Study of Zn6Al6Ag Alloy Application in Ultrasonic Soldering of Al2O3–(Al/Al2O3) Joints
Abstract
:1. Introduction
2. Experimental
- A composite substrate from Fraunhofer Ltd. (Dresden, Germany) composed of an Al matrix, reinforced with ceramic particles of Al2O3 with an average particle size of 30 μm in the form of square plates, with dimensions of 10 × 10 × 3 mm. The volume fraction of Al2O3 particles in the composite was 50%;
- Al2O3 ceramic substrate from Flocculus Ltd. (Libina, Czech Republic) in the form of discs Ø15 × 3 mm and in a shape of square plates with dimensions of 10 mm × 10 mm × 3 mm with 3N purity;
- 4N purity metallic Cu substrate in the form of discs Ø15 × 3 mm;
- Al alloy Al7075 substrate in the form of discs Ø15 × 3 mm.
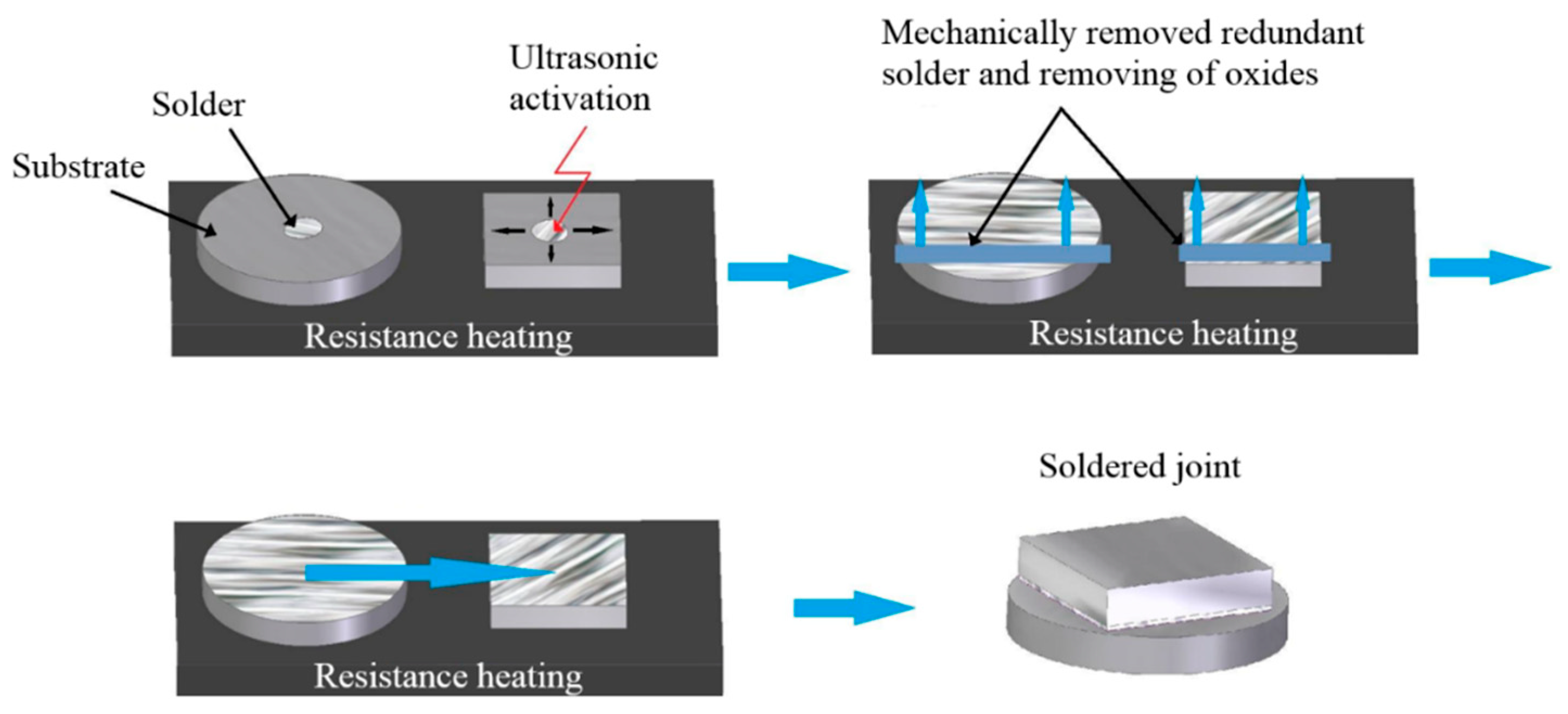
- Cleaning and degreasing soldered materials;
- Laying the substrate on a hot plate;
- Heating the hot plate to 430 °C;
- Deposition of a small amount of solder with thickness of 600 μm on the surfaces of the substrates;
