Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid
Abstract
1. Introduction
2. Experimental Section
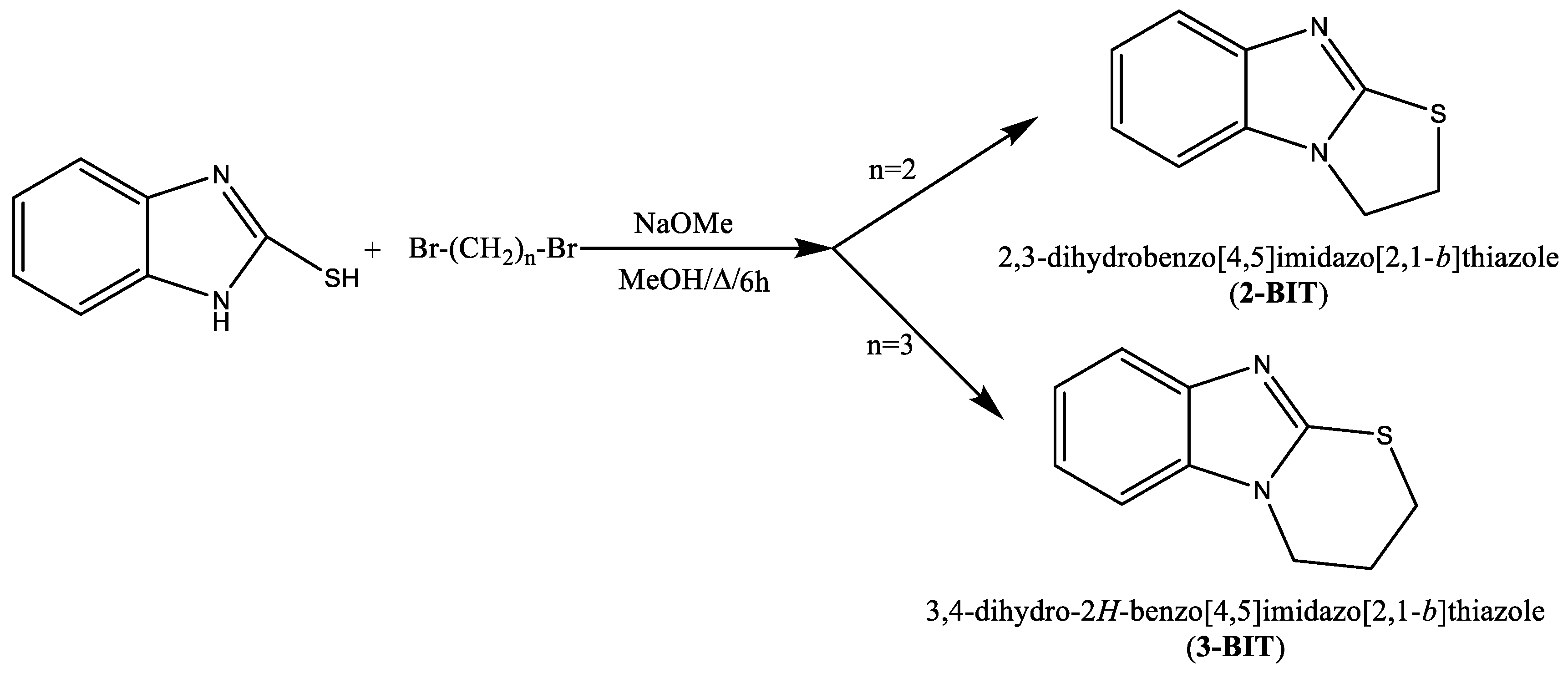
2.1. Synthesis of Benzimidazole Derivatives
2.2. Test Specimens and Solutions Used
2.3. Weight Loss Measurements
2.4. Electrochemical Techniques
2.5. Surface Study
3. Results and Discussion
3.1. Gravimetric Measurements
3.1.1. Effect of Temperature
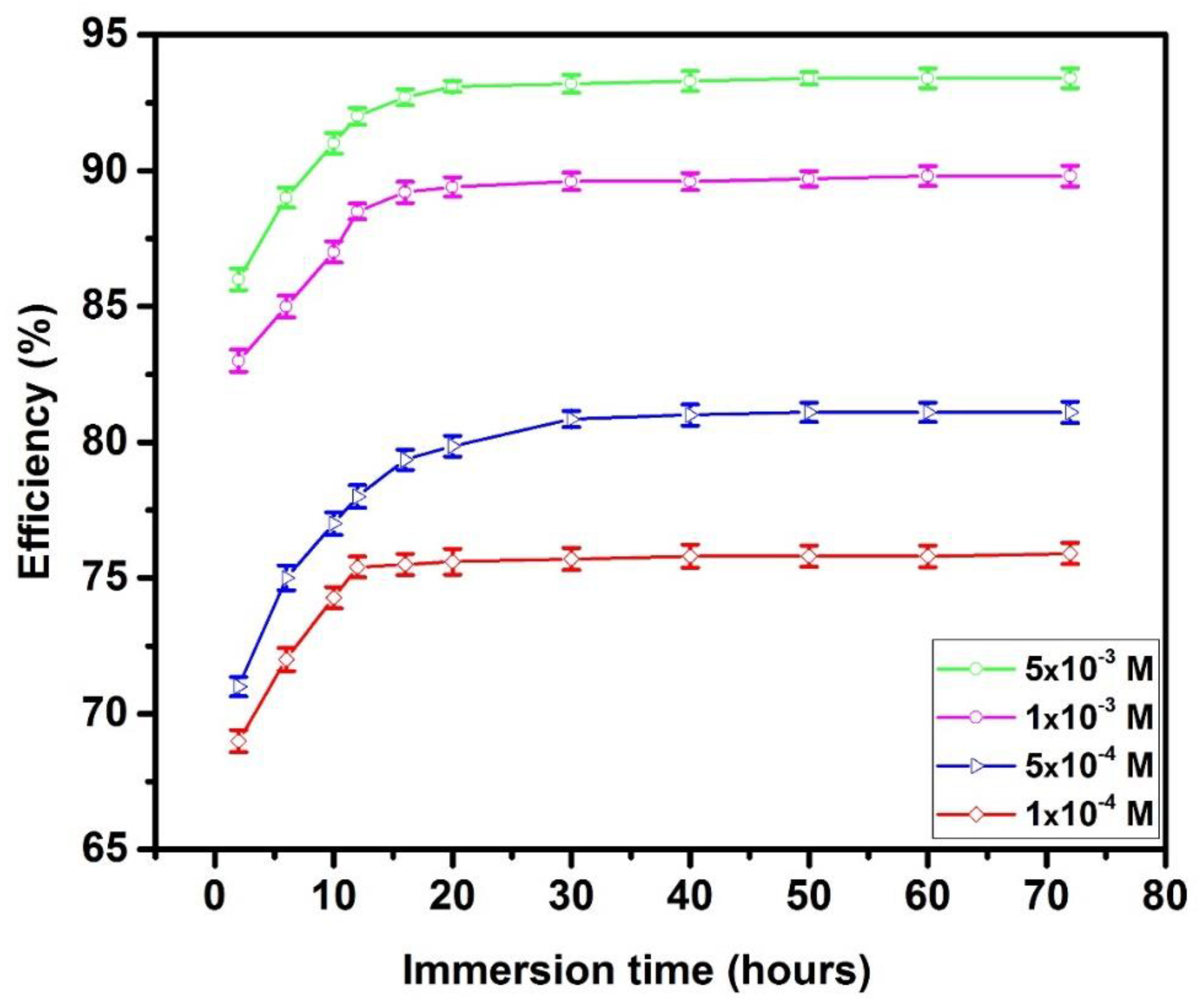
3.1.2. Effect of Immersion Time
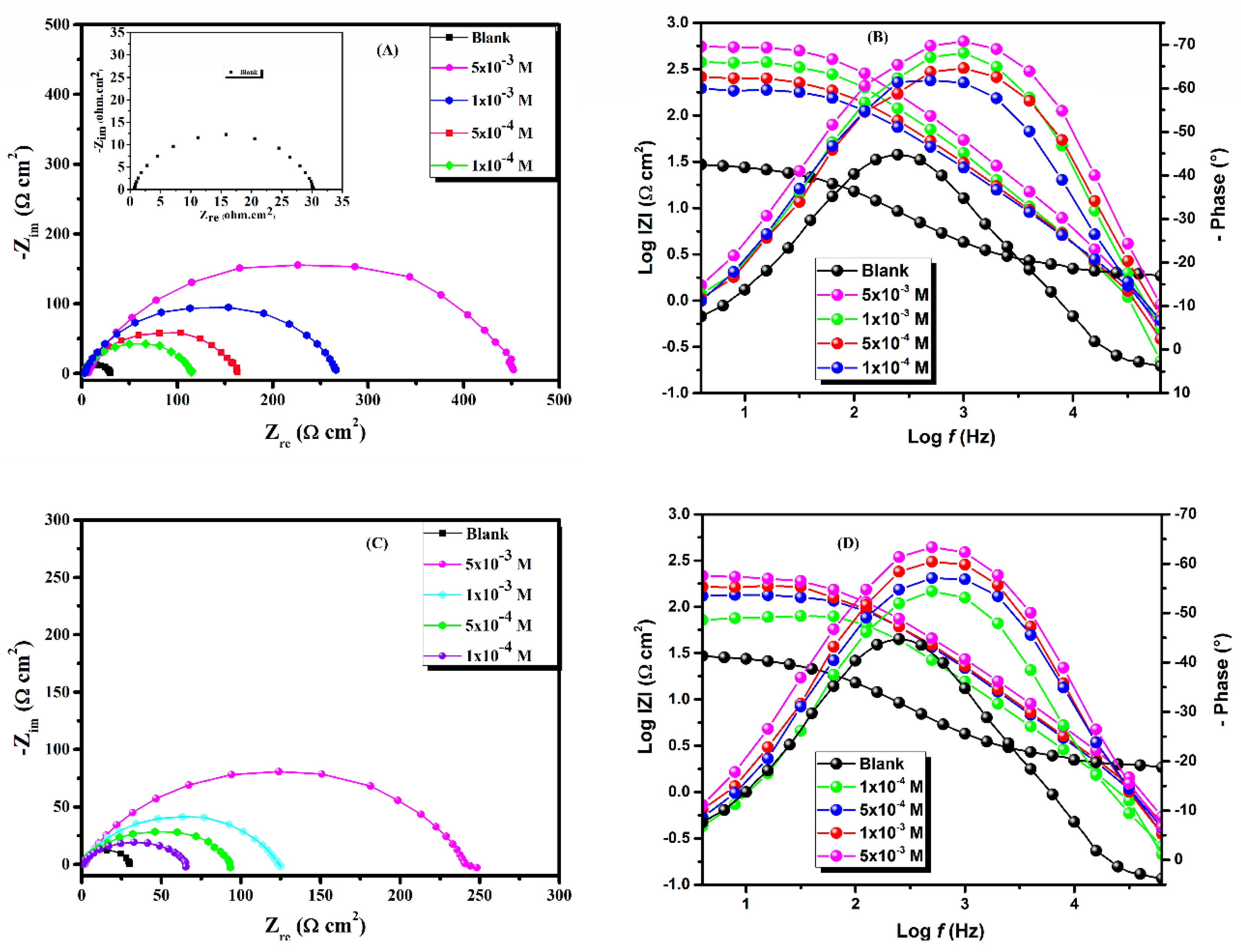
3.2. Electrochemical Impedance Spectroscopy (EIS)
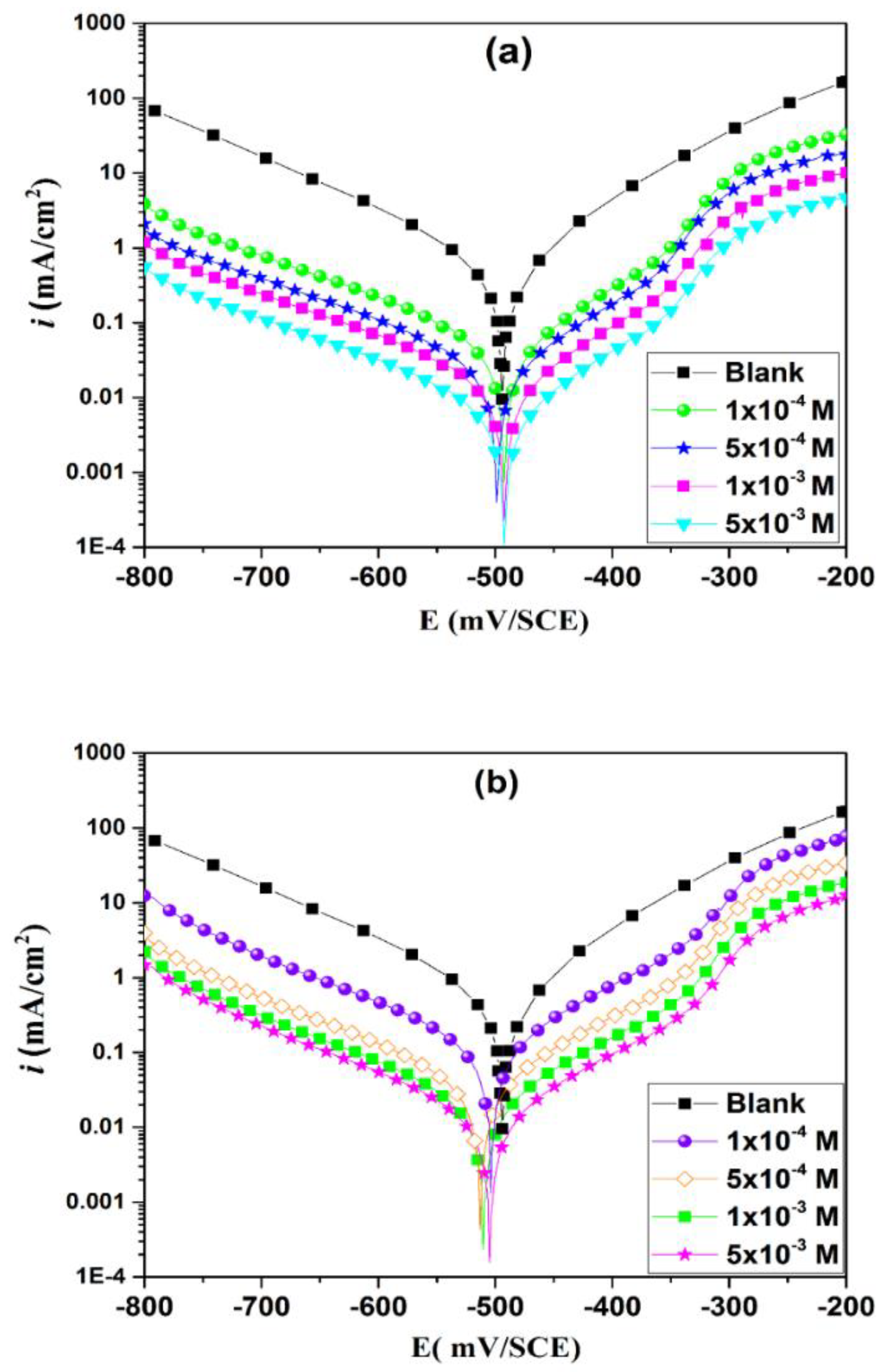
3.3. Polarization Curves
3.4. Adsorption Isotherm
3.5. Surface Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aljourani, J.; Golozar, M.A.; Raeissi, K. The inhibition of carbon steel corrosion in hydrochloric and sulfuric acid media using some benzimidazole derivatives. Mater. Chem. Phys. 2010, 121, 320–325. [Google Scholar] [CrossRef]
- Lindsay, R.; Kokalj, A. Corrosion Inhibition. Metals 2018, 8, 821. [Google Scholar] [CrossRef]
- Ehsani, A.; Nasrollahzadeh, M.; Mahjani, M.G.; Moshrefi, R.; Mostaanzadeh, H. Electrochemical and quantum chemical investigation of inhibitory of 1,4-Ph(OX)2(Ts)2 on corrosion of 1005 aluminum alloy in acidic medium. J. Ind. Eng. Chem. 2014, 20, 4363–4370. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Detailed macro-/micro-scale exploration of the excellent active corrosion inhibition of a novel environmentally friendly green inhibitor for carbon steel in acidic environments. J. Taiwan Inst. Chem. Eng. 2019, 100, 239–261. [Google Scholar] [CrossRef]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ramezanzadeh, B.; Dehghani, A.; Ramezanzadeh, M. Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala seed extract for mild steel corrosion in HCl solution: Detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq. 2019, 283, 174–195. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.; Bahadur, I.; Obot, I.; Quraishi, M. 5-(Phenylthio)-3H-pyrrole-4-carbonitriles as effective corrosion inhibitors for mild steel in 1 M HCl: Experimental and theoretical investigation. J. Mol. Liq. 2015, 212, 209–218. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Singh, A. 2-Amino-5-nitro-4, 6-diarylcyclohex-1-ene-1, 3, 3-tricarbonitriles as new and effective corrosion inhibitors for mild steel in 1 M HCl: Experimental and theoretical studies. J. Mol. Liq. 2015, 212, 804–812. [Google Scholar] [CrossRef]
- Daoud, D.; Douadi, T.; Hamani, H.; Chafaa, S.; Al-Noaimi, M. Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study. Corros. Sci. 2015, 94, 21–37. [Google Scholar] [CrossRef]
- Kubba, R.M.; Alag, A.S. Experimental and Theoretical Evaluation of new Quinazolinone Derivative as Organic Corrosion Inhibitor for Carbon Steel in 1M HCl Solution. Int. J. Sci. Res. IJSR6 2017, 6, 1832–1842. [Google Scholar]
- Bentiss, F.; Lagrenee, M.; Traisnel, M.; Hornez, J. The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros. Sci. 1999, 41, 789–803. [Google Scholar] [CrossRef]
