Abstract
Modern methods of metal and metal-containing materials production involve a serious consideration of the impact on the environment. Emissions of greenhouse gases and the efficiency of energy use have been used as starting points for more sustainable production for several decades, but a more complete analysis can be made using life cycle assessment (LCA). In this paper, three examples are described: the production of precipitated calcium carbonate (PCC) from steelmaking slags, the fixation of carbon dioxide (CO2) from blast furnace top gas into magnesium carbonate, and the production of metallic nanoparticles using a dry, high-voltage arc discharge process. A combination of experimental work, process simulation, and LCA gives quantitative results and guidelines for how these processes can give benefits from an environmental footprint, considering emissions and use and reuse of material resources. CO2 mineralization offers great potential for lowering emissions of this greenhouse gas. At the same time, valuable solid materials are produced from by-products and waste streams from mining and other industrial activities.
1. Introduction
Metal production and subsequent production, use, and disposal of metallic (or metal-containing) products have a significant and worldwide environmental impact. Besides the immediate impact of extraction of rock and ores, a large contribution to emissions of carbon dioxide (CO2) resulting from a vast use of energy, a very significant use of fresh water and the effect of disposing enormous amounts of tailings, other mining residues and by-products/wastes of metal processing can be mentioned. On top of this, an increasing world population requests ever increasing amounts of materials and products while facing the challenges of climate change and environmental pollution in the world that has finite resources.
The greatest potential for reversing the trends is offered by methods and assessment tools that address several problems at the same time. Three of these methods from the field of metal and metal-containing products manufacturing are addressed in this paper, describing and summarizing the work done in Finland, mostly as PhD thesis works supervised by the author, during the last fifteen to twenty years. These three methods are as following:
1.1. Steelmaking Slags Valorization
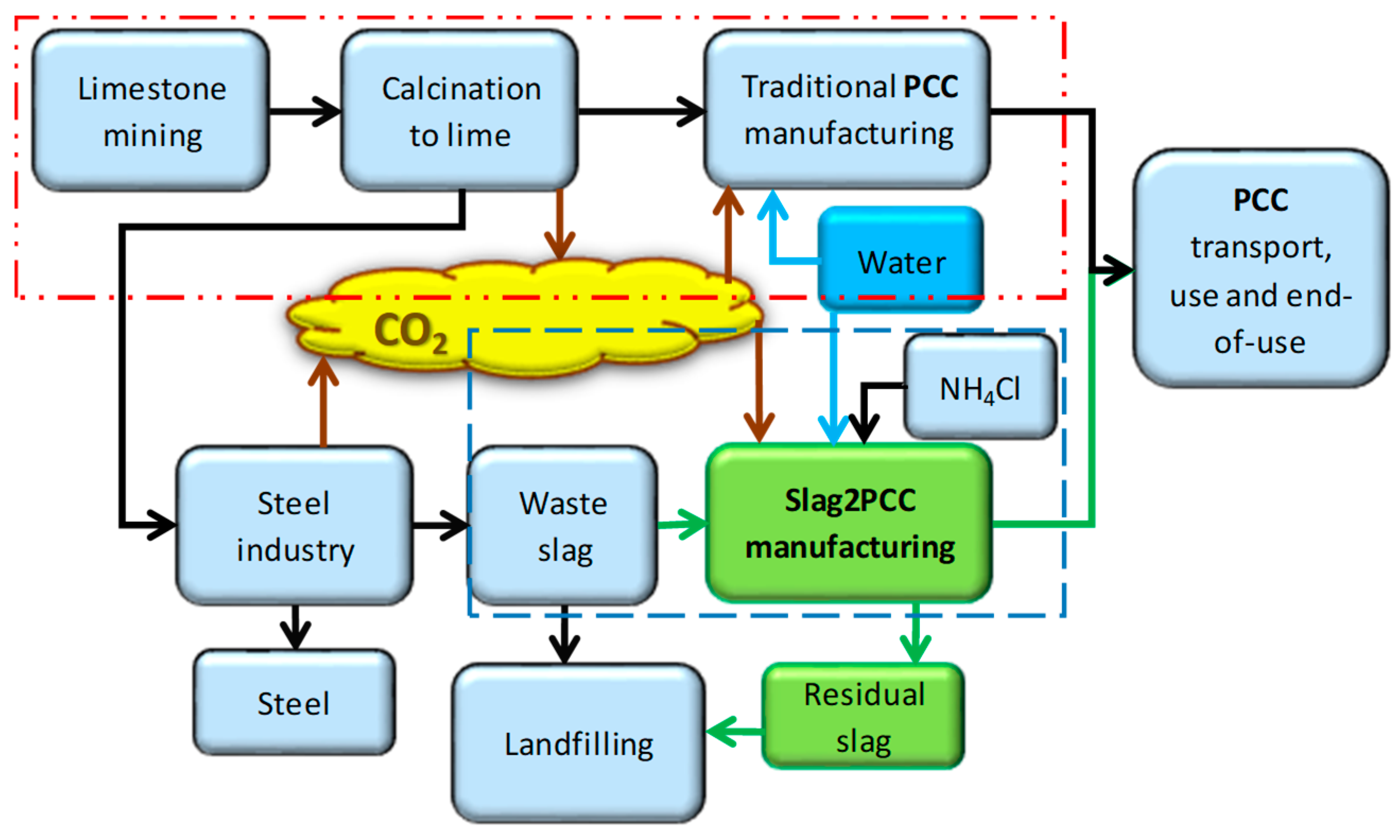
Steelmaking slags, more specific steel converter (basic oxygen furnace, BOF) slags can be converted with CO2 into precipitated calcium carbonate (PCC) with market value. This route also known as the slag2PCC concept [1,2,3,4,5,6,7,8,9].
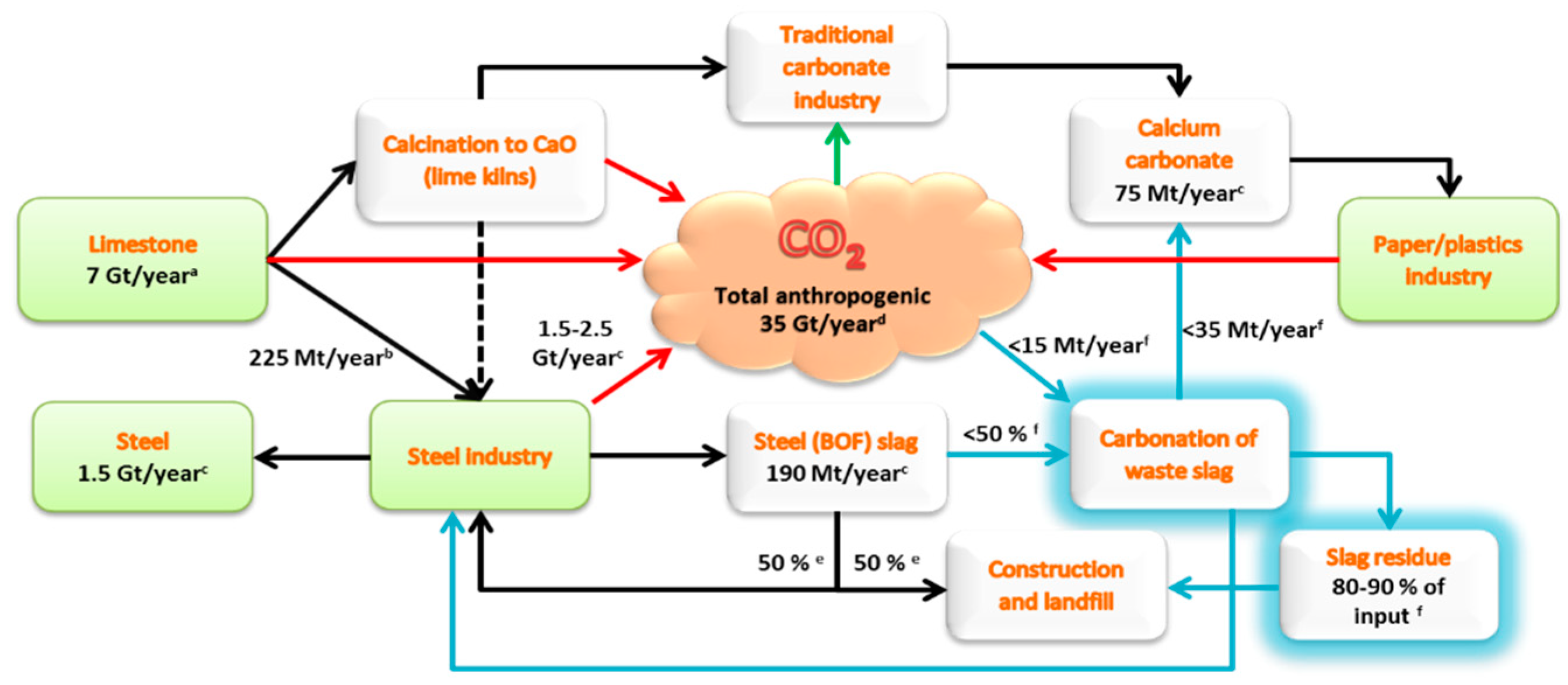
Figure 1 gives an overview of how the sectors of iron- and steelmaking, paper/plastics and the mining of limestone are interconnected with typical global annual mass flows indicated [6]. With significant fixed amounts of CO2, steel converter (BOF) slags can be diverted from landfill and processed into valuable PCC, which reduces the need for the mining and calcination of limestone. (It may be argued that producing PCC binds the same amount of CO2 as what was released during limestone calcination, but it should be noted that CO2 emissions from fuels used for limestone calcination, which stands for approximately 1/3 of the CO2 emissions, are avoided when reusing calcium-rich slags.)
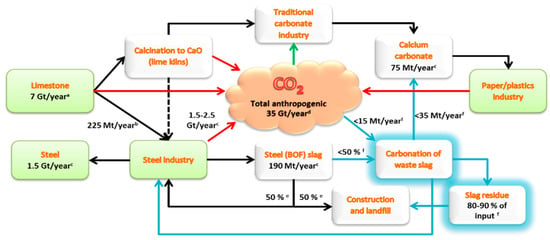
Figure 1.
Typical annual mass flows of anthropogenic CO2 and steelmaking slags, paper/plastics and limestone rock from a viewpoint of precipitated calcium carbonate (PCC) production.
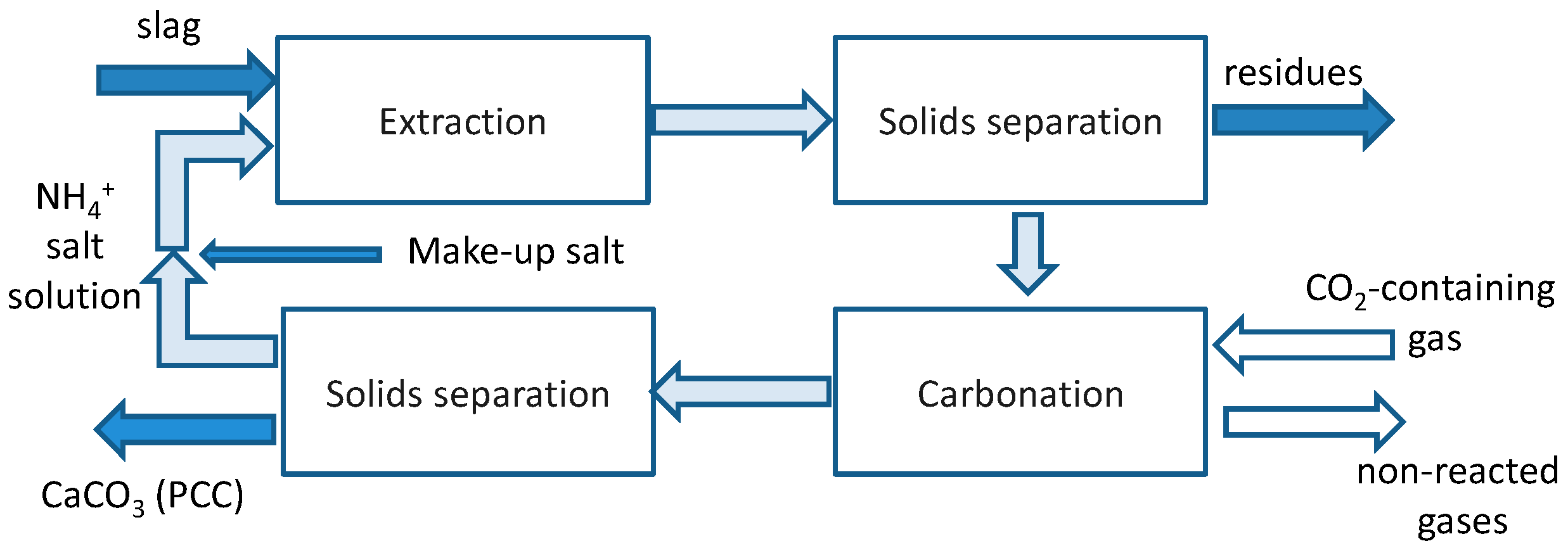
Figure 2 summarizes the slag2PCC concept that may operate on CO2-containing gas directly (if the target PCC quality allows for it) without a need for a separate CO2 capture step. This opportunity is one of the main strengths of CO2 mineralization as a CO2 capture and storage (CCS) technology, since the capture step gives a very significant energy penalty (see [10]). The overall chemistry can be summarized by Reactions (R1)–(R3), where ion “X” is nitrate—NO3−, chloride—Cl−, or acetate—CH3COO−:
CaO(s) + 2NH4X(aq) + H2O(l) ↔ CaX2(aq) + 2NH4OH(aq),
CO2(g) + 2NH4OH(aq) ↔ (NH4)2CO3(aq) + H2O(l),
(NH4)2CO3(aq) + CaX2(aq) ↔ CaCO3(s) + NH4X(aq).