- Placing the ultrasonic device sonotrode into the molten solder for 5 s;
- After ultrasound activation, removing the surface oxides on the molten solder by use of a stainless-steel plate;
- Bringing the prepared substrates with the solder on their surface into contact, and leaving them for approximately 1 min.
3. Experimental Results
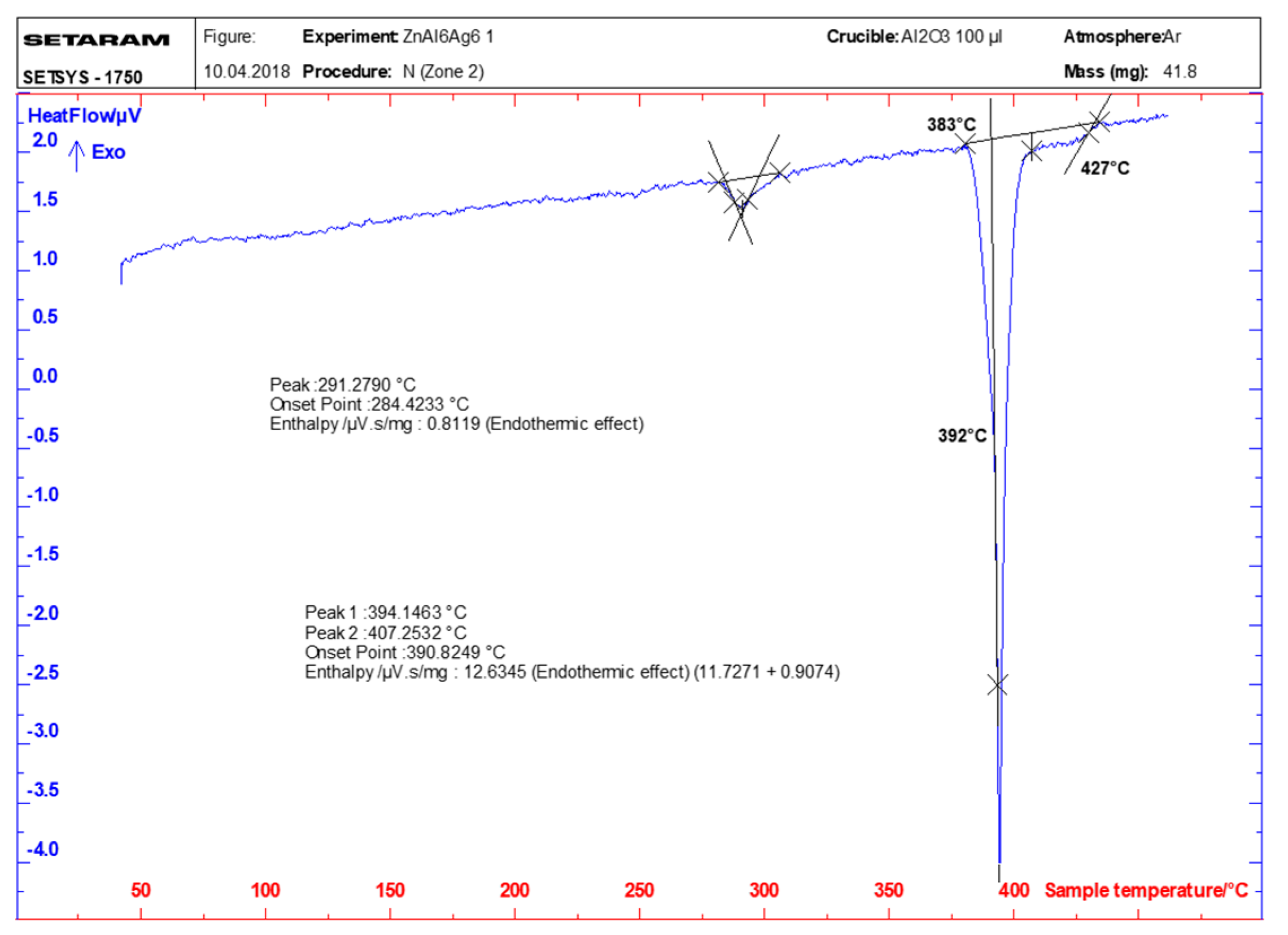
3.1. DSC/DTA Analysis
3.2. Microstructure of Zn–Al–Ag Solder
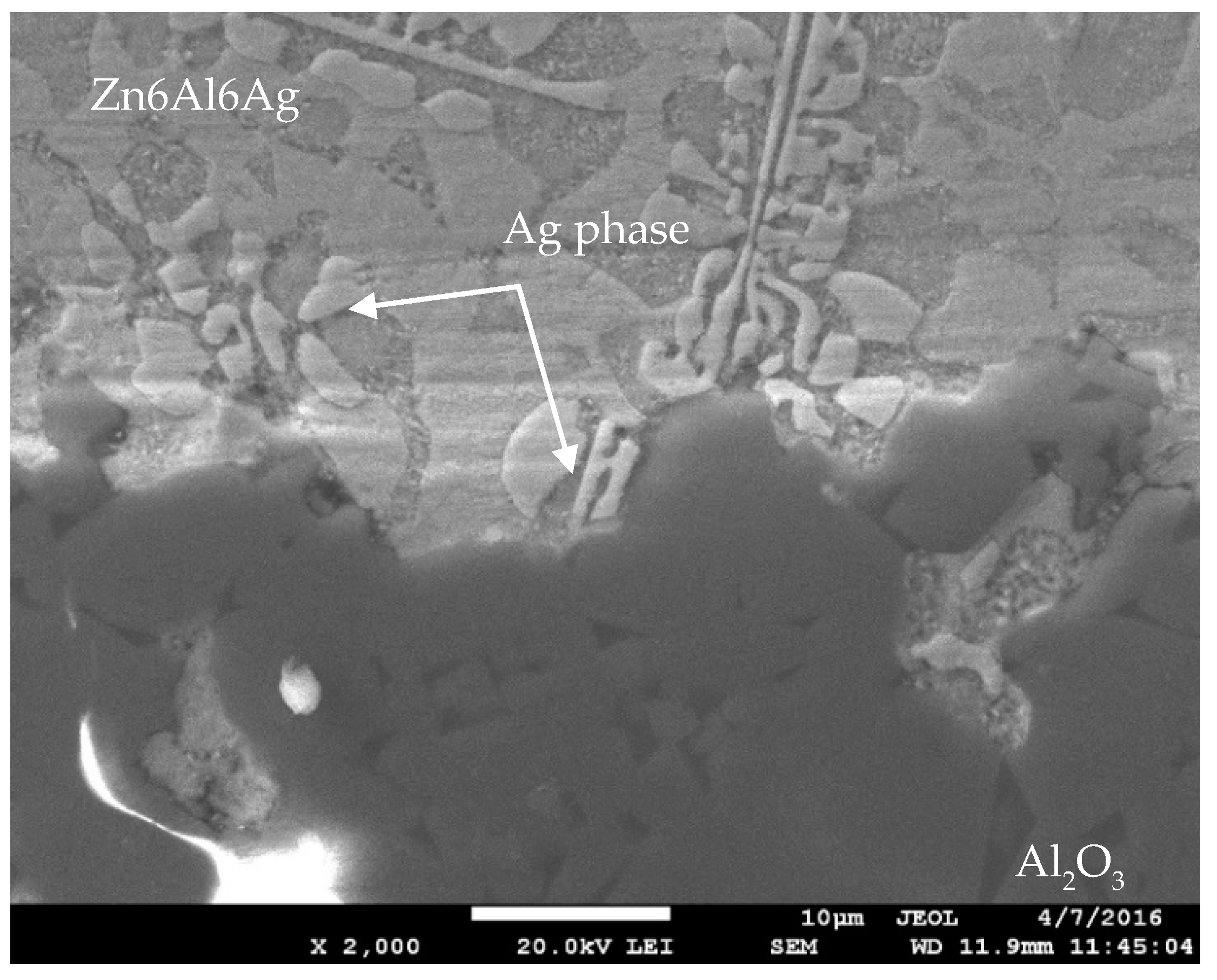
3.3. Analysis of Soldered Joint on the Boundary of MMC/Zn6Al6Ag Joint