- Baskar, R.; Kesavan, D.; Gopiraman, M.; Subramanian, K. Corrosion inhibition of mild steel in 1.0M hydrochloric acid medium by new photo-cross-linkable polymers. Prog. Org. Coat. 2014, 77, 836–844. [Google Scholar]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Fouda, A.S.; Attia, A.A.; Negm, A. Some thiophene derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solution. J Met. 2014, 2014, 1–15. [Google Scholar]
- Awad, M.K. Semiempirical investigation of the inhibition efficiency of thiourea derivatives as corrosion inhibitors. J. Electroanal. Chem. 2004, 567, 219–225. [Google Scholar] [CrossRef]
- Torres, V.; Rayol, V.; Magalhães, M.; Viana, G.; Aguiar, L.; Machado, S.; Orofino, H.; D’Elia, E. Study of thioureas derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution. Corros. Sci. 2014, 79, 108–118. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Belghiti, M.E.; Hammouti, B.; Jodeh, S.; Hamed, O.; Lgaz, H.; Salghi, R. Adsorption and corrosion inhibition effect of 2-mercaptobenzimidazole (surfactant) on a carbon steel surface in an acidic medium: Experimental and monte carlo simulations. Port. Electrochimica Acta 2018, 36, 197–212. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M.; Raicheva, S.; Sokolova, E. Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corros. Sci. 2004, 46, 1333–1350. [Google Scholar] [CrossRef]
- Zhang, G.A.; Hou, X.M.; Hou, B.S.; Liu, H.F. Benzimidazole derivatives as novel inhibitors for the corrosion of mild steel in acidic solution: Experimental and theoretical studies. J. Mol. Liq. 2019, 278, 413–427. [Google Scholar] [CrossRef]