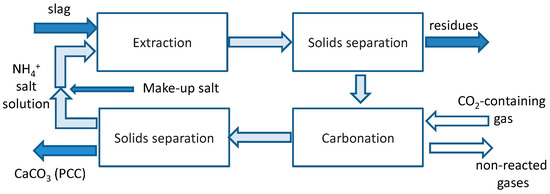
Figure 2.
Schematic overview of the slag2PCC process concept.
As shown, the solvent solution is recycled for reuse, although some makeup salt is needed depending on PCC separation from the solvent and subsequent washing.
1.2. Blast Furnace Top Gas Processing
The second concept implies fixing CO2 from a blast furnace (BF) top gas into solid carbonate using magnesium extracted from serpentinite rock. This is based on the first one of several so-called Åbo Akademi (ÅA) routes for step-wise carbonation of serpentinite rock. Mineral sequestration offers a large-scale CCS option for Finland and many other countries, where the underground storage of CO2 is not possible [10]. While a significant volume of the literature using ÅA routes for CO2-containing exhaust gases from heat and power production exists, metal production or mineral processing [11,12,13,14,15,16,17,18] a special opportunity arises for the processing of a BF top gas with Mg(OH)2 that can be obtaineded from thevast(more than needed) natural resources of magnesium silicate rock available worldwide [19]. BF top gas, containing CO2 as well as CO in more or less equal amounts of approximately 20 vol.%, can be processed by sequential mineralization of CO2 followed by conversion of CO into CO2 and H2 via the CO/water shift reaction [20]:
which (depending on H2O pressure and temperature) competes with the following reaction:
Two different conversion efficiencies can be defined from the CO2 conversion and the carbonation of Mg(OH)2, respectively, shown as:
As shown, the overall chemistry gives not only the fixation of CO2 in thermodynamically stable magnesite, but MgCO3 produces hydrogen at the same time. The latter can be easily separated from a gas stream using a membrane. Magnesite may be hydrated to nesquehonite, MgCO3∙3H2O, which may be used as a thermal energy storage (TES) material in a cyclic magnesite/nesquehonite conversion process [21]. As the first analysis of this potential, an optimization study was made for BF top gas processing as a function of temperature, pressure, and Mg(OH)2 vs. CO + CO2 conversion [20]. A similar approach was followed in experimental work in China using calcium oxides for carbonation in parallel with CO/water shift reaction in a fluidized bed reactor [22]. While this method has the advantage of atmospheric pressure operation and somewhat higher temperatures than used with Mg(OH)2, the fixed CO2 is eventually released in order to regenerate CaO, which gives it rather a calcium looping character.
1.3. Metallic Nanoparticle Production
The third concept involves production of metallic nanoparticles (NPs) using a production route with a potentially lower environmental impact than conventional methods. Development of a list of metals and alloys (Ag, Al, Au, Cu, Ni, Zn and FeCr, and NiCu) in the unit of kilograms per day was the task of the recent EU FP7 project BUONAPART-E [23]. One objective was to reduce the environmental impact of metallic NP production by avoiding the use of complex mixes of toxic or hazardous chemical solutions (for reducing oxidized metallic salts), resulting in a significant postprocessing of by-products and wastes.
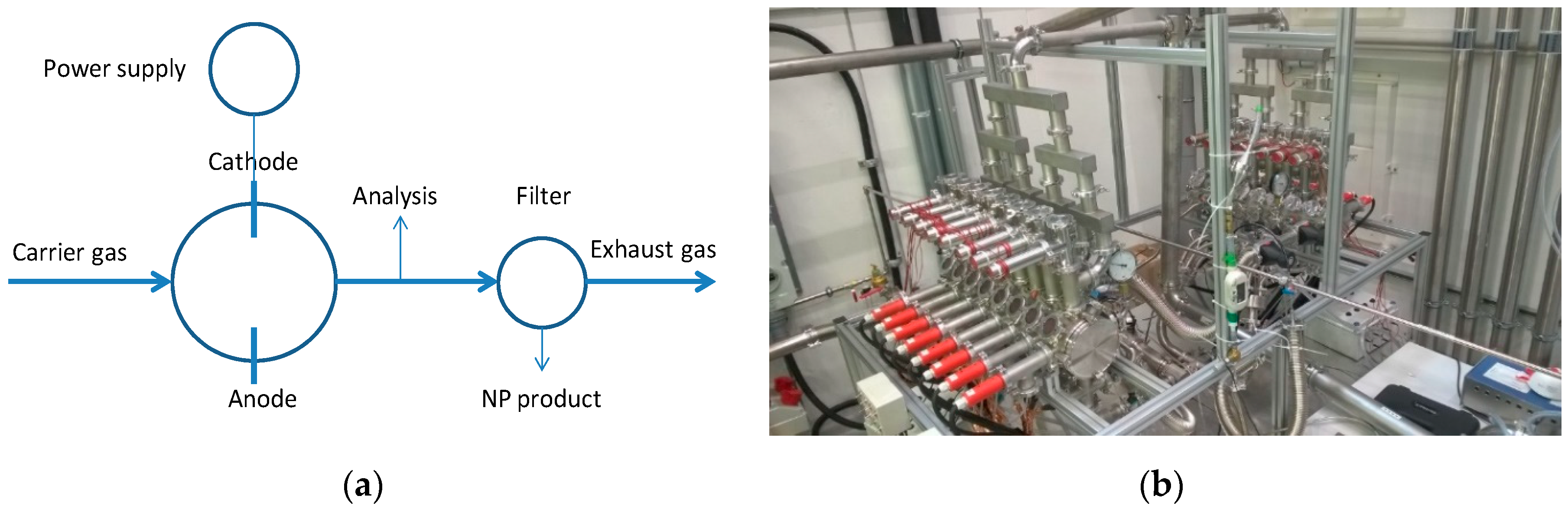
The BUONAPART-E NP production route involves the use of high-voltage arc discharges for melting and evaporation of metal, followed by condensation and solidification in an inert gas atmosphere (N2, Ar, Ne, and N2/H2 mixture (95%/5% vol./vol.)). A simplified process diagram is shown in Figure 3a, Figure 3b displays a 2 × 8 operational units set-up at the University of Duisburg-Essen (UDE), Germany. The units were shown to be capable of producing (using 15 of the 16 electrode pairs) Cu NPs with a primary particle size of 79 nm at a production rate of 69 g/h at a specific electricity consumption (SEC) of 170 kWh/kg in a N2 carrier gas. NP sizes down to 14 nm were obtained by adjusting gas composition, gas flow, or applied electric power [24].
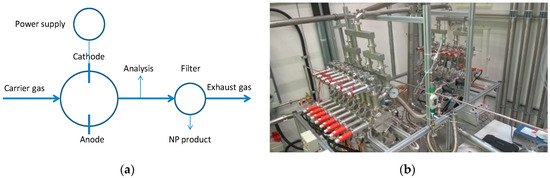
Figure 3.
(a) Schematic overview of the BUONAPART-E concept for metallic nanoparticle production. (b) Multiple operating units (2 × 8) system at the University of Duisburg-Essen (UDE), Duisburg, Germany.
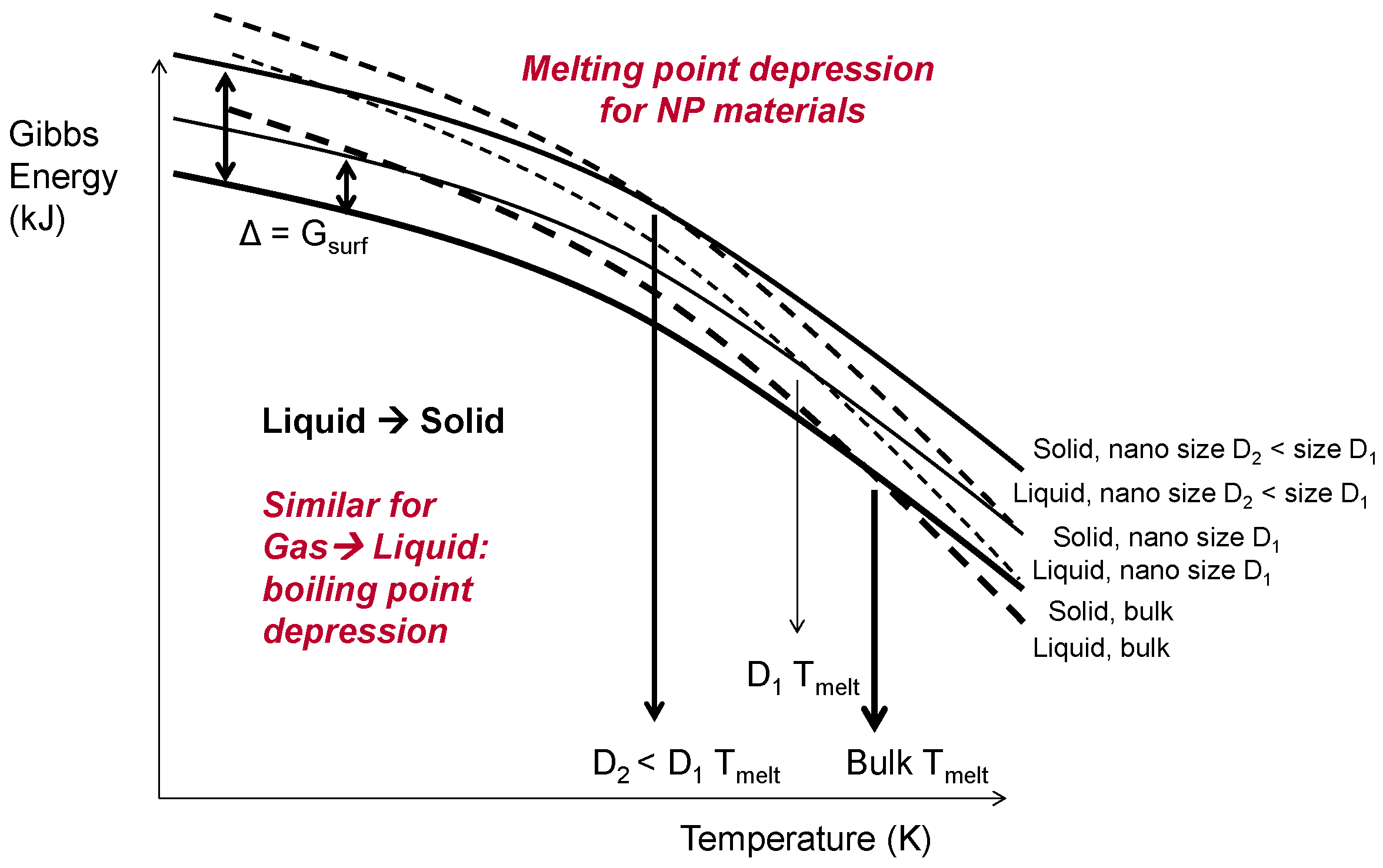
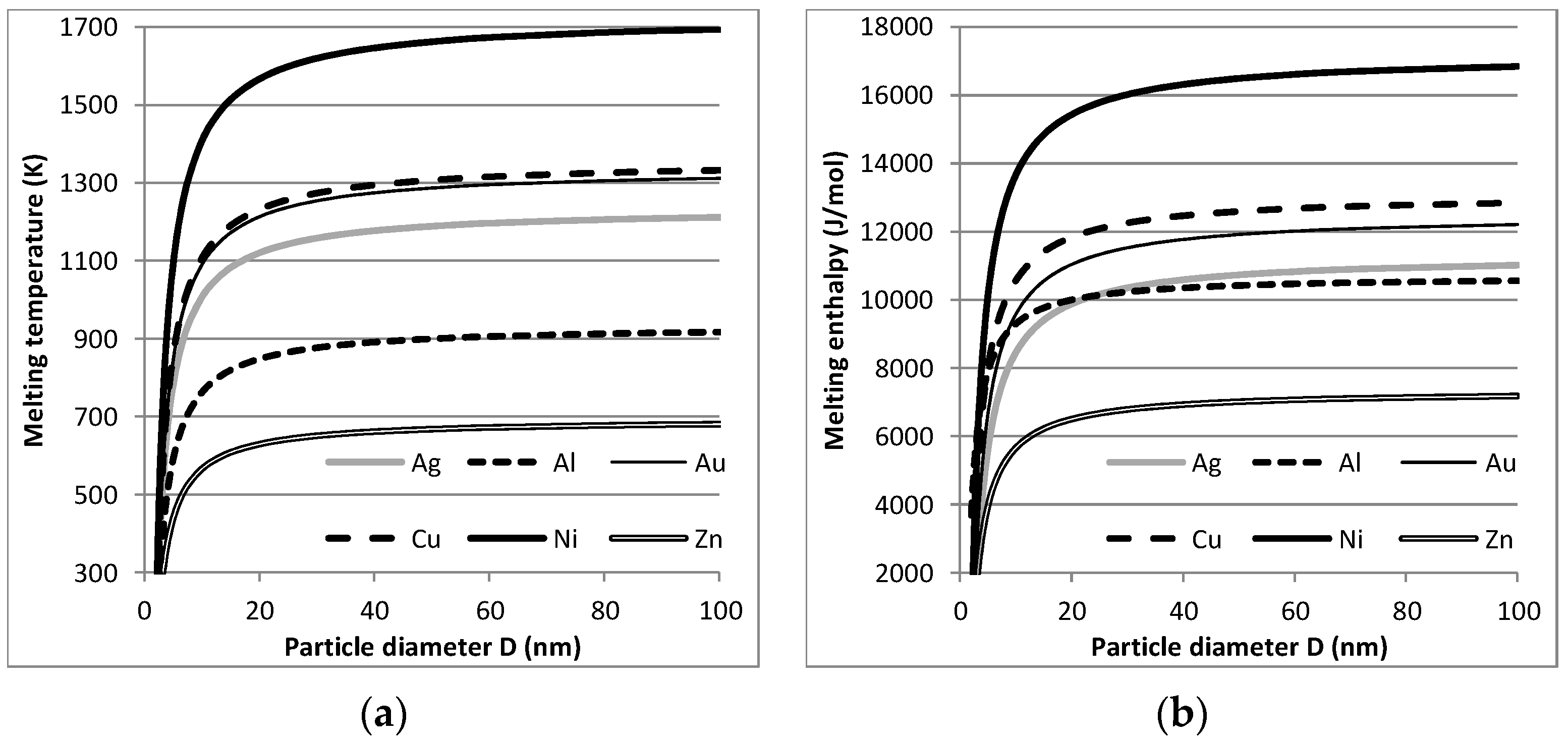