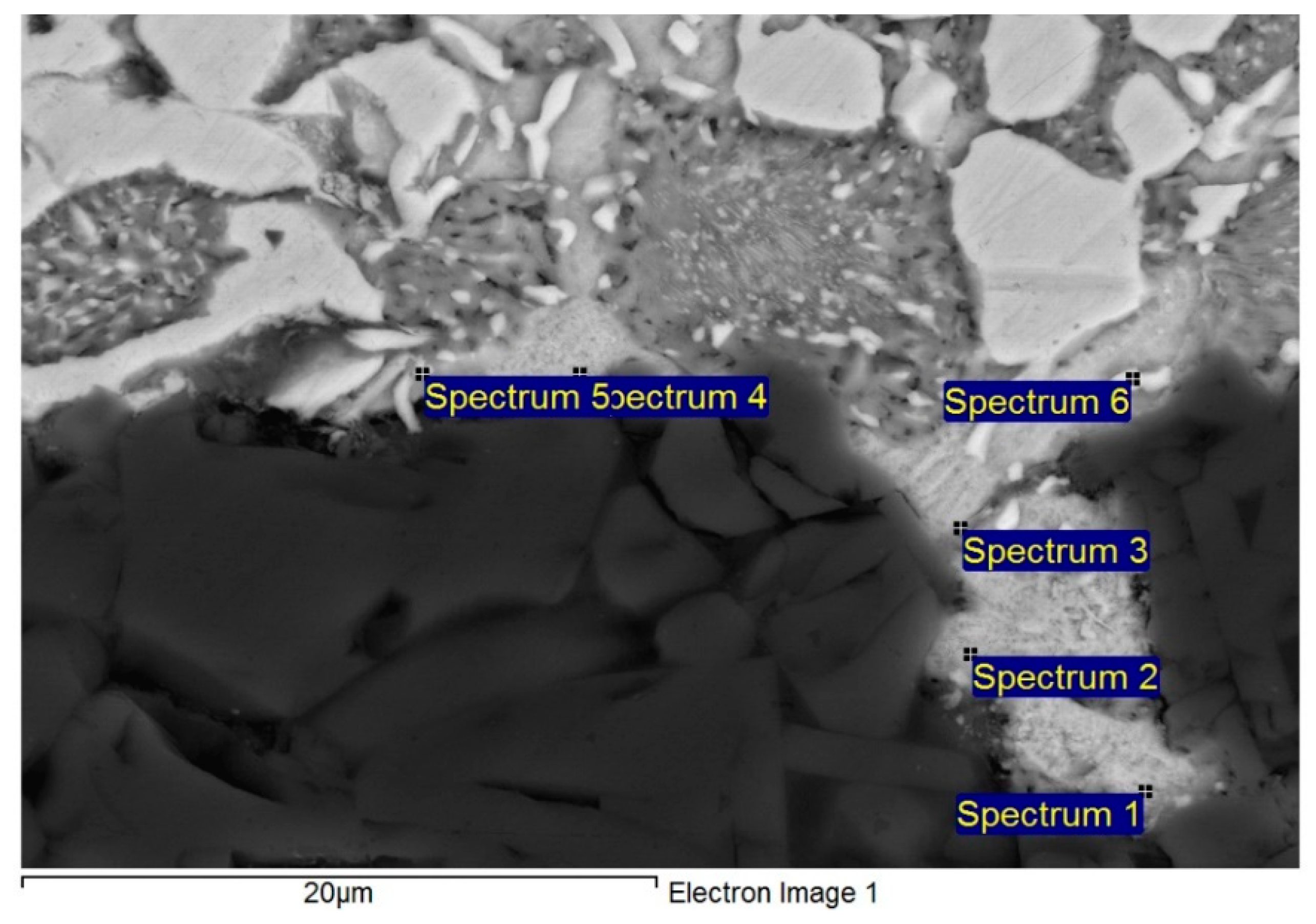
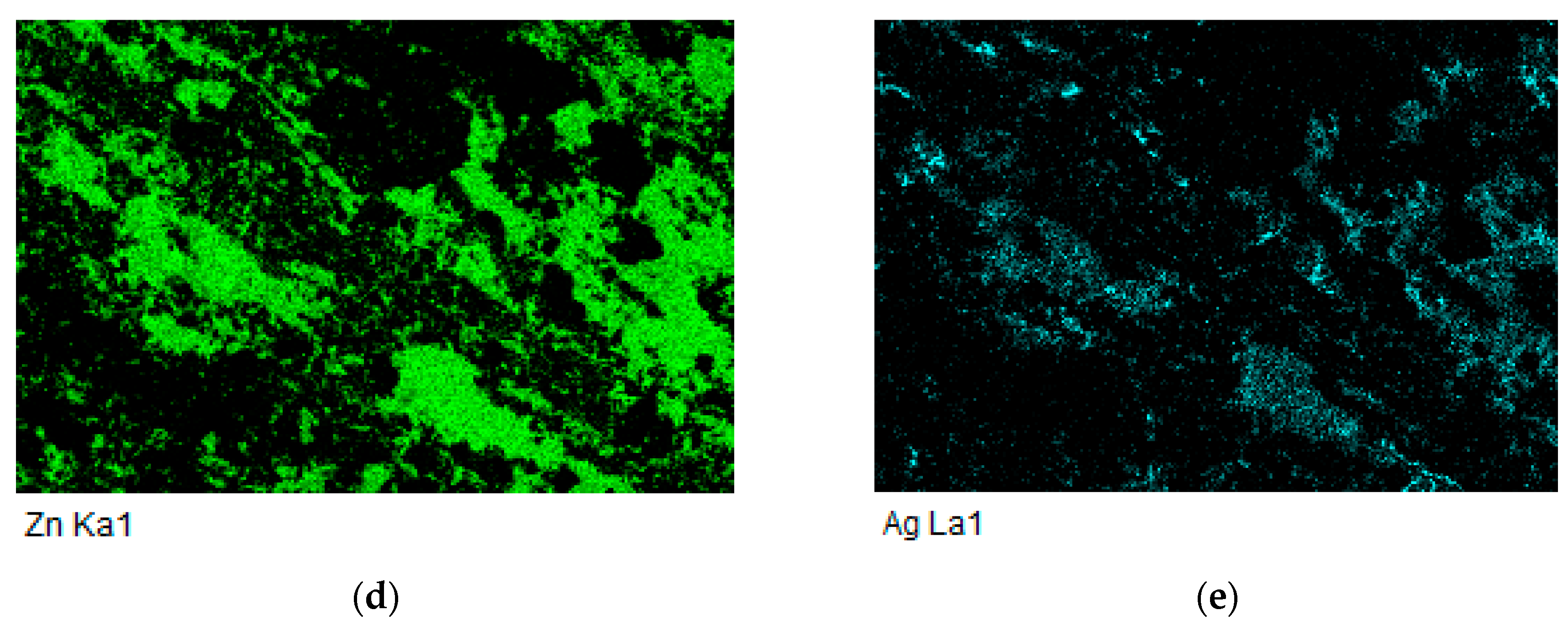
3.4. Analysis of Soldered Bond in the Boundary of Al2O3/Zn6Al6Ag Joint
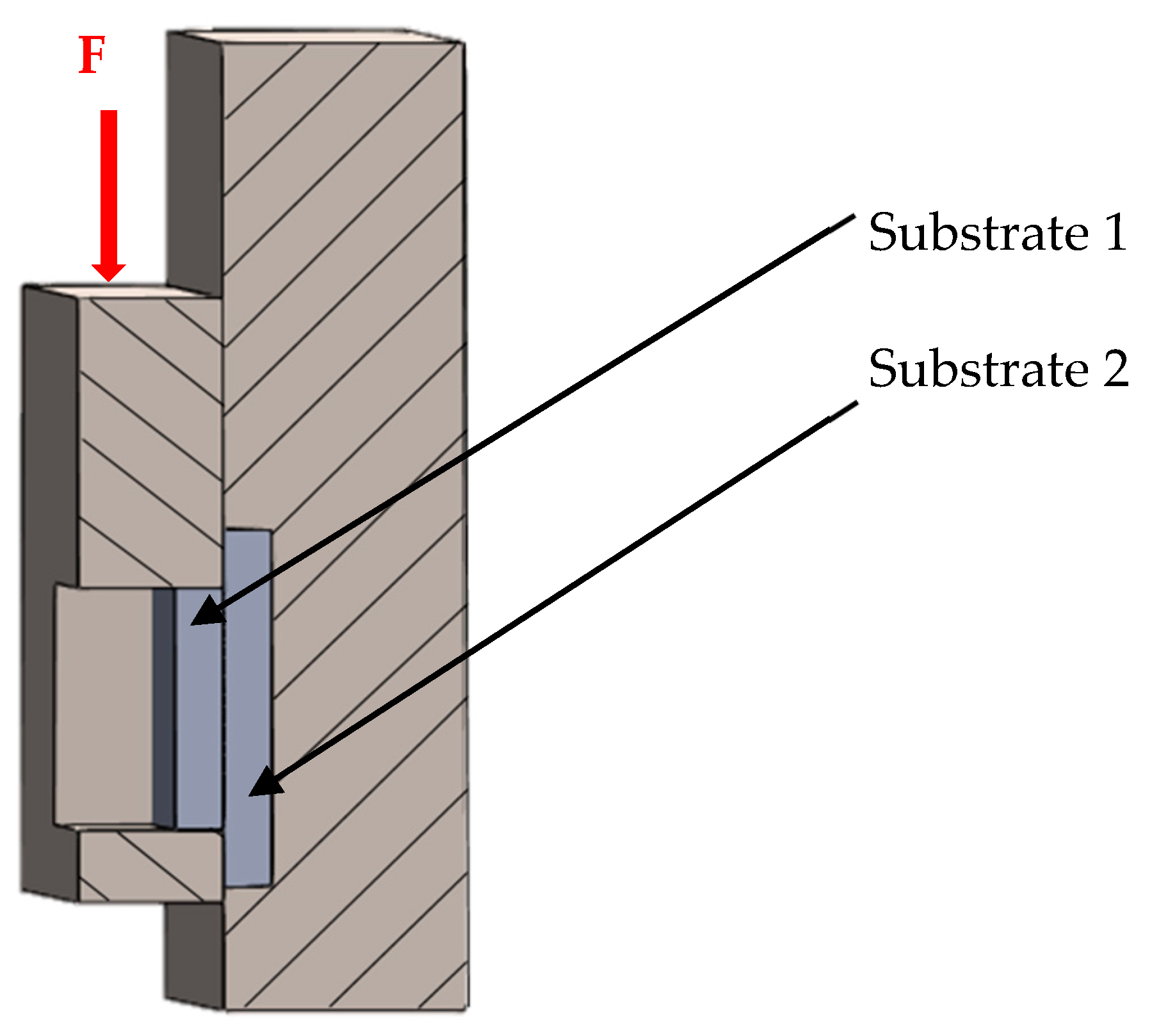
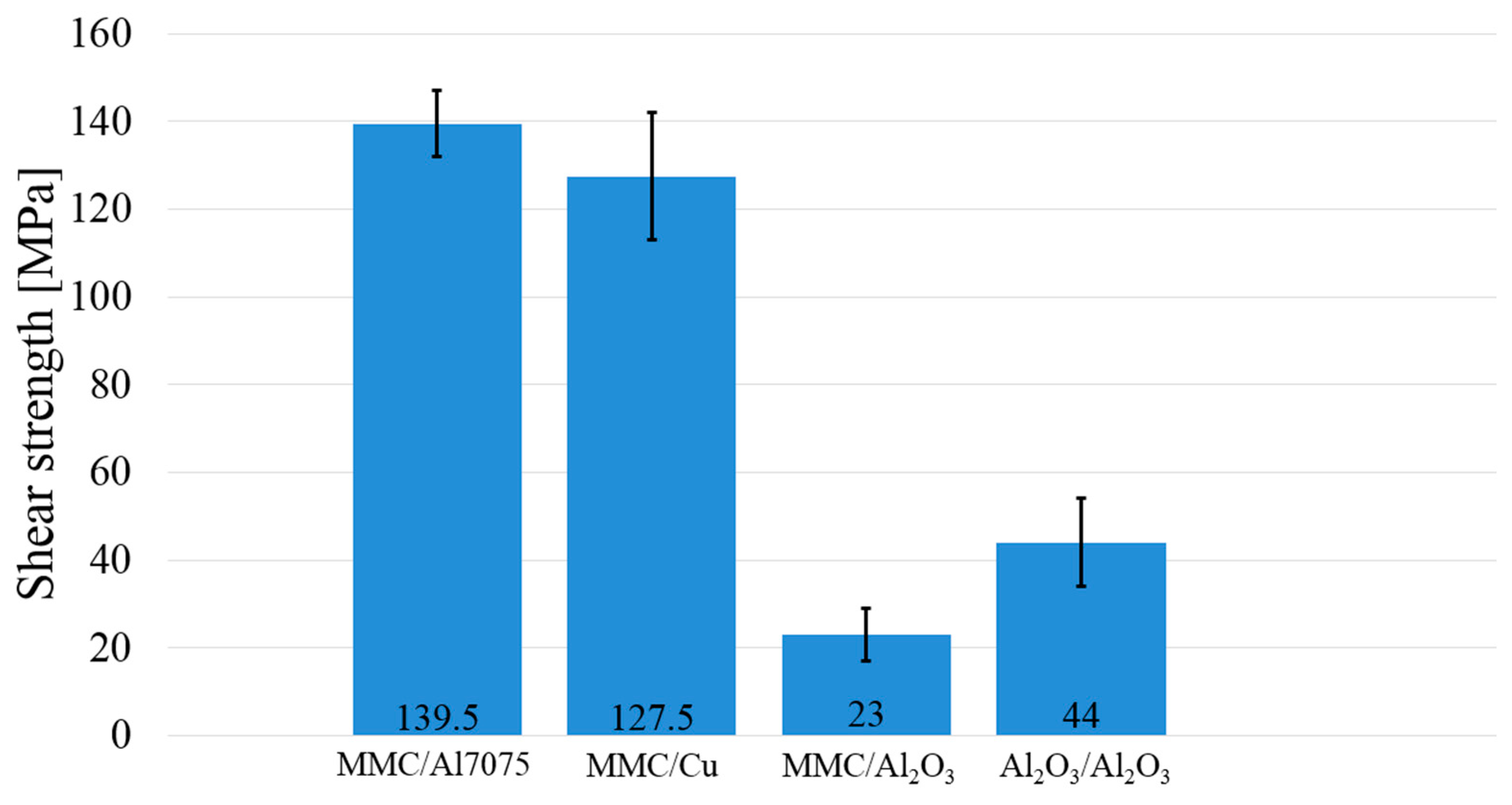
3.5. Measurement of Shear Strength in Soldered Joints
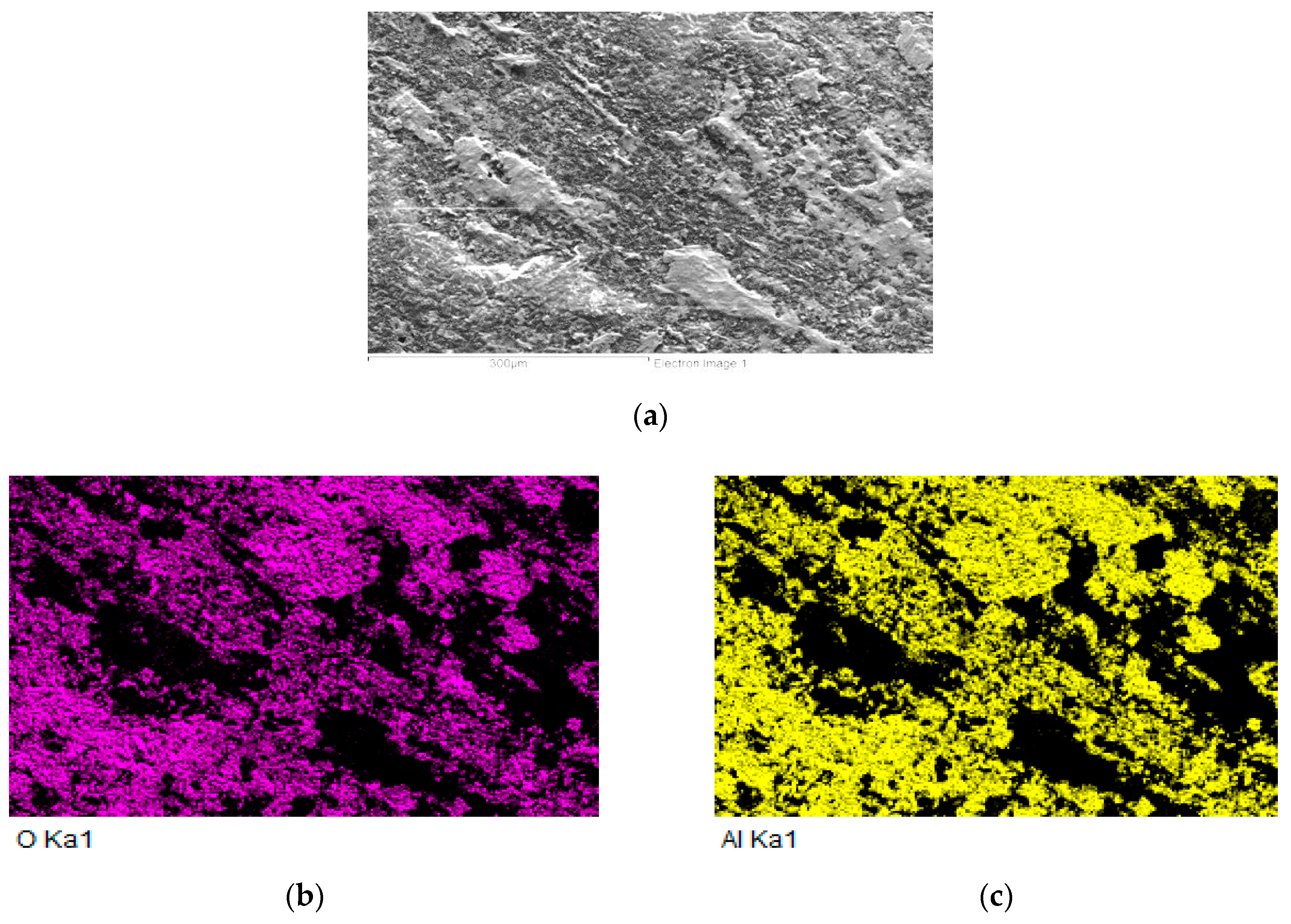
3.6. Analysis of Fractured Surfaces
4. Conclusions
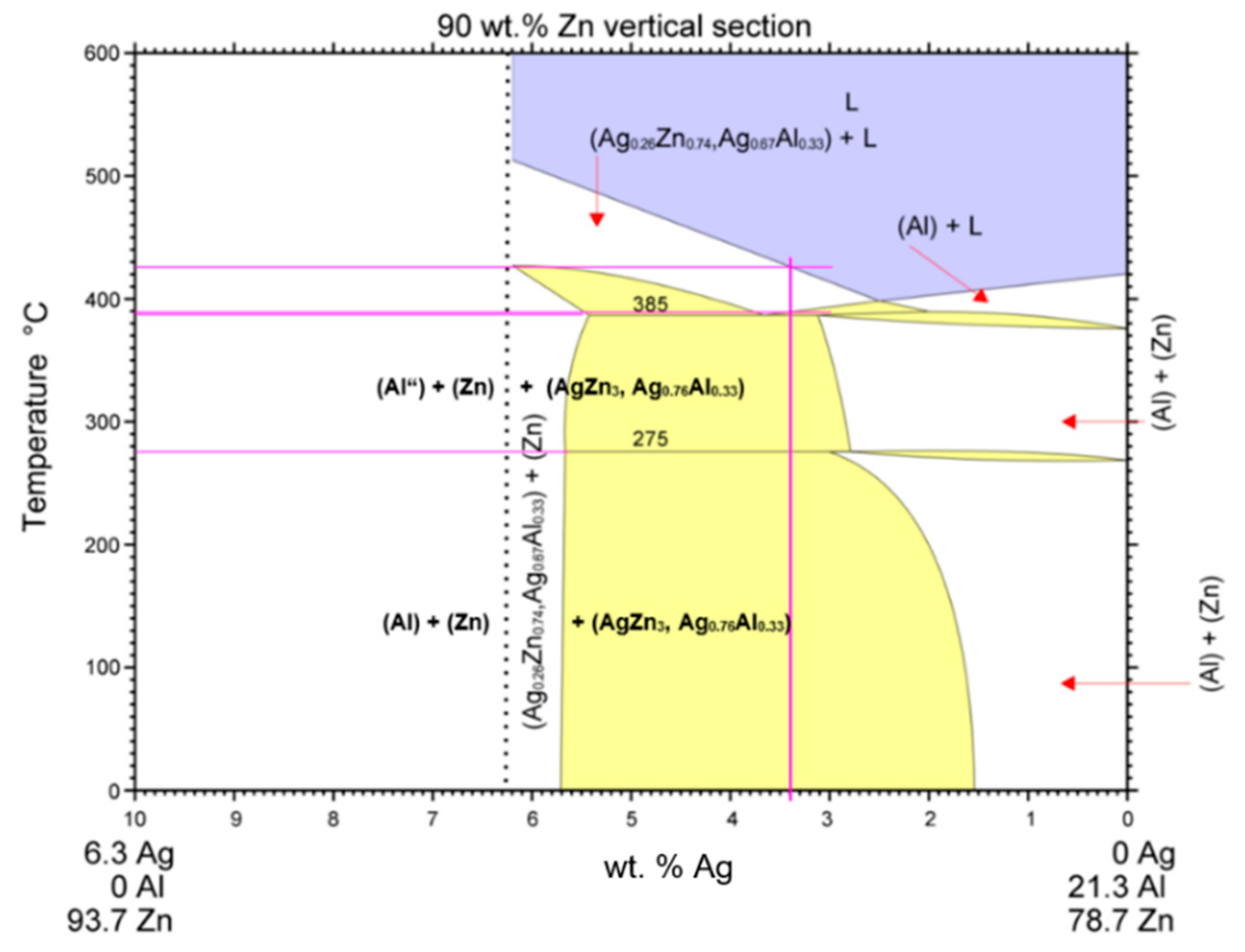
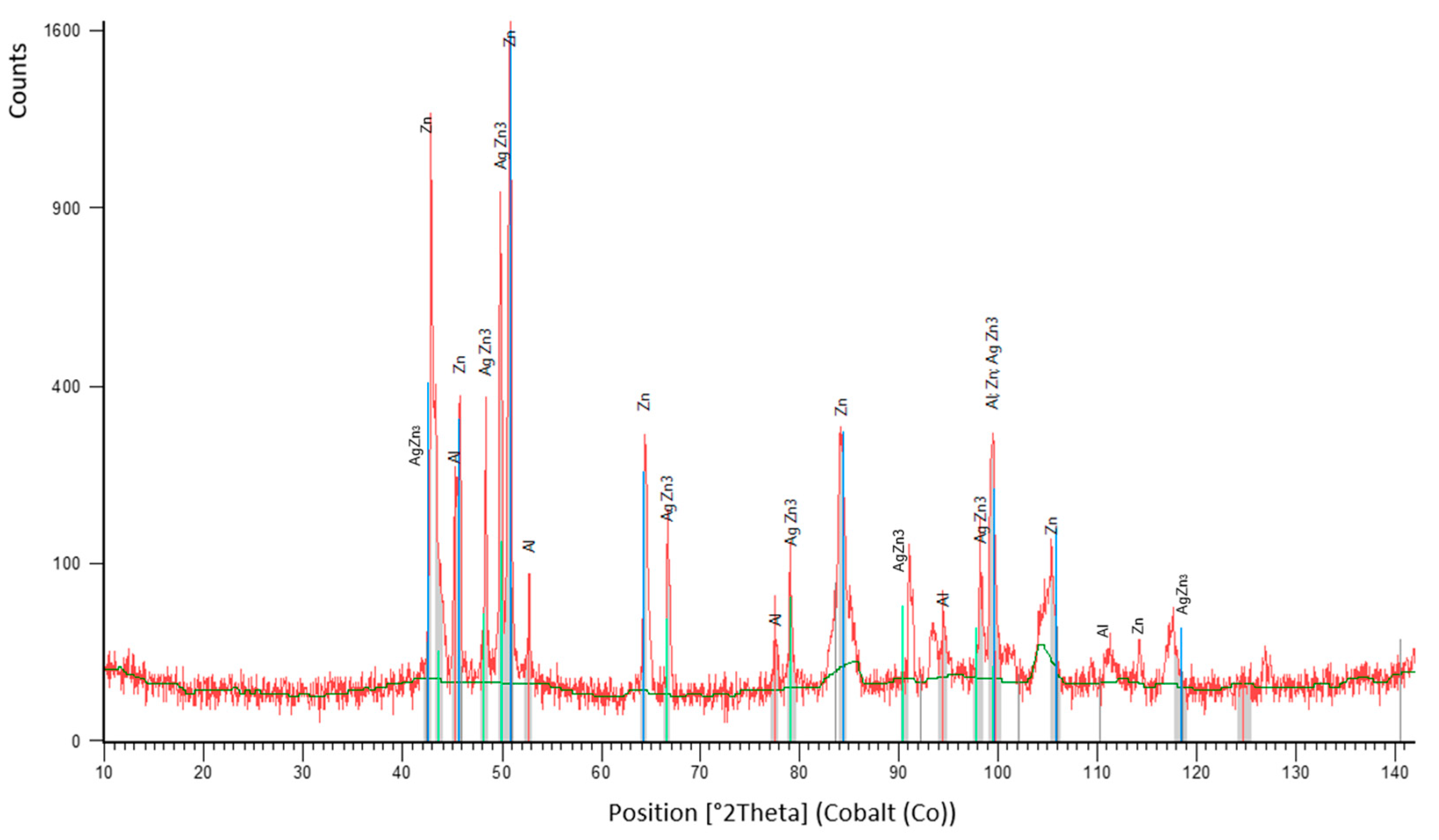
- DSC analysis was used to determine the melting point of the solder. The DSC curve contained two main peaks with four thermal effects in the Zn–Al–Ag system. The first and smaller peak corresponded to the eutectoid reaction at a characteristic temperature of 275 °C. The second and larger peak corresponded to a eutectic reaction at the characteristic temperature of 385 °C. Next, the reaction at a temperature of 405 °C corresponded to segregation of the AgZn3 phase. The last reaction characterized the melting point of solder at a temperature of 427 °C.