- Morales-Gil, P.; Walczak, M.S.; Cottis, R.A.; Romero, J.M.; Lindsay, R. Corrosion inhibitor binding in an acidic medium: Interaction of 2-mercaptobenizmidazole with carbon-steel in hydrochloric acid. Corros. Sci. 2014, 85, 109–114. [Google Scholar] [CrossRef]
- Wang, L. Evaluation of 2-mercaptobenzimidazole as corrosion inhibitor for mild steel in phosphoric acid. Corros. Sci. 2001, 43, 2281–2289. [Google Scholar] [CrossRef]
- Mahdavian, M.; Ashhari, S. Corrosion inhibition performance of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole compounds for protection of mild steel in hydrochloric acid solution. Electrochimica Acta 2010, 55, 1720–1724. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Scully, J.; Baboian, R. Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM: Philadelphia, PA, USA, 1995; p. 110. [Google Scholar]
- ASTM, E. 647: Standard test method for measurement of fatigue crack growth rates. Annu. Book ASTM Stand. 2011, 3, 591–630. [Google Scholar]
- Eduok, U.M.; Khaled, M.M. Corrosion protection of non-alloyed AIAI 316L concrete steel metal grade in aqueous H2SO4: Electroanalytical and surface analyses with Metiamide. Constr. Build. Mater. 2014, 68, 285–290. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Lgaz, H.; Chung, I.-M. Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium. J. Colloid Interface Sci. 2020, 560, 225–236. [Google Scholar] [CrossRef]
- Singh, D.K.; Kumar, S.; Udayabhanu, G.; John, R.P. 4 (N, N-dimethylamino) benzaldehyde nicotinic hydrazone as corrosion inhibitor for mild steel in 1M HCl solution: An experimental and theoretical study. J. Mol. Liq. 2016, 216, 738–746. [Google Scholar] [CrossRef]
