Tantalum Recycling by Solvent Extraction: Chloride Is Better than Fluoride
Abstract
1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Preparation of TaCl5 and TaF5 Solutions
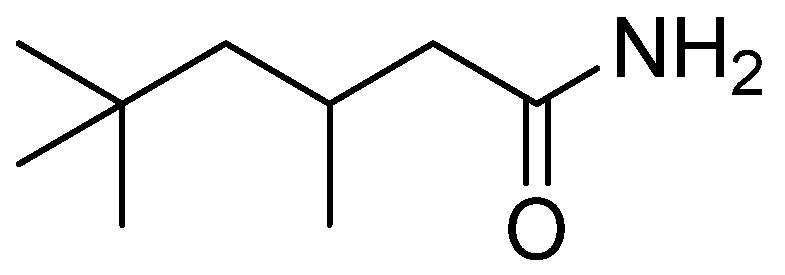
2.3. General Solvent Extraction Procedure
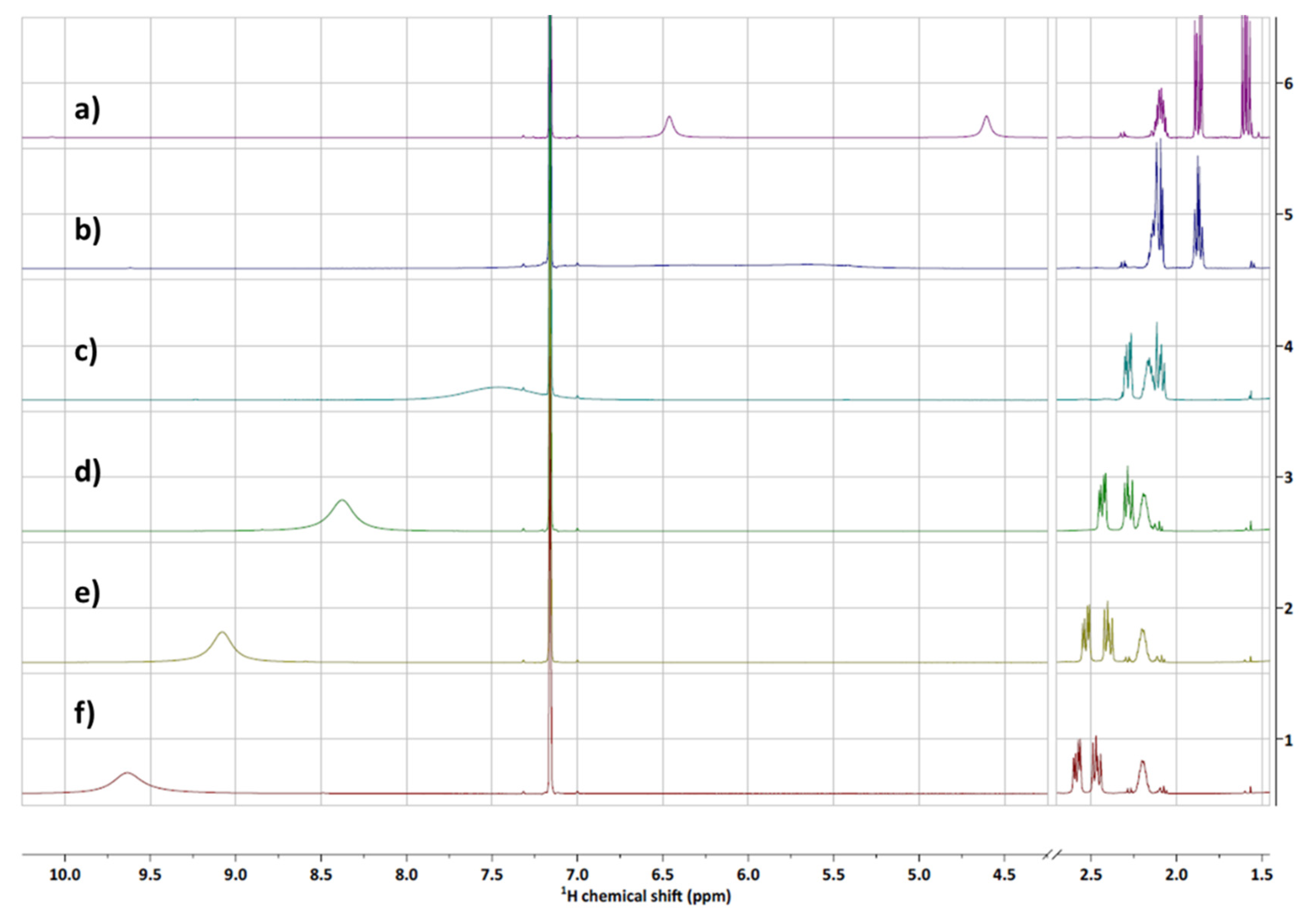
2.4. NMR Data for [H(C11H23NO)2]2[SnCl6].
3. Results and Discussion
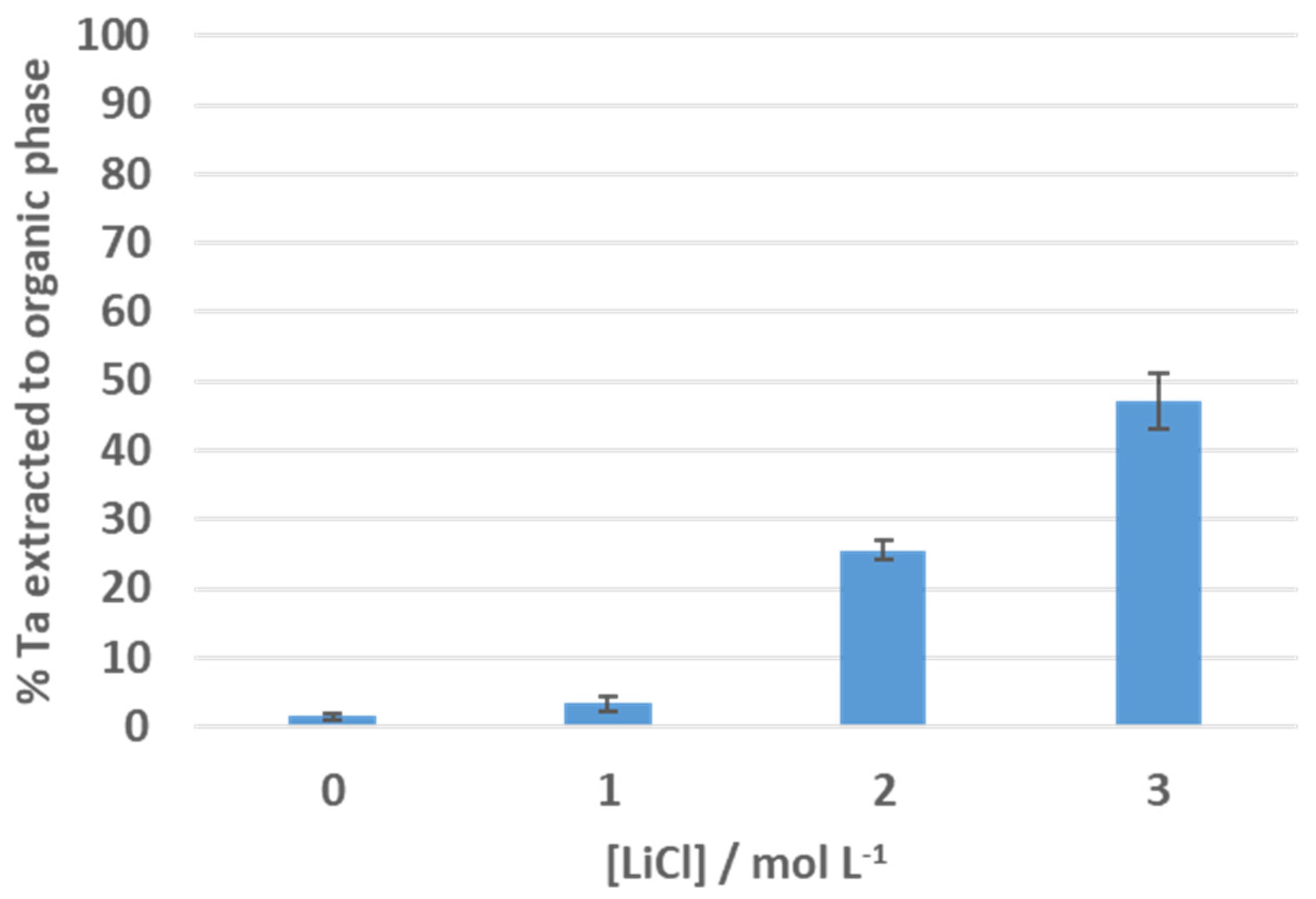
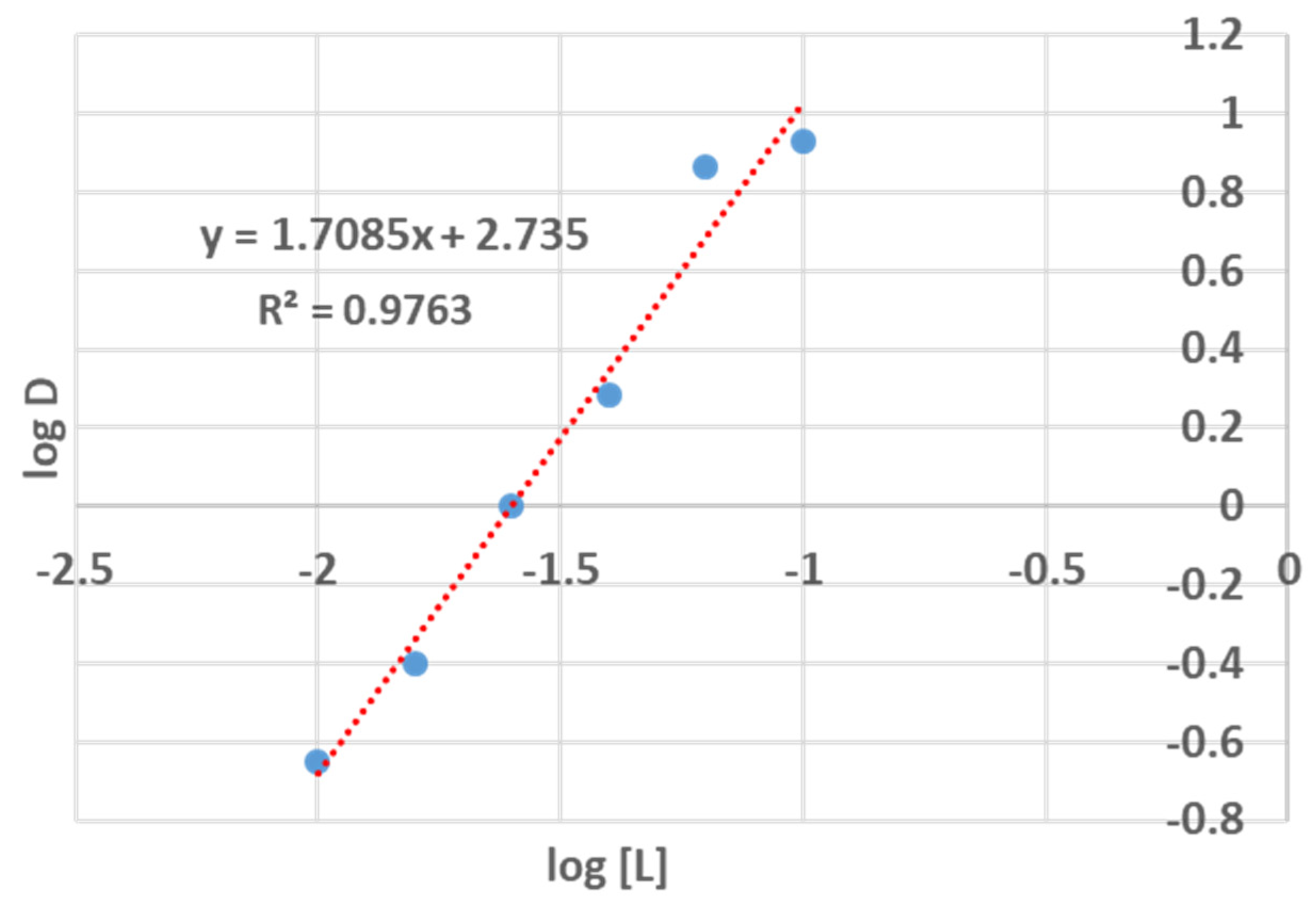
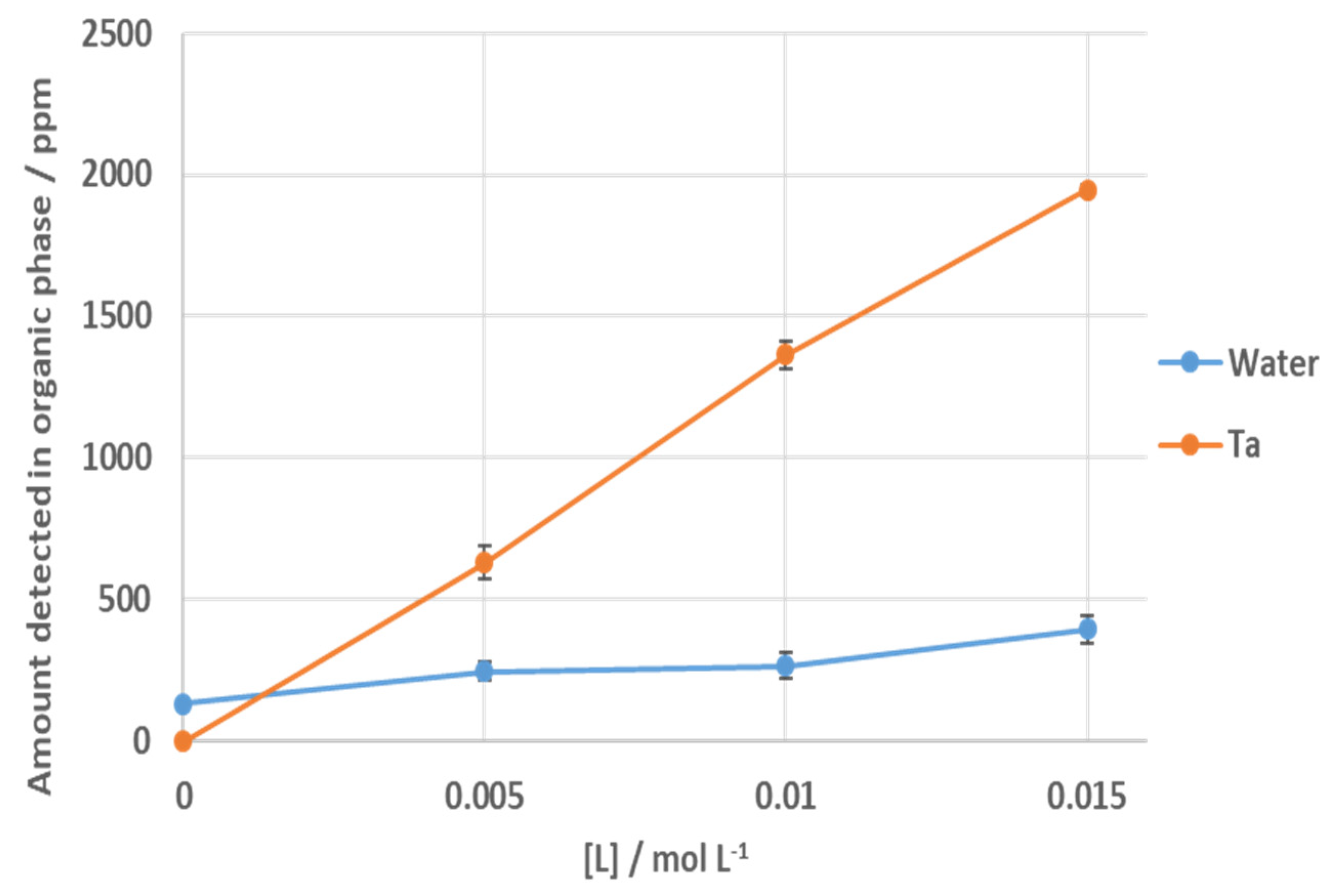
3.1. Solvent Extraction of Tantalum Pentachloride
