Reduction and Nitridation of Iron/Vanadium Oxides by Ammonia Gas: Mechanism and Preparation of FeV45N Alloy
Abstract
1. Introduction
2. Experimental
2.1. Experimental Procedure
2.2. Analytical Method
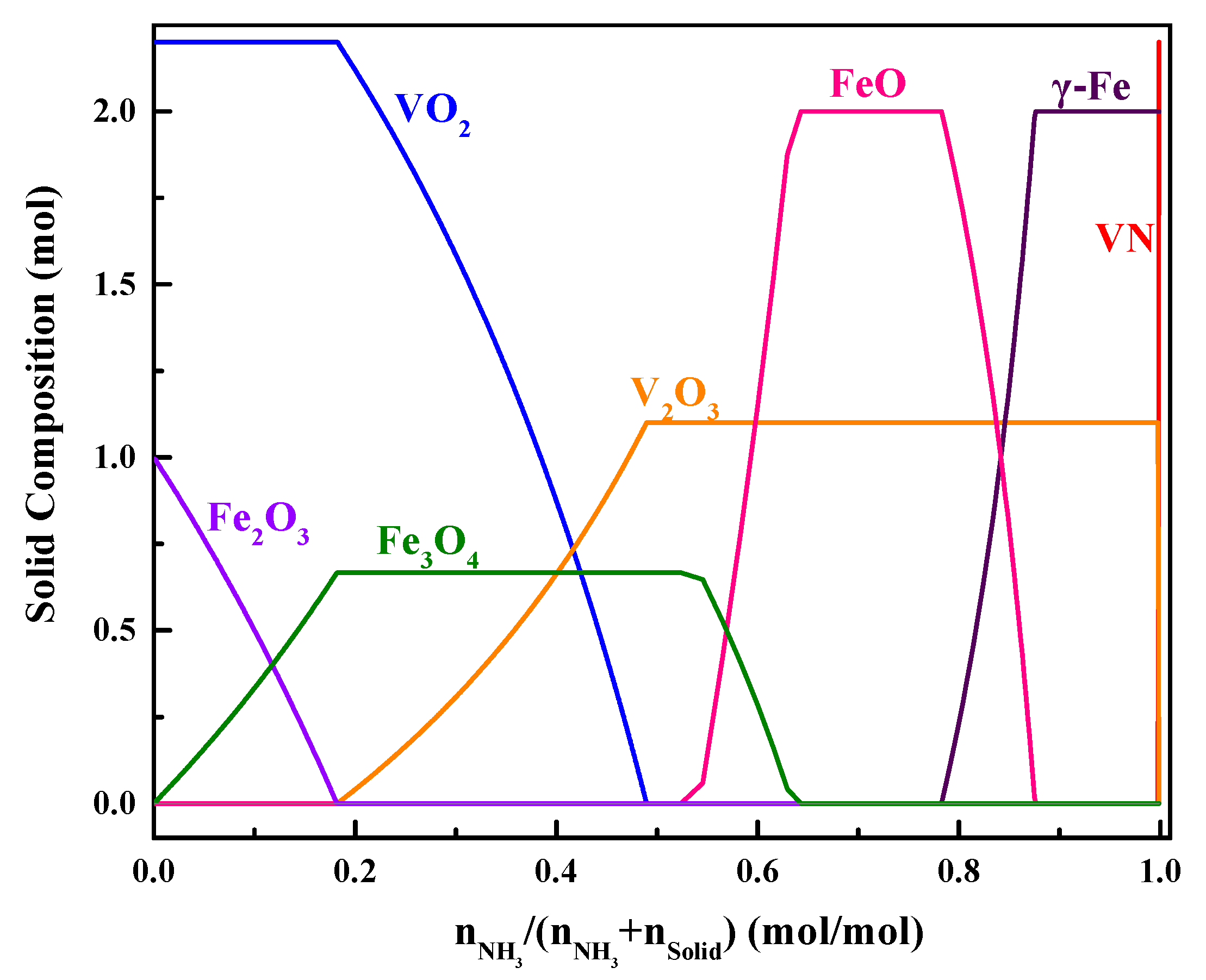
3. Thermodynamic Analysis
4. Results and Discussion
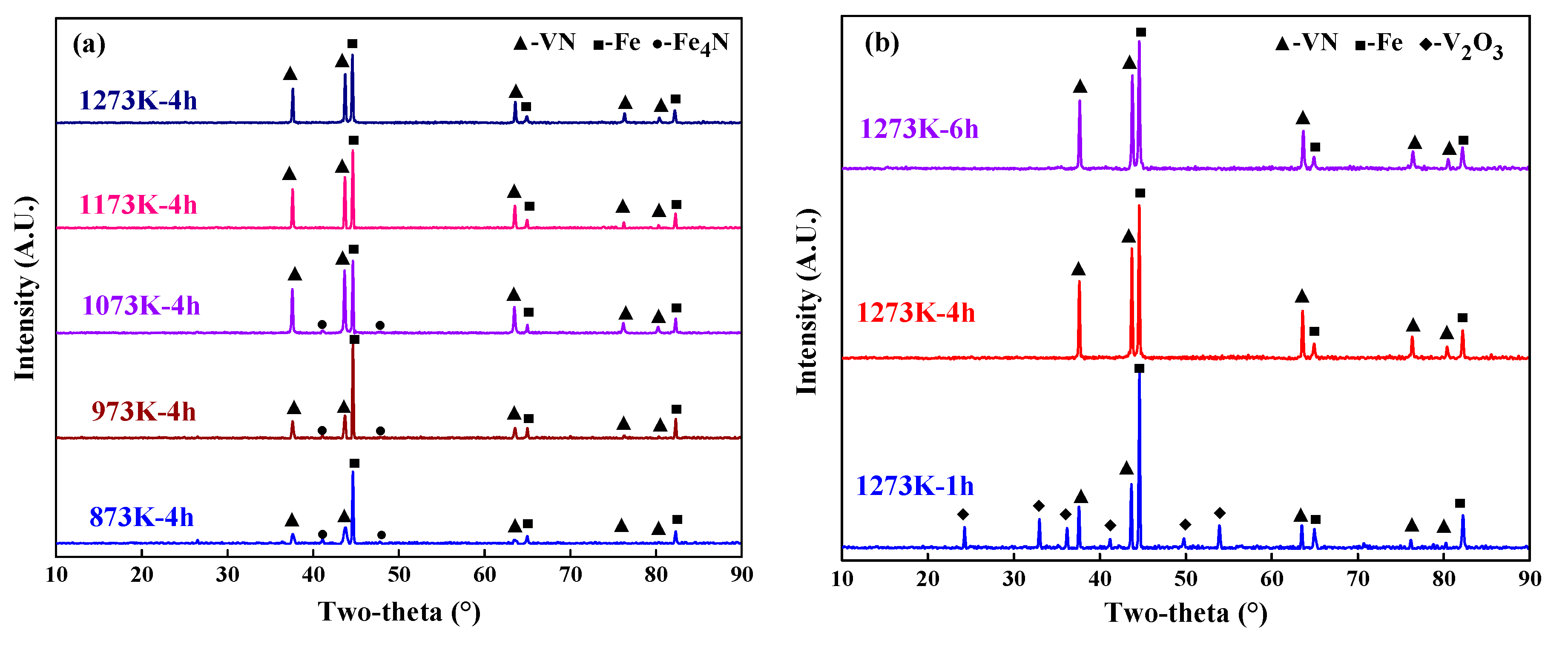
4.1. Phase Evolution during Reduction with Ammonia
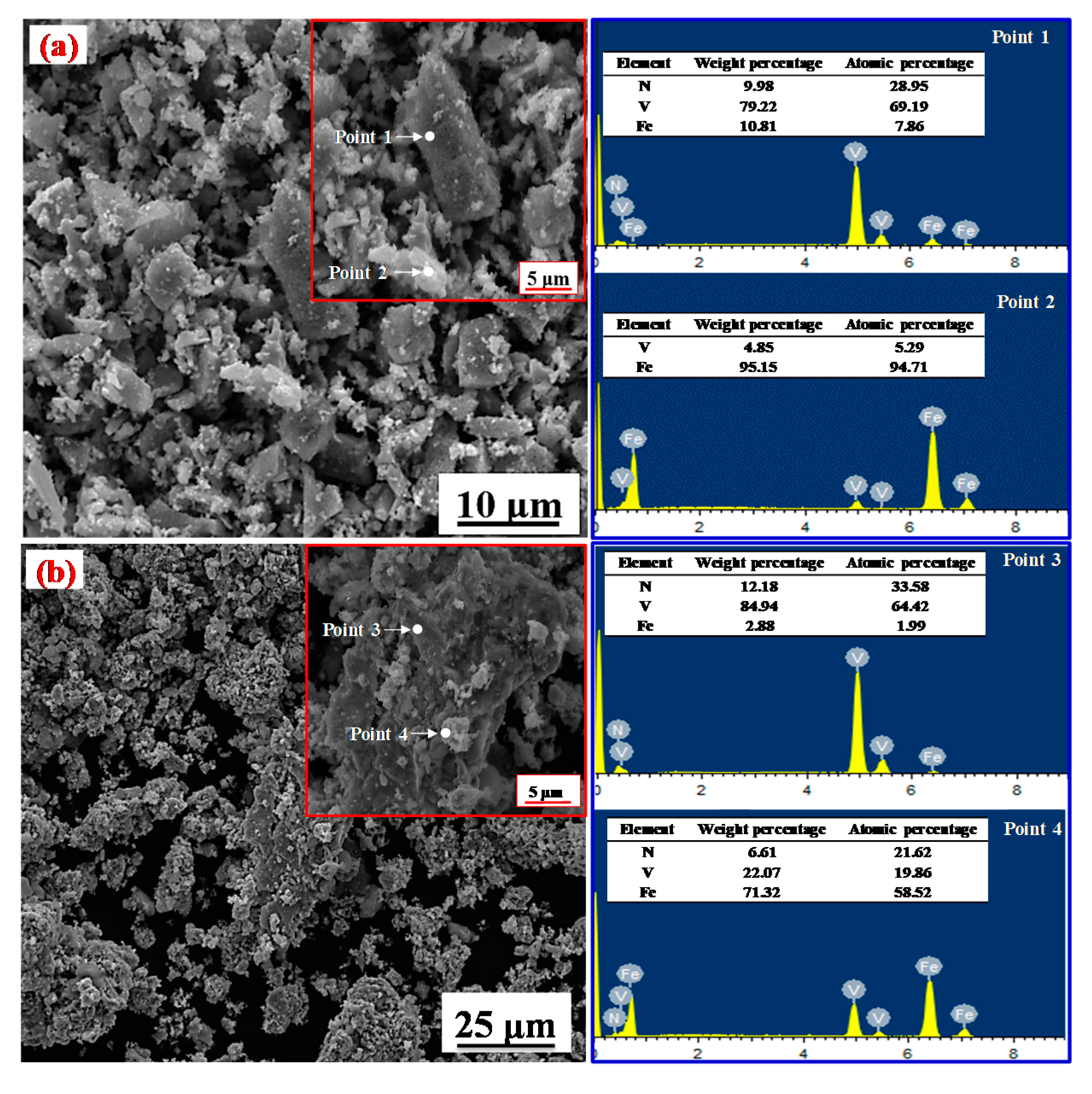
4.2. Morphological Analysis
4.3. Characterization of the Products
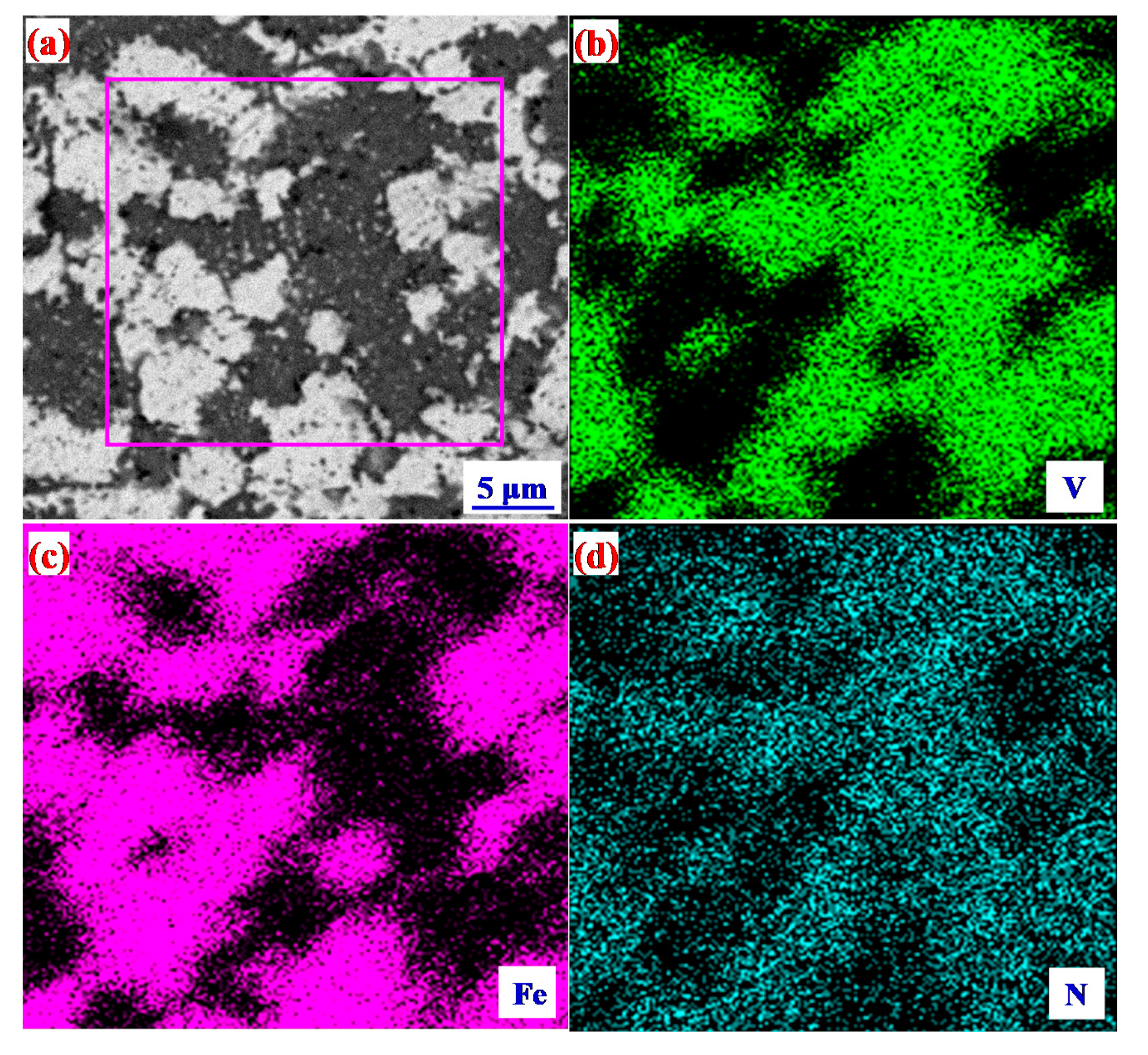
4.4. Microstructure of the Alloy after Sintering
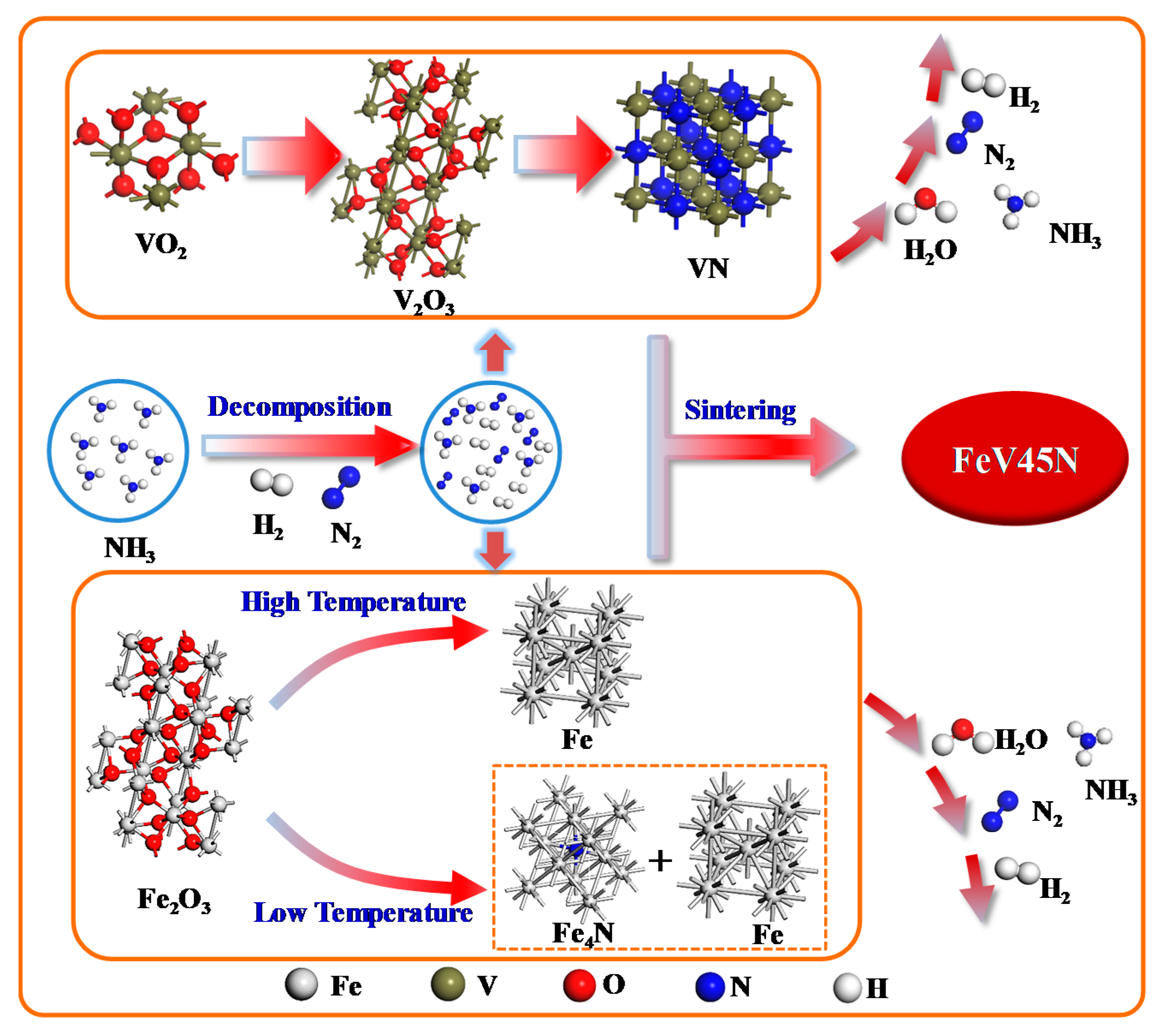
4.5. Reaction Mechanism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baker, T.N. Processes, microstructure and properties of vanadium microalloyed steels. J. Mater. Sci. Technol. 2009, 25, 1083–1107. [Google Scholar] [CrossRef]
- Vervynckt, S.; Verbeken, K.; Lopez, B.; Jonas, J.J. Modern HSLA steels and role of non–recrystallisation temperature. Int. Mater. Rev. 2012, 57, 187–207. [Google Scholar] [CrossRef]