- Gopi, D.; Sherif, E.-S.M.; Surendiran, M.; Jothi, M.; Kumaradhas, P.; Kavitha, L. Experimental and theoretical investigations on the inhibition of mild steel corrosion in the ground water medium using newly synthesised bipodal and tripodal imidazole derivatives. Mater. Chem. Phys. 2014, 147, 572–582. [Google Scholar] [CrossRef]
- Guadalupe, H.J.; Garcia-Ochoa, E.; Maldonado-Rivas, P.J.; Cruz, J.; Pandiyan, T. A combined electrochemical and theoretical study of N, N′-bis (benzimidazole-2yl-ethyl)-1, 2-diaminoethane as a new corrosion inhibitor for carbon steel surface. J. Electroanal. Chem. 2011, 655, 164–172. [Google Scholar] [CrossRef]
- Dehri, İ.; Özcan, M. The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater. Chem. Phys. 2006, 98, 316–323. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Qi, S.; Dong, D.; Cang, H.; Lu, G. The inhibition performance of long-chain alkyl-substituted benzimidazole derivatives for corrosion of mild steel in HCl. Corros. Sci. 2016, 102, 517–522. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Zhang, L.; Feng, Y.; Zhai, H. Studies of protection of self-assembled films by 2-mercapto-5-methyl-1, 3, 4-thiadiazole on iron surface in 0.1 M H2SO4 solutions. J. Electroanal. Chem. 2008, 612, 257–268. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G.; Çulha, M.; Yazıcı, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochimica Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. 