Characteristics and Formation Tendency of Freckle Segregation in Electroslag Remelted Bearing Steel
Abstract
1. Introduction
2. Materials and Methods
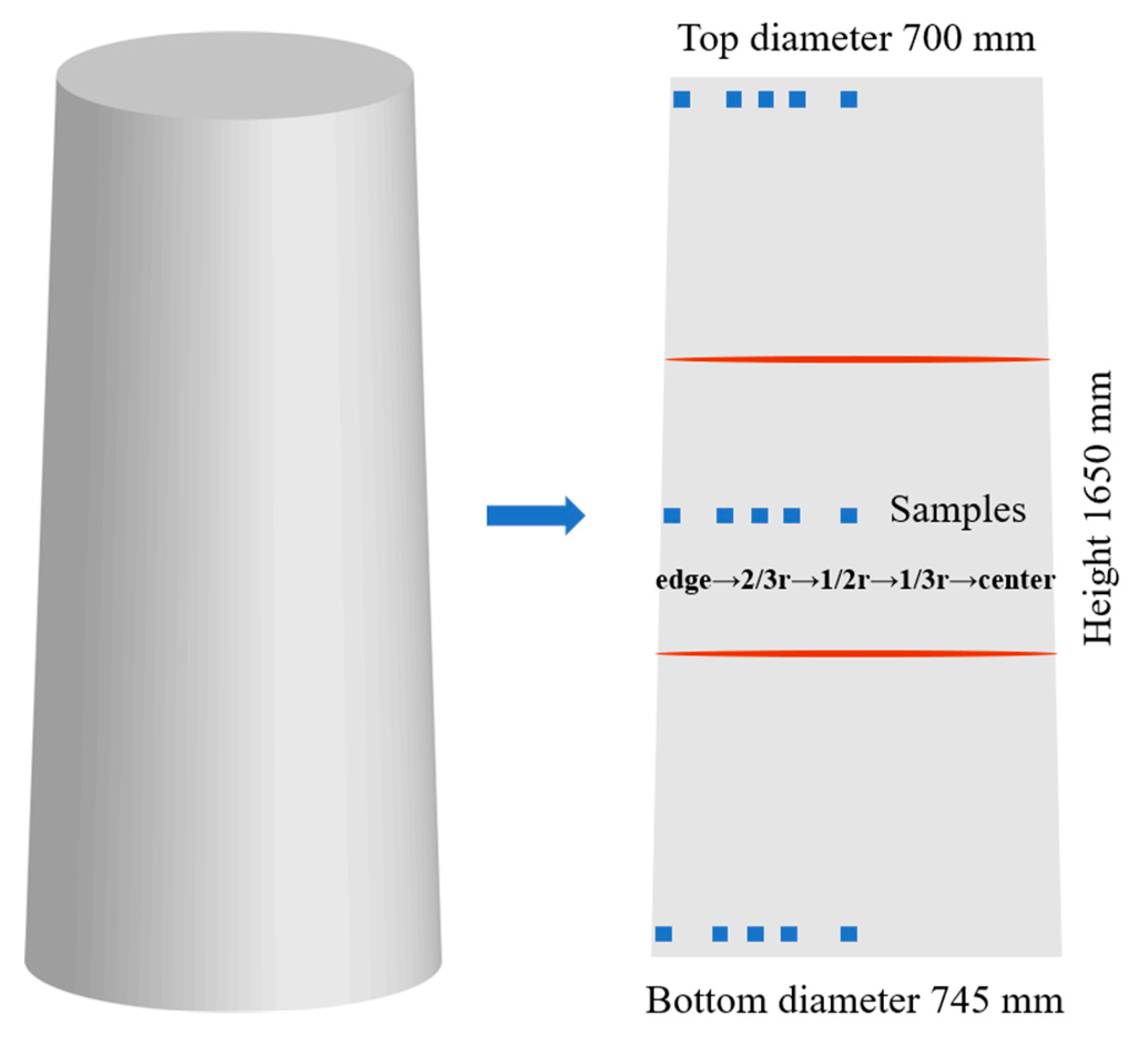
2.1. Materials Preparation and Characterization
2.2. Thermodynamic and Thermo-Physical Calculation
3. Results and Discussion
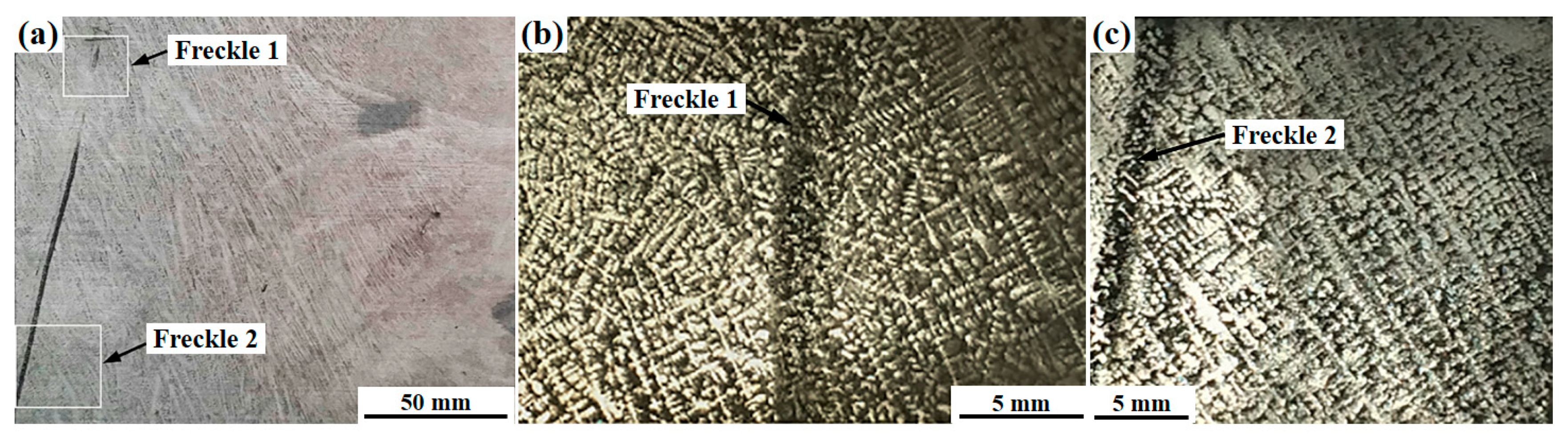
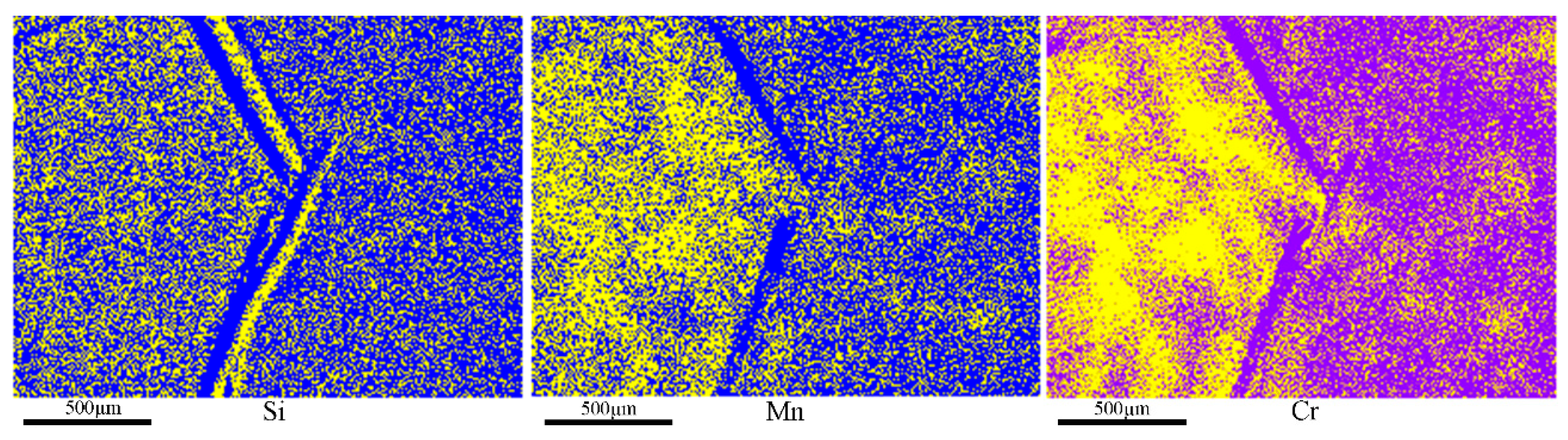
3.1. Characteristics of Freckles
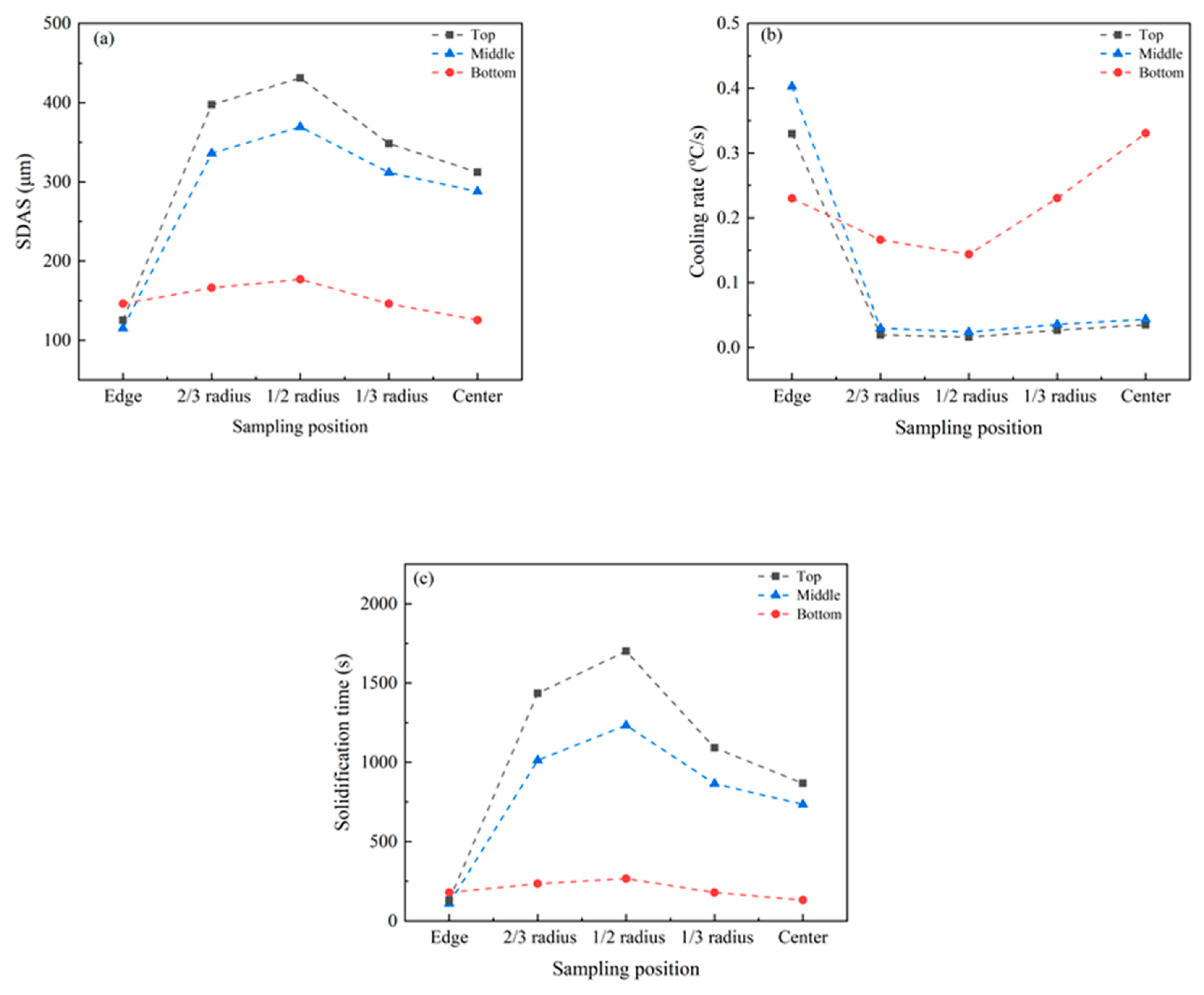
3.2. SDAS and Solidification front Angle
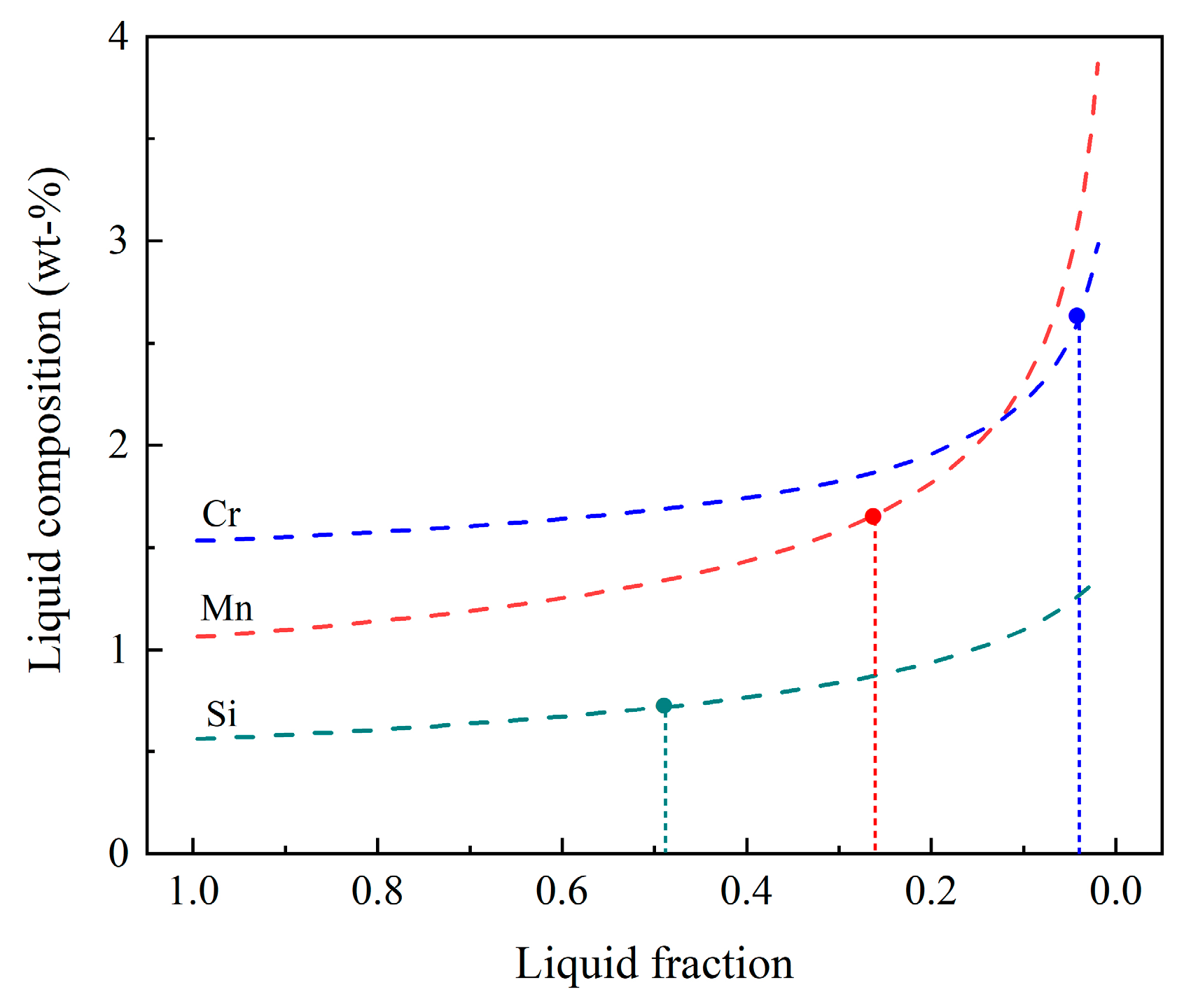
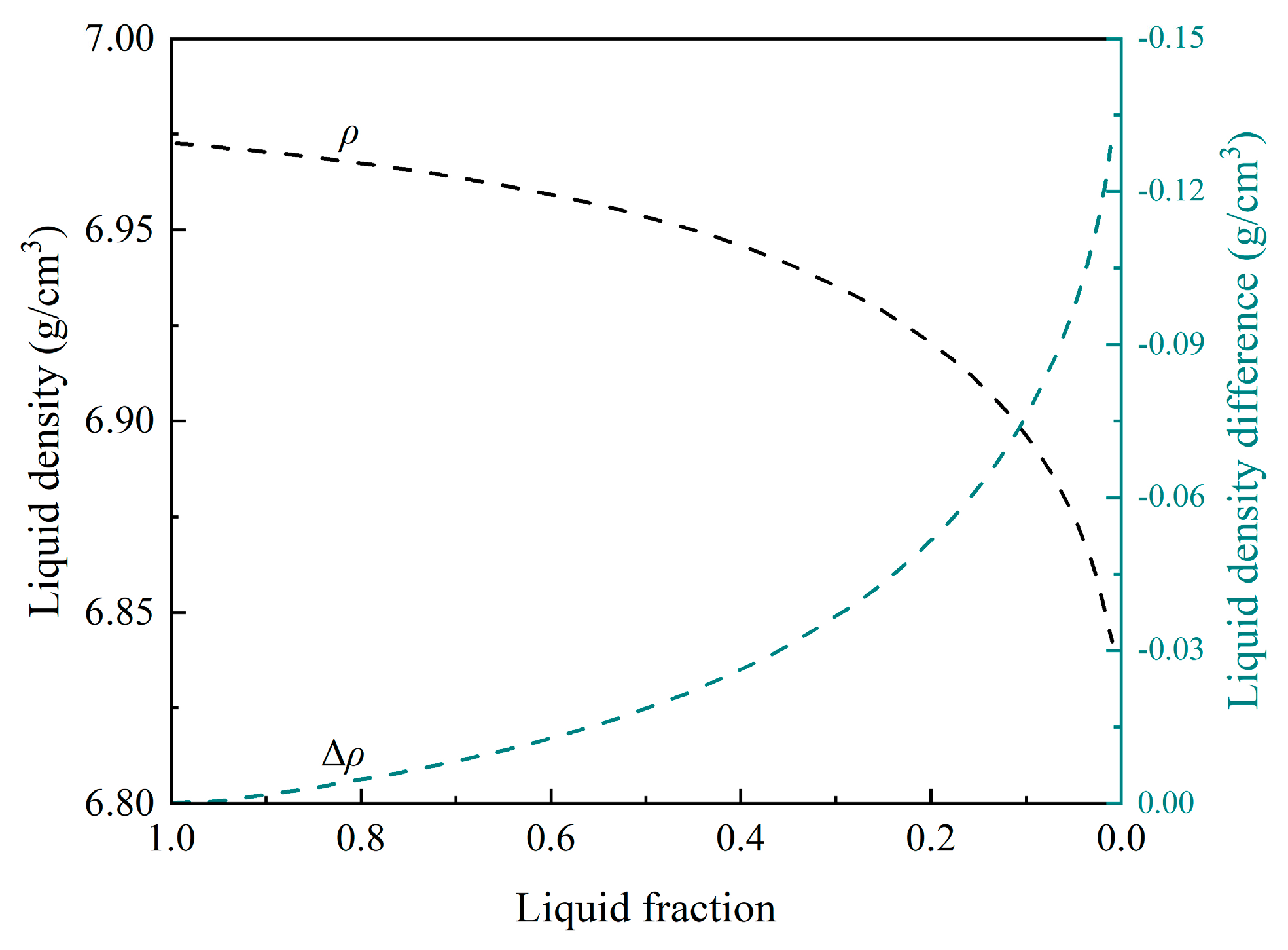
3.3. Compositions and Thermal-Physical Properties of Liquid
3.4. Prediction of Freckles using Rayleigh Number
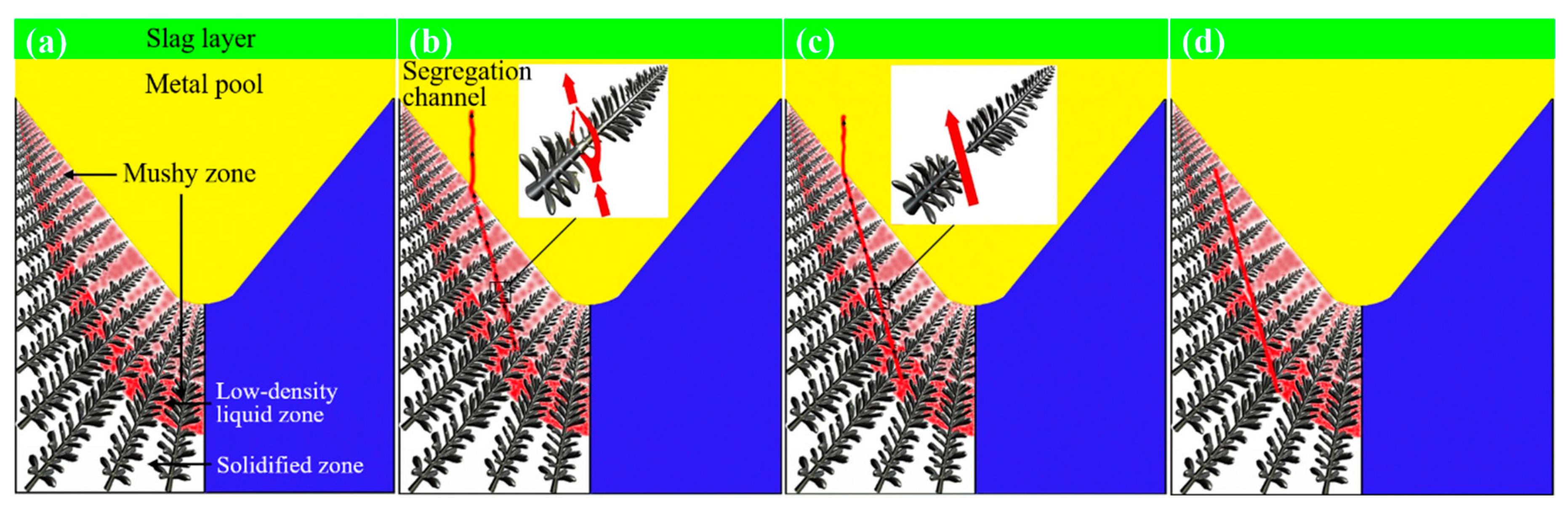
3.5. Formation Mechanism of Freckles in ESR Bearing Steel Ingot
4. Conclusions
- (1)
- Freckles were identified to be of a (Si, Mn, Cr)-enriched equiaxed grain structure with a higher microhardness, and formed around the 1/2–2/3 radius region at the middle upper zone of the GCr15SiMn ESR ingot, where SDAS reaches its maximum but cooling reaches its minimum.
- (2)
- Similar liquid fractions of 0.3–0.5, corresponding to the range of the strongest freckle formation, were obtained based on both freckle composition and relative Ra, using thermodynamic approaches.
- (3)