- Baker, T.N. Microalloyed steels. Ironmaking Steelmaking 2016, 43, 264–307. [Google Scholar] [CrossRef]
- Vdovin, K.; Pesin, A.; Feoktistov, N.; Gorlenko, D. Surface wear in hadfield steel castings DOPED with nitrided vanadium. Metals 2018, 8, 845. [Google Scholar] [CrossRef]
- Li, H.Z.; Tong, W.P.; Cui, J.J.; Zhang, H.; Chen, L.Q.; Zuo, L. The influence of deep cryogenic treatment on the properties of high-vanadium alloy steel. Mater. Sci. Eng. A 2016, 662, 356–362. [Google Scholar] [CrossRef]
- MacKenzie, M.; Craven, A.J.; Collins, C.L. Nanoanalysis of very fine VN precipitates in steel. Scr. Mater. 2006, 54, 1–5. [Google Scholar] [CrossRef]
- Crooks, M.J.; Garratt, R.A.J.; Vander, S.J.B.; Owen, W.S. The isothermal austenite–ferrite transformation in some deformed vanadium steels. Metall. Trans. A 1982, 13, 1347–1353. [Google Scholar] [CrossRef]
- Yücel, O.; Cinar, F.; Addemir, O.; Tekin, A. The preparation of ferroboron and ferrovanadium by aluminothermic reduction. High Temp. Mater. Processes (London) 1996, 15, 103–110. [Google Scholar]