2-Mercaptopyrimidine as an effective inhibitor for the corrosion of cold rolled steel in HNO3 solution. Corros. Sci. 2017, 118, 202–216. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Chung, I.-M.; Ali, I.; Gaonkar, S.L.; Bhat, K.; Salghi, R.; Oudda, H.; Khan, M. Understanding corrosion inhibition of mild steel in acid medium by new benzonitriles: Insights from experimental and computational studies. J. Mol. Liq. 2018, 266, 603–616. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N.; Eseola, A. Anticorrosion potential of 2-mesityl-1H-imidazo [4,5-f][1,10] phenanthroline on mild steel in sulfuric acid solution: Experimental and theoretical study. Ind. Eng. Chem. Res. 2011, 50, 2098–2110. [Google Scholar] [CrossRef]
- Quraishi, M. Electrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid medium. Corros. Sci. 2013, 70, 161–169. [Google Scholar]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach. Phys. Chem. Chem. Phys. 2016, 18, 17898–17911. [Google Scholar] [CrossRef]
- El Aoufir, Y.; Aslam, R.; Lazrak, F.; Marzouki, R.; Kaya, S.; Skal, S.; Ghanimi, A.; Ali, I.H.; Guenbour, A.; Lgaz, H.; et al. The effect of the alkyl chain length on corrosion inhibition performances of 1,2,4-triazole-based compounds for mild steel in 1.0 M HCl: Insights from experimental and theoretical studies. J. Mol. Liq. 2020, 303, 112631. [Google Scholar] [CrossRef]
- Yadav, D.K.; Quraishi, M. Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind. Eng. Chem. Res. 2012, 51, 8194–8210. [Google Scholar] [CrossRef]