- A threshold value of relative Ra was determined to be –0.023 for freckle formation in the GCr15SiMn ESR ingot based on experimental and simulation results.
Author Contributions
Funding
Conflicts of Interest
References
- Moore, J.; Shah, N. Mechanisms of formation of A- and V-segregation in cast steel. Int. Mat. Rev. 1983, 28, 338–356. [Google Scholar] [CrossRef]
- Ma, D.; Wu, Q.; Buehrig-Polaczek, A. Some new observations on freckle formation in directionally solidified superalloy components. Metall. Mater. Trans. B 2012, 43, 344–353. [Google Scholar] [CrossRef]
- Hong, J.; Ma, D.; Wang, J.; Wang, F.; Sun, B.; Dong, A.; Li, F.; Bührig-Polaczek, A. Freckle defect formation near the casting interfaces of directionally solidified superalloys. Materials 2016, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, L.; Hu, X.; Ge, B.; Zhang, J.; Fu, H. Review of freckle defects under directional solidification of Nickel-based superalloys. J. Mater. Sci. Eng. 2010, 28, 145. [Google Scholar]
- Copley, S.; Giamei, A.; Johnson, S.; Hornbecker, M. The origin of freckles in unidirectionally solidified castings. Metall. Trans. 1970, 1, 2193–2204. [Google Scholar] [CrossRef]
- Mehrabian, R.; Keane, M.; Flemings, M. Experiments on macrosegregation and freckle formation. Metall. Trans. 1970, 1, 3238–3241. [Google Scholar]
