Recovery of Metal Values from Ni-Cd Cake Waste Residue of an Iranian Zinc Plant by Hydrometallurgical Route
Abstract
1. Introduction
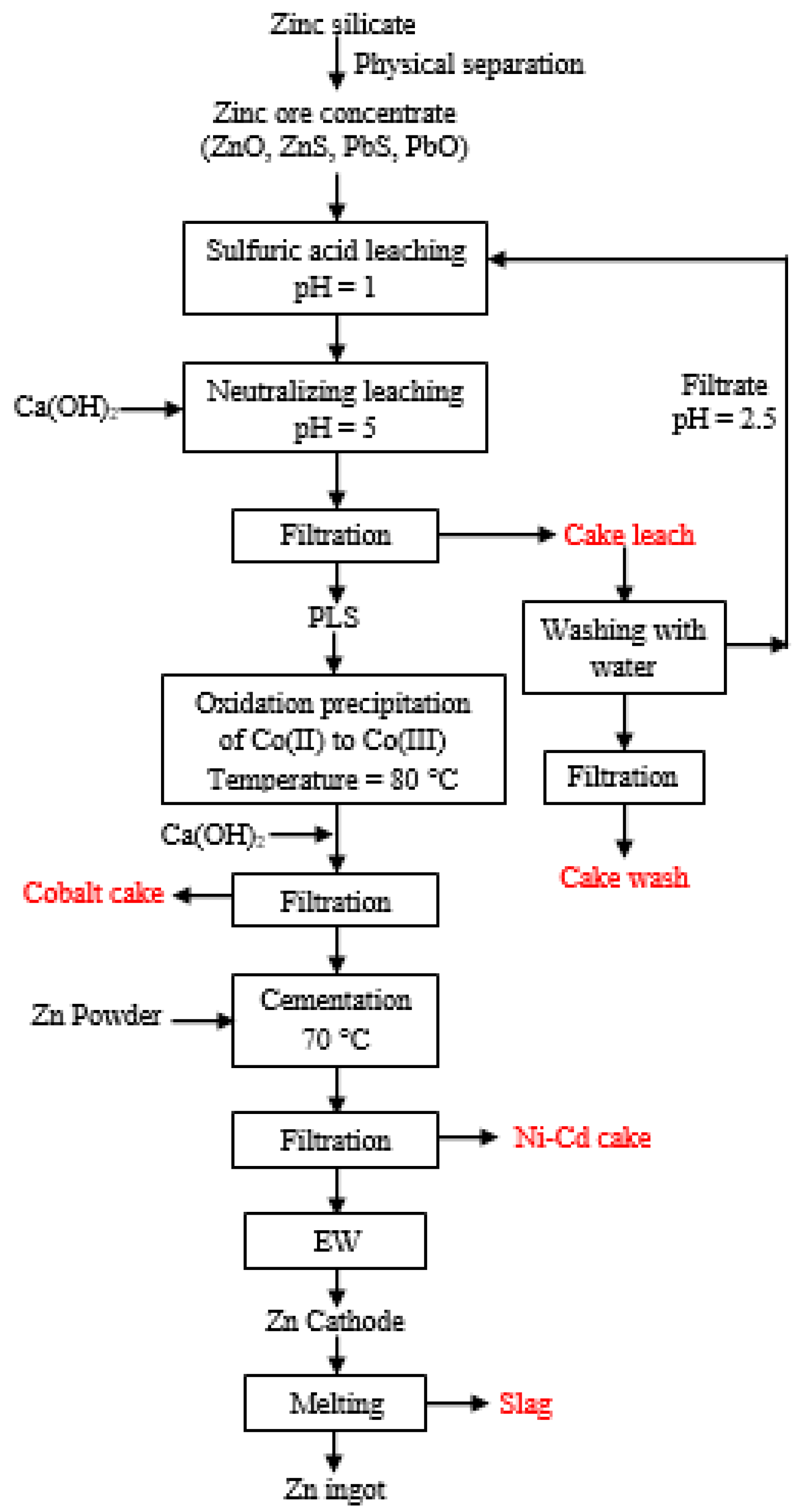
2. Experiments
3. Results and Discussion
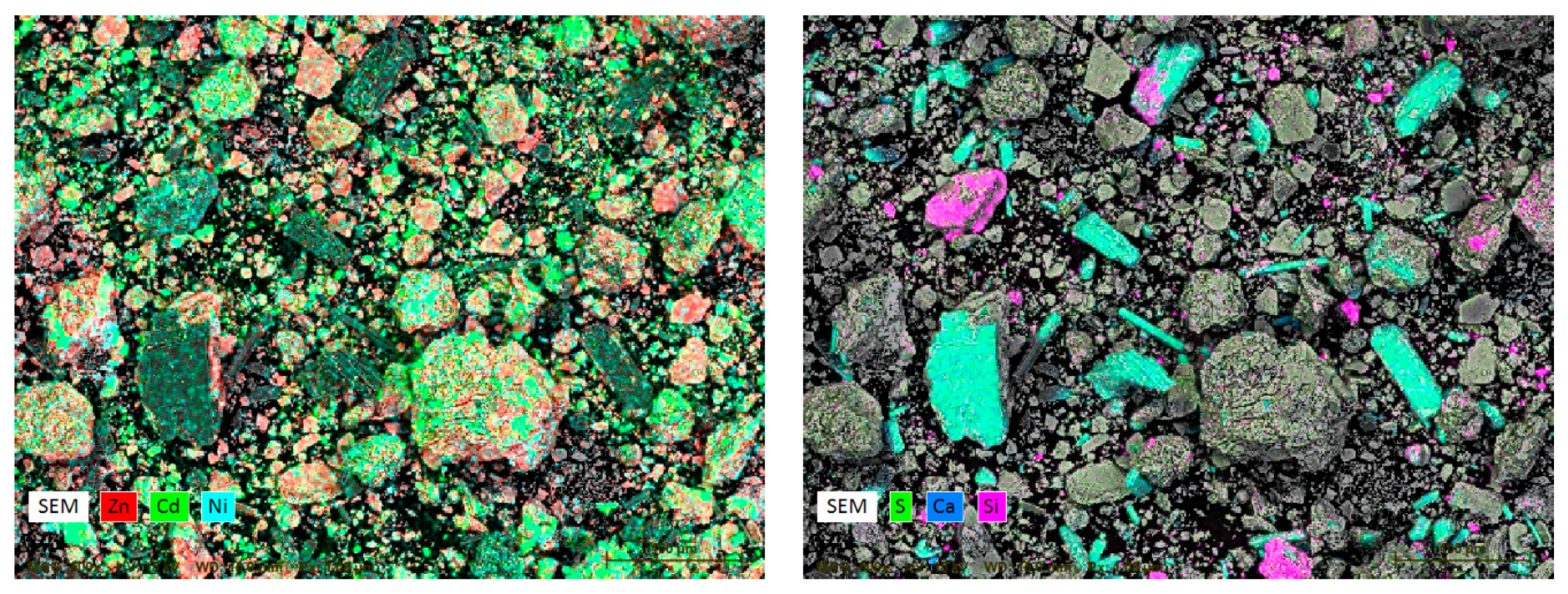
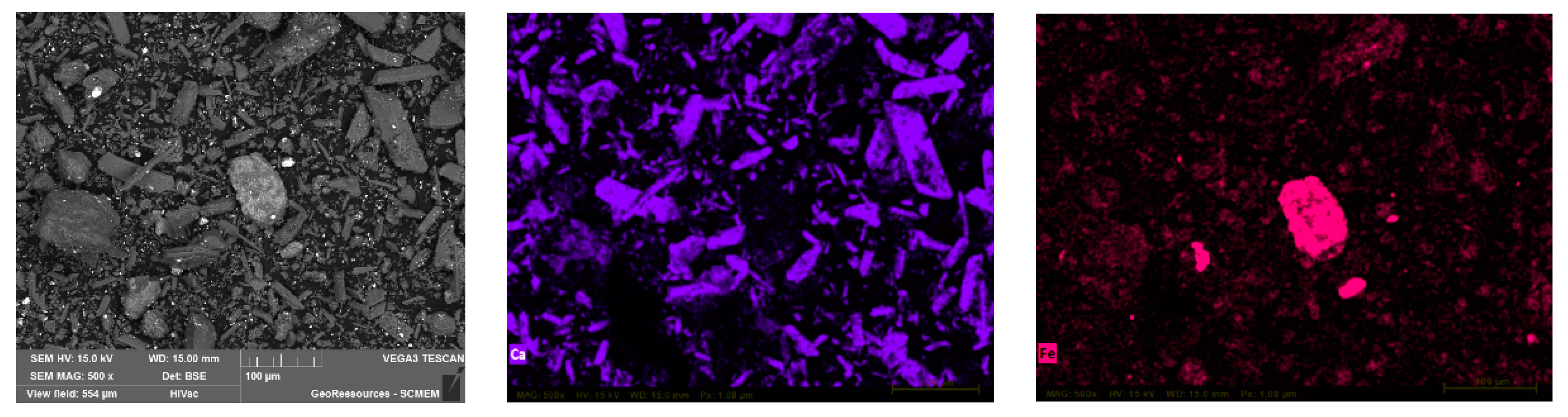
3.1. Characterization of Ni-Cd Cake Waste Residue
3.2. Preliminary Studies on the Recovery of Metal Values from Ni-Cd Cake
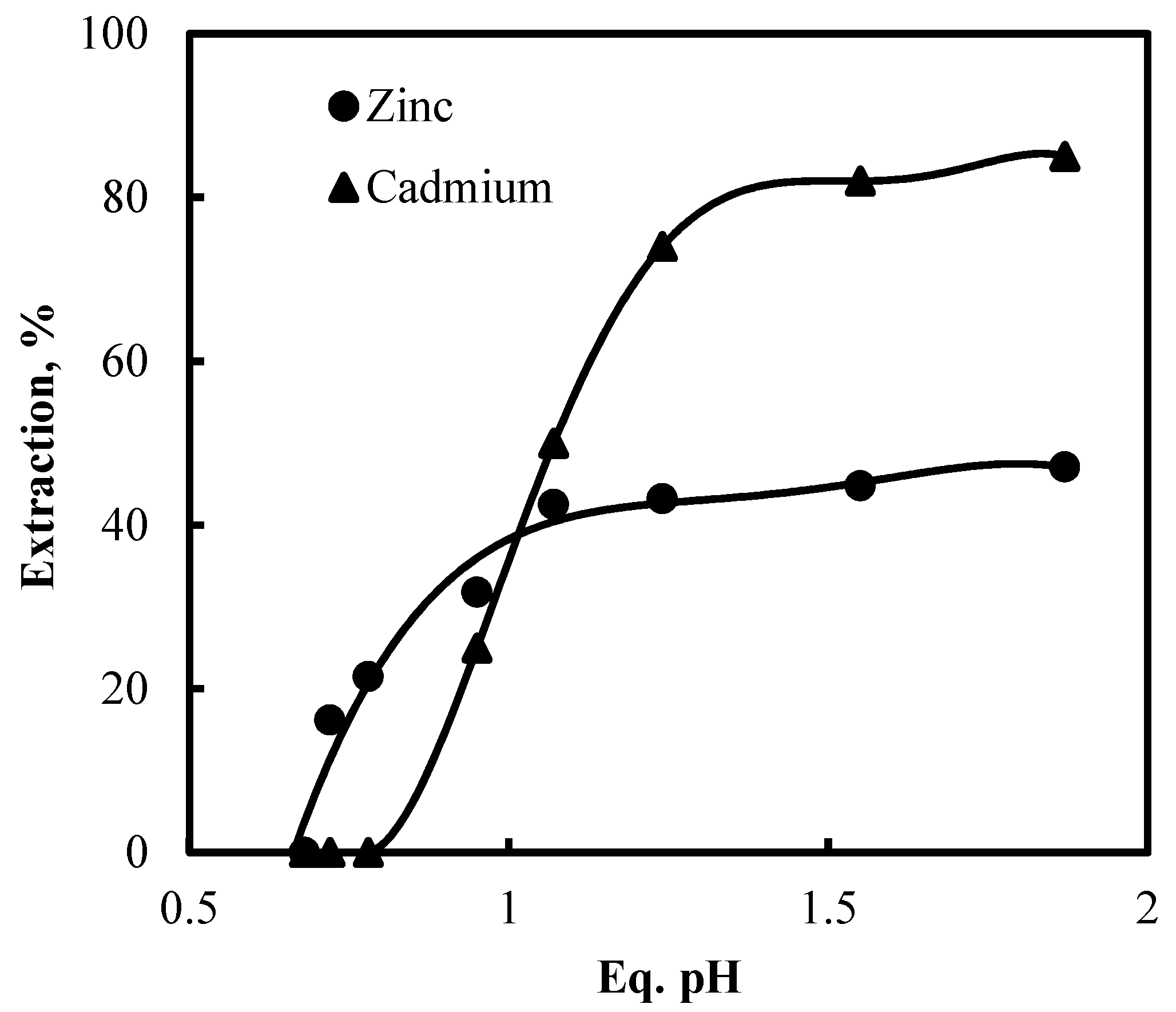
3.2.1. Sulfuric Acid Leaching of Metal Values from Ni-Cd Cake Waste Residue and their Separation by Solvent Extraction
3.2.2. Chloride Leaching of Ni-Cd Cake Waste Residue and Recovery of Metal Values by Solvent Extraction
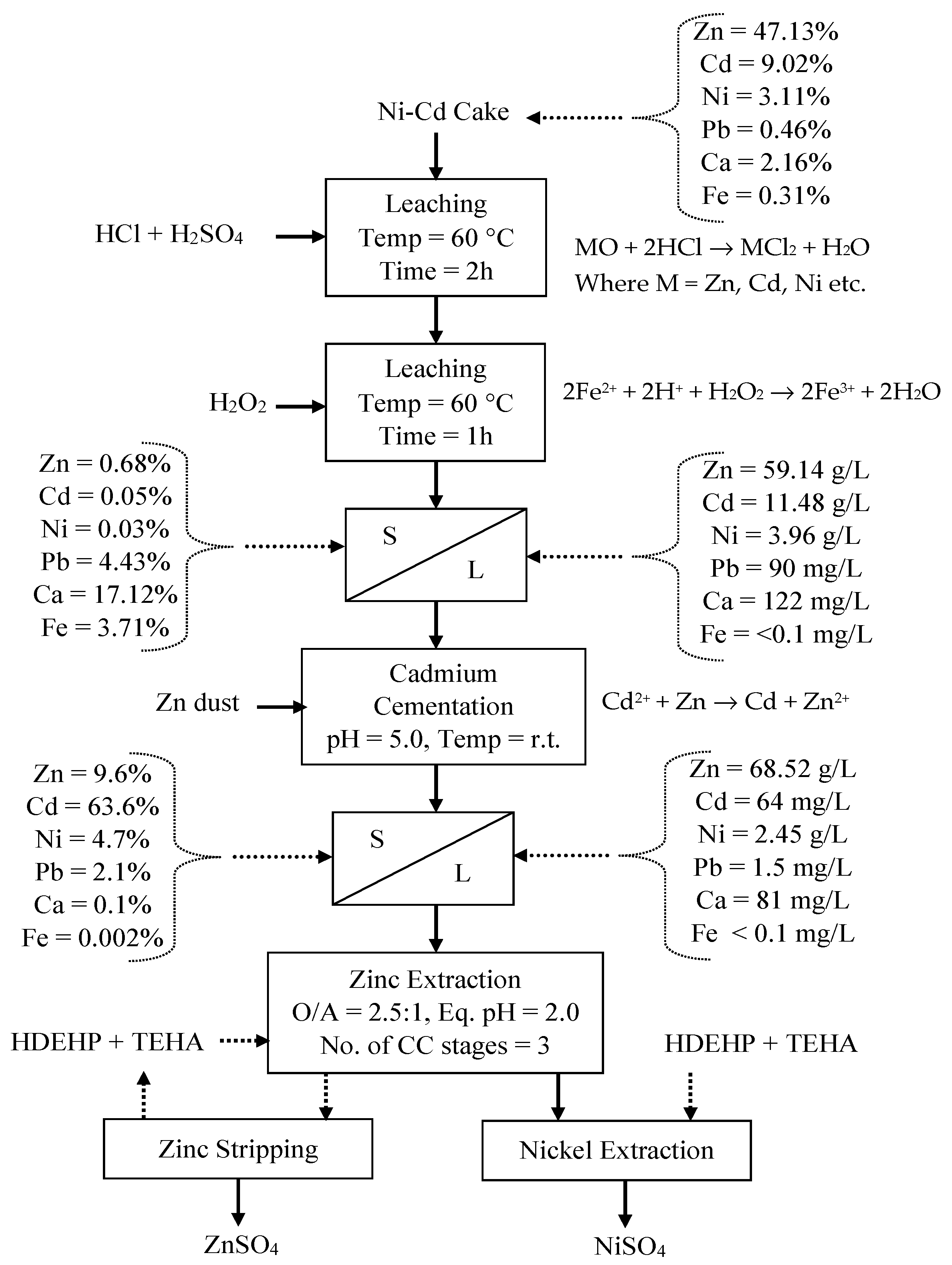
3.3. Optimization of Ni-Cd Cake Waste Residue Leaching
3.4. Purification of Leach Solution