- Lgaz, H.; Saha, S.K.; Chaouiki, A.; Bhat, K.S.; Salghi, R.; Shubhalaxmi; Banerjee, P.; Ali, I.H.; Khan, M.I.; Chung, I.-M. Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies. Constr. Build. Mater. 2020, 233, 117320. [Google Scholar] [CrossRef]
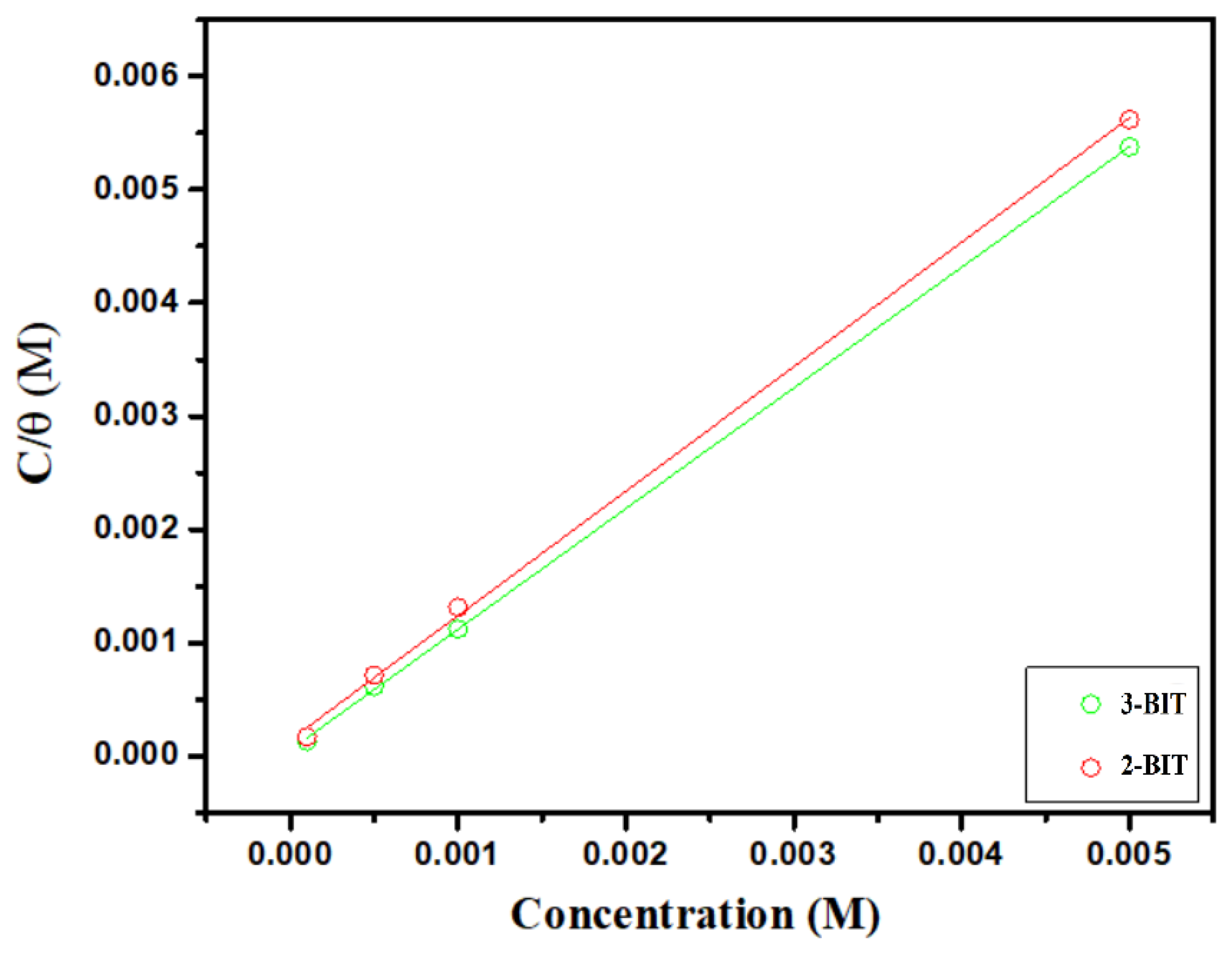
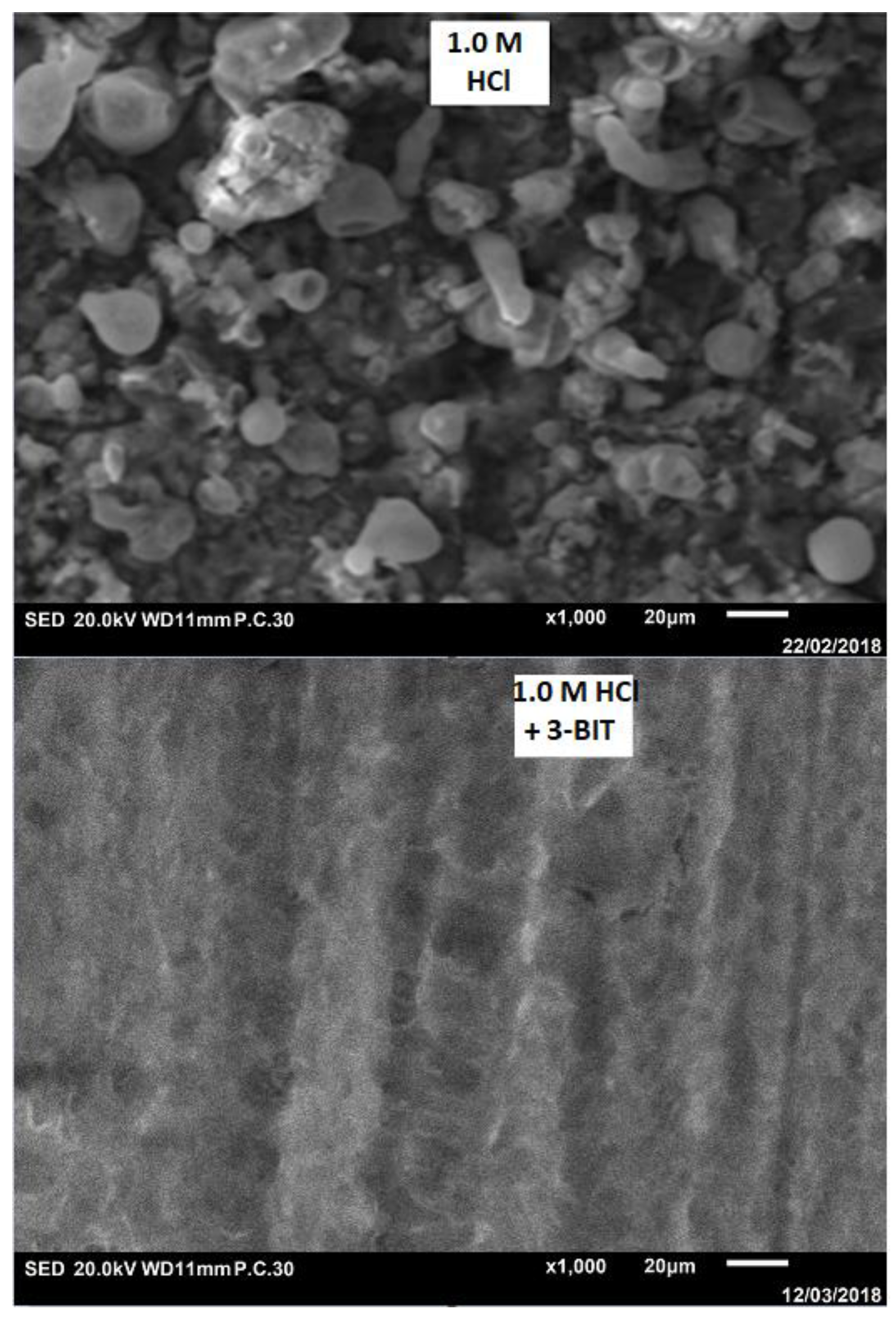
| Inhibitor | Concentration (mol/L) | (mg/cm2 × h) | (%) |
|---|---|---|---|
| Blank | 1.0 | 1.135 | - |
| 3-BIT | 0.074 | 93 | |
| 0.115 | 90 | ||
| 0.214 | 81 | ||
| 0.273 | 76 | ||
| 2-BIT | 0.123 | 89 | |
| 0.262 | 77 | ||
| 0.332 | 70 | ||
| 0.473 | 58 |
| Concentration (mol/L) | 303 K | 313 K | 323 K | 333 K | ||||
|---|---|---|---|---|---|---|---|---|
(mg/cm2 × h) | (%) | (mg/cm2 × h) | (%) | (mg/cm2 × h) | (%) | (mg/cm2 × h) | (%) | |
| Blank | 1.1350 | - | 1.4162 | - | 1.9981 | - | 2.5392 | - |
| 5 × 10−3 | 0.074 | 93 | 0.147 | 89 | 0.383 | 81 | 0.553 | 78 |
| 1 × 10−3 | 0.115 | 90 | 0.276 | 80 | 0.473 | 76 | 0.726 | 71 |
| 5 × 10−4 | 0.214 | 81 | 0.317 | 77 | 0.517 | 74 | 0.769 | 70 |
| 1 × 10−4 | 0.273 | 76 | 0.385 | 73 | 0.599 | 70 | 0.858 | 66 |
| Inhibitor | Concentration (mol/L) | (%) | |||||
|---|---|---|---|---|---|---|---|
| Blank | 1.0 | 29 | 0.89 | 1.761 | 91 | - | - |
| 3-BIT | 453 | 0.80 | 0.398 | 14 | 93 | 0.93 | |
| 266 | 0.76 | 0.589 | 15 | 89 | 0.89 | ||
| 162 | 0.78 | 0.712 | 20 | 82 | 0.82 | ||
| 115 | 0.81 | 1.085 | 38 | 74 | 0.74 | ||
| 2-BIT | 248 | 0.78 | 0.583 | 17 | 88 | 0.88 | |
| 125 | 0.79 | 0.659 | 18 | 76 | 0.76 | ||
| 94 | 0.80 | 0.896 | 27 | 69 | 0.69 | ||
| 69 | 0.82 | 1.146 | 39 | 57 | 0.57 |
| Inhibitor | Concentration (mol/L) | (mV/SCE) | (mV dec−1) | (mV dec−1) | (μA cm−2) | (%) | Ɵ |
