Recovery of Chromium from Slags Leachates by Electrocoagulation and Solid Product Characterization
Abstract
1. Introduction
- Formation of coagulants by electrolytic oxidation of the anode.
- Destabilization of the contaminants, particulate suspension and breaking of emulsions.
- Aggregation of the destabilized phases to form flocks.
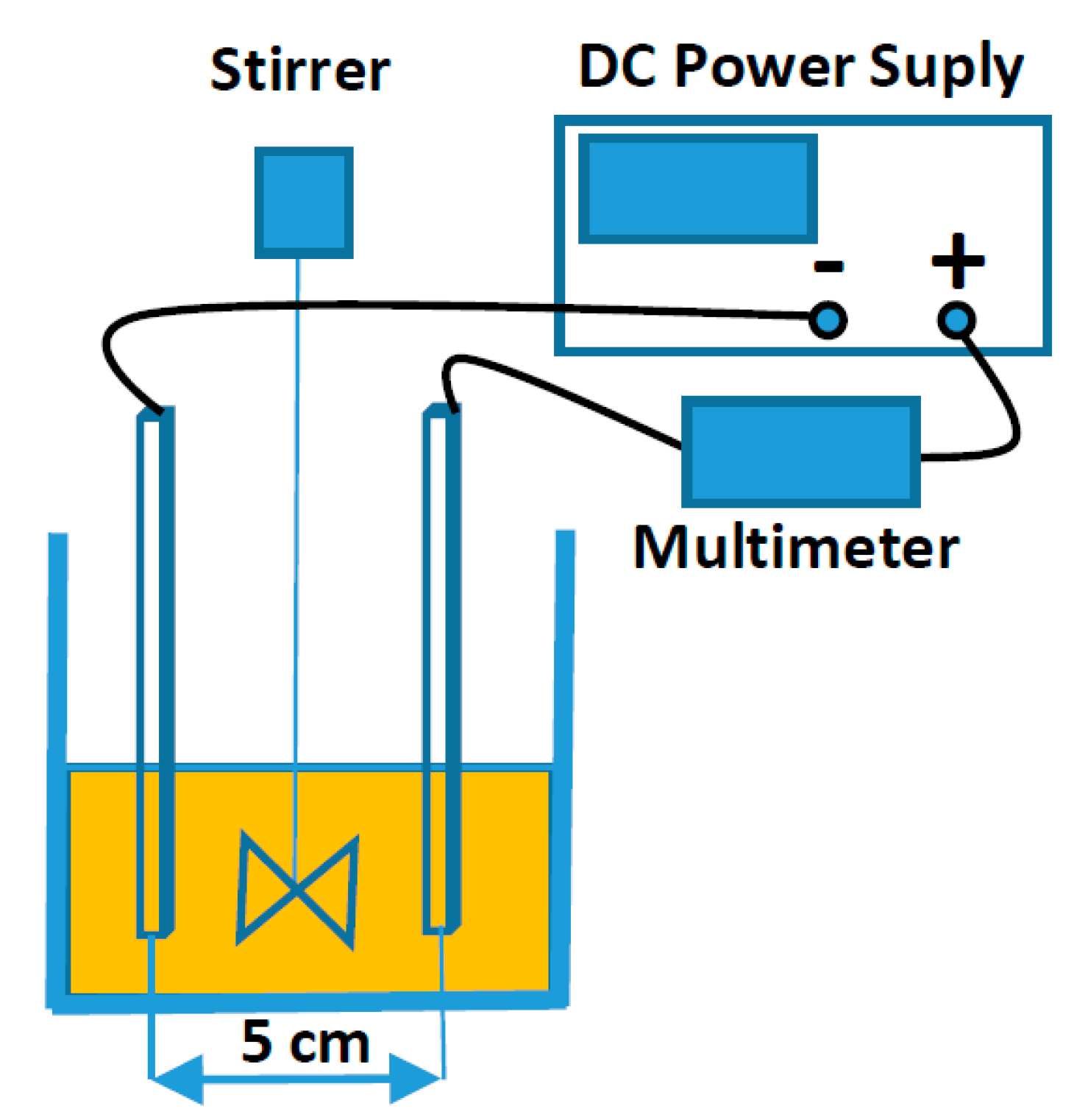
2. Materials and Methods
2.1. Leachates Preparation
2.2. EC Process Conditions
2.3. Methods Used for Solid Product Characterization
3. Results and Discussion
3.1. Influence of pH
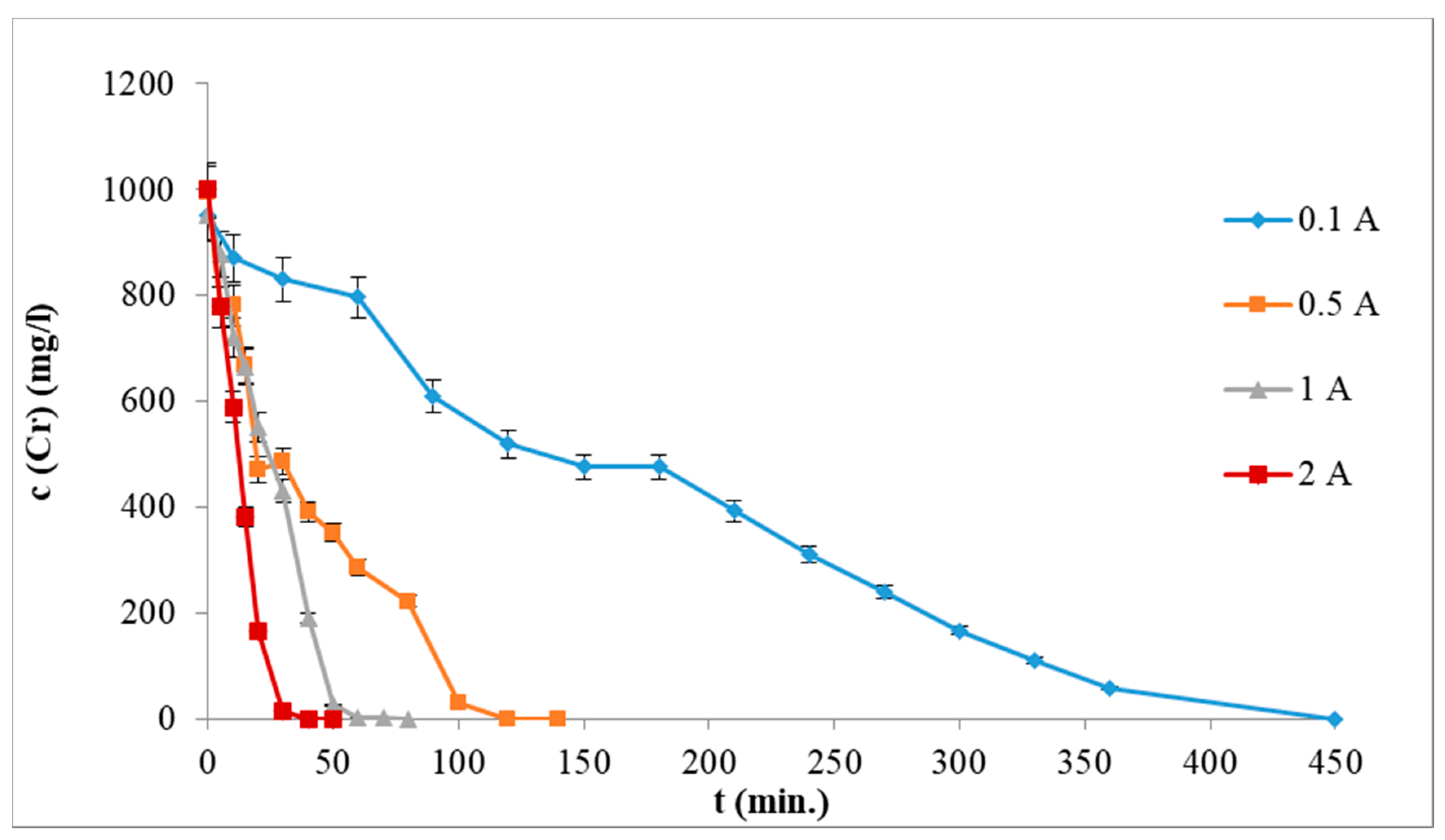
3.2. Influence of Current Intensity
3.3. Influence of NaCl