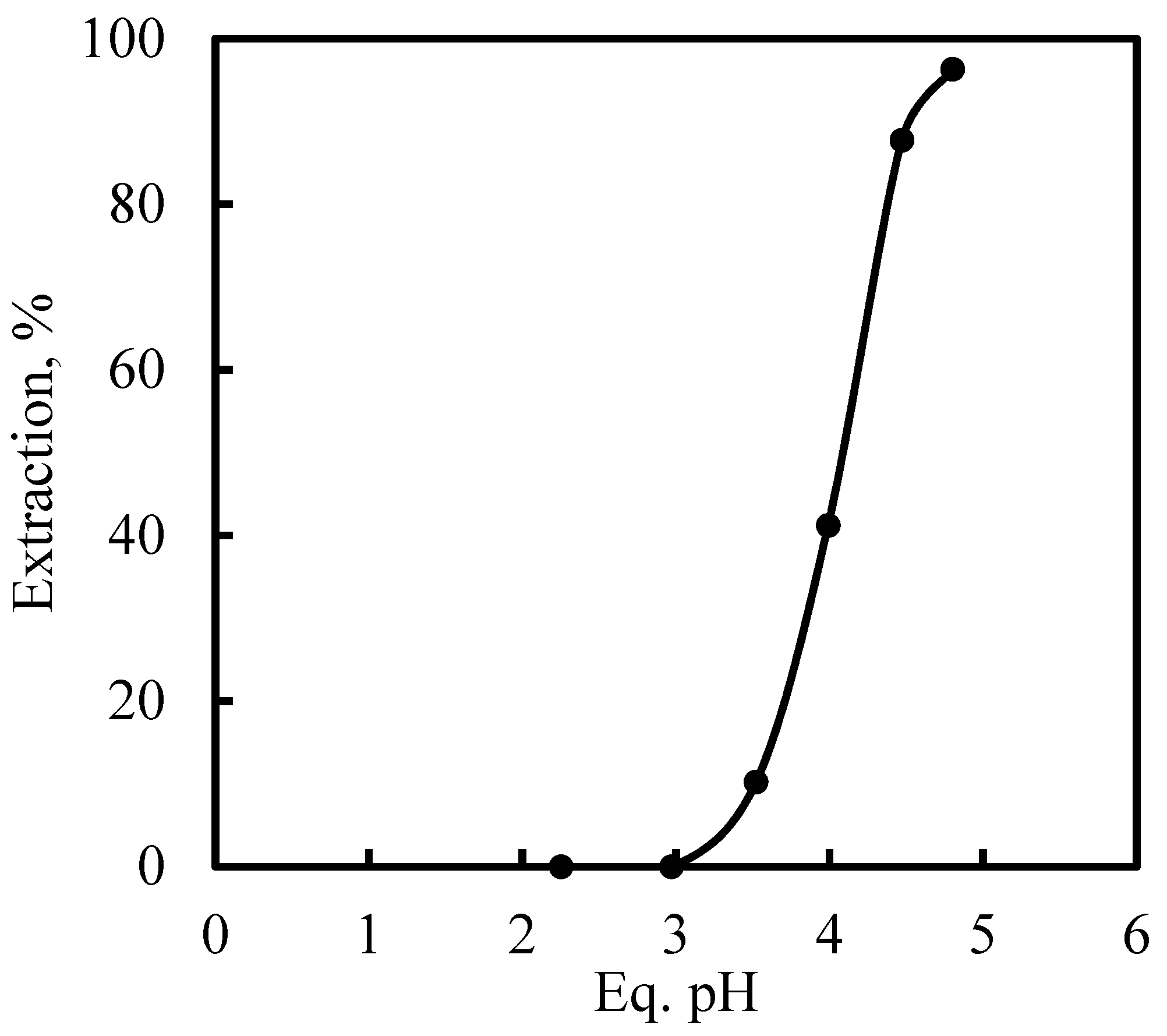
3.5. Recovery of Zinc and Nickel from the Purified Leach Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial waste. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Sahu, K.K.; Agrawal, A.; Pandey, B.D. Recent trends and current practices for secondary processing of zinc and lead. Part II: Zinc recovery from secondary sources. Waste Manag. Res. 2004, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kul, M.; Topkaya, Y. Recovery of germanium and other valuable metals from zinc plant residues. Hydrometallurgy 2008, 92, 87–94. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Hydrometallurgical process for recovery of cobalt from zinc plant residue. Hydrometallurgy 2002, 63, 225–234. [Google Scholar] [CrossRef]