At ÅA, the efficiency of energy (i.e., electricity) use and the environmental footprint of the dry, arc discharge NP production route were analysed and compared with those of more conventional production routes based on aqueous solution chemistry. One feature that makes the energy efficiency assessment very interesting is the fact that below a size of approximately 50 nm the thermodynamic properties of (metallic) NPs start to change: melting and evaporation temperatures as well as heat (i.e., enthalpy) for that were lowered. This is illustrated by Figure 4 for the melting/solidification of NPs with both sizes, D1 and D2, of <<100 nm (D2 < D1), compared with the properties of bulk materials, which was confirmed by the data for 5 nm Ag NPs in [25].
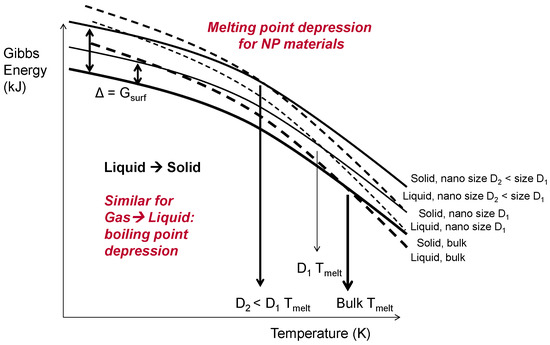
Figure 4.
Gibbs energies of nanosized droplets and solid particles vs. those of bulk size materials, showing melting point depression for the diameter D1 of <<100 nm and D2 < D1.
This can be found in [26], where an analysis of energy needs for (metallic) NP production is based on the surface free energy (SFE) of atoms at the surface compared to those in the bulk volume of a material. (For liquid–gas and liquid–liquid interfaces, SFE is commonly referred to as surface tension.) As in fact work is needed, here in the form of electricity, to increase or form an interface with the surroundings, energy efficiency assessments based on exergy (see below for more details) are straightforward, once data on SFE are found. The work by Xiong et al. [27,28] reported on diameter-dependent thermodynamic properties of metallic NPs, such as temperatures and enthalpies for melting and evaporation and their temperature dependences.
Using the scarce literature, for six metallic elements, the relations between NP size and melting temperature and melting heat could be calculated as given in Figure 5 (see also [26]). It should be noted that entropy change as the ratio melting heat/melting temperature is unchanged. The same was found for the evaporation/condensation process. As reported below, this had significant consequences for the energy use of the dry, arc discharge process for (metallic) NP production and the life cycle footprint of this production route.
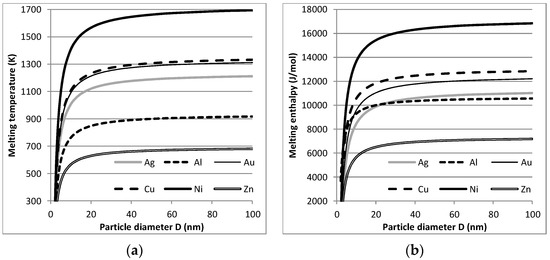
Figure 5.
Size-dependent melting points (a) and melting enthalpy (b) for six metallic nanoparticles (NPs).
These three concepts will be evaluated for their potential when aiming at reduced CO2 emissions, less production of wastes or an overall smaller environmental footprint when compared to “business as usual” or other profitable production. Prohibitive legislation and/or regulation may be on the horizon. Nonetheless, however promising a “better” method may seem and regardless of evidence that these methods are from many points of view superior to existing methods, large-scale implementation will not occur until industry recognizes an effective business model. Minimizing or lowering costs is not sufficient, since profit must be made at all times while guaranteeing some level of growth, with new products promptly available in the market place.
The red line of this paper is new technologies and tools for production of metals and metal-containing products, resulting in a smaller environmental footprint. This is illustrated by three project-driven and independent studies with each having a narrower focus. For this reason, readers are encouraged to consult the original papers as referenced for more details on background, methods and results.
2. Materials and Methods
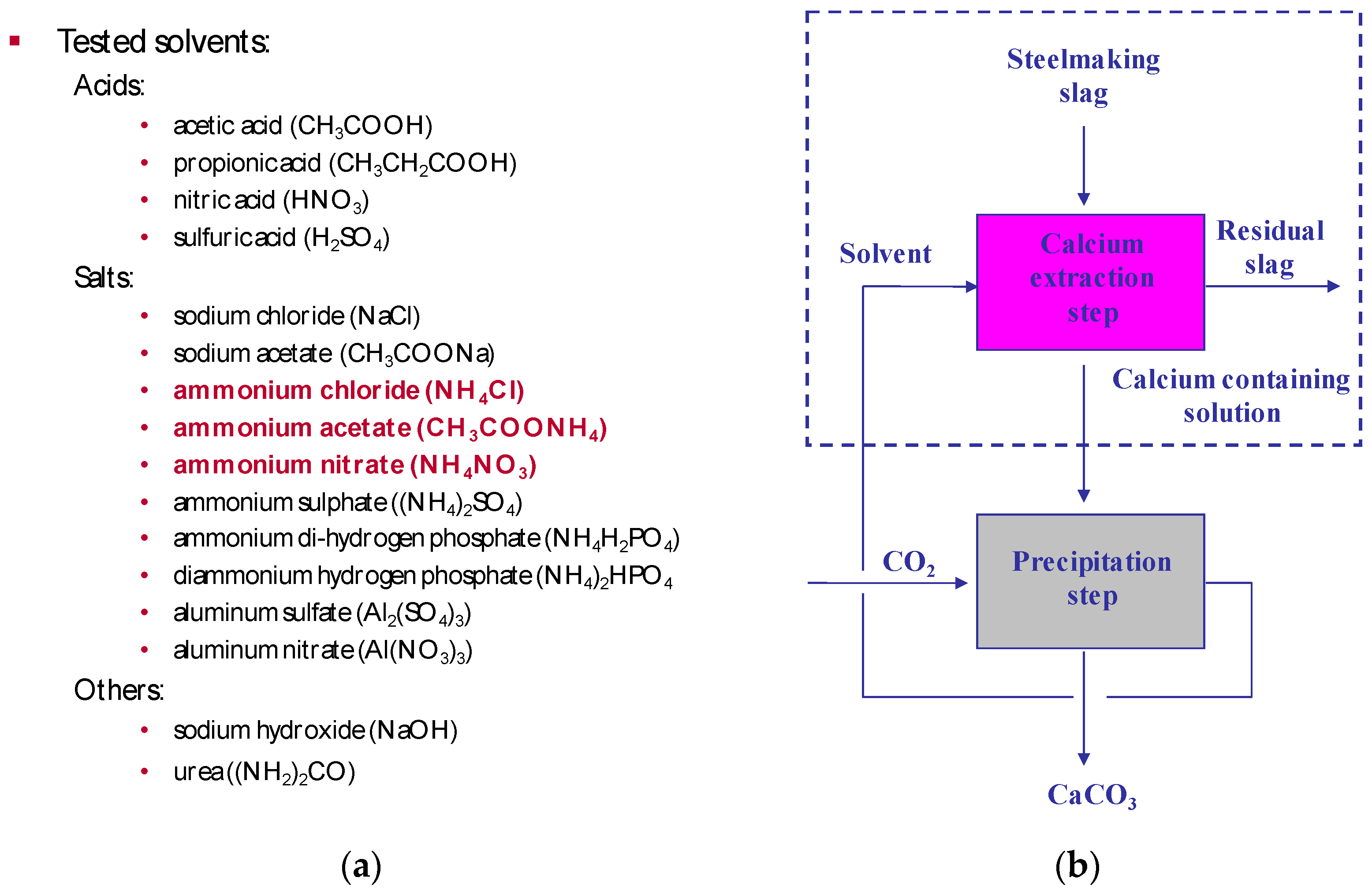
Since 2005, the development of the slag2PCC concept has involved experimental work later followed by process simulation (Aspen Plus) on steel converter (BOF) slags from Finnish iron- and steelmaking industry. A range of possible solvent salts was tested in aqueous solutions at ambient pressure and low temperature (20–30 °C). In a few cases, heating was used until up to 70 °C [2,3,4,5,6,7,8], in order to selectively leach calcium from slags (see Figure 6 [4]).
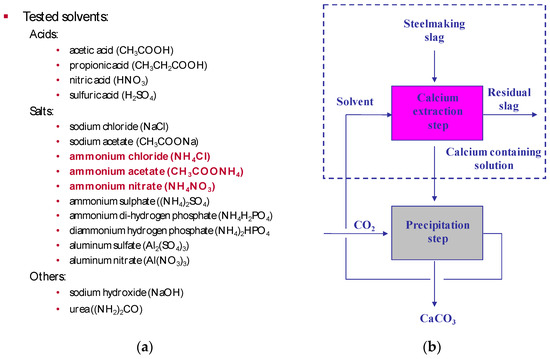
Figure 6.
(a) Solvents tested as aqueous solutions for selective leaching of calcium from steel converter (basic oxygen furnace (BOF)) slags with three successful solvents highlighted. (b) Carbonation to produce PCC with the recycling of the leaching solvent.
Successful experiments were done with ammonium salts, mostly ammonium chloride. Initial tests were done with 1 L solutions, and later tests were conducted with 2 × 28 L vessels (made of Perspex) connected with separators and circulation pumps [5,6]. At the same time, Aalto University in Espoo Finland put up a demonstration plant with 200 L reactor vessels [7,8]. Later, the work involved life circle assessment (LCA) simulations using SimaPro with Ecoinvent and other databases [9] and process scale-up and integration with a focus on separation methods for PCC product solids and spent slags from aqueous dispersions [1,6].