- Cao, H.; Shen, H.; Liu, B. Numerical simulation of freckle formation in directional solidification of Nickel-based superalloy. Rare Metal Mater. Eng. 2006, 35, 1849–1853. [Google Scholar]
- Guillemot, G.; Gandin, C.; Bellet, M. Interaction between single grain solidification and macrosegregation: Application of a cellular automaton—Finite element model. J. Cryst. Growth 2007, 303, 58–68. [Google Scholar] [CrossRef]
- Schneider, M.; Gu, J.; Beckermann, C.; Boettinger, W.; Kattner, U. Modeling of micro- and macrosegregation and freckle formation in single-crystal nickel-base superalloy directional solidification. Metall. Mater. Trans. A 1997, 28, 1517–1531. [Google Scholar] [CrossRef]
- Zaloznik, M.; Kumar, A.; Combeau, H. An operator splitting scheme for coupling macroscopic transport and grain growth in a two-phase multiscale solidification model: Part II—Application of the model. Comp. Mater. Sci. 2010, 48, 11–21. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Li, D.; Liu, H.; Fu, P. Comparison of channel segregation formation in model alloys and steels via numerical simulations. Metall. Mater. Trans. A 2016, 47, 2927–2939. [Google Scholar] [CrossRef]
- Torabi Rad, M.; Kotas, P.; Beckermann, C. Rayleigh number criterion for formation of A-segregates in steel castings and ingots. Metall. Mater. Trans. A 2013, 44, 4266–4281. [Google Scholar]