- Moradkhani, D.; Sedaghat, B.; Khodakarami, M.; Ataei, I. Recovery of valuable metals from zinc plant residue through separation between manganese and cobalt with N-N reagent. Physicochem. Probl. Miner. Process. 2014, 50, 735–746. [Google Scholar]
- Gharabaghi, M.; Irannajad, M.; Azadmehr, A.R. Acid leaching of cadmium from zinc plant residue. Physicochem. Probl. Miner. Process. 2011, 47, 91–104. [Google Scholar]
- Palencar, M.; Kukurgya, F.; Miskufova, A. Processing of solution after leaching of steel making dust by cementation. Acta Metall. Slovaca 2015, 21, 142–153. [Google Scholar] [CrossRef]
- Altundoğan, H.S.; Erdem, M.; Orhan, R.; Ozer, A.; Tumen, F. Heavy metal pollution potential of zinc leach residues discarded in Cinkur plant. Turk. J. Eng. Environ. Sci. 1998, 22, 167–177. [Google Scholar]
- Safarzadeh, M.S.; Moradkhani, D.; Ilkhchi, M.O.; Golshan, N.H. Determination of the optimum conditions for the leaching of Cd–Ni residues from electrolytic zinc plant using statistical design of experiments. Sep. Purif. Technol. 2008, 58, 367–376. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moradkhani, D.; Ilkhchi, M.O. Kinetics of sulfuric acid leaching of cadmium from Cd–Ni zinc plant residues. J. Hazard. Mater. 2009, 163, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Yoo, K. Leaching behavior of valuable metals from by-product generated during purification of zinc electrolyte. Geosyst. Eng. 2016, 19, 312–316. [Google Scholar] [CrossRef]
- Sinha, M.K.; Sahu, S.K.; Meshram, P.; Pandey, B.D. Solvent extraction and separation of zinc and iron from spent pickle liquor. Hydrometallurgy 2014, 147–148, 103–111. [Google Scholar] [CrossRef]