3.2. Structure Elucidation
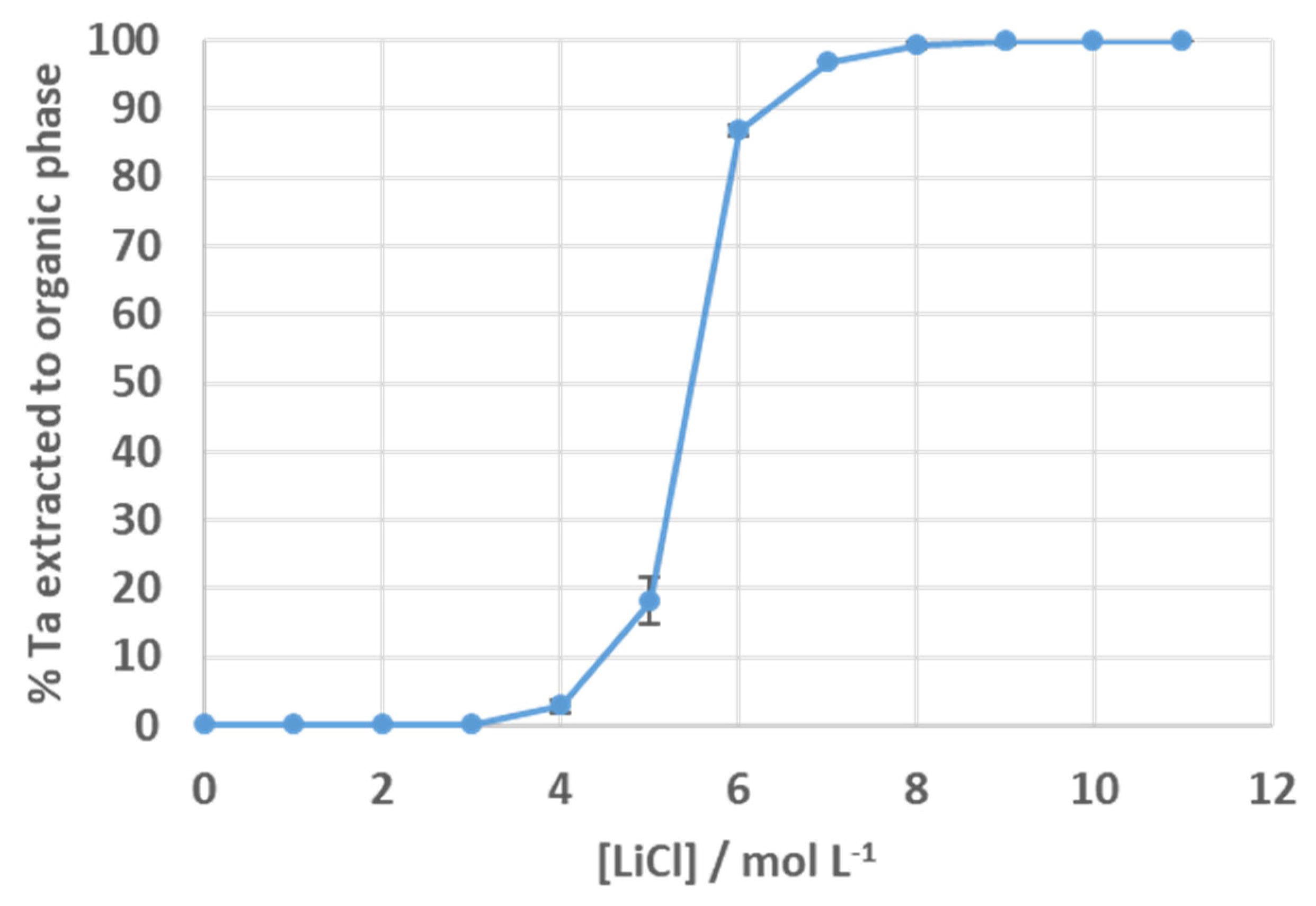
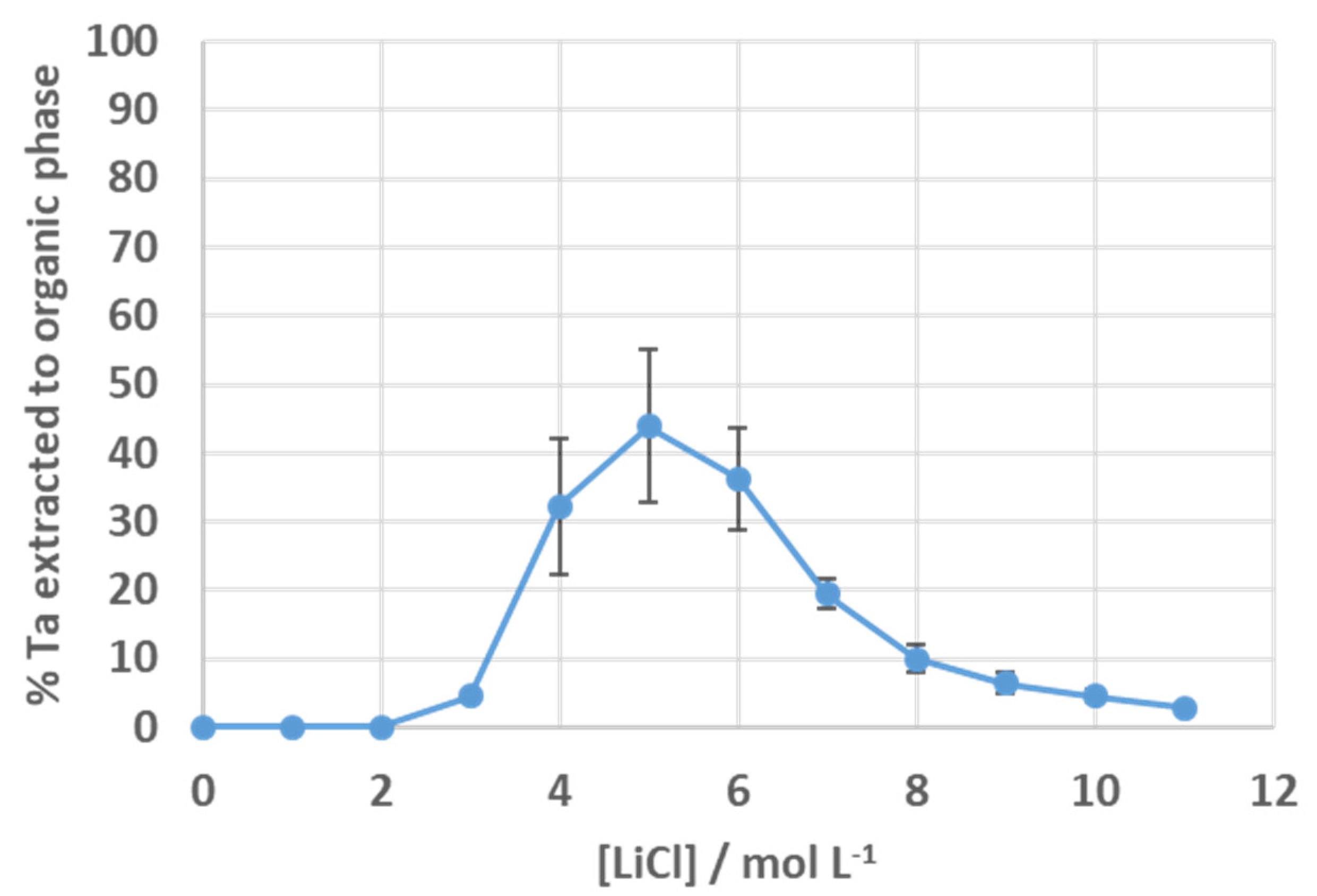
3.3. Solvent Extraction of Tantalum Fluoride
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Commission: Critical raw materials fact sheets. Available online: https://ec.europa.eu/info/index_en (accessed on 13 January 2020). [CrossRef]
- Ogunseitan, O.A.; Schoenung, J.M.; Saphores, J.D.M.; Shapiro, A.A. The electronics revolution: From E-wonderland to E-wasteland. Science 2009, 326, 670–671. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef]
- Zeng, X.; Mathews, J.A.; Li, J. Urban mining of E-waste is becoming more cost-effective than virgin mining. Environ. Sci. Technol. 2018, 52, 4835–4841. [Google Scholar] [CrossRef]
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-waste Monitor-2017, 2017. United Nations University (UNU), International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Vienna. Available online: https://www.itu.int/en/ITU-D/Climate-Change/Documents/GEM%202017/Global-E-waste%20Monitor%202017%20-%20Executive%20Summary.pdf (accessed on 13 January 2020).