|---|---|---|---|---|---|---|---|
| Blank | 1.0 | 496 | 150 | 132 | 564 | - | - |
| 3-BIT | 505 | 170 | 89 | 41 | 92 | 0.92 | |
| 513 | 172 | 98 | 69 | 87 | 0.87 | ||
| 524 | 167 | 98 | 99 | 82 | 0.82 | ||
| 504 | 177 | 96 | 146 | 74 | 0.74 | ||
| 2-BIT | 498 | 163 | 97 | 62 | 89 | 0.89 | |
| 496 | 168 | 99 | 134 | 76 | 0.76 | ||
| 503 | 162 | 93 | 169 | 70 | 0.70 | ||
| 494 | 154 | 95 | 233 | 58 | 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lgaz, H.; Masroor, S.; Chafiq, M.; Damej, M.; Brahmia, A.; Salghi, R.; Benmessaoud, M.; Ali, I.H.; Alghamdi, M.M.; Chaouiki, A.; et al. Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Metals 2020, 10, 357. https://doi.org/10.3390/met10030357
Lgaz H, Masroor S, Chafiq M, Damej M, Brahmia A, Salghi R, Benmessaoud M, Ali IH, Alghamdi MM, Chaouiki A, et al. Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Metals. 2020; 10(3):357. https://doi.org/10.3390/met10030357
Chicago/Turabian StyleLgaz, Hassane, Sheerin Masroor, Maryam Chafiq, Mohamed Damej, Ameni Brahmia, Rachid Salghi, Mohammed Benmessaoud, Ismat H. Ali, Majed M. Alghamdi, Abdelkarim Chaouiki, and et al. 2020. "Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid" Metals 10, no. 3: 357. https://doi.org/10.3390/met10030357
APA StyleLgaz, H., Masroor, S., Chafiq, M., Damej, M., Brahmia, A., Salghi, R., Benmessaoud, M., Ali, I. H., Alghamdi, M. M., Chaouiki, A., & Chung, I.-M. (2020). Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Metals, 10(3), 357. https://doi.org/10.3390/met10030357