- Alizadeh, R.; Rashchi, F.; Vahidi, E. Recovery of zinc from leach residues with minimum iron dissolution using oxidative leaching. Waste Manag. Res. 2010, 29, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.K.; El-Ashtoukhy, E.-S.Z.; Abdelwahab, O. Rate of cadmium ions removal from dilute solutions by cementation on zinc using a rotating fixed bed reactor. Hydrometallurgy 2007, 89, 224–232. [Google Scholar] [CrossRef]
- Aurousseau, M.; Pham, N.T.; Ozil, P. Effects of ultrasound on the electrochemical cementation of cadmium by zinc powder. Ultrason. Sonochemistry 2004, 11, 23–26. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moradkhani, D.; Ilkhchi, M.O. Determination of the optimum conditions for the cementation of cadmium with zinc powder in sulfate medium. Chem. Eng. Process. Process Intensif. 2007, 46, 1332–1340. [Google Scholar] [CrossRef]
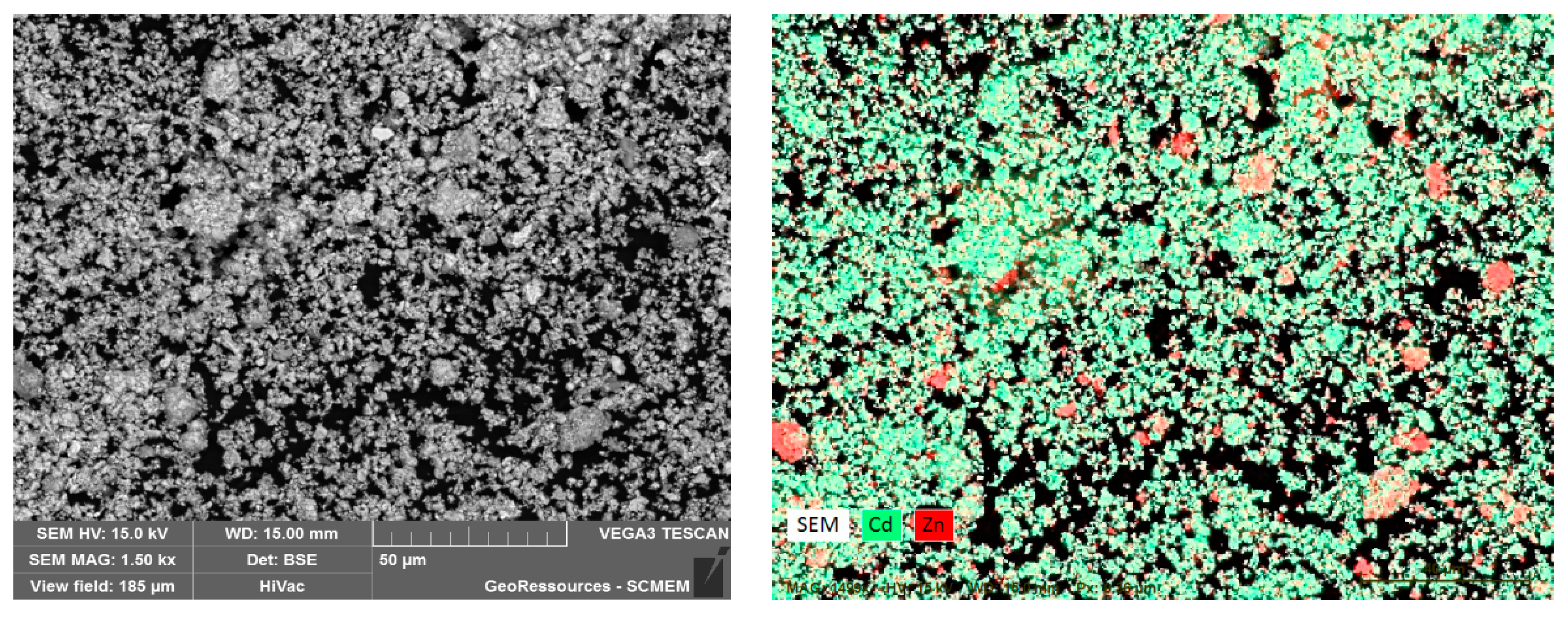
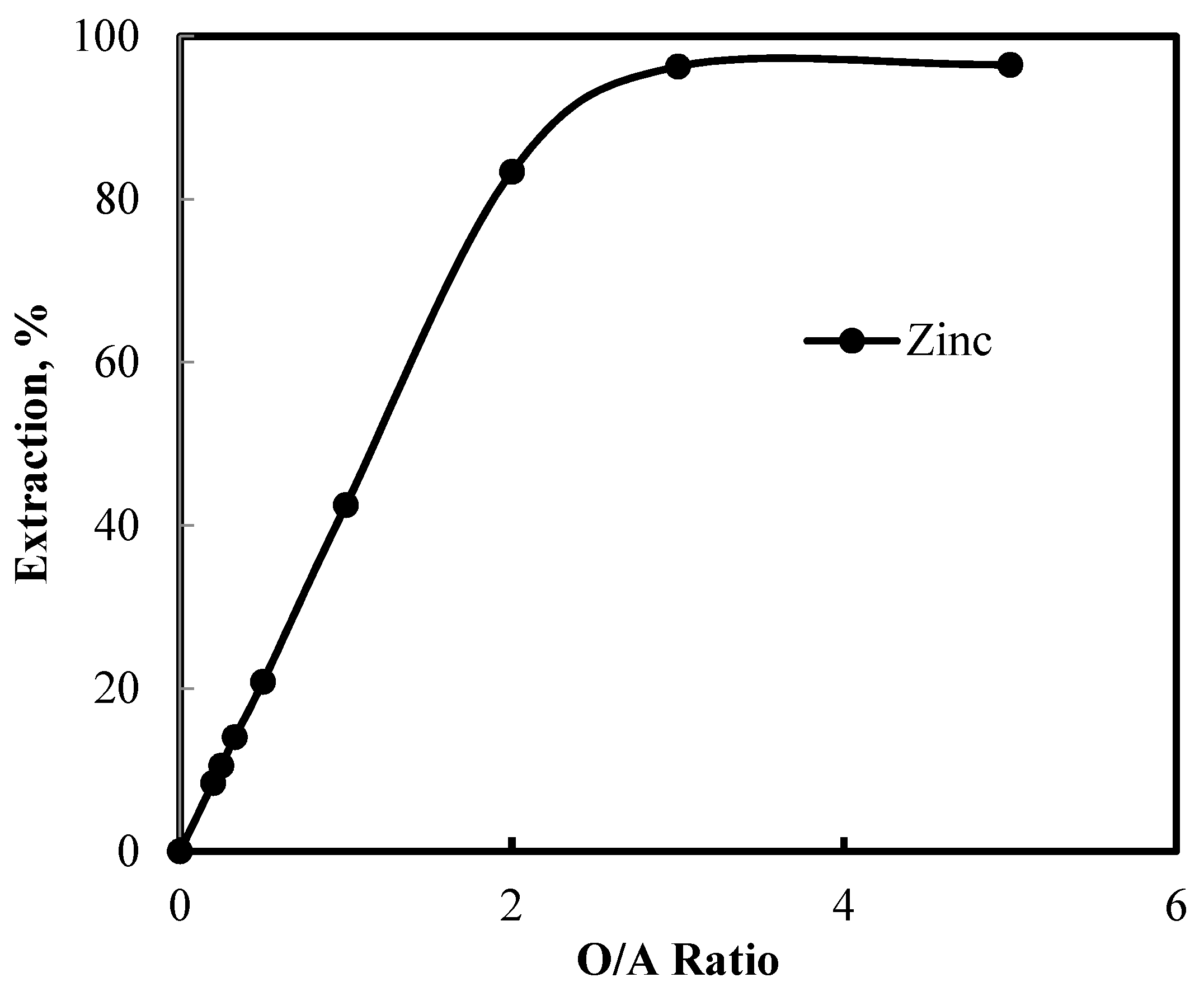
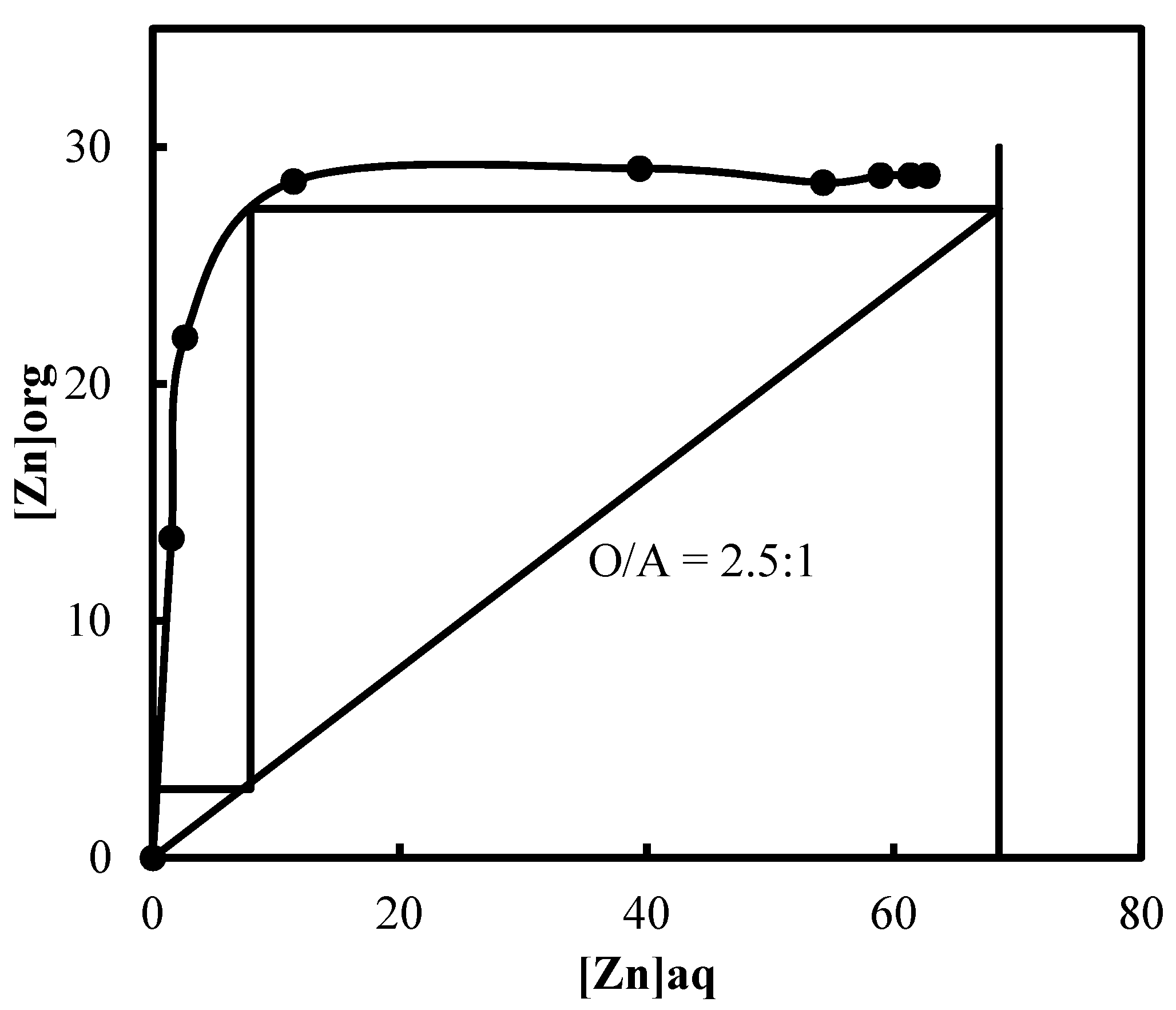
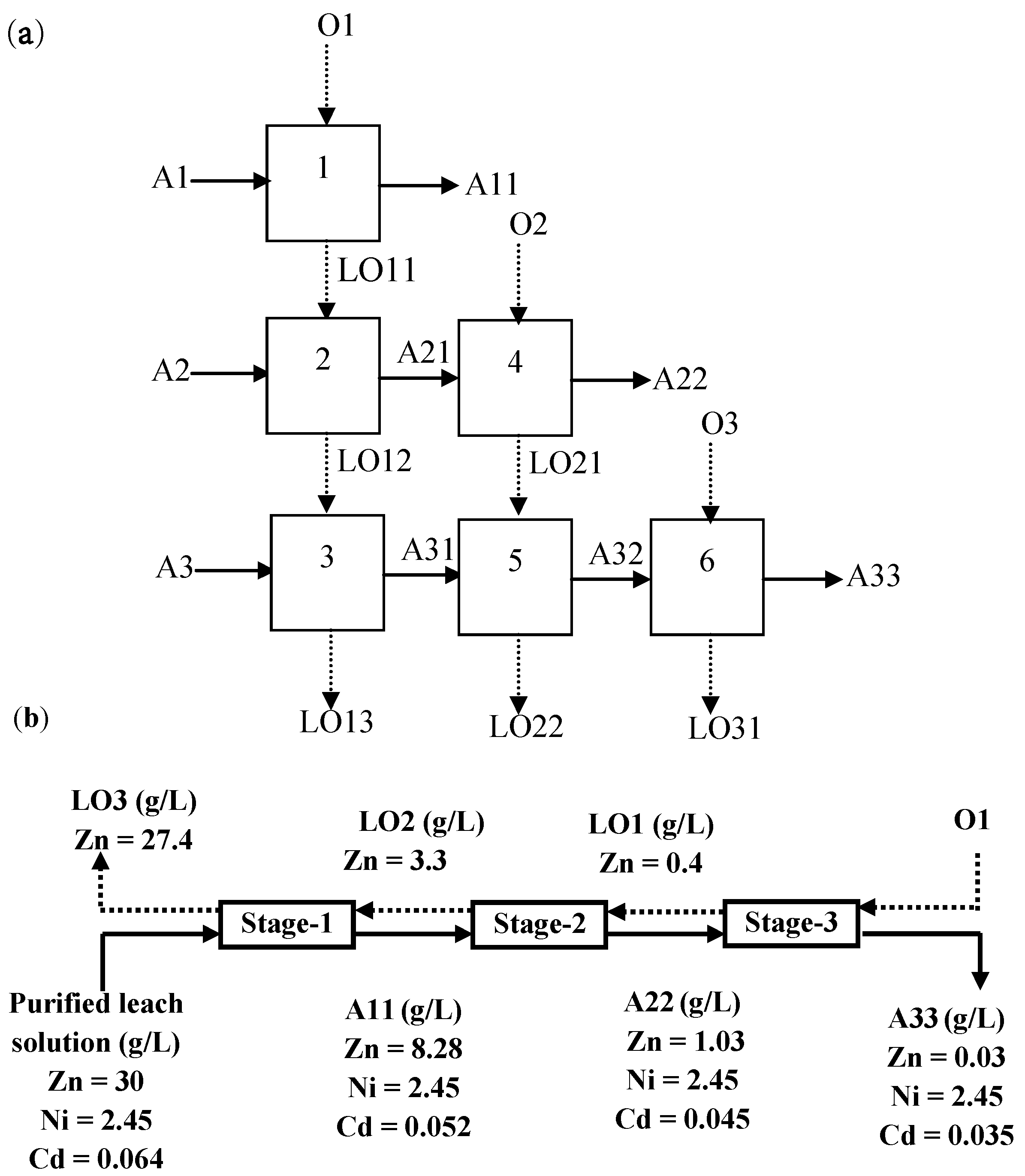
| Name | Ti | Fe | Mn | Ca | Mg | Ni | Cd | Zn | Pb | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| Cake leach | 0.167 | 5.19 | 0.23 | 13.96 | 0.78 | 0.03 | 0.089 | 4.94 | 2.96 | 0.030 |
| Cake wash | 0.185 | 5.85 | 0.08 | 15.35 | 0.74 | 0.02 | 0.047 | 2.04 | 3.05 | 0.012 |
| Cobalt cake | 0.007 | 0.32 | 11.35 | 9.62 | 1.28 | 0.20 | 0.361 | 19.94 | 0.12 | 1.372 |
| Ni-Cd cake | 0.011 | 0.314 | 0.311 | 2.161 | 0.499 | 3.113 | 9.017 | 47.131 | 0.46 | 0.019 |
| Slag | 0.005 | 0.12 | 0.04 | 0.34 | 0.40 | 0.00 | 0.004 | 77.96 | 0.08 | 0.002 |
| Element | Ni-Cd Cake Waste Residue (%) | Leach Solution (mg/L) | Leach Residue (%) |
|---|---|---|---|
| Ti | 0.01 | <0.1 | 0.13 |