- Han, J.L.; Zhang, Y.M.; Liu, T.; Huang, J.; Xue, N.N.; Hu, P.C. Preparation of vanadium nitride using a thermally processed precursor with coating structure. Metals 2017, 7, 360. [Google Scholar] [CrossRef]
- Zajac, S.; Siwecki, T.; Hutchinson, W.B.; Lagneborg, R. Strengthening mechanisms in vanadium microalloyed steels intended for long products. ISIJ Int. 1998, 38, 1130–1139. [Google Scholar] [CrossRef]
- Cai, Z.; Mao, X.P.; Bao, S.Q.; Zhao, G.; Xu, Y.W. Influence of vanadium microalloying on deformation-induced pearlite transformation of eutectoid steel. Metals 2019, 9, 268. [Google Scholar] [CrossRef]
- Grishchenko, S.G.; Matvienko, V.A.; Sarankin, V.A. Development and introduction of the production of nitrided ferrovanadium at zaporozhe ferroalloy plant. Stal 1982, 7, 42–44. [Google Scholar]
- Franke, H.; Breuer, F.; Fuchs, A. Nitrogen-bearing sintering products and melted alloys. NEUE HUTTE 1966, 11, 604–606. [Google Scholar]
- Krastev, D. Nitriding of ferroalloys. In Proceedings of the VI-th International Metallurgical Congress, Ohrid, Macedonia, 29 May–1 June 2014. [Google Scholar]