- prevention of electrode passivation,
- anode corrosion agent, liberating Fe(II) ions into the solution,
- ameliorating the conductivity of the solution.
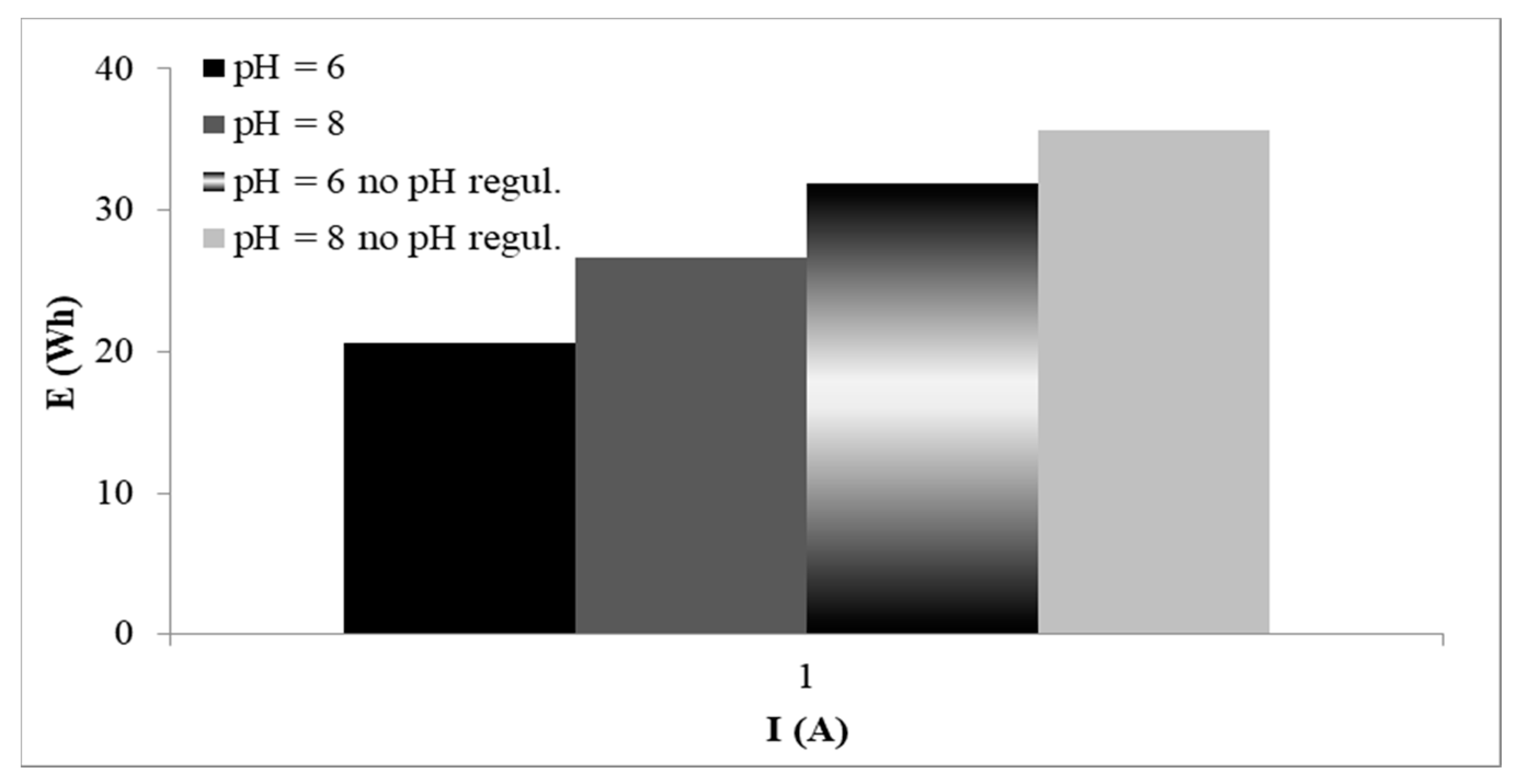
3.4. Energy Consumption of EC Process
- an optimized pH of 6,
- optimized energy consumption and presence of iron in the product requires a moderate concentration of NaCl of around 3000 mg/L,
- to maximize the Cr/Fe ratio, low current intensities (0.1–0.5 A) are needed (energy consumption is always lowered by decreasing the current intensity),
- to remove all of Cr from solution in shorter time, high current intensities (2 A) must be employed.