| Fe | 0.31 | <0.1 | 3.71 |
| Mn | 0.31 | 369 | 0.12 |
| Ca | 2.16 | 122 | 17.12 |
| Mg | 0.50 | 566 | 0.55 |
| Ni | 3.11 | 3960 | 0.03 |
| Cd | 9.02 | 11480 | 0.05 |
| Zn | 47.13 | 59140 | 0.68 |
| Pb | 0.46 | 90 | 4.43 |
| Co | 0.02 | 20 | 0.001 |
| Element | Leach Solution (mg/L) | Purified Leach Solution (mg/L) | Cement Material (%) |
|---|---|---|---|
| Ti | <0.1 | <0.1 | 0.001 |
| Fe | <0.1 | <0.1 | 0.002 |
| Mn | 369 | 366 | 0.003 |
| Ca | 122 | 81 | 0.1 |
| Mg | 566 | 562 | 0.005 |
| Ni | 3960 | 2450 | 4.7 |
| Cd | 11,480 | 64 | 63.6 |
| Zn | 59,140 | 68,520 | 9.6 |
| Pb | 90 | 1.5 | 2.1 |
| Co | 20 | 20 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar Sahu, S.; Kargar Razi, M.; Beuscher, M.; Chagnes, A. Recovery of Metal Values from Ni-Cd Cake Waste Residue of an Iranian Zinc Plant by Hydrometallurgical Route. Metals 2020, 10, 655. https://doi.org/10.3390/met10050655
Kumar Sahu S, Kargar Razi M, Beuscher M, Chagnes A. Recovery of Metal Values from Ni-Cd Cake Waste Residue of an Iranian Zinc Plant by Hydrometallurgical Route. Metals. 2020; 10(5):655. https://doi.org/10.3390/met10050655
Chicago/Turabian StyleKumar Sahu, Sushanta, Maryam Kargar Razi, Mathieu Beuscher, and Alexandre Chagnes. 2020. "Recovery of Metal Values from Ni-Cd Cake Waste Residue of an Iranian Zinc Plant by Hydrometallurgical Route" Metals 10, no. 5: 655. https://doi.org/10.3390/met10050655
APA StyleKumar Sahu, S., Kargar Razi, M., Beuscher, M., & Chagnes, A. (2020). Recovery of Metal Values from Ni-Cd Cake Waste Residue of an Iranian Zinc Plant by Hydrometallurgical Route. Metals, 10(5), 655. https://doi.org/10.3390/met10050655