- Bogdan, O. Influence of ingot size and mold design on macro-segregation in AISI 4340 forging ingots. In Proceedings of the 1st International Conference on Ingot Casting, Rolling and Forging, Aachen, Germany, 3–7 June 2012. [Google Scholar]
- Gu, J.; Beckermann, C. Simulation of convection and macrosegregation in a large steel ingot. Metall. Mater. Trans. A 1999, 30, 1357–1366. [Google Scholar] [CrossRef]
- Pollock, T.; Murphy, W. The breakdown of single-crystal solidification in high refractory nickel-base alloys. Metall. Mater. Trans. A 1996, 27, 1081–1094. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T. Study on the formation of-A segregates in steel ingot. Trans. Iron Steel Inst. Jpn. 1978, 18, 80–89. [Google Scholar] [CrossRef]
- Beckermann, C.; Gu, J.; Boettinger, W. Development of a freckle predictor via Rayleigh number method for single-crystal Nickel-base superalloy castings. Metall. Mater. Trans. A 2000, 31, 2545–2557. [Google Scholar] [CrossRef]
- Ramirez, J.; Beckermann, C. Evaluation of a rayleigh-number-based freckle criterion for Pb-Sn alloys and Ni-base superalloys. Metall. Mater. Trans. A 2003, 34, 1525–1536. [Google Scholar] [CrossRef]
- Yang, W.; Chen, W.; Chang, K. Freckles in remelted niobium containning superalloys. In Proceedings of the 130th Annual Meeting & Exhibition of The Minerals, Metals & Materials Society, New Orleans, LA, USA, 11–15 February 2001; pp. 113–122. [Google Scholar]