- Liu, W.; Dong, K.; Zhu, R. Preparation of Nitrogenous Ferrovanadium by Gaseous Nitriding in the Liquid Phase Ferrovanadium. Proceeding of the 5th International Symposium on High–Temperature Metallurgical Processing, San Diego, CA, USA, 3 March 2014; pp. 185–192. [Google Scholar]
- Ziatdinov, M.K.; Sha tokhin, I.M. Self–propagating high–temperature synthesis of ferrovanadium nitride for use in smelting high–strength low–alloy steels. Steel Transl. 2009, 39, 1005–1011. [Google Scholar] [CrossRef]
- Pomarin, Y.M.; Grigorenko, G.M.; Lakomskiy, V.I. Solubility of nitrogen in iron alloys with vanadium and niobium. Russ. Metall. 1975, 5, 61–65. [Google Scholar]
- Cheng, J.; Shen, H. Research on Nitrogen Solubility of Fe–Cr–Mn–V–N System Alloys in Liquid and Solid Phases. Trans. Indian Inst. Met. 2018, 71, 2433–2442. [Google Scholar] [CrossRef]
- Wu, Y.D.; Zhang, G.H.; Chou, K.C. A novel process to synthesize high-quality ferrovanadium nitride. Metall. Mater. Trans. B 2016, 47, 3405–3412. [Google Scholar] [CrossRef]