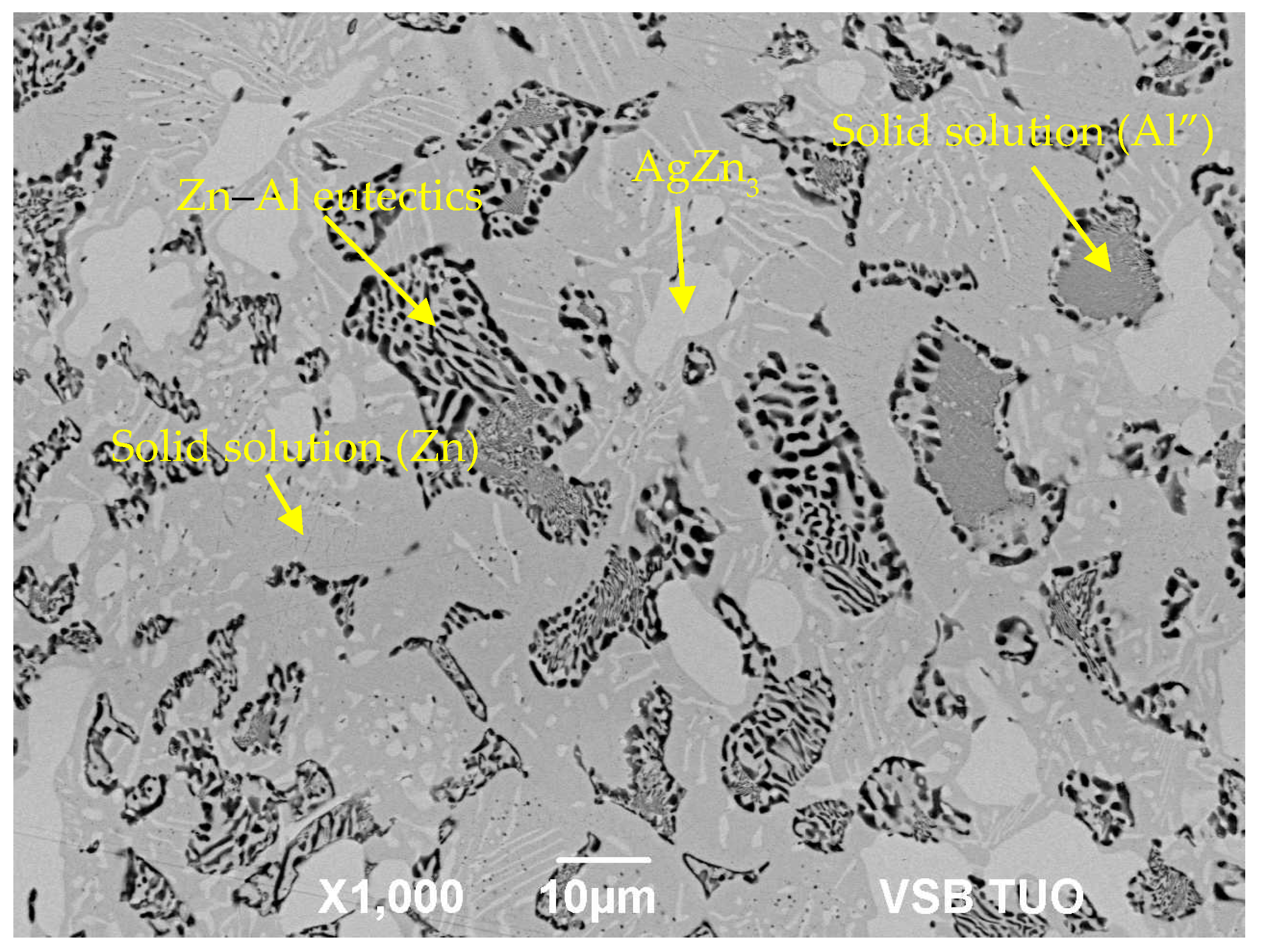
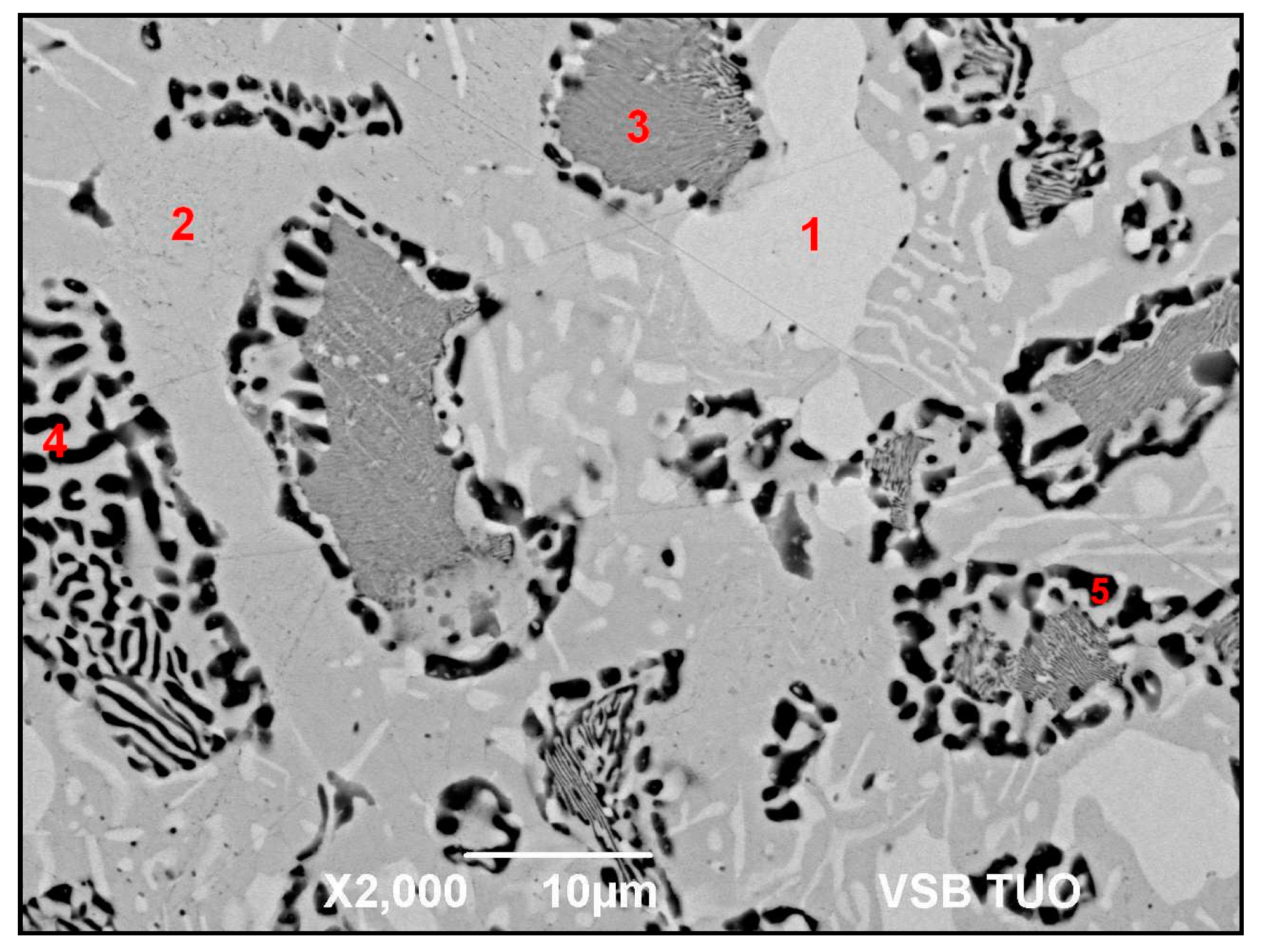
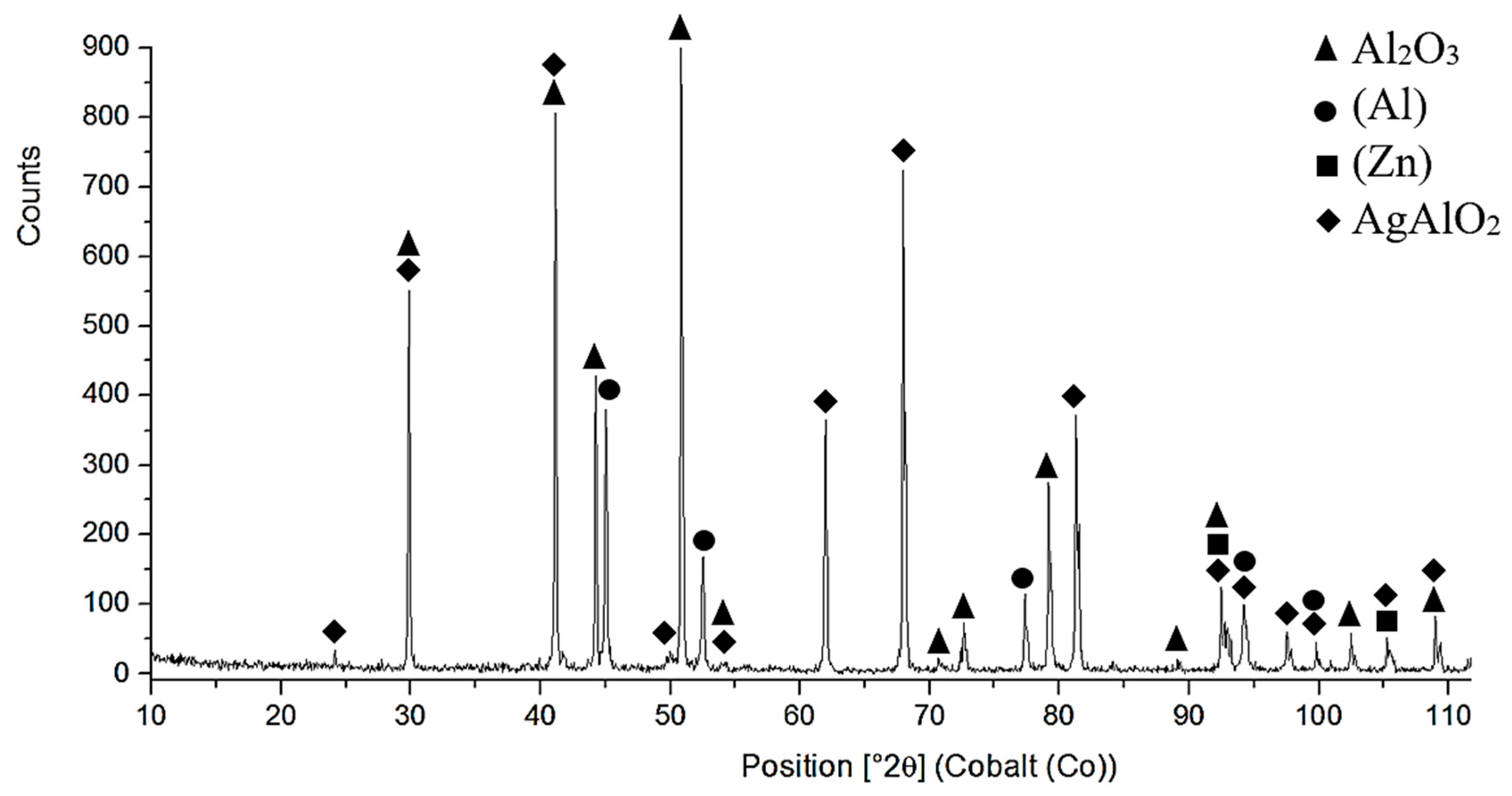
- The microstructure of Zn6Al6Ag solder consisted of solid solution (Al), solid solution (Zn), and ε–AgZn3 phase, which was proven by EDX and XRD analyses.