For the analysis of carbonation of Mg(OH)2 with CO2 in the BF top gas, where in parallel the CO/water shift reaction supplies CO2 for subsequent mineralization, a simulation study was made using Aspen Plus (AspenTech, Bedford, MA, USA) (v.8.2 and later v.9.0). Pressures, temperatures, and Mg(OH)2 feed vs. gas feed were optimized for maximum CO2 as well as combined CO2 + CO conversion efficiencies while minimizing production of reactive MgO and energy input requirements. LCA studies that include the production of Mg(OH)2 as part of serpentinite carbonation for treatment of flue gases from a power plant or a lime kiln are given elsewhere [14,17].
For the analysis of the efficiency of energy use with a dry, arc discharge (BUONAPART-E) process route for metallic NP production, the production of waste heat was measured and quantified at the 2 × 8 operational single-unit production facility at the UDE, Germany, using an infrared camera (Fluke Ti9, Fluke Europe, Eindhoven, The Netherlands) and an infrared thermometer (Testo Quicktemp 860-2, Sensorcell, Helsinki, Finland) (see [14,29]). Exergy analysis was used for assessing how much electricity was used for creating NP surface energy and how much was dissipated as waste heat. While for electric power P the exergy Ex(P) is P, the exergy of heat Q depends on its absolute temperature T and that of the surroundings T° and can be written as Ex(Q) = Q∙(1 − T°/T). Szargut et al. [30] gave more details on exergy analysis, a concept that follows the second law of thermodynamics. The (Carnot) factor (1 − T°/T) quantifies the quality of heat, and thus the exergy of energy sources normalizes these to the capacity of doing work.
Besides electricity, also materials and other resources are used or consumed by NP production processes. Here, LCA simulations were made using SimaPro with Ecoinvent and other databases for comparing NP production routes and the use of metallic NPs in consumer products. These benefits were from special properties obtained already when using very small amounts of NPs. Examples are silver NPs in hospital cotton (bed sheets and lab coats), gold NPs in solar energy collectors, nickel NPs for catalysts, zinc NPs as flame redardants in plastics like PP, or copper NPs in water giving a nanofluid with improved cooling performance. Besides the summary in [14], more details on LCA work were given in [29] and [31] with focuses on energy use and comparisons of conventional methods (wet chemistry) for metallic NP production, respectively.
3. Results and Discussion
3.1. Steelmaking Slags Carbonation: the Slag2PCC Concept
3.1.1. Experimental Results and Process Scale-up
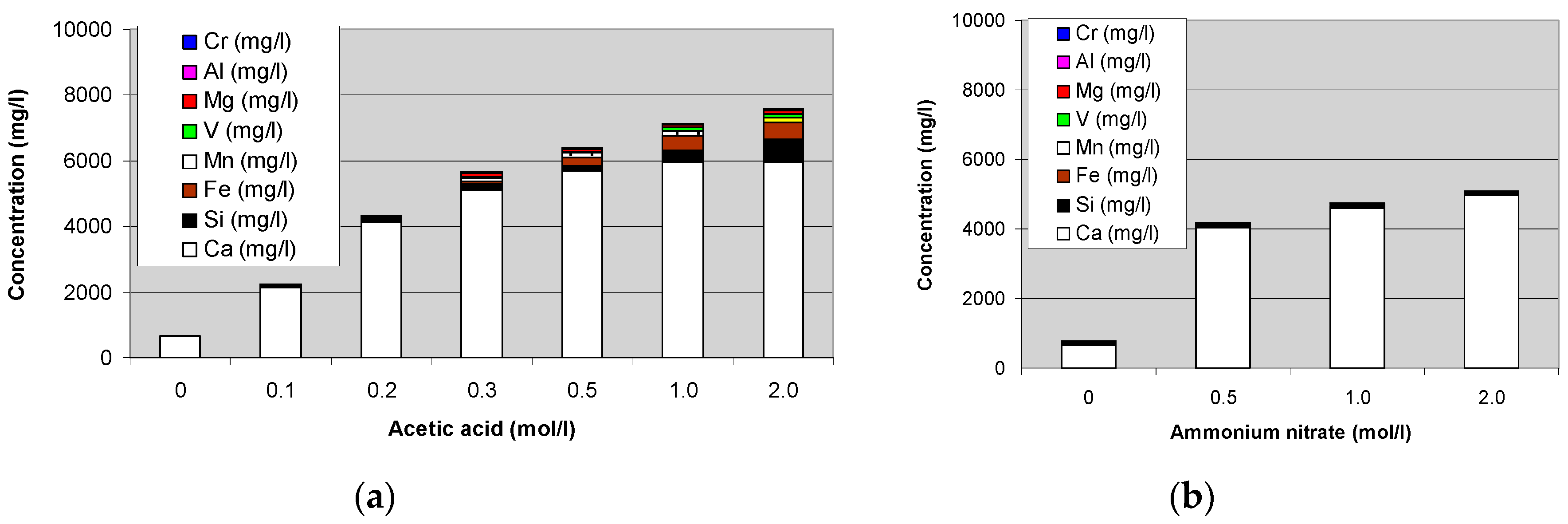
Steel converter slag leaching tests with a range of solvents as shown in Figure 6 gave for most cases no good results for calcium leaching and its selectivity. A good leaching (~90% after 1 h, 1 g per 50 mL at ambient conditions for particle sizes of 74–125 µm) without significant formation of precipitates was obtained with acetic acid, but unfortunately with significant leaching of other species was obtained as well, primarily silicon, iron, and manganese, as shown in Figure 7a. Leaching with ammonium salts gave much more selective leaching of calcium, with only very little silicon, as can be seen from Figure 7b. Leaching with ammonium nitrate gave a 40–45% leaching efficiency for calcium under the same conditions as for the acetic acid tests. Although the obtained leached calcium was less than with acidic solutions, the selectivity achieved with ammonium salts solvents further benefited from the alkalinity of the final solution. This made the precipitation of calcium carbonate possible with a pH buffering effect when adding CO2. After the precipitation, the solvent solution was returned to the leaching reactor. More details are given elsewhere [4].
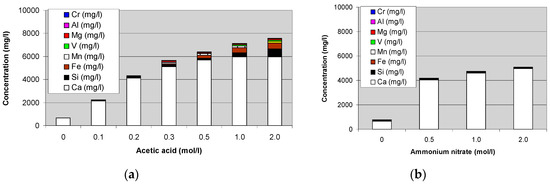
Figure 7.
Selective leaching of calcium and other elements from steel converter (BOF) slags at ambient conditions using acetic acid (a) and ammonium nitrate (b) solutions with different strengths.
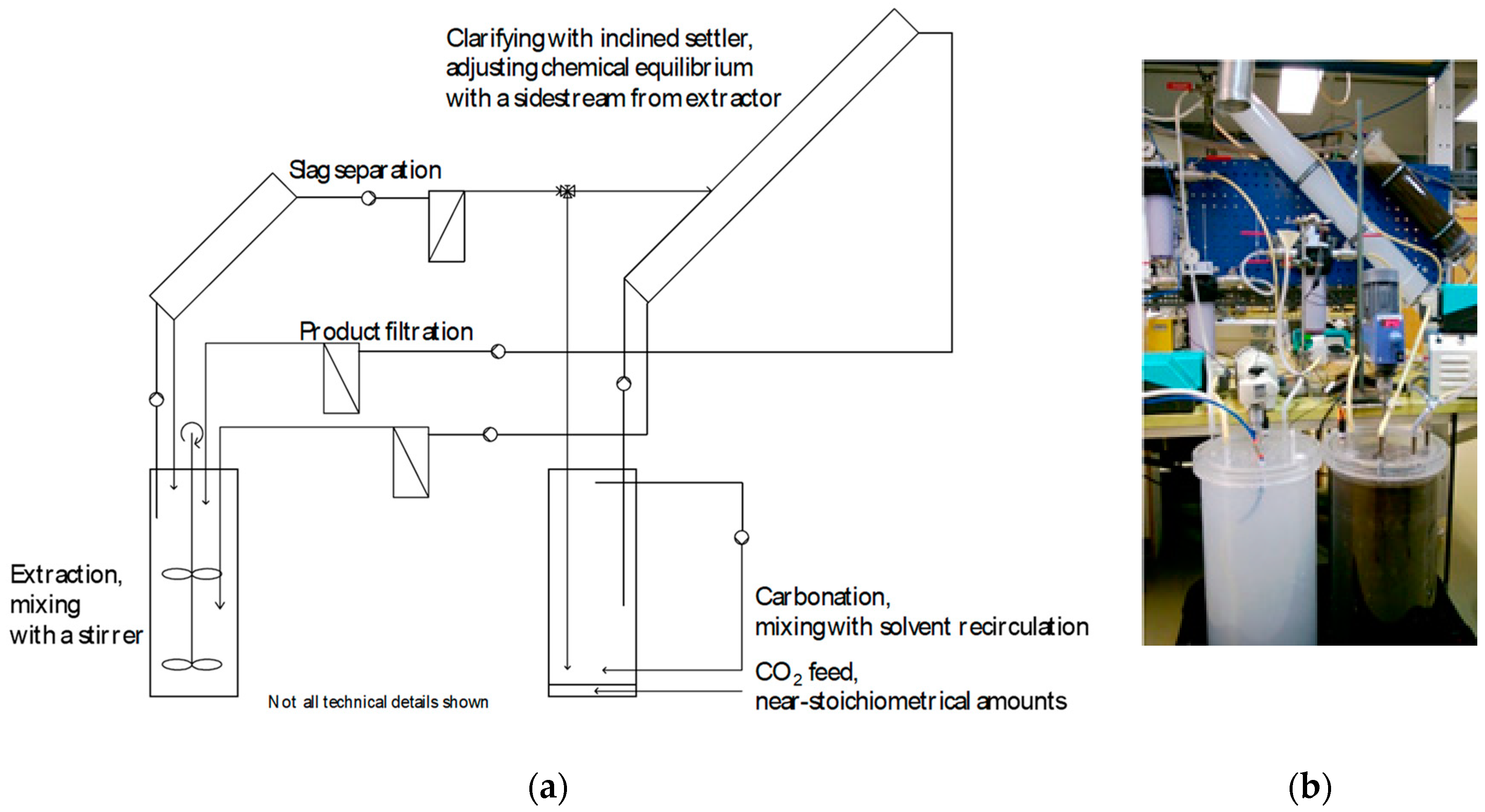
Having identified a suitable and selective solvent that allows for PCC production at (near) ambient conditions, the work proceeded with scale-up and operation in a continuous process mode. Compared to conventional PCC production, which typically implies a batch process (starting with lime, CaO), a continuous process offers more flexibility for varying composition, calcium content, and calcium compounds of industrial steel converter slags. A schematic of the process set-up at ÅA, constructed of Perspex parts, is given in Figure 8, with two 28 L reactors for extraction and carbonation, respectively. The schematic also shows the two tubular-inclined (45°) settlers that removed ~99% of the dispersed solids from the solution (inner diameter: 0.1 m; length: 1.1 m for PCC removal; length: 0.5 m for spent slag removal) upstream of candle filters for further cleaning. The inset photo in Figure 8 shows the reactors with white PCC and black slag dispersions, respectively. A set of pumps circulated the dispersions between the reactor vessels at a rate of 1.5 L/min, while temperatures and pH values of the dispersions were measured on-line and logged.
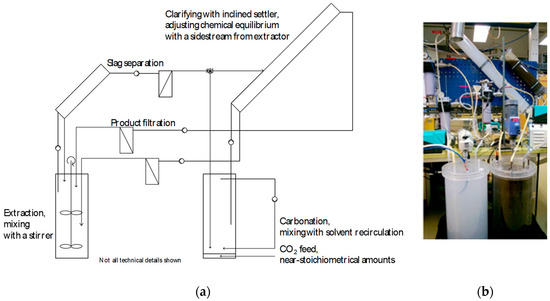
Figure 8.
(a) Schematic of the continuous lab-scale slag2PCC process set-up with two 28 L reactors and inclined settlers followed by candle filter separators for particle product and residue removal. In (b) the reactor vessel with black liquids is the extractor with dispersed steel converter slags, and the reactor with a white solution is the carbonator with dispersed PCC particles.