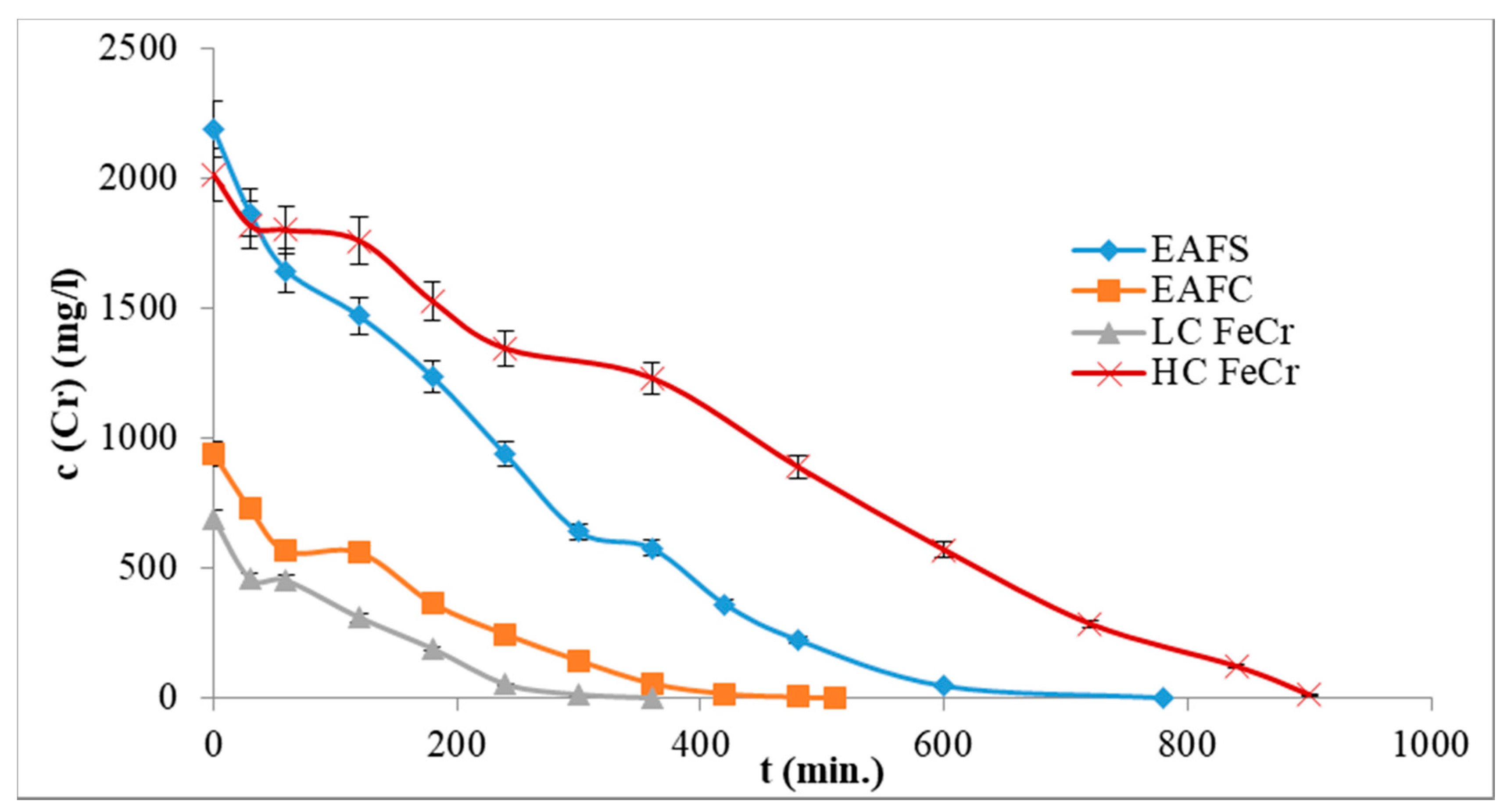
3.5. Real Leachates
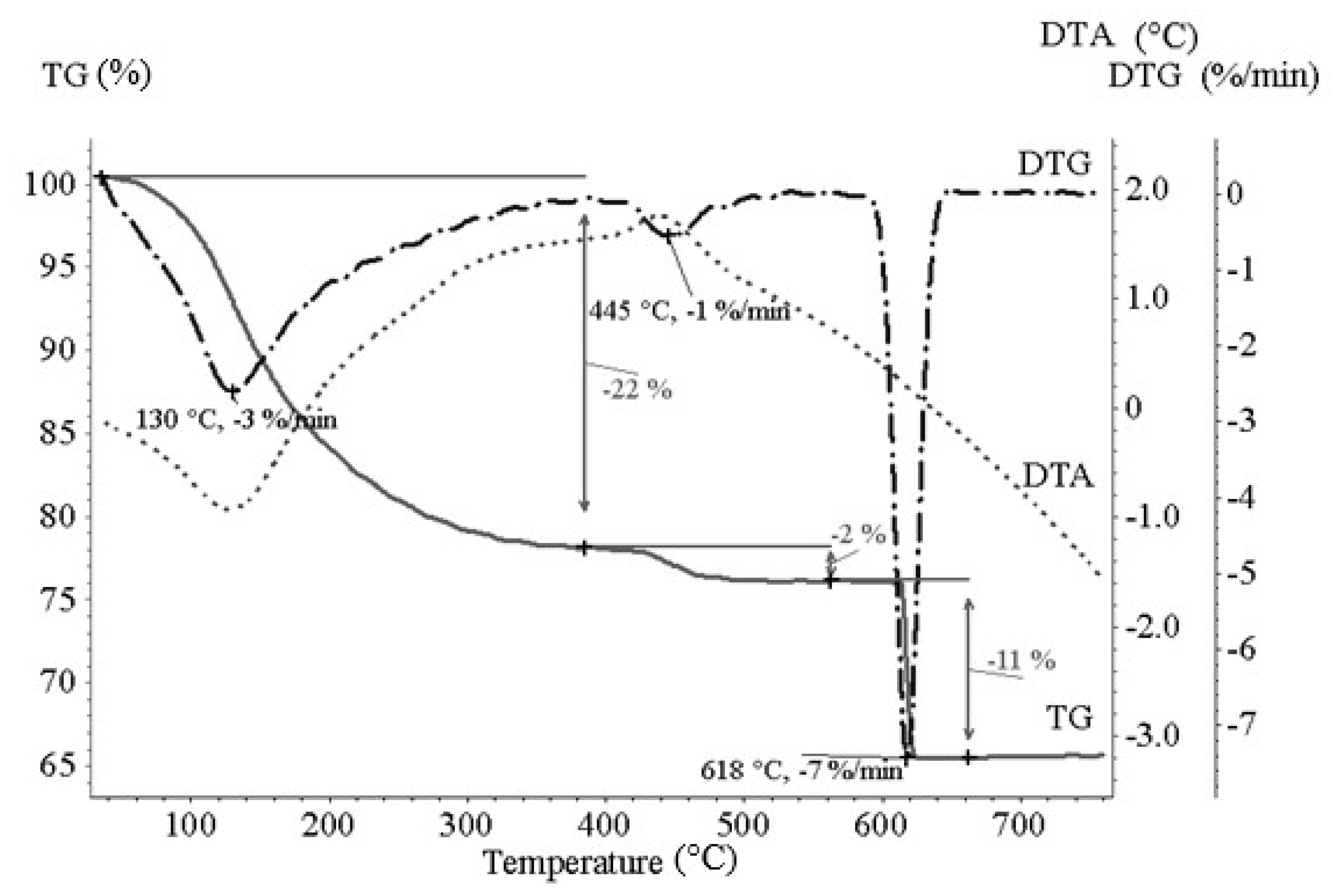
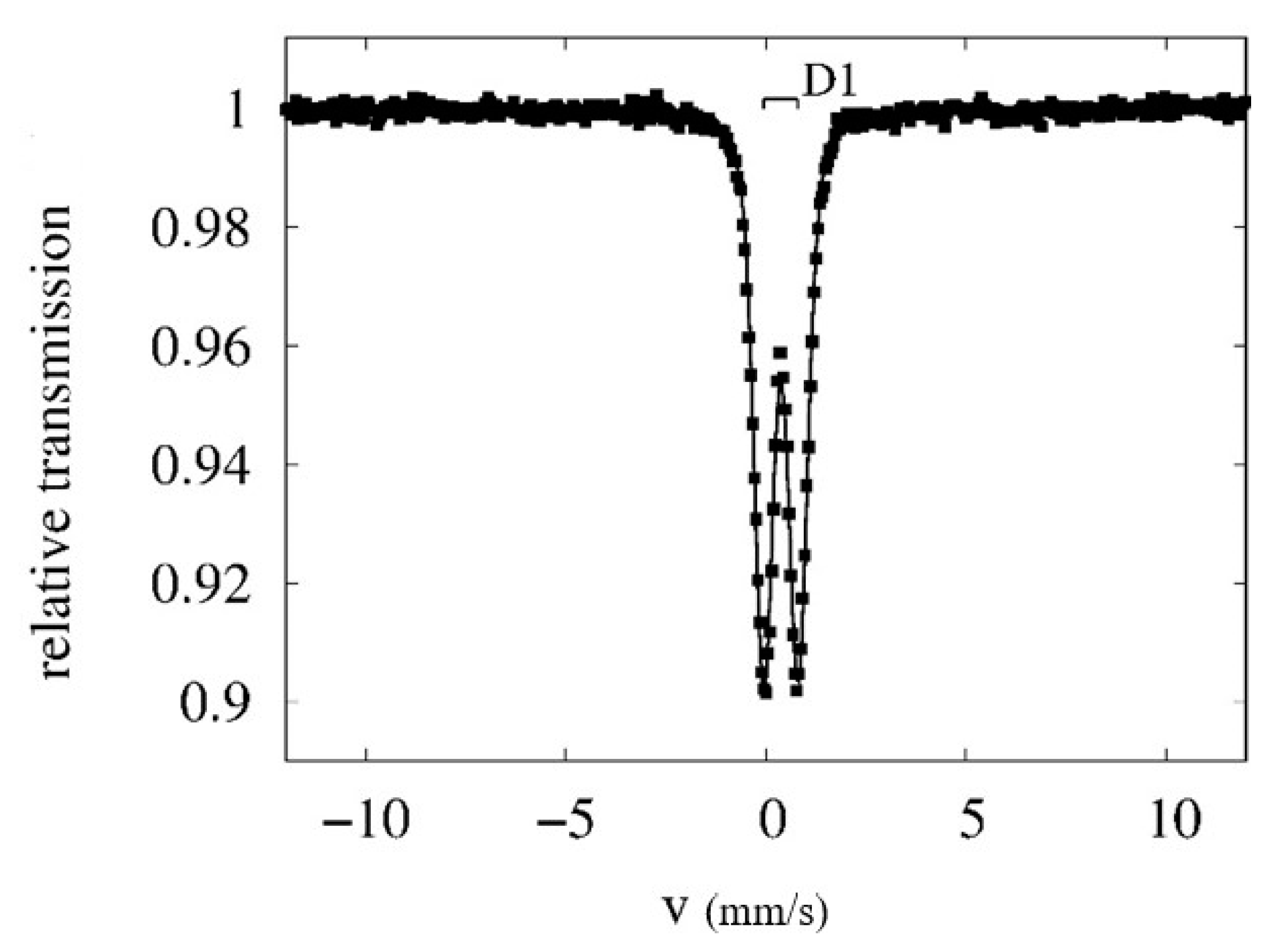
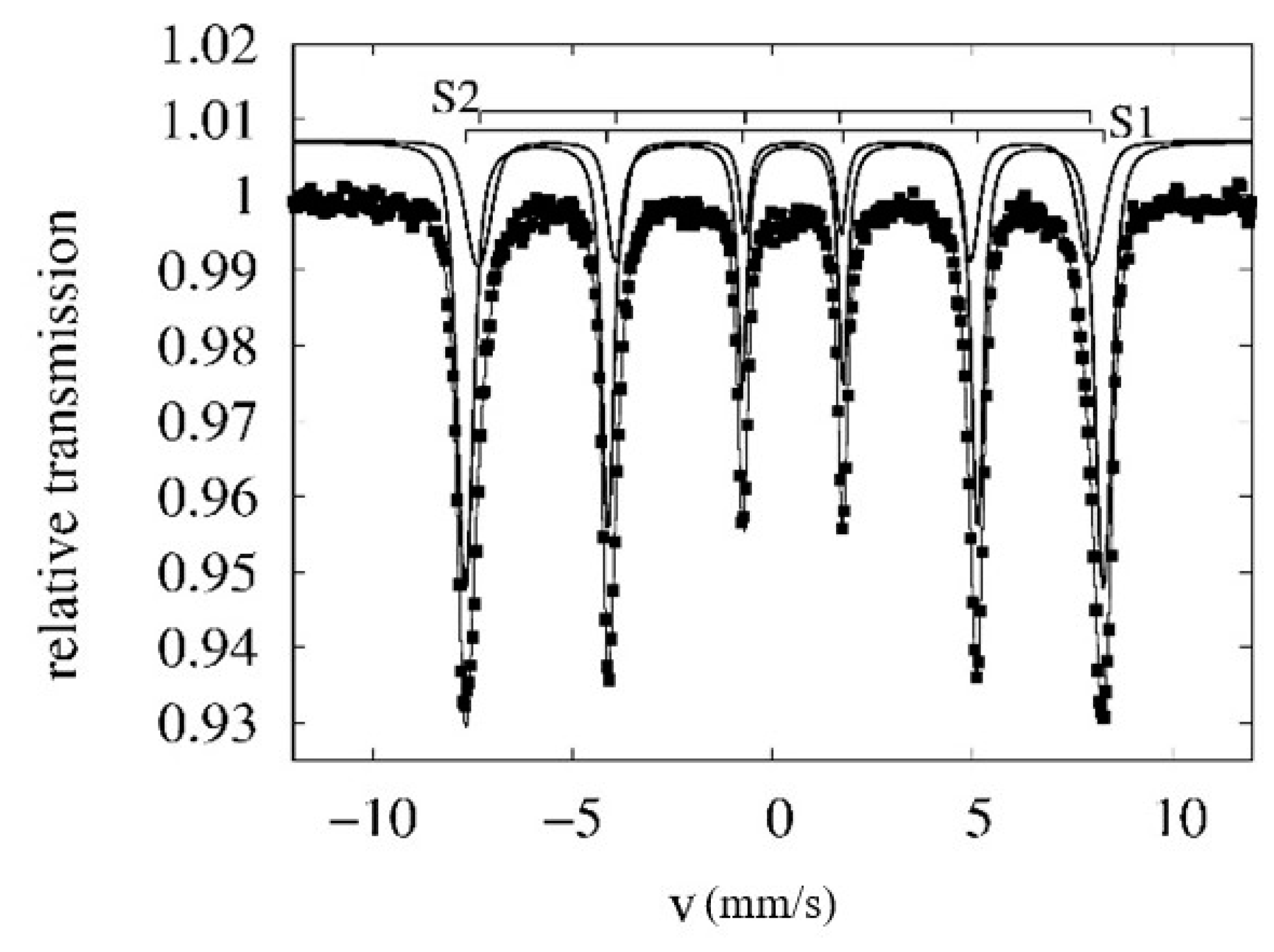
3.6. EC solid Product Characterisation
4. Conclusions
- Electrocoagulation is an effective tool for removing chromium from the leachates by concentrating in a solid product useful in the production of chromium.
- pH value of 6 was optimal for EC procedure, due to the reduction of the experiment duration for total precipitation of Cr and thus minimization the energy consumption.Concentration of NaCl, under the same conditions of pH and current intensity, had no influence on the experiment duration. This parameter affected only the proportion of Fe in the solid product and the energy consumption during experiments.
- Application of higher current intensities (1 or 2 A) led to shortening the time of EC process necessary for the total Cr removal from solution but it led to solution overheating up to 60 °C. 100% efficiency of chromium removal was achieved for 0.5 A after 140 min and for 0.1 A after 430 min of EC process.
- Thermogravimetric analysis, XRD analysis and Mössbauer spectroscopy of solid products provided description of key product parameters in detail.
- Final EC solid products reached up to 20% of chromium in the form of substituted hematite. Initial amount of 2–5% of chromium in slags used for leachates preparation is stated for comparison.
- EC demands application of high current intensities when it is used as waste water treatment technology (solid product composition is insignificant).
- Metals recovery from secondary raw materials could be achieved by employing of low current intensities (metal concentration increases in the final solid product).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Worldsteel Association. Steel Industry Co-Products. Available online: https://www.worldsteel.org/en/dam/jcr:1b916a6d-06fd-4e84-b35d-c1d911d18df4/Fact_By-products_2018.pdf (accessed on 20 January 2020).