- Velenturf, A.P.M.; Jopson, J.S. Making the business case for resource recovery. Sci. Total Environ. 2019, 648, 1031–1041. [Google Scholar] [CrossRef]
- Young, S.B. Responsible sourcing of metals: certification approaches for conflict minerals and conflict-free metals. Int. J. Life Cycle Assess. 2018, 23, 1429–1447. [Google Scholar] [CrossRef]
- Nete, M.; Purcell, W.; Nel, J.T. Hydrometallurgical separation of niobium and tantalum: A fundamental approach. JOM 2016, 68, 556–566. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, C.Y. Solvent extraction technology for the separation and purification of niobium and tantalum: A review. Hydrometallurgy 2011, 107, 1–12. [Google Scholar] [CrossRef]
- Kabangu, M.J.; Crouse, P.L. Separation of niobium and tantalum from Mozambican tantalite by ammonium bifluoride digestion and octanol solvent extraction. Hydrometallurgy 2012, 129–130, 151–155. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A review on the separation of niobium and tantalum by solvent extraction. Miner. Process. Extr. Metall. Rev. 2019, 40, 265–277. [Google Scholar] [CrossRef]
- Nelson, J.J.M.; Schelter, E.J. Sustainable inorganic chemistry: metal separations for recycling. Inorg. Chem. 2019, 58, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Chowdhury, R.; Ghosh, S.K. Sustainability of metal recovery from E-waste. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Love, J.B.; Miguirditchian, M.; Chagnes, A. New insights into the recovery of strategic and critical metals by solvent extraction: The effects of chemistry and the process on performance. In Ion Exchange and Solvent Extraction: Changing the Landscape in Solvent Extraction; Moyer, B.A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; Volume 23, pp. 1–44. ISBN 9781315114378. [Google Scholar]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.P.; Bengio, D.; Beltrami, D.; Bélair, S.; Cote, G.; Chagnes, A. A fluoride-free liquid-liquid extraction process for the recovery and separation of niobium and tantalum from alkaline leach solutions. Sep. Purif. Technol. 2019, 215, 634–643. [Google Scholar] [CrossRef]
- Deblonde, G.J.P.; Weigel, V.; Bellier, Q.; Houdard, R.; Delvallée, F.; Bélair, S.; Beltrami, D. Selective recovery of niobium and tantalum from low-grade concentrates using a simple and fluoride-free process. Sep. Purif. Technol. 2016, 162, 180–187. [Google Scholar] [CrossRef]
- Deblonde, G.J.P.; Chagnes, A.; Bélair, S.; Cote, G. Solubility of niobium(V) and tantalum(V) under mild alkaline conditions. Hydrometallurgy 2015, 156, 99–106. [Google Scholar] [CrossRef]
- Turgis, R.; Arrachart, G.; Michel, S.; Legeai, S.; Lejeune, M.; Draye, M.; Pellet-Rostaing, S. Ketone functionalized task specific ionic liquids for selective tantalum extraction. Sep. Purif. Technol. 2018, 196, 174–182. [Google Scholar] [CrossRef]
- Ungerer, M.J.; Van Der Westhuizen, D.J.; Lachmann, G.; Krieg, H.M. Comparison of extractants for the separation of TaF5 and NbF5 in different acidic media. Hydrometallurgy 2014, 144–145, 195–206. [Google Scholar] [CrossRef]
- Ungerer, M.J.; van Sittert, C.G.C.E.; van der Westhuizen, D.J.; Krieg, H.M. Molecular modelling of tantalum penta-halides during hydrolysis and oxidation reactions. Comput. Theor. Chem. 2016, 1090, 112–119. [Google Scholar] [CrossRef]