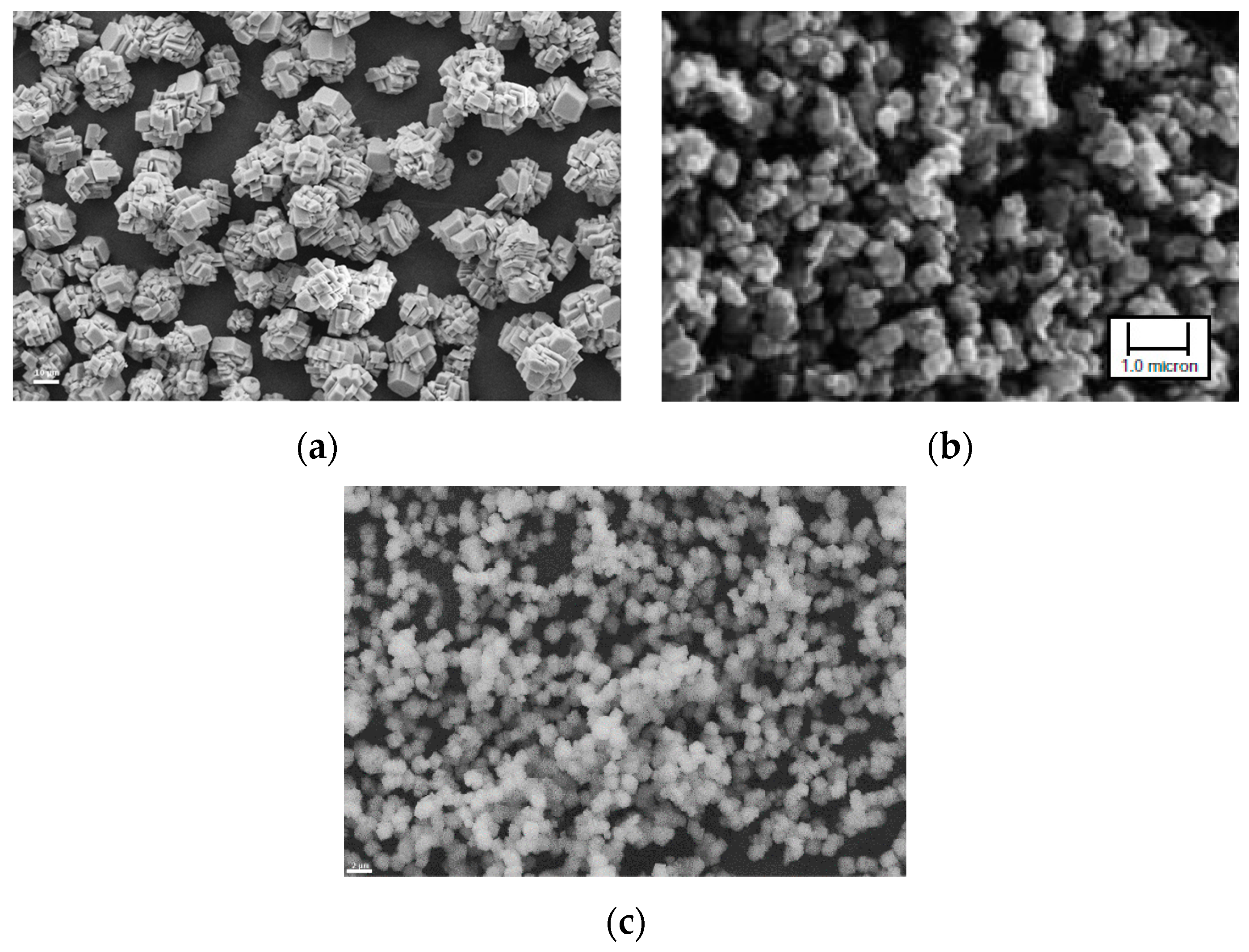
Using this set-up, PCC products with varying particle size and crystal shape were produced, depending on pH levels of the dispersions. Examples are given as SEM photos in Figure 9.
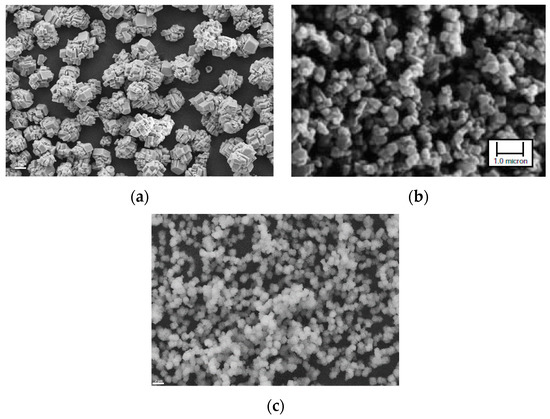
Figure 9.
PCC products produced from steel converter (BOF) slag using the slag2PCC concept with a 1 M ammonium chloride solvent: (a) rhombohedral calcite particles; (b) cubic calcite particles; and (c) spherical vaterite particles. Scale bar in (b): 1 µm.
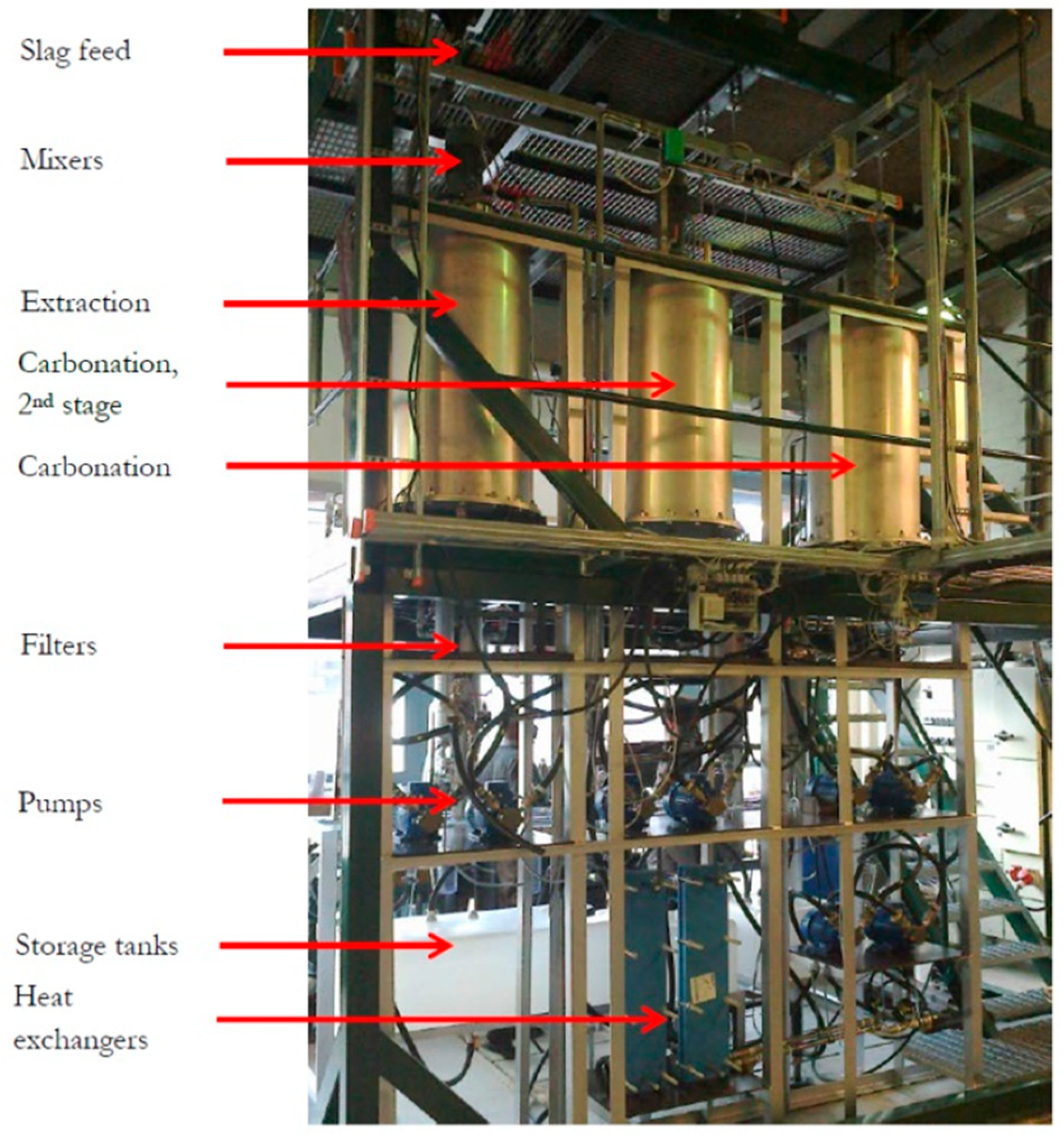
The results of the lab-scale experimental work combined with the parallel work at Aalto University resulted in the design and construction of a pilot-scale test set-up at Aalto University. The reporting by Said et al. [7,8] gives a description of this set-up, composed of 200 L reactor vessels, pumps, a feeding silo, and candle filters for the separation of particles from aqueous dispersions. Figure 10 gives an impression of the test facility. The objectives of ongoing work are scale-up to yet a larger scale than ~100 kg/h this pilot unit can handle, debottlenecking with respect to solid particles separation and increasing the amount of calcium extracted from the slags while guaranteeing the continuous production of PCC with preselected properties. This shall eventually take the technology into large-scale use and commercialization (following the patent in 2008).
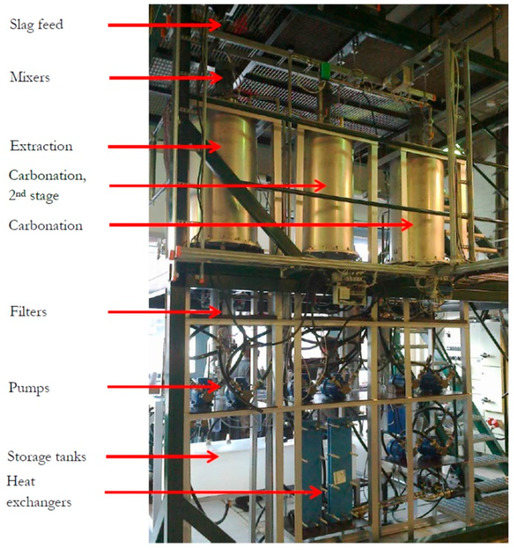
Figure 10.
Slag2PCC pilot-scale test facility at Aalto University, Espoo Finland. The photo was taken by the author.
3.1.2. LCA of PCC Production
A wide-scope analysis of whether a certain product or process is more preferable than another from an environmental footprint point of view can be made using LCA. A comparison of the slag2PCC process route with conventional PCC production was made, as illustrated by Figure 11. SimaPro software (v. 7.3.2) with several life cycle inventory (LCI) datasets (Ecoinvent v3, European Life Cycle Data (ELCD), EU and Danish Input Output Library, and Swiss Input Output) were used for the calculations (see [6,9] for more details). Four impact categories being climate change, human health, ecosystem quality, and resources were considered. A cradle-to-gate assessment was made, excluding the product use and the end-of-life phase, as these would be the same as for conventional PCC production.
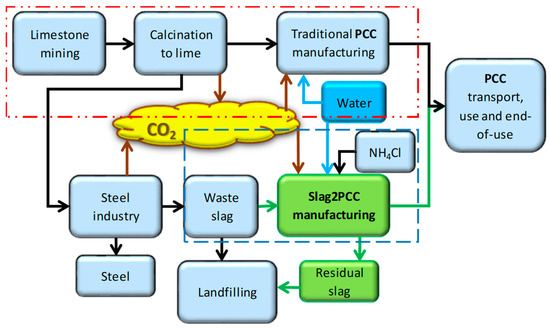
Figure 11.
System boundaries for PCC production using traditional processing (red striped line box) and the use of the slag2PCC concept (blue striped line box).