- Garcia-Ramos, E.; Romero-Serrano, A.; Zeifert, B.; Flores-Sanchez, P.; Hallen-Lopez, M.; Palacios, E.G. Immobilization of chromium in slags using MgO and Al2O3. Steel Res. Int. 2008, 79, 332–339. [Google Scholar] [CrossRef]
- Panda, C.R.; Mishra, K.K.; Nayak, B.D.; Rao, D.S.; Nayak, B.B. Release behaviour of chromium from ferrochrome slag. Int. J. Environ. Technol. Manag. 2012, 15, 261–274. [Google Scholar] [CrossRef]
- Qing, Z.; Chengjun, L.; Longhu, C.; Xiang, Z.; Maofa, J. Effect of Lime on Stability of Chromium in Stainless Steel Slag. Minerals 2018, 8, 424. [Google Scholar] [CrossRef]
- Bayramoglu, M.; Eyvaz, M.; Kobya, M. Treatment of the textile wastewater by electrocoagulation: Economical evaluation. Chem. Eng. J. 2007, 128, 155–161. [Google Scholar] [CrossRef]
- Wang, C.T.; Chou, W.L. Performance of COD removal from oxide chemical mechanical polishing wastewater using iron electrocoagulation. J. Environ. Sci. Health Part A 2009, 44, 1289–1297. [Google Scholar] [CrossRef]
- Fernandes, A.; Spranger, P.; Fonseca, A.D.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Effect of electrochemical treatments on the biodegradability of sanitary landfill leachates. Appl. Catal. B Environ. 2014, 144, 514–520. [Google Scholar] [CrossRef]
- Kobya, M.; Ciftci, C.; Bayramoglu, M.; Sensoy, M.T. Study on the treatment of waste metal cutting fluids using electrocoagulation. Sep. Purif. Technol. 2008, 60, 285–291. [Google Scholar] [CrossRef]
- Zodi, S.; Louvet, J.N.; Michon, C.; Potier, O.; Pons, M.N.; Lapicque, F.; Leclerc, J.P. Electrocoagulation as a tertiary treatment for paper mill wastewater: Removal of non-biodegradable organic pollution and arsenic. Sep. Purif. Technol. 2011, 81, 62–68. [Google Scholar] [CrossRef]
- Behloul, M.; Grib, H.; Drouiche, N.; Abdi, N.; Lounici, H.; Mameri, N. Removal of Malathion Pesticide from Polluted Solutions by Electrocoagulation: Modeling of Experimental Results using Response Surface Methodology. Sep. Sci. Technol. 2013, 48, 664–672. [Google Scholar] [CrossRef]
- Inan, H.; Alaydin, E. Phosphate and nitrogen removal by iron produced in electrocoagulation reactor. Desalin. Water Treat. 2014, 52, 1396–1403. [Google Scholar] [CrossRef]
- Aroyo, M.G.; Pérez-Herranz, V.; Montanés, M.T.; García-Antón, J.; Guinón, J.L. Effect of pH and chloride concentration on the removal of hexavalent chromium in a batch electrocoagulation reactor. J. Hazard. Mater. 2009, 169, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Keshmirizadeh, E.; Yousefi, S.; Rofouei, M.K. An investigation on the new operational parameter effective in Cr(VI) removal efficiency: A study on electrocoagulation by alternating pulse current. J. Hazard. Mater. 2011, 190, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Yang, Z.H.; Luo, Y.L.; Zeng, G.M.; Huang, J.; Wanga, L.; Song, P.P.; Yang, X. A novel approach to sustain Fe0-electrocoagulation for Cr(VI) removal by optimizing chloride ion. Sep. Purif. Technol. 2015, 156, 200–206. [Google Scholar] [CrossRef]
- Janin, A.; Zaviska, F.; Drogui, P.; Blais, J.F.; Mercier, G. Selective recovery of metals in leachate from chromated copper arsenate treated wastes using electrochemical technology and chemical precipitation. Hydrometallurgy 2009, 96, 318–326. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Removal of Cr3+ by electrocoagulation with multiple electrodes: Bipolar and monopolar configurations. J. Hazard. Mater. 2007, 141, 653–661. [Google Scholar] [CrossRef]
- Khan, S.U.; Islam, D.T.; Farooqi, I.H.; Ayub, S.; Basheer, S. Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation. Process Saf. Environ. Prot. 2019, 122, 118–130. [Google Scholar] [CrossRef]
