. [Google Scholar] [CrossRef]
- Nicol, M.; Basson, P. The anodic behaviour of covellite in chloride solutions. Hydrometallurgy 2017, 172, 60–68. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The leaching of sulphide minerals in chloride media. Hydrometallurgy 1992, 29, 1–45. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Quezada, V.; Velásquez, L.; Roca, A.; Benavente, O.; Melo, E.; Keith, B. Effect of curing time on the dissolution of a secondary copper sulphide ore using alternative water resources. IOP Conf. Ser. Mater. Sci. Eng. 2018, 427, 26–29. [Google Scholar] [CrossRef]
- Herreros, O. Dissolution kinetics of copper, white metal and natural chalcocite in Cl2-Cl y media. Metals 1999. [Google Scholar] [CrossRef]
- Fisher, W.W.; Flores, F.A.; Henderson, J.A. Comparison of chalcocite dissolution in the oxygenated, aqueous sulfate and chloride systems. Miner. Eng. 1992, 5, 817–834. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).