- Wu, Y.D.; Zhang, G.H.; Chou, K.C. Synthesis of high–quality FeV55N alloy by carbonitrothermic reduction of vanadium pentoxide–rerric oxide mixture. JOM 2017, 69, 676–1681. [Google Scholar] [CrossRef]
- Claridge, J.B.; York, A.P.E.; Brungs, A.J.; Green, M.L.H. Study of the temperature–programmed reaction synthesis of early transition metal carbide and nitride catalyst materials from oxide precursors. Chem. Mater. 2000, 12, 132–142. [Google Scholar] [CrossRef]
- Glushenkov, A.M.; Jurcakova, D.H.; Llewellyn, D.; Lu, G.Q.; Chen, Y. Structure and capacitive properties of porous nanocrystalline VN prepared by temperature–programmed ammonia reduction of V2O5. Chem. Mater. 2009, 22, 914–921. [Google Scholar] [CrossRef]
- Dong, S.M.; Chen, X.; Gu, L.; Zhou, X.H.; Wang, H.B.; Liu, Z.H.; Han, P.X.; Yao, J.H.; Wang, L.; Cui, G.L.; et al. TiN/VN composites with core/shell structure for supercapacitors. Mater. Res. Bull. 2011, 46, 835–839. [Google Scholar] [CrossRef]
- Colling, C.W.; Choi, J.G.; Thompson, L.T. Molybdenum nitride catalysts: II. H2 temperature programmed reduction and NH3 temperature programmed desorption. J. Catal. 1996, 160, 35–42. [Google Scholar] [CrossRef]