- Cherifi, M.; Hazourli, S.; Pontvianne, S.; Lapicque, F.; Leclerc, J.P. Electrokinetic removal of aluminum and chromium from industrial wastewater electrocoagulation treatment sludge. Desalin. Water Treat. 2015, 57, 1–16. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) presentin aqueous solutions by aluminium electrocoagulation. J. Hazard. Mater. 2008, 152, 934–941. [Google Scholar] [CrossRef]
- Zewail, T.M.; Yousef, N.S. Chromium ions (Cr6+ & Cr3+) removal from synthetic wastewaterby electrocoagulation using vertical expanded Fe anode. J. Electroanal. Chem. 2014, 735, 123–128. [Google Scholar] [CrossRef]
- Mouedhen, G.; Feki, M.; De Petris-Wery, M.; Ayedi, H.F. Electrochemical removal of Cr(VI)from aqueous media using iron and aluminum as electrode materials: Towards a better understanding of the involved phenomena. J. Hazard. Mater. 2009, 168, 983–991. [Google Scholar] [CrossRef]
- Kakakhel, L.; Lutfullah, G.; Bhanger, M.I.; Shah, A.; Niaz, A. Electrolytic recovery of chromium salts from tannery wastewater. J. Hazard. Mater. 2007, 148, 560–565. [Google Scholar] [CrossRef]
- Benhadji, A.; Ahmed, M.T.; Maachi, R. Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination 2011, 277, 128–134. [Google Scholar] [CrossRef]
- Lakshmipathiraj, P.; Raju, G.B.; Basariya, M.R.; Parvathy, S.; Prabhakar, S. Removal of Cr(VI) by electrochemical reduction. Sep. Purif. Technol. 2008, 60, 96–102. [Google Scholar] [CrossRef]
- Jin, W.; Du, H.; Zheng, S.; Zhang, Y. Electrochemical processes for the environmental remediation of toxic Cr(VI): A review. Electrochim. Acta 2016, 191, 1044–1055. [Google Scholar] [CrossRef]
- Kim, J.D.; Pyun, S.I. The effects of applied potential and chloride ion on the repassivation kinetics of pure iron. Corros. Sci. 1996, 38, 1093–1102. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Studies on the Al-Zn-In-alloy as anode material for the removal of chromium from drinking water in electrocoagulation process. Desalination 2011, 275, 260–268. [Google Scholar] [CrossRef]
- Barrera-Diaz, C.; Lugo-Lugo, V.; Roa-Morales, G.; Natividad, R.; Martinez-Delgadillo, S.A. Enhancing the electrochemical Cr(VI) reduction in aqueous solution. J. Hazard. Mater. 2011, 185, 1362–1368. [Google Scholar] [CrossRef]
- Horckmans, L.; Möckel, R.; Nielsen, P.; Kukurugya, F.; Vanhoof, C.; Morillon, A.; Algermissen, D. Multi-Analytical Characterization of Slags to Determine the Chromium Concentration for a Possible Re-Extraction. Minerals 2019, 9, 646. [Google Scholar] [CrossRef]
- Lutterotti, L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Žák, T.; Jirásková, Y. CONFIT: Mössbauer spectra fitting program. Surf. Interface Anal. 2006, 38, 710–714. [Google Scholar] [CrossRef]
- Parga, J.R.; Cocke, D.L.; Valverde, V.; Gomes, J.A.G.; Kesmez, M.; Moreno, H.; Weir, M.; Mencer, D. Characterization of Electrocoagulation for Removal of Chromium and Arsenic. Chem. Eng. Technol. 2005, 28, 605–612. [Google Scholar] [CrossRef]
- Parga, J.R.; Vazquez, V.; Gonzalez, G.; Cisneros, M.M. Thermodynamic Studies of Chromium Adsorption on Iron Species Generated by Electrocoagulation. Chem. Eng. Technol. 2010, 33, 1582–1590. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Hartridge, A.; Mallick, K.K.; Majumdar, C.K.; Das, D.; Chintalapudi, S.N. An X-ray diffraction and Mössbauer study of nanocrystalline Fe2O3–Cr2O3 solid solutions. J. Mater. Sci. 1997, 32, 557–560. [Google Scholar] [CrossRef]
- Yogi, A.; Varshney, D. Magnetic and structural properties of pure and Cr-doped haematite: α-Fe2-xCrxO3 (0 ≤ x ≤ 1). J. Adv. Ceram. 2013, 2, 360–369. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Cahn, R.W.; Flemings, M.C.; Ilschner, B.; Kramer, E.J.; Mahajan, S.; Veyssière, P. Production of Chromium Ferroalloys. In Encyclopedia of Materials: Science and Technology; Elsevier Ltd.: Oxford, UK; Pergamon: Oxford, UK, 2001; ISBN 978-0-08-043152-9. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutiona affiliations. |


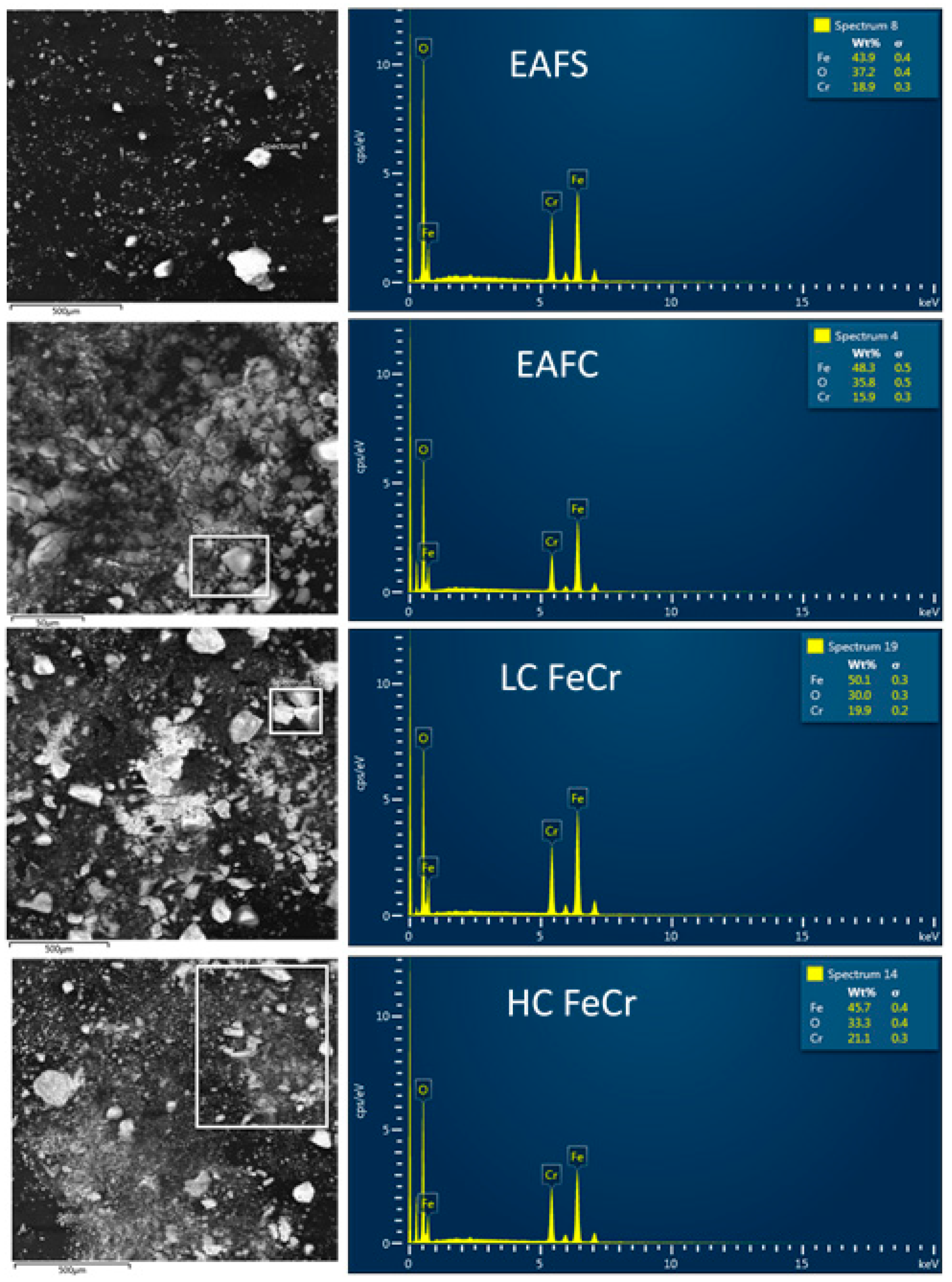