- The bond between the ceramic composite and Zn6Al6Ag solder was formed as follows: firstly, the surface oxides on the MMC substrate were disrupted under the molten layer of zinc solder. Subsequently, the dissolution of the aluminum surface of the MMC substrate took place, owing to the activity of the molten Zn solder. On dissolution of the aluminum matrix of the substrate in the zinc solder, the Al2O3 particles, which were initially in the MMC substrate, were also put into motion. A transition zone was, thus, formed (a band formed of a new composite).
- The bond of Al2O3 with Zn6Al6Ag solder was formed as follows: in soldering with ultrasound assistance, the surface of the ceramic was firstly disrupted. Portions of Zn and Al were oxidized during soldering in air. Owing to ultrasound activation, the oxidized Zn and Al particles were distributed to the boundary of the ceramic material. The Zn, Al, and Ag particles combined with the oxides on the surfaces of ceramic materials, resulting in wetting and penetration of solder into the recesses on the substrate surfaces.
- In the case of the MMC/Al2O3 combination, the average shear strength attained was 23 MPa. In the case of soldering Al2O3 ceramics, no new phase at the ceramic/solder boundary was formed, and diffusion did not take place; therefore, the bond was merely of an adhesive character.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaczmar, J.W.; Pietrzak, K.; Włosiński, W. The production and application of metal matrix composite materials. J. Mater. Process. Technol. 2000, 106, 58–67. [Google Scholar] [CrossRef]
- Mallik, S.; Ekere, N.; Best, C.; Bhatti, R. Investigation of thermal management materials for automotive electronic control units. Appl. Therm. Eng. 2011, 31, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Zweben, C. Metal-matrix composites for electronic packaging. JOM 1992, 44, 15–23. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.W.; Lee, H.M. Thermodynamics aided alloy design and evaluation of Pb-free solders for high-temperature applications. Mater. Trans. 2002, 43, 1873–1878. [Google Scholar] [CrossRef] [Green Version]
- Chidambaram, V.; Hald, J.; Hattel, J. Development of Au-Ge based candidate alloys as an alternative to high-lead content solders. J. Alloys Compd. 2010, 490, 170–179. [Google Scholar] [CrossRef]
- Kroupa, A.; Andersson, D.; Hoo, N.; Pearce, J.; Watson, A.; Dinsdale, A.; Mucklejohn, S. Current Problems and Possible Solutions in High-Temperature Lead-Free Soldering. J. Mater. Eng. Perform. 2011, 21, 629–637. [Google Scholar] [CrossRef]
- Rettenmayr, M.; Lambracht, P.; Kempf, B.; Graff, M. High melting Pb-free solder alloys for die-attach applications. Adv. Eng. Mater. 2005, 7, 965–969. [Google Scholar] [CrossRef]
- Suganuma, K.; Kim, S.J.; Kim, K.S. High-Tepmerature Lead-Free Solders: Properties and Possibilities. JOM 2009, 61, 64–71. [Google Scholar] [CrossRef]
- Cay, F.; Kurnaz, C. Hot tensile and fatigue of zinc-aluminium alloys produced by gravity and squeeze casting. Mater. Des. 2005, 26, 479–485. [Google Scholar] [CrossRef]
- Prach, M.; Kolenak, R. Soldering of Copper with High-Temperature Zn-Based Solders. Proceeding of the 25th DAAAM International Symposium on Intelligent Manufacturing and Automation, Vienna, Austria, 26–29 November 2014; pp. 1370–1375. [Google Scholar]
- Haque, A.; Lim, B.H.; Haseeb, A.S.M.A.; Masjuki, H.H. Die attach properties of Zn-Al-Mg-Ga based high-temperature lead-free solder on Cu lead-frame. J. Mater. Sci. Mater. Electron. 2012, 23, 115–123. [Google Scholar] [CrossRef]
- Cheng, F.; Gao, F.; Wang, Y.; Wu, Y.; Ma, Z.; Yang, J. Sn addition on the tensile properties of high temperature Zn-4Al-3Mg solder alloys. Microelectron. Reliab. 2012, 52, 579–584. [Google Scholar] [CrossRef]
- Shimizu, T.; Ishikawa, H.; Ohnuma, I.; Ishida, K. Zn-Al-Mg-Ga Alloys as Pb-Free Solder for Die-Attaching Use. J. Electron. Mater. 1999, 28, 1172–1175. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.; Ren, S.; Wang, Q.; Wei, J.; Fan, G. Microstructure, mechanical properties, and bonding mechanism of ultrasonic-assisted brazed joints of SiC ceramics with ZnAlMg filler metals in air. Ceram. Int. 2014, 40, 683–689. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Chen, X.; Cui, Y. Ultrasonic dissolution of brazing of 55% SiCp/A356 composites. Trans. Nonferrous Met. Soc. China 2010, 20, 746–750. [Google Scholar] [CrossRef]
- Jinbin, L.; Yunchao, M.; Xiangwei, L.; Jintal, N. A New Method For Soldering Particle-reinforced aluminum metal matrix composites. Mater. Sci. Eng., B 2012, 177, 1759–1763. [Google Scholar]