- Wang, L.; Dong, J.; Tian, Y.; Zhang, L. Microsegregation and Rayleigh number variation during the solidification of superalloy Inconel 718. J. Univ. Sci. Technol. Beijing 2008, 15, 594–599. [Google Scholar] [CrossRef]
- Valdés, J.; King, P.; Liu, X. On the formulation of a freckling criterion for Ni-based superalloy vacuum arc remelting ingots. Metall. Mater. Trans. A 2010, 41, 2408–2416. [Google Scholar] [CrossRef]
- Yang, W.; Chang, K.; Chen, W.; Mannan, S.; Barbadillo, J. Freckle criteria for the upward directional solidification of alloys. Metall. Mater. Trans. A 2001, 32, 397–406. [Google Scholar] [CrossRef]
- Han, D.; Jiang, W.; Xiao, J.; Li, K.; Lu, Y.; Zheng, W.; Zhang, S.; Lou, L. Investigation on freckle formation and evolution of single-crystal nickel-based superalloy specimens with different thicknesses and abrupt cross-section changes. J. Alloys Compd. 2019, 805, 218–228. [Google Scholar] [CrossRef]
- Giamei, A.; Kear, B. On the nature of freckles in nickel base superalloys. Metall. Trans. 1970, 1, 2185–2192. [Google Scholar] [CrossRef]
- Li, Q.; Shen, J.; Qin, L.; Xiong, Y. Investigation on local cooling in reducing freckles for directionally solidified superalloy specimens with abruptly varying cross-sections. Mater. Char. 2017, 130, 139–148. [Google Scholar] [CrossRef]