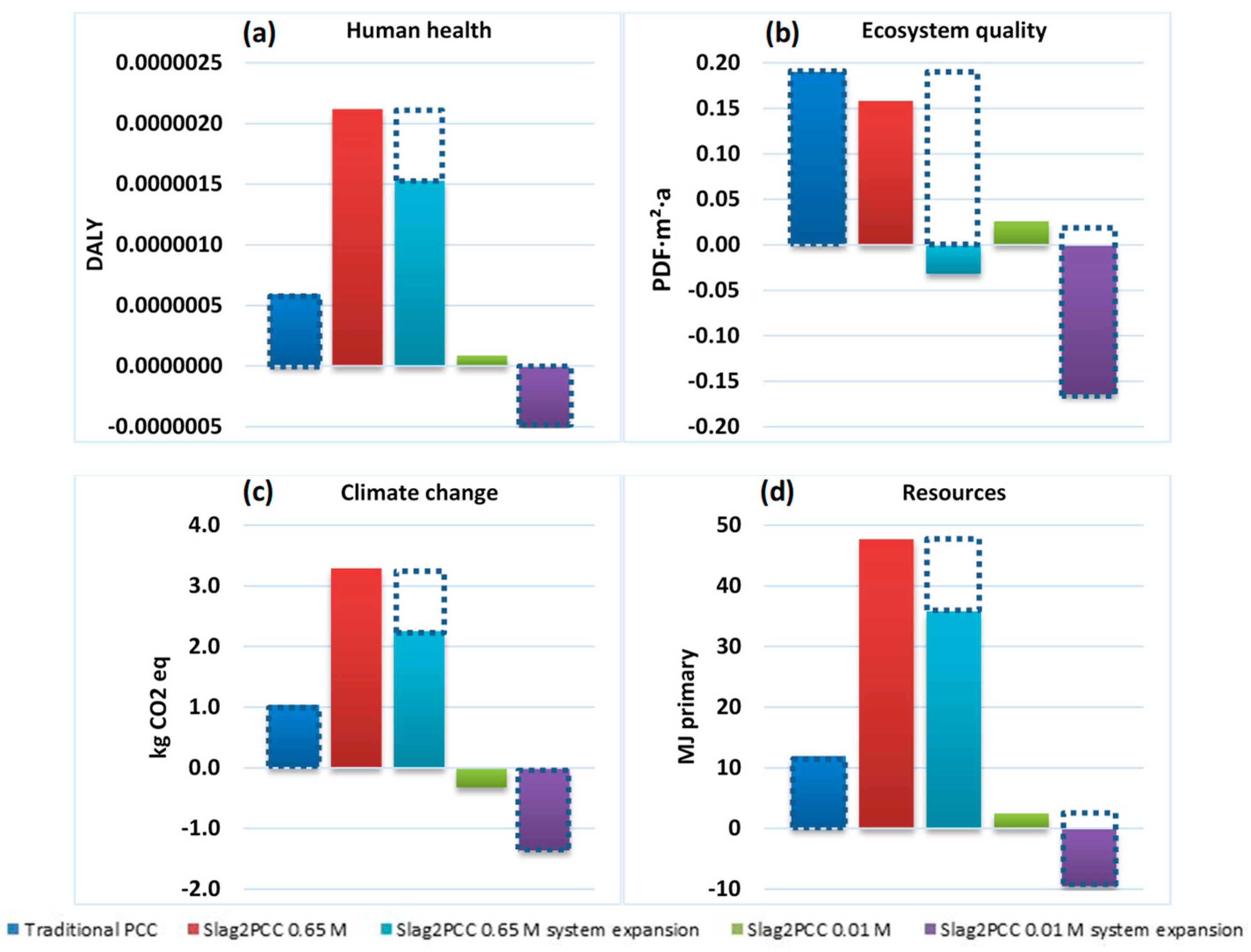
The results are given in Figure 12, initially for a comparison between conventional PCC production and slag2PCC operating with a 0.65 M ammonium solvent solution and a 0.1 kg/L slag/liquid loading, which from a process performance point of view was found to be optimal. However, this gave a larger environmental impact than the conventional PCC production, as the solvent leaving the process attached to the PCC product required a significant energy input (steam) for its recovery. The outcome was better using a “system expansion” consideration that took into account the fact that the PCC market has a limited volume. Producing PCC using an alternative route lowered the impact of the conventional process route, most likely with a location elsewhere, which was partly taken out of business (see [9]). This is illustrated by the dotted rectangles in Figure 12.
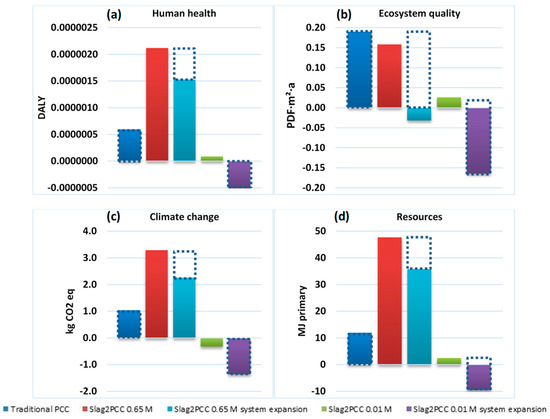
Figure 12.
Life cycle assessment (LCA) results for PCC production using a traditional method versus the use of the slag2PCC concept at different solvent concentrations and/or as a system expansion concept (see the text) for four impact categories: Human health (a), Ecosystem quality (b), Climate change (c), Resources (d) DALY: disability adjusted life years. PDF∙m2∙a: potentially disappeared fraction of species times area and time.
Nonetheless, a strong environmental benefit was obtained by diluting solutions in the slag2PCC process to 0.01 M NH4Cl while keeping the slag loading similar to 0.65 M operation, after which much less product washing was needed. A significant negative environmental footprint was obtained for the system expansion approach, replacing the market volume and the environmental footprint of conventionally produced PCC. In practice, operating the slag2PCC route at lower solvent concentrations implies that dispersions may make several cycles to obtain full conversion. More details on the consequences of operating the slag2PCC process under such conditions are given in [6,9].
3.2. Mineralization of CO2 from BF Top Gas Using Magnesium Hydroxide
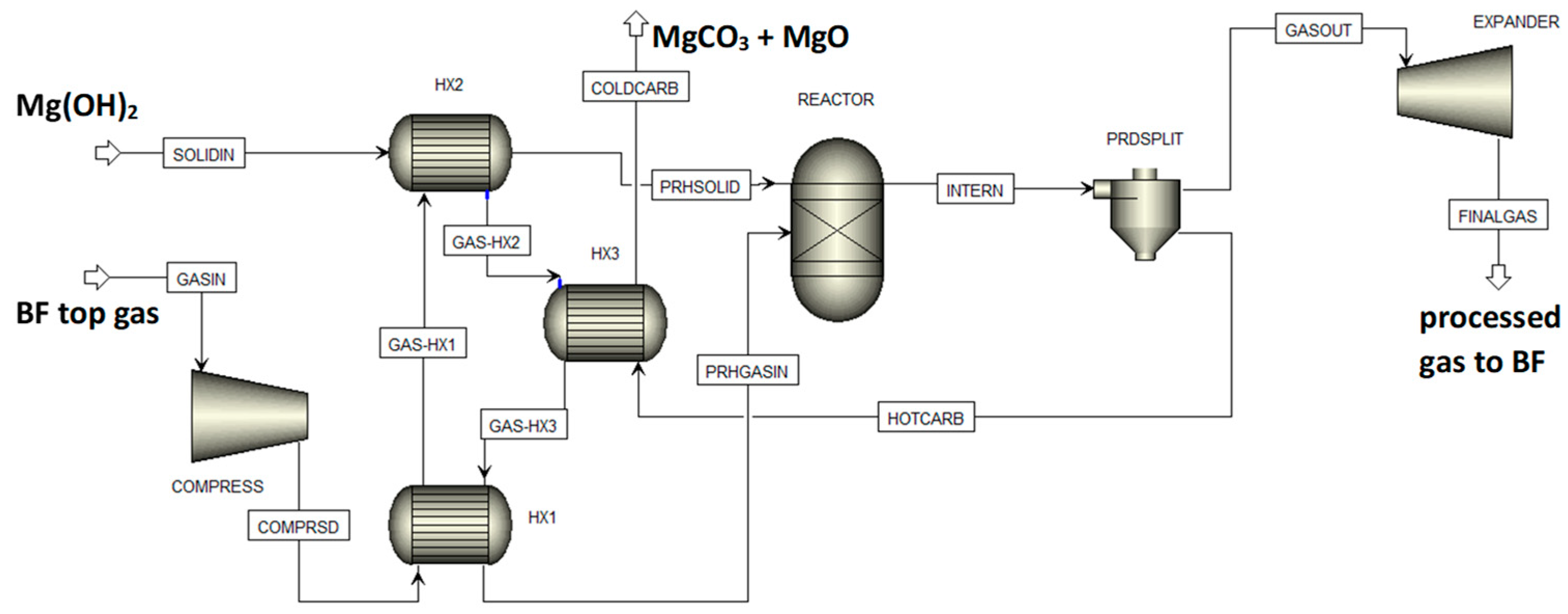
For the assessment of the feasibility of integrated mineralization of CO2 from BF top gas using Mg(OH)2 produced from magnesium silicate rock and CO/water shift reaction supplying CO2 from CO, a BF top gas composed of 20.7 vol% CO2, 20.7vol% CO, 3.8 vol% H2, 6 vol% H2O, and 48.8 vol% N2 was assumed [32]. The flow sheet for the Aspen Plus model used for the simulations is shown in Figure 13, with SOLIDIN as the Mg(OH)2 supply that eventually gives solid products COLDCARB and BF top gas stream feed GASIN that is compressed to process conditions eventually giving product gas FINALGAS after expansion. Three gas/solid heat exchangers, a gas/solid (chemical equilibrium) reactor, and a gas/solid separator made up the final process equipment [20]. This model may be readily added to or integrated with Aspen Plus models for BFs or other sections of iron- and steelmaking processes, such as for example given in [33,34].
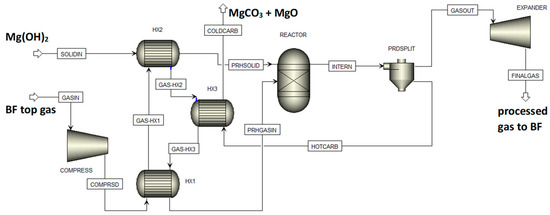
Figure 13.
The Aspen Plus flow sheet for BF top gas processing.
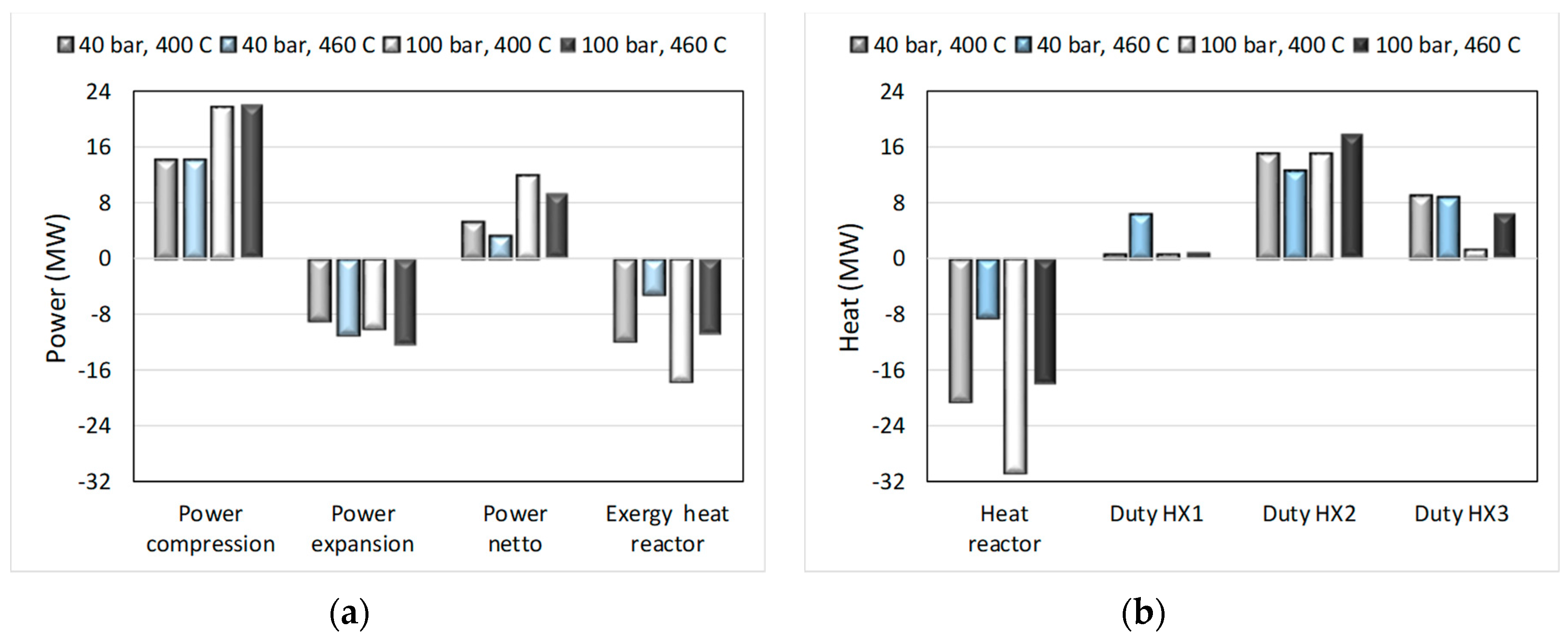
A gas feed of 1 kmol/s with the abovementioned composition was used with an excess of 17% Mg(OH)2 for the conversion, corresponding to 0.3 mol Mg per mol input gas. Temperature and pressure for the reactor were varied in the ranges of 400–500 °C and 40–100 bar, respectively. This was based on preliminary calculations using Gibbs energy minimization and the earlier work at ÅA on Mg(OH)2 carbonation using CO2-containing exhaust gases from other processes. The simulation results showed that in all cases Mg(OH)2 was converted not only to MgCO3 but also to MgO. At 40 bar, the carbonation efficiency dropped from 93% at 400 °C to 66% at 460 °C. For 100 bar, the carbonation efficiency changed to 97% at 400 °C and 87% at 460 °C. The values for the CO + CO2 conversion efficiency were similar to the values for the carbonation efficiency. Interestingly, there was little effect of process temperature and pressure on the amount of H2 produced, which eventually left the system at approximately six times the amount entering with the feed gas. One feature of the process is that with increasing temperature the CO/water shift reaction equilibrium caused CO to be more stable while the corresponding higher H2O partial pressure could not prevent MgO formation. Experimental work under the preferable process conditions can be a next step, and the required suitable equipment (e.g., a pressurized fluidized bed as in [11]) is available at ÅA.