- Ungerer, M.J.; van Sittert, C.G.C.E.; van der Westhuizen, D.J.; Krieg, H.M. DFT modelling of tantalum pentafluoride extraction with phosphorus-based extractants–A molecular dynamics study. J. Phys. Chem. Solids 2019, 135, 109121. [Google Scholar] [CrossRef]
- Doidge, E.D.; Carson, I.; Tasker, P.A.; Ellis, R.J.; Morrison, C.A.; Love, J.B. A simple primary amide for the selective recovery of gold from secondary resources. Angew. Chem. Int. Ed. 2016, 55, 12436–12439. [Google Scholar] [CrossRef]
- Doidge, E.D.; Kinsman, L.M.M.; Ji, Y.; Carson, I.; Duffy, A.J.; Kordas, I.A.; Shao, E.; Tasker, P.A.; Ngwenya, B.T.; Morrison, C.A.; et al. Evaluation of simple amides in the selective recovery of gold from secondary sources by solvent extraction. ACS Sustain. Chem. Eng. 2019, 7, 15019–15029. [Google Scholar] [CrossRef]
- Nash, K.L. A review of the basic chemistry and recent developments in trivalent f-element separations. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]
- Carson, I.; MacRuary, K.J.; Doidge, E.D.; Ellis, R.J.; Grant, R.A.; Gordon, R.J.; Love, J.B.; Morrison, C.A.; Nichol, G.S.; Tasker, P.A.; et al. Anion receptor design: Exploiting outer-sphere coordination chemistry to obtain high selectivity for chloridometalates over chloride. Inorg. Chem. 2015, 54, 8685–8692. [Google Scholar] [CrossRef]
- Turkington, J.R.; Bailey, P.J.; Love, J.B.; Wilson, A.M.; Tasker, P.A. Exploiting outer-sphere interactions to enhance metal recovery by solvent extraction. Chem. Commun. 2013, 49, 1891–1899. [Google Scholar] [CrossRef]
- Bartalucci, N.; Bortoluzzi, M.; Pampaloni, G.; Pinzino, C.; Zacchini, S.; Marchetti, F. Stable coordination complexes of α-diimines with Nb(V) and Ta(V) halides. Dalton Trans. 2018, 47, 3346. [Google Scholar] [CrossRef]
- Kiyota, Y.; Kadoya, T.; Yamamoto, K.; Iijima, K.; Higashino, T.; Kawamoto, T.; Takimiya, K.; Mori, T. Benzothienobenzothiophene-based molecular conductors: High conductivity, large thermoelectric power factor, and one-dimensional instability. J. Am. Chem. Soc. 2016, 138, 3920–3925. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinsman, L.M.M.; Crevecoeur, R.A.M.; Singh-Morgan, A.; Ngwenya, B.T.; Morrison, C.A.; Love, J.B. Tantalum Recycling by Solvent Extraction: Chloride Is Better than Fluoride. Metals 2020, 10, 346. https://doi.org/10.3390/met10030346
Kinsman LMM, Crevecoeur RAM, Singh-Morgan A, Ngwenya BT, Morrison CA, Love JB. Tantalum Recycling by Solvent Extraction: Chloride Is Better than Fluoride. Metals. 2020; 10(3):346. https://doi.org/10.3390/met10030346
Chicago/Turabian StyleKinsman, Luke M. M., Rosa A. M. Crevecoeur, Amrita Singh-Morgan, Bryne T. Ngwenya, Carole A. Morrison, and Jason B. Love. 2020. "Tantalum Recycling by Solvent Extraction: Chloride Is Better than Fluoride" Metals 10, no. 3: 346. https://doi.org/10.3390/met10030346
APA StyleKinsman, L. M. M., Crevecoeur, R. A. M., Singh-Morgan, A., Ngwenya, B. T., Morrison, C. A., & Love, J. B. (2020). Tantalum Recycling by Solvent Extraction: Chloride Is Better than Fluoride. Metals, 10(3), 346. https://doi.org/10.3390/met10030346