- Kaya, H.; Boyuk, U.; Cadirli, E.; Marasli, N. Measurements of the microhardness, electrical and thermal properties of the Al-Ni eutectic alloy. Mater Des. 2012, 34, 707–712. [Google Scholar] [CrossRef]
- El-Bealy, M.; Thomas, B. Prediction of dendrite arm spacing for low alloy steel casting processes. Metall. Mater. Trans. B 1996, 27, 689–693. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T. Influence of alloy elements on the formation of-A segregates in steel ingot. Trans. Iron Steel Inst. Jpn. 1980, 1, 377–383. [Google Scholar]
- Auburtin, P.; Cockcroft, S.; Mitchell, A. Liquid density inversion during the solidification of superalloys and their relationship to freckle formation in castings. In Proceedings of the 8th Int. Symp. on Superalloys (Superalloys 1996), Anaheim, CA, USA, 22–26 September 1996; pp. 443–450. [Google Scholar]
- Long, Z.; Liu, X.; Yang, W.; Chang, K.; Barbero, E. Thermodynamic assessment of liquid composition change during solidification and its effect on freckle formation in superalloys. Mat. Sci. Eng. A 2004, 386, 254–261. [Google Scholar] [CrossRef]
- Valdes, J.; Liu, X.; King, P.; Cowen, C.; Jablonski, P. Solidification front tilt angle effect on potential nucleation sites for freckling in the remelt of Ni-base superalloys. In Proceedings of the 7th Int. Symp. on Superalloy 718 and Derivatives, Seattle, WA, USA, 10–13 October 2010; pp. 79–94. [Google Scholar]