- Galesic, I.; Reusch, U.; Angelkort, C.; Lewalter, H.; Berendes, A.; Schweda, E.; Kolbesen, B.O. Nitridation of vanadium in molecular nitrogen: a comparison of rapid thermal processing (RTP) and conventional furnace annealing. Vacuum 2001, 61, 479–484. [Google Scholar] [CrossRef]
- Wexler, D.; Calka, A.; Mosbah, A.Y. Ti–TiN hard metals prepared by in situ formation of TiN during reactive ball milling of Ti in ammonia. J. Alloy. Compd. 2000, 309, 201–207. [Google Scholar] [CrossRef]
- Chen, H.Y.; Nambu, A.; Wen, W.; Graciani, J.; Zhong, Z.; Hanson, J.C.; Fujita, E.; Rodriguez, J.A. Reaction of NH3 with titania: N–doping of the oxide and TiN formation. J. Phys. Chem. C 2007, 111, 1366–1372. [Google Scholar] [CrossRef]
- Li, Y.G.; Gao, L.; Li, J.G.; Yan, D.S. Synthesis of nanocrystalline chromium nitride powders by direct nitridation of chromium oxide. J. Am. Ceram. Soc. 2002, 85, 1294–1296. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, Y.; Zhang, Y.; You, Z.X.; Lv, X.W. Mechanism on Reduction and Nitridation of Micron–Sized Titania with Ammonia Gas. J. Am. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Rao, K.J. Synthesis of Ti, Ga, and V nitrides: Microwave-assisted carbothermal reduction and nitridation. Chem. Mater. 1997, 9, 1196–1200. [Google Scholar] [CrossRef]
- Wu, Y.D.; Zhang, G.H.; Chou, K.C. Preparation of high-quality FeV55N using ammonia as a reductant and nitrogen source. JOM 2018, 70, 2493–2498. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances; Springer: Berlin, Germany, 1977. [Google Scholar]
- Stull, D.R.; Prophet, H. JANAF Thermochemical Tables; U.S. Department of Commerce: Washington, DC, USA, 1985.
- Arabczyk, W.; Rafat, P. Studies of the kinetics of two parallel reactions: ammonia decomposition and nitriding of iron catalyst. J. Phys. Chem. A 2008, 113, 411–416. [Google Scholar] [CrossRef]
- Qin, M.L.; Wu, H.Y.; Cao, Z.Q.; Zhang, D.Y.; Qu, X.H. A novel method to synthesize vanadium nitride nanopowders by ammonia reduction from combustion precursors. J. Alloy. Compd. 2019, 772, 808–813. [Google Scholar] [CrossRef]

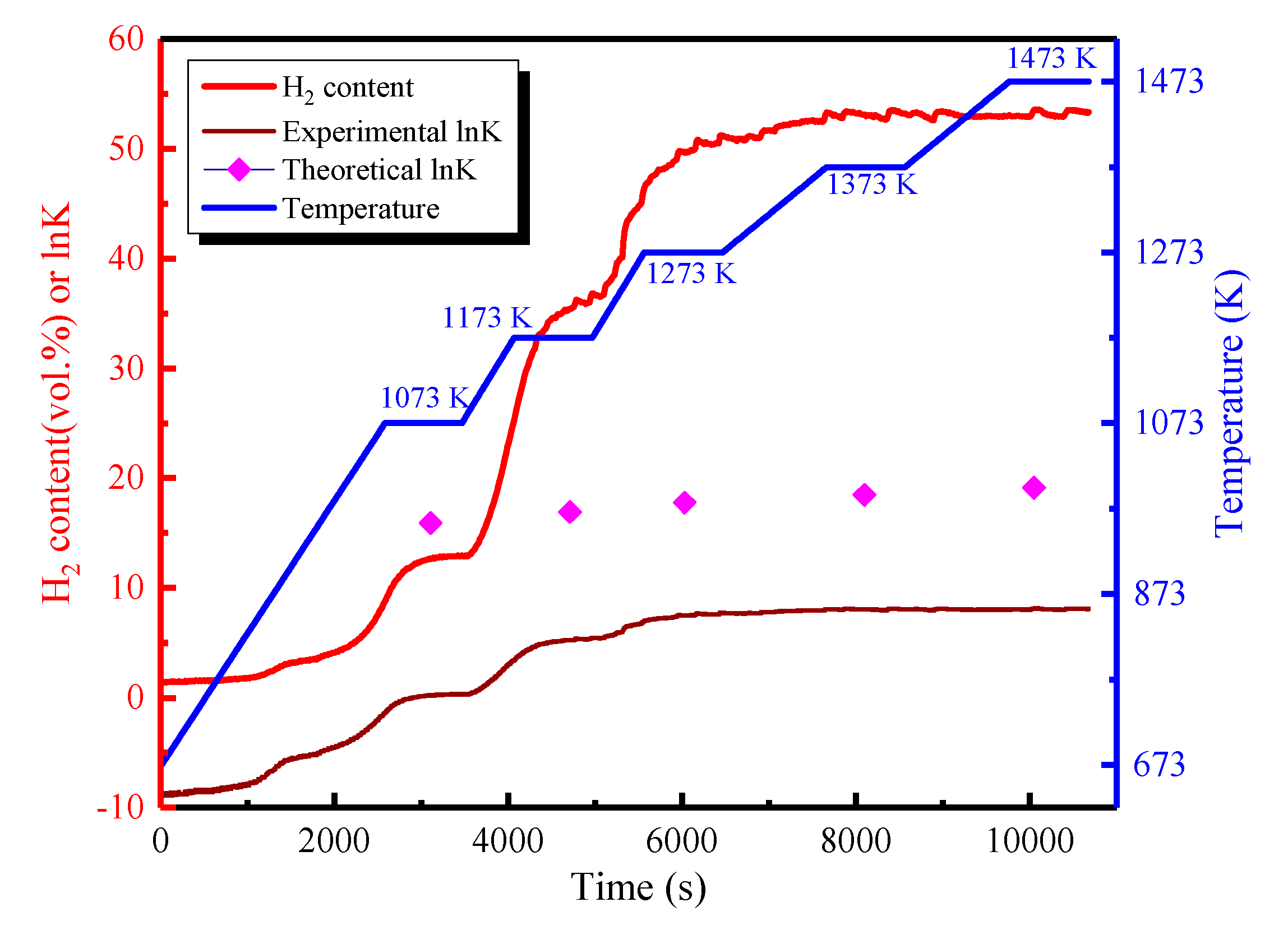