The study was finalized by making an analysis of energy input/output and exchanger duties, with results as shown below in Figure 14. It can be seen that power requirements may be compensated for by heat that is produced by the overall process with the surroundings temperature T° and the process unit temperatures Ti and the exergies of the combined heat outputs, Qi, calculated by ΣQi∙(1 − T°/Ti), was larger than the required netto power input. See [20] for more details on these results and [30] for the use of exergy analysis, which based on the second law of thermodynamics allows recalculating energy flows of different forms (here are power and heat) into the equal denominator of useful work.
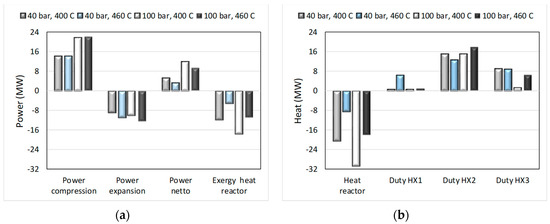
Figure 14.
Energy input and output and heat exchanger duties for process conditions as indicated for the process shown in Figure 13. Power in/out and exergy of reaction heat (a) and heat of reaction and heat duties of heat exchangers (b).
3.3. Metallic NP Production Using an Arc Discharge Route
3.3.1. Specific Electricity Consumption (SEC)
Analyzing the SEC for the production of metallic NPs leads to a significant penalty resulting from the NP size dependence and the energies (enthalpies) of melting/solidification, evaporation/condensation and the temperatures for these. Melting and evaporation (volatilization) requires heats Qm and Qv at temperatures Tm and Tv, respectively, while later the heat released by condensation Qc is smaller than Qv for condensation temperature Tc < Tv and the heat released by solidification Qs is smaller than Qm for solidification temperature Ts < Tm. Input exergies were significantly larger than output exergies calculated as Q∙(1 – T°/T) for the heating and cooling processes involving the material that produces NPs.
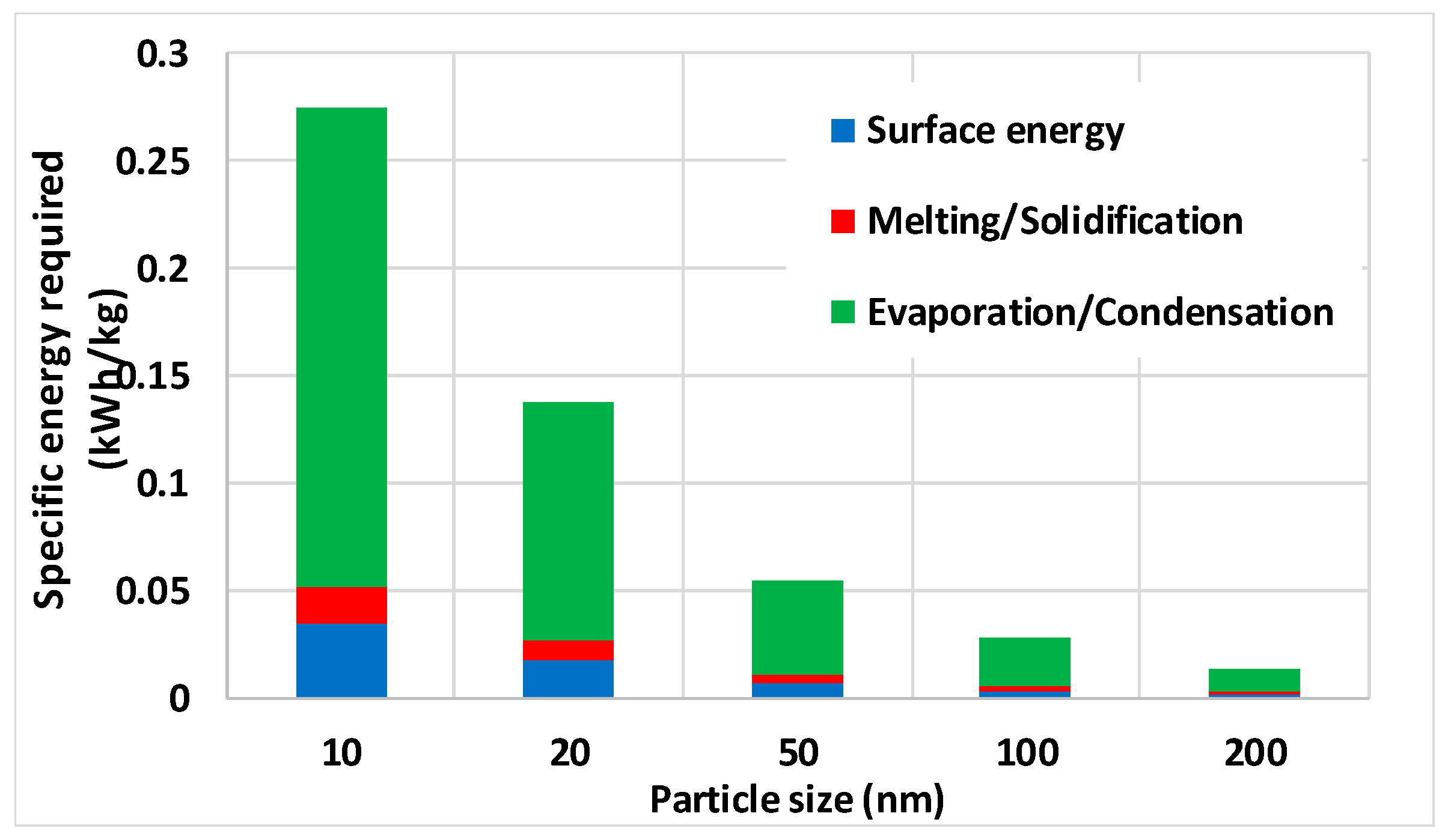
For copper NPs, the results are illustrated by Figure 15, showing the SEC for several NP sizes as a result of exergy input for evaporation not being returned as exergy of condensation and exergy input for melting not being returned as exergy of solidification. This comes on top of the exergy needed for producing increased surface energies of atoms in NPs compared to those of atoms inside the material. It was clearly shown that the evaporation/condensation exergy consumption is by far the most important one of the three processes, which come on top of the SEC that arises from pumping around the carrier gas [14,29]. The values given in Figure 15 are several orders of magnitude lower than the 170 kWh/kg reported for experimental production of 79 nm Cu [29].
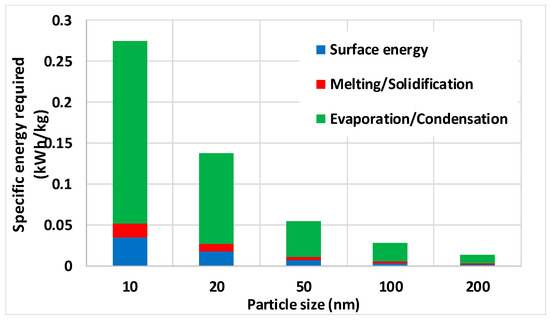
Figure 15.
Specified electricity consumption (SEC) for copper NP production considering surface energy and losses due to melting/solidification and evaporation/condensation.
3.3.2. LCA of NP Production
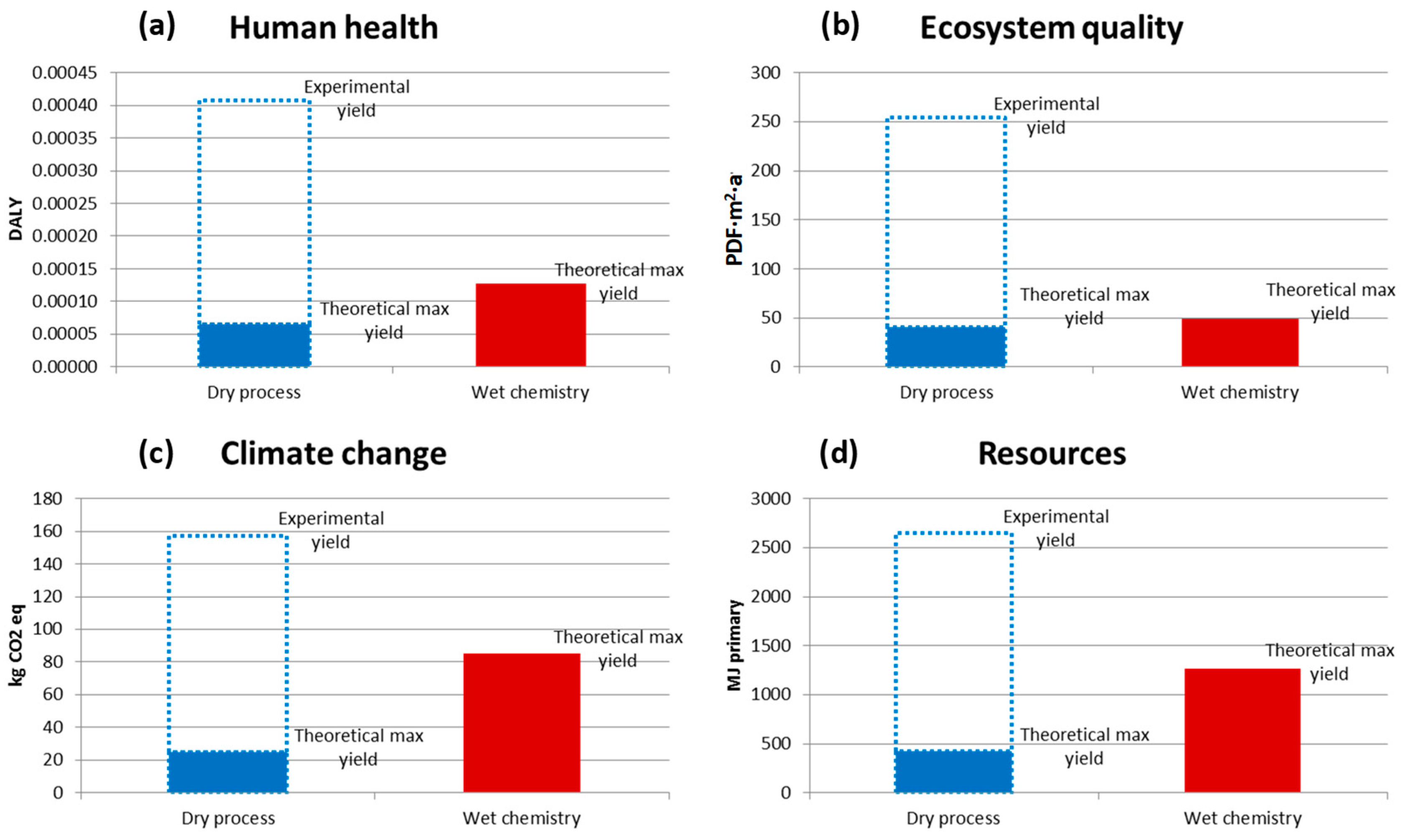
Besides the energy efficiency analysis, a wider analysis of the environmental footprint was made of metallic NP production via the dry, arc discharge process versus more conventional wet, aqueous solution metallic oxide salt reduction. For copper NPs, the results are given in Figure 16, illustrating that the BUONAPART-E concept is preferable only if more of the input metal material is obtained as NP products rather than remaining as deposits inside the production facility. Although NP metal production and LCA results were reported, metal NP yield needs to be improved [24].