- Hirai, M. Estimation of viscosities of liquid alloys. ISIJ Int. 1993, 33, 251–258. [Google Scholar] [CrossRef]
- Kumar, A.; Založnik, M.; Combeau, H.; Goyeau, B.; Gobin, D. Channel segregation during columnar solidification: Influence of inertia. In Proceedings of the 4th Int. Conf. on Porous Media and Its Applications, Potsdam, Germany, 17–22 June 2012; pp. 1–6. [Google Scholar]
- Yuan, L.; Lee, P. A new mechanism for freckle initiation based on microstructural level simulation. Acta Mater. 2012, 60, 4917–4926. [Google Scholar] [CrossRef]
- Gao, Z.; Jie, W.; Liu, Y.; Zheng, Y.; Luo, H. A model for coupling prediction of inverse segregation and porosity for up-vertical unidirectional solidification of Al-Cu alloys. J. Alloys Compd. 2019, 797, 514–522. [Google Scholar] [CrossRef]
- Poirier, D. Permeability for flow of interdendritic liquid in columnar-dendritic alloys. Metall. Trans. B 1987, 18, 245–255. [Google Scholar] [CrossRef]
- Auburtin, P.; Wang, T.; Cockcroft, S. A Mitchell. Freckle formation and freckle criterion in superalloy castings. Metall. Mater. Trans. B 2000, 31, 801–811. [Google Scholar] [CrossRef]


| C | Si | Mn | Cr | S | P | Ni | Cu | Mo | Ti | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 0.56 | 1.06 | 1.53 | 0.001 | 0.018 | 0.04 | 0.05 | 0.06 | 0.012 | 0.014 | Bal |