| Temperature (K) | Reaction time (h) | O content (wt. %) | N content (wt. %) |
|---|---|---|---|
| 1073 | 6 | 1.16 | 11.74 |
| 1173 | 4 | 1.11 | 11.75 |
| 1273 | 4 | 0.74 | 11.79 |
| 1273 | 6 | 0.25 | 11.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; You, Z.; Lv, X. Reduction and Nitridation of Iron/Vanadium Oxides by Ammonia Gas: Mechanism and Preparation of FeV45N Alloy. Metals 2020, 10, 356. https://doi.org/10.3390/met10030356
Liu Y, Wang Y, You Z, Lv X. Reduction and Nitridation of Iron/Vanadium Oxides by Ammonia Gas: Mechanism and Preparation of FeV45N Alloy. Metals. 2020; 10(3):356. https://doi.org/10.3390/met10030356
Chicago/Turabian StyleLiu, Yongjie, Yue Wang, Zhixiong You, and Xuewei Lv. 2020. "Reduction and Nitridation of Iron/Vanadium Oxides by Ammonia Gas: Mechanism and Preparation of FeV45N Alloy" Metals 10, no. 3: 356. https://doi.org/10.3390/met10030356
APA StyleLiu, Y., Wang, Y., You, Z., & Lv, X. (2020). Reduction and Nitridation of Iron/Vanadium Oxides by Ammonia Gas: Mechanism and Preparation of FeV45N Alloy. Metals, 10(3), 356. https://doi.org/10.3390/met10030356