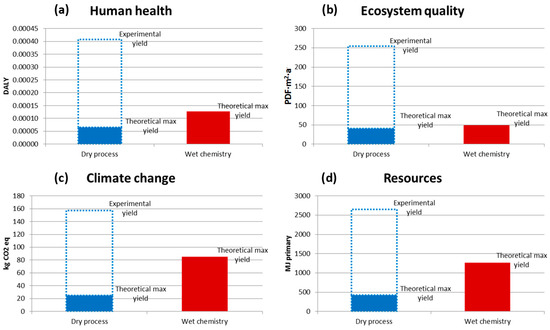
Figure 16.
Life cycle impact (LCI) comparisons of dry, arc discharge NP production and chemical reduction methods for copper particles for four impact categories: Human health (a), Ecosystem quality (b), Climate change (c), Resources (d). See Figure 12 for the explanations of abbreviations.
The LCA studies on metal NP production were expanded to products that contain metallic NPs, such as silver NPs in cotton used in hospitals and copper NPs in water, so as to give a nano-cooling fluid. These assessments can be found elsewhere [14,35] and showed great potential and special properties of consumer product materials containing metallic NPs. The increased demand for NP materials obviously calls for scale-up of production routes, while at the same time negative impacts need to be addressed, as was the objective of the work reported here. Note, however, that the LCA studies were cradle-to-gate assessments, i.e., from producing metal from ore to products containing NP particles leaving production. Lack of data on end-of-life product handling makes a full cradle-to-gate LCA impossible, although a first step in this direction was recently presented [36].
4. Conclusions
The work reported addresses modern trends seen in development of more sustainable process routes for iron- and steelmaking as well as nonferrous materials and products in which these are used. CO2 emissions reduction is obviously on top of many industrial production agendas together with energy efficiency, followed by water use and waste and by-product disposal. This faces the facts of limited resources and an increasing need for more circular economies for materials, resources, and processes that allow producing them. Experimental methods combined with modern theoretical tools such as exergy assessment for energy efficiency analysis and LCA give quantitative information on whether improved processes or products actually are an improvement vis-à-vis profitability. Three examples were given in this paper.
Slag2PCC is a proven concept developed in Finland for valorization of steelmaking (BOF) slags, binding CO2 while producing high-value PCC. Scale-up and commercialization is ongoing, after the lab-scale proof-of-concept was demonstrated followed by successful operation of a pilot plant. The LCA results showed that water use may be a critical factor.
CO2 (and CO) from/in BF top gas can be converted with Mg(OH)2 that can be produced from abundant serpentinite rock to MgCO3, hydrogen and steam, integrated with CO/water shift. The process simulation studies showed under the process conditions (pressure: 40 bar; temperature: 400 °C) good conversion levels were obtained, yet to be experimentally verified.
The dry production of metallic NPs using high-voltage (arc discharge) evaporation has many benefits, including life cycle impact, compared to with wet methods for NP production, and appears to allow for easier scale-up to production levels of kilograms per hour. The LCA calculations showed the overall benefit from an environmental footprint of metallic NP production as well as the production of products that, with very small amounts of NP, have a variety of beneficial properties.
5. Patents
The slag2PCC concept described in this paper has been patented under Finnish patent 122348 and US patent 8603428.
Author Contributions
The author presented this work at the International Process Metallurgy Symposium IPMS2019 event in Espoo Finland 5–6 November 2019 and produced this manuscript based on that presentation.
Funding
The development work of the slag2PCC concepts was possible, during 2005–2016 with primarily Tekes funded projects Slag2PCC, Slag2PCC+ and Cleen Oy/Clic Oy CCSP and Finland’s Graduate School for Chemical Engineering (2010–2014). The work on metal nanoparticulates was conducted during 2012–2016 funded by EU FP7 project BUONAPART-E (grant agreement: 280765) and Finland’s earlier Graduate School of Energy Engineering and Systems (2012–2015).
Acknowledgments
Daniel Lindberg of Aalto University, Espoo Finland is acknowledged for inviting the author to present the work at International Process Metallurgy Symposium IPMS2019, and Lauri Holappa of Aalto University is acknowledged for the invitation to produce this manuscript. The author acknowledges his earlier co-workers, currently postdoctoral researchers in most cases, for intensive and productive cooperation, a great deal of which is summarized in this paper.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Teir, S.; Kotiranta, T.; Pakarinen, J.; Mattila, H.-P. Case study for production of calcium carbonate from carbon dioxide in flue gases and steelmaking slag. J. CO2 Util. 2016, 14, 37–46. [Google Scholar] [CrossRef]
- Teir, S. Fixation of Carbon Dioxide by Producing Carbonates from Minerals and Steelmaking Slags. Ph.D. Thesis, Helsinki University of Technology, Espoo, Finland, 2 June 2008. Available online: http://lib.tkk.fi/Diss/2008/isbn9789512293537/ (accessed on 19 February 2020).
- Eloneva, S.; Said, A.; Fogelholm, C.-J.; Zevenhoven, R. Preliminary assessment of a method utilizing Carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Eloneva, S. Reducing CO2 Emissions by Mineral Carbonation: Steelmaking Slags As Raw Material with a Pure Calcium Carbonate End-Product. Ph.D. Thesis, Aalto University, Espoo, Finland, 26 November 2010. Available online: http://lib.tkk.fi/Diss/2010/isbn9789526034577/ (accessed on 19 February 2020).
- Mattila, H.-P.; Zevenhoven, R. Designing a continuous process setup for precipitated calcium carbonate production from steel converter slag. ChemSusChem 2014, 7, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.-P. Utilization of Steelmaking Waste Materials for Production of Calcium Carbonate (CaCO3). Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 10 October 2014. Available online: https://www.doria.fi/handle/10024/99011 (accessed on 19 February 2020).
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Said, A. CO2 Sequestration by Steelmaking Slags for the Production of Precipitated Calcium Carbonate—From Laboratory to Demonstration Stage. Ph.D. Thesis, Aalto University, Espoo, Finland, 12 May 2017. Available online: https://aaltodoc.aalto.fi/handle/123456789/25401 (accessed on 19 February 2020).
- Mattila, H.-P.; Hudd, H.; Zevenhoven, R. Cradle-to-gate life cycle assessment of precipitated calcium carbonate production from steel converter slag. J. Clean. Prod. 2014, 84, 611–617. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Romão, I.S. CO2 mineralisation as a route to energy-efficient CO2 sequestration or materials with market value. In CO2 Sequestration by Ex-Situ Mineral Carbonation; Sanna, A., Maroto-Valer, M.M., Eds.; World Scientific Publ. Co.: London, UK, 2017; pp. 41–90. [Google Scholar]
- Fagerlund, J. Carbonation of Mg(OH)2 in a Pressurized Fluidized Bed for CO2 Sequestration. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2 March 2012. Available online: https://www.doria.fi/handle/10024/74477 (accessed on 19 February 2020).
- Nduagu, E.I. Production of Mg(OH)2 from Mg-Silicate Rock for CO2 Mineral Sequestration. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 13 December 2012. Available online: https://www.doria.fi/handle/10024/86170 (accessed on 19 February 2020).
- Soares Romão, I.S. Production of Magnesium Carbonates from Serpentinites for CO2 Mineral Sequestration: Optimisation towards Industrial Application. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, University of Coimbra, Coimbra, Portugal, 17 December 2015. Available online: https://www.doria.fi/handle/10024/117766 (accessed on 19 February 2020).
- Slotte, M. Two Process Case Studies on Energy Efficiency, Life Cycle Assessment and Process Scale-up. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 11 January 2017. Available online: https://www.doria.fi/handle/10024/130097 (accessed on 19 February 2020).
- Lavikka (n. Sjöblom), S. Geological and Mineralogical Aspects on Mineral Carbonation. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 3 January 2017. Available online: https://www.doria.fi/handle/10024/130096 (accessed on 19 February 2020).
- Koivisto, E. Membrane Separations, Extractions and Precipitations in Aqueous Solutions for CO2 Mineralisation. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 11 October 2019. Available online: https://www.doria.fi/handle/10024/171340 (accessed on 19 February 2020).
- Zevenhoven, R.; Slotte, M.; Koivisto, E.; Erlund, R. Serpentinite carbonation process routes using ammonium sulphate and integration in industry. Energy Technol. 2017, 5, 945–954. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Slotte, M.; Åbacka, J.; Highfield, J. A comparison of CO2 mineral carbonation processes involving a dry or wet carbonation step. ENERGY 2016, 117, 604–611. [Google Scholar] [CrossRef]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Zevenhoven, R.; Virtanen, M. CO2 mineral sequestration integrated with water-shift reaction. ENERGY 2017, 141, 2484–2489. [Google Scholar] [CrossRef]
- Erlund, R.; Zevenhoven, R. Thermal energy storage (TES) capacity of a lab-scale magnesium hydrocarbonates/silica gel system. J. Energy Storage 2019, 25, 100907. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, L.; Cai, N. Effect of sorbent type on the sorption enhanced water gas shift process in a fluidized bed reactor. Ind. Eng. Chem. Res. 2012, 51, 11989–11997. [Google Scholar] [CrossRef]
- EU FP7 Project Better Upscaling and Optimization of Nanoparticle and Nanostructure Production by Means of Electrical Discharges BUONAPART-E (2012–2016). Available online: http://www.buonapart-e.eu/ (accessed on 3 January 2020).
- Stein, M.; Kruis, F.E. Scaling-up metal nanoparticle production by transferred arc discharge. Adv. Powder Technol. 2018, 29, 3138–3144. [Google Scholar] [CrossRef]
- Hu, W.; Xiao, S.; Deng, H.; Luo, W.; Deng, L. Thermodynamic properties of nano-silver and alloy particles. In Silver Nanoparticles; In-Tech: Vukovar, Croatia, 2010; pp. 1–34. [Google Scholar]
- Zevenhoven, R.; Beyene, A. The exergy of nano-particulate materials. Int. J. Thermodyn. 2014, 17, 145–151. [Google Scholar] [CrossRef]
- Xiong, S.Y.; Qi, W.H.; Cheng, Y.J.; Huang, B.Y.; Wang, M.P.; Li, Y.J. Modeling size effects on the surface free energy of metallic nanoparticles and nanocavities. Phys. Chem. Chem. Phys. 2011, 13, 10648–10651. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.Y.; Qi, W.H.; Cheng, Y.J.; Huang, B.Y.; Wang, M.P.; Li, Y.J. Universal relation for size dependent thermodynamic properties of metallic nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 10652–10660. [Google Scholar] [CrossRef] [PubMed]
- Slotte, M.; Zevenhoven, R. Energy efficiency and scalability of metallic nanoparticle production using arc/spark discharge. ENERGIES 2017, 10, 1065. [Google Scholar] [CrossRef]
- Szargut, J.; Morris, D.; Steward, F.R. Exergy Analysis of Thermal, Chemical and Metallurgical Processes; Hemisphere Publishing Co.: New York, NY, USA, 1988. [Google Scholar]
- Slotte, M.; Mehta, G.; Zevenhoven, R. Life cycle indicator comparison of copper, silver, zinc and aluminum nanoparticle production through electric arc/spark evaporation or chemical reduction. Int. J. Energy Environ. Eng. (IJEEE) 2015, 6, 233–243. [Google Scholar] [CrossRef]
- Saxén, H. (Åbo Akademi University, Thermal and Flow Eng., Turku, Finland). Personal Communication, 13 October 2015.
- Schultmann, F.; Engels, B.; Rentz, O. Flowsheeting-based simulation of recycling concepts in the metal industry. J. Clean. Prod. 2004, 12, 737–751. [Google Scholar] [CrossRef]
- Porzio, G.F.; Colla, V.; Fornai, B.; Vannucci, M.; Larsson, M.; Stripple, H. Process integration analysis and some economic-environmental implications for an innovative environmentally friendly recovery and pre-treatment of steel scrap. Appl. Energy 2016, 161, 656–672. [Google Scholar] [CrossRef]
- Slotte, M.; Zevenhoven, R. Energy requirements and LCA of production and product integration of silver, copper and zinc nanoparticles. J. Clean. Prod. 2017, 148, 948–957. [Google Scholar] [CrossRef]
- Zevenhoven, R. Energy requirements for recovery of (metallic) nanoparticulate material from waste. In Proceedings of the 2019 World Resources Forum (WRF) Conference, Geneva, Switzerland, 22–24 October 2019; Available online: http://users.abo.fi/rzevenho/SS7-5-Zevenhoven.pdf (accessed on 31 January 2020).
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).