| Element | Content (SD), wt pct | HV (SD), MPa | ||
|---|---|---|---|---|
| Si | Mn | Cr | ||
| Freckle | 0.72 (0.054) | 1.65 (0.17) | 2.70 (0.23) | 271.5 (21.4) |
| Matrix | 0.67 (0.070) | 1.20 (0.14) | 1.72 (0.16) | 200.9 (10.3) |
| Position | Edge | 2/3-radius | 1/2-radius | 1/3-radius | Center |
|---|---|---|---|---|---|
| Top | 46 | 38 | 40 | 26 | 20 |
| Middle | 50 | 35 | 35 | 30 | 25 |
| Bottom | 65 | 60 | 50 | 23 | 18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Zhang, Y.; Chen, W.; Li, J. Characteristics and Formation Tendency of Freckle Segregation in Electroslag Remelted Bearing Steel. Metals 2020, 10, 246. https://doi.org/10.3390/met10020246
Yan W, Zhang Y, Chen W, Li J. Characteristics and Formation Tendency of Freckle Segregation in Electroslag Remelted Bearing Steel. Metals. 2020; 10(2):246. https://doi.org/10.3390/met10020246
Chicago/Turabian StyleYan, Wei, Yang Zhang, Weiqing Chen, and Jing Li. 2020. "Characteristics and Formation Tendency of Freckle Segregation in Electroslag Remelted Bearing Steel" Metals 10, no. 2: 246. https://doi.org/10.3390/met10020246
APA StyleYan, W., Zhang, Y., Chen, W., & Li, J. (2020). Characteristics and Formation Tendency of Freckle Segregation in Electroslag Remelted Bearing Steel. Metals, 10(2), 246. https://doi.org/10.3390/met10020246