| Real Leach. Mark | c (Cr)(mg/L) | Original pH | V (HCl 1:1) Added to 100 mL of Leachate for pH Adjusting to 6 (mL) | NaCl Produced from 100 mL of Leachate (g) | G *(mS) |
|---|---|---|---|---|---|
| EAFS | 2191 | 12.5 | 2.36 | 0.414 | 17.37 |
| EAFC | 942 | 12.6 | 1.69 | 0.416 | 12.67 |
| LC FeCr | 687 | 12.6 | 1.33 | 0.333 | 9.4 |
| HC FeCr | 2013 | 10.5 | 0.67 | - | 8.8 |
| Sample | c (Cr) (mg/L) | I (A) | U (V) | c (NaCl) (mg/L) | Cr (g/kg) * | Fe (g/kg) * | Ratio Cr:Fe | Energy Consumption (kWh/m3) |
|---|---|---|---|---|---|---|---|---|
| model 1 | 1000 | 0.1 | 2.6 | 3000 | 138.9 | 384.3 | 0.3614 | 5.57 |
| model 2 | 1000 | 0.5 | 8.5 | 3000 | 107.5 | 367.9 | 0.2922 | 24.65 |
| model 3 | 1000 | 1 | 16 | 3000 | 95.8 | 388.3 | 0.2467 | 59.00 |
| model 4 | 1000 | 2 | 25 | 3000 | 79.6 | 437.8 | 0.1818 | 107.52 |
| model 5 | 1000 | 0.5 | 4 | 10,000 | 79.5 | 364.3 | 0.2182 | 13.83 |
| model 6 | 1000 | 0.5 | 2.5 | 30,000 | 84.3 | 384.5 | 0.2192 | 12.98 |
| model 7 | 1000 | 0.5 | 2 | 50,000 | 98.3 | 727.8 | 0.1351 | 6.76 |
| EAFS | 2191 | 0.1 | 1.8 | not add. | 156.6 | 357.9 | 0.4376 | 6.64 |
| EAFC | 942 | 0.1 | 1.8 | not add. | 140 | 340.6 | 0.4110 | 4.21 |
| LC FeCr | 687 | 0.1 | 2.2 | not add. | 141.1 | 346.1 | 0.4077 | 3.74 |
| HC FeCr | 2013 | 0.1 | 2.5 | not add. | 111.1 | 222.2 | 0.500 | 10.99 |
| Sample | Fe | Cr | Cl | S | Mg | Ʃ Elem. | Balance | Total % | Ratio Cr/Fe |
|---|---|---|---|---|---|---|---|---|---|
| model 1 | 44.88 | 14.93 | 0.351 | 3.53 | 3.85 | 70.87 | 29.00 | 99.87 | 0.3327 |
| model 1 * | 51.72 | 16.96 | 0.070 | 0.464 | 4.65 | 77.43 | 22.48 | 99.90 | 0.3279 |
| EAFS | 42.31 | 15.56 | 2.05 | 1.04 | 2.98 | 72.30 | 32.87 | 99.79 | 0.3678 |
| EAFS * | 46.75 | 17.39 | 0.075 | 0.988 | 4.03 | 73.77 | 27.35 | 99.84 | 0.3720 |
| EAFC | 41.87 | 15.36 | 1.46 | 0.578 | 3.76 | 74.15 | 34.15 | 99.89 | 0.3668 |
| EAFC * | 48.30 | 16.79 | 0.069 | 0.123 | 3.69 | 73.63 | 27.75 | 99.84 | 0.3476 |
| LC FeCr | 43.20 | 15.13 | 1.53 | 0.305 | 4.22 | 74.86 | 32.58 | 99.85 | 0.3502 |
| LC FeCr * | 50.97 | 17.79 | 0.084 | 0.262 | 4.19 | 74.00 | 23.34 | 99.85 | 0.3490 |
| HC FeCr | 34.28 | 15.77 | 0.823 | 0.503 | 3.11 | 73.22 | 42.60 | 99.84 | 0.4600 |
| HC FeCr * | 43.59 | 20.11 | 0.070 | 0.466 | 3.02 | 73.87 | 29.49 | 99.84 | 0.4613 |
| Conditions | <IS> [mm/s] | <QS> [mm/s] | <Bhf> [T] |
|---|---|---|---|
| RT | 0.37 ± 0.02 | 0.87 ± 0.02 | - |
| 4.2 K | 0.47 ± 0.02 | 0.00 ± 0.02 | 46.2 ± 0.3 |
| Conditions | <IS> [mm/s] | <QS> [mm/s] | <Bhf> [T] | |
|---|---|---|---|---|
| RT | S1 | 0.40 ± 0.02 | −0.21 ± 0.02 | 49.6 ± 0.3 |
| S2 | 0.40 ± 0.02 | −0.21 ± 0.02 | 47.6 ± 0.3 | |
| 4.2 K | S1 | 0.48 ± 0.02 | −0.20 ± 0.02 | 52.6 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikna, L.; Hezelova, M.; Morillon, A.; Algermissen, D.; Milkovic, O.; Findorak, R.; Cesnek, M.; Briancin, J. Recovery of Chromium from Slags Leachates by Electrocoagulation and Solid Product Characterization. Metals 2020, 10, 1593. https://doi.org/10.3390/met10121593
Pikna L, Hezelova M, Morillon A, Algermissen D, Milkovic O, Findorak R, Cesnek M, Briancin J. Recovery of Chromium from Slags Leachates by Electrocoagulation and Solid Product Characterization. Metals. 2020; 10(12):1593. https://doi.org/10.3390/met10121593
Chicago/Turabian StylePikna, Lubomir, Maria Hezelova, Agnieszka Morillon, David Algermissen, Ondrej Milkovic, Robert Findorak, Martin Cesnek, and Jaroslav Briancin. 2020. "Recovery of Chromium from Slags Leachates by Electrocoagulation and Solid Product Characterization" Metals 10, no. 12: 1593. https://doi.org/10.3390/met10121593
APA StylePikna, L., Hezelova, M., Morillon, A., Algermissen, D., Milkovic, O., Findorak, R., Cesnek, M., & Briancin, J. (2020). Recovery of Chromium from Slags Leachates by Electrocoagulation and Solid Product Characterization. Metals, 10(12), 1593. https://doi.org/10.3390/met10121593