- Gancarz, T.; Pstruś, J.; Fima, P.; Mosinska, S. Thermal properties and wetting behavior of high temperature Zn-Al-In solders. J. Mater. Eng. Perform. 2012, 21, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, W.; Leng, X.; Fu, Q.; Wang, L.; Yan, J. Microstructure evolution and mechanical properties of ultrasonic-assisted soldering of 2024 aluminum alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 1937–1943. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Liu, L. Study of low temperature brazing of magnesium alloy to aluminum alloy using Sn-xZn solders. Mater. Des. 2012, 39, 14–19. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, H.; Li, M.; Kim, J. Ultrasound-assisted brazing of Cu/Al dissimilar metals using a Zn-3Al filler metal. Mater. Des. 2013, 52, 740–747. [Google Scholar] [CrossRef]
- Zhang, L.X.; Shi, J.M.; Li, H.W.; Tian, X.Y.; Feng, J.C. Interfacial microstructure and mechanical properties of ZrB2-SiC-C ceramic and GH99 superalloy joints brazed with a Ti-modified FeCoNiCrCu high-entropy alloy. Mater. Des. 2016, 97, 230–238. [Google Scholar] [CrossRef]
- Chen, B.; Xiong, H.; Cheng, Y.; Mao, W.; Wu, S. Microstructure and property of AlN joint brazed with Au-Pd-Co-Ni-V brazing filler. J. Mater. Sci. Technol. 2015, 31, 1034–1038. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, R.; Dandapat, N.; Pal, K.S.; Datta, S.; Basu, D. Characterization of alumina–alumina/graphite/monel superalloy brazed joints. Ceram. Int. 2012, 38, 663–670. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, Y.; Wang, L.; Qu, P.; Kang, H.; Chu, P.K. Microstructure evolution and mechanical properties of vacuum-brazed C/C composite with AgCuTi foil. Mater. Sci. Eng., A 2013, 564, 192–198. [Google Scholar] [CrossRef]
- Shi, J.M.; Feng, J.C.; Liu, H.; Tian, X.Y.; Zhang, L.X. Vacuum brazing of the Gr/2024Al composite and TC4 alloy using AgCuTi filler alloy with Ni-Al interlayer as auxiliary heat source. J. Alloys Compd. 2017, 694, 672–681. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]



| Specimen | Charge (wt.%) | ICP-AES (wt.%) | ||||
|---|---|---|---|---|---|---|
| Zn | Al | Ag | Zn | Al | Ag | |
| Zn6Al6Ag | 88.0 | 6.0 | 6.0 | 88.38 | 6.14 ± 0.31 | 5.48 ± 0.55 |
| Parameters | Unit | Value |
|---|---|---|
| Ultrasound Power | W | 400 |
| Working frequency | kHz | 40 |
| Amplitude | μm | 2 |
| Soldering temperature | °C | 430 |
| Time of ultrasound activation | s | 5 |
| Alloy | Heat | |||
|---|---|---|---|---|
| TL | T1 | T2 | T3 | |
| ZnAl6Ag6 | 427 | 406 | 391 | 285 |
| 424 | 405 | 385 | 280 | |
| Spectrum. | Zn (wt.%) | Al (wt.%) | Ag (wt.%) | Solder Component |
|---|---|---|---|---|
| Spectrum 1 | 78.2 | - | 21.8 | phase AgZn3 |
| Spectrum 2 | 98.7 | - | 1.3 | solid solution (Zn) |
| Spectrum 3 | 77.2 | 20.9 | 1.9 | solid solution (Al″) |
| Spectrum 4 | 63.6 | 35.5 | 0.9 | Zn–Al eutectic |
| Spectrum 5 | 41.6 | 58.1 | 0.3 | solid solution (Al″) |
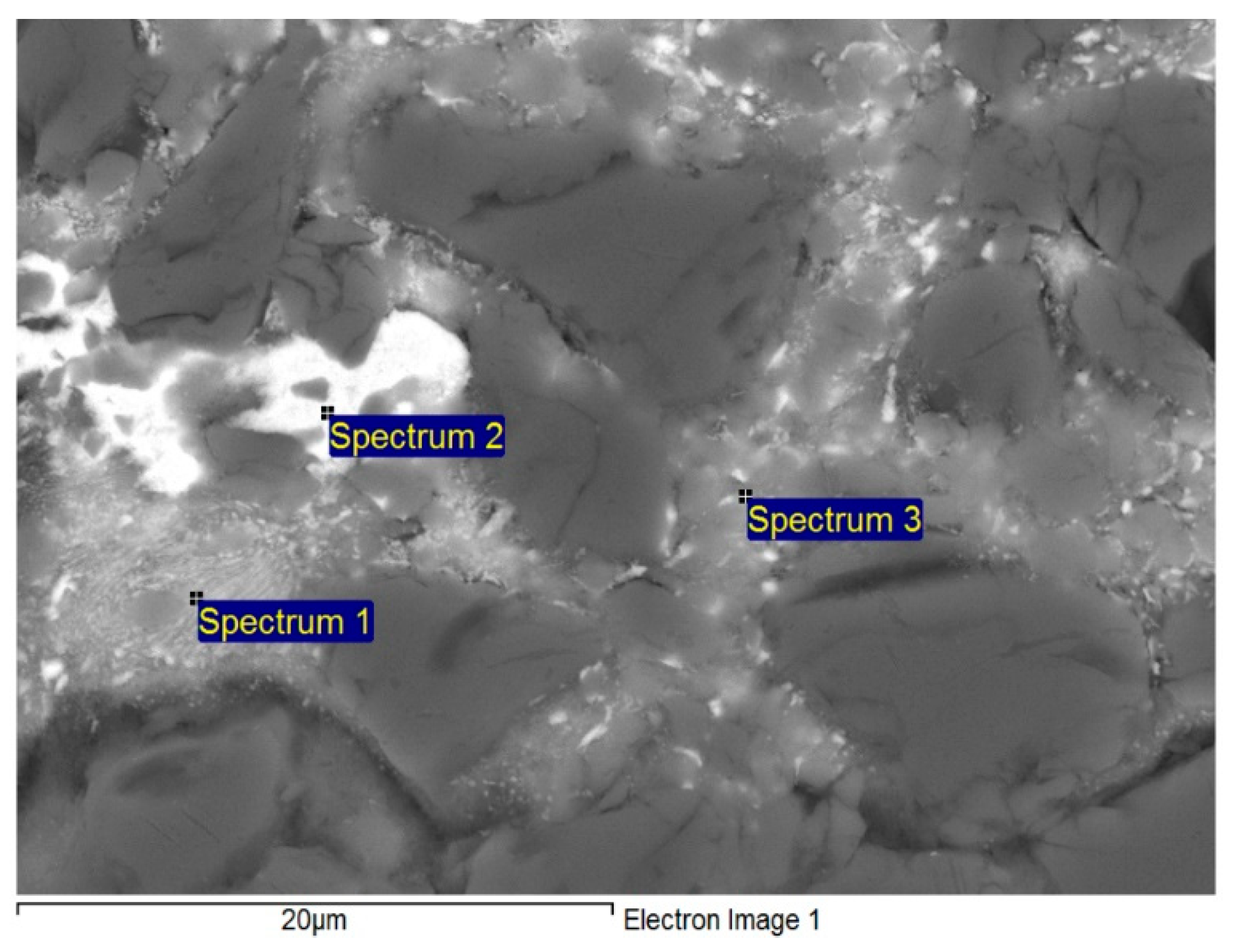
| Spectrum | Zn (wt.%) | Al (wt.%) | Ag (wt.%) | Solder Component |
|---|---|---|---|---|
| Spectrum 1 | 47.93 | 44.80 | 7.26 | phase (Al) |
| Spectrum 2 | 76.28 | 5.07 | 18.65 | Phase ε–AgZn3 |
| Spectrum 3 | 48.23 | 48.23 | 3.54 | phase (Al) |
| Spectrum. | Zn (wt.%) | Al (wt.%) | Ag (wt.%) | O (wt.%) |
|---|---|---|---|---|
| Spectrum 1 | 72.17 | 9.90 | 7.04 | 10.89 |
| Spectrum 2 | 73.76 | 10.83 | 5.75 | 9.66 |
| Spectrum 3 | 64.95 | 25.20 | 2.80 | 7.04 |
| Spectrum 4 | 69.02 | 14.64 | 2.84 | 13.51 |
| Spectrum 5 | 62.02 | 25.69 | 4.23 | 8.06 |
| Spectrum 6 | 57.42 | 14.80 | 11.51 | 16.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolenak, R.; Kostolny, I.; Drapala, J.; Babincova, P.; Pasak, M. Study of Zn6Al6Ag Alloy Application in Ultrasonic Soldering of Al2O3–(Al/Al2O3) Joints. Metals 2020, 10, 343. https://doi.org/10.3390/met10030343
Kolenak R, Kostolny I, Drapala J, Babincova P, Pasak M. Study of Zn6Al6Ag Alloy Application in Ultrasonic Soldering of Al2O3–(Al/Al2O3) Joints. Metals. 2020; 10(3):343. https://doi.org/10.3390/met10030343
Chicago/Turabian StyleKolenak, Roman, Igor Kostolny, Jaromir Drapala, Paulina Babincova, and Matej Pasak. 2020. "Study of Zn6Al6Ag Alloy Application in Ultrasonic Soldering of Al2O3–(Al/Al2O3) Joints" Metals 10, no. 3: 343. https://doi.org/10.3390/met10030343
APA StyleKolenak, R., Kostolny, I., Drapala, J., Babincova, P., & Pasak, M. (2020). Study of Zn6Al6Ag Alloy Application in Ultrasonic Soldering of Al2O3–(Al/Al2O3) Joints. Metals, 10(3), 343. https://doi.org/10.3390/met10030343





