Effect of Alloying Mn by Selective Laser Melting on the Microstructure and Biodegradation Properties of Pure Mg
Abstract
1. Introduction
2. Experiments
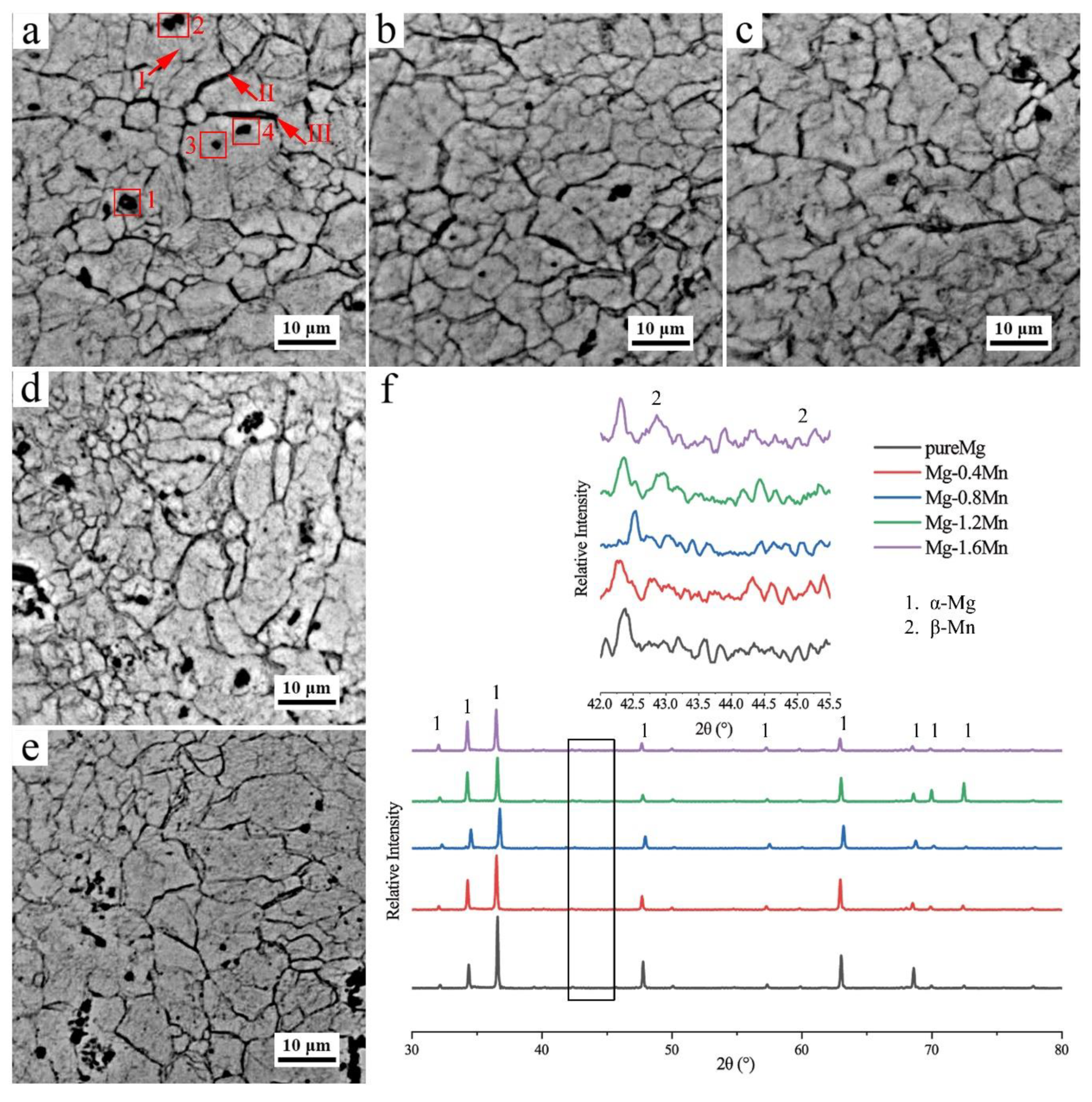
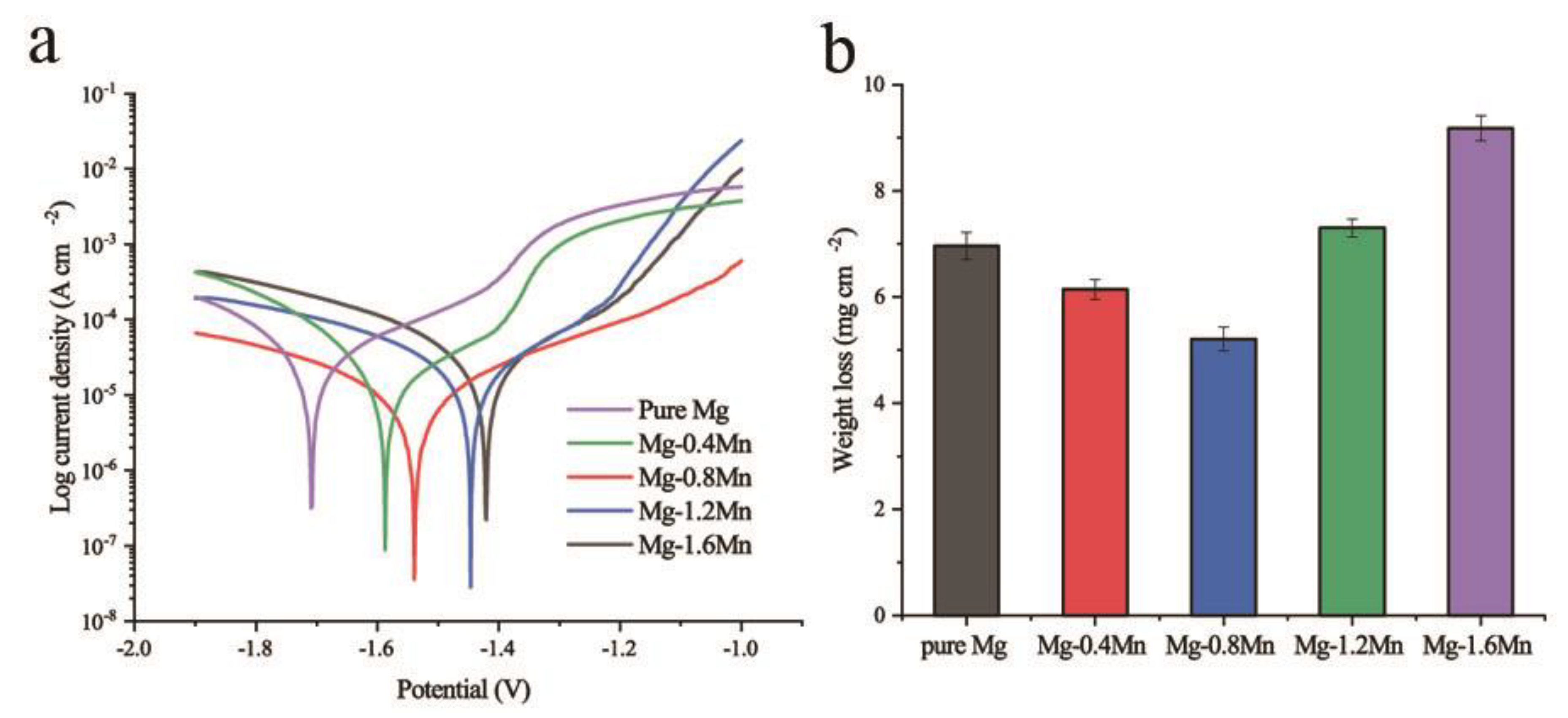
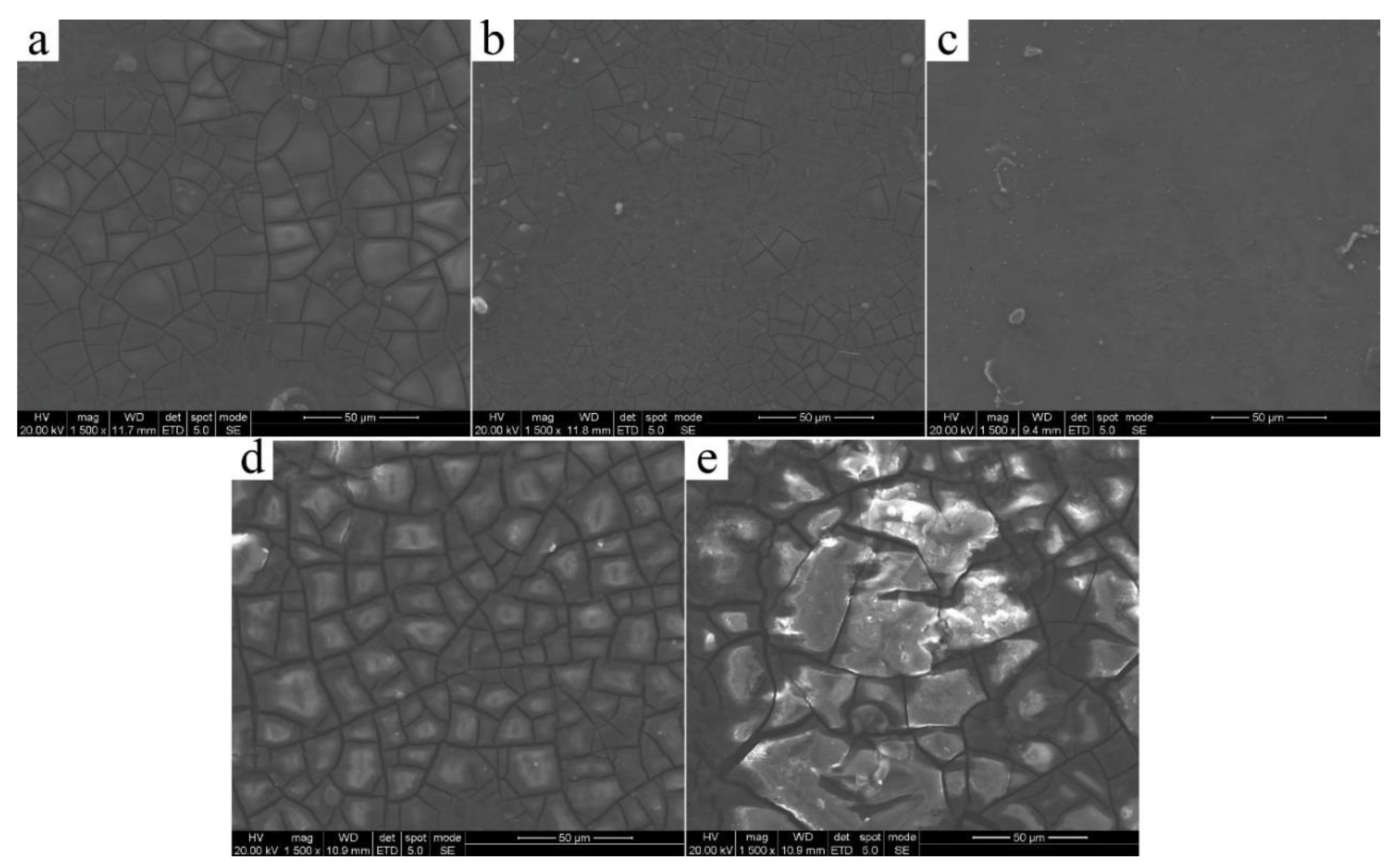
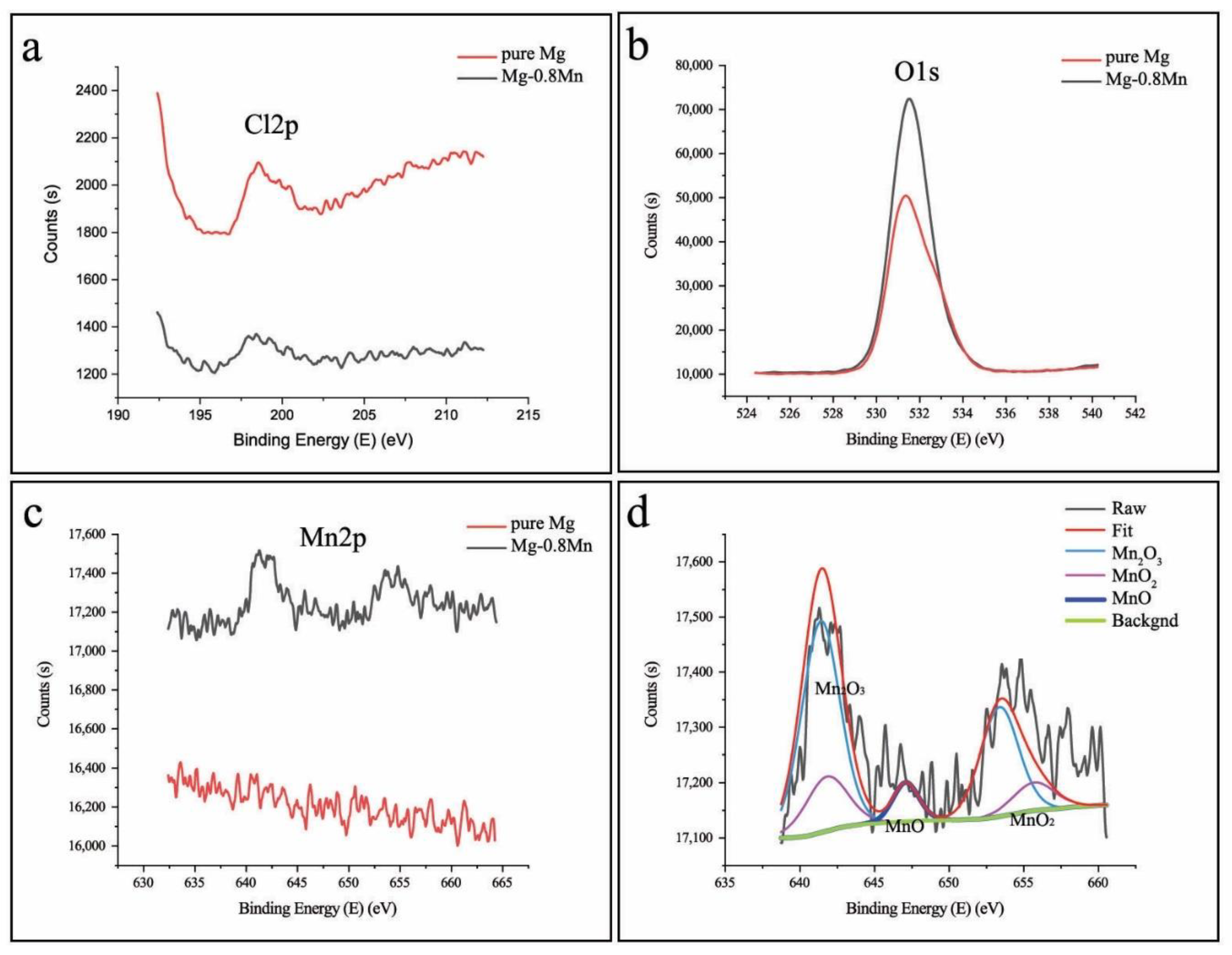
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Ibrahim, M.; Etim, I.P.; Tan, L.; Yang, K. In vitro degradation and antibacterial property of a copper-containing micro-arc oxidation coating on Mg-2Zn-1Gd-0.5Zr alloy. Colloids Surf. B Biointerfaces 2019, 179, 77–86. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; et al. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective: Magnesium Biomaterials for Orthopedic Application. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Tao, J.-X.; Zhao, M.-C.; Zhao, Y.-C.; Yin, D.-F.; Liu, L.; Gao, C.; Shuai, C.; Atrens, A. Influence of graphene oxide (GO) on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting. J. Magnes. Alloys 2020, 8, 952–962. [Google Scholar] [CrossRef]
- Liu, Y.; Koltick, D.; Byrne, P.; Wang, H.; Zheng, W.; Nie, L.H. Development of a transportable neutron activation analysis system to quantify manganese in bone in vivo: Feasibility and methodology. Physiol. Meas. 2013, 34, 1593–1609. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Wang, Q.; Wu, H.; Liu, Y.; Deng, Y.; Zhou, Y.; Shuai, C. The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting. Materials 2016, 9, 216. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kim, H.J.; Baek, S.-M.; Kim, B.; Sohn, S.-D.; Shin, H.-J.; Jeong, H.Y.; Park, S.H.; Yim, C.D.; You, B.S.; et al. Improved corrosion resistance of extruded Mg–8Sn–1Zn–1Al alloy by microalloying with Mn. Scr. Mater. 2015, 109, 38–43. [Google Scholar] [CrossRef]
- Metalnikov, P.; Ben-Hamu, G.; Templeman, Y.; Shin, K.S.; Meshi, L. The relation between Mn additions, microstructure and corrosion behavior of new wrought Mg-5Al alloys. Mater. Charact. 2018, 145, 101–115. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, B.W.; Park, J.Y.; Cho, K.M.; Park, I.M. Effect of Mn addition on corrosion properties of biodegradable Mg-4Zn-0.5Ca-xMn alloys. J. Alloys Compd. 2017, 695, 1166–1174. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Birbilis, N. Influence of Mn and Zr on the Corrosion of Al-Free Mg Alloys: Part 2—Impact of Mn and Zr on Mg Alloy Electrochemistry and Corrosion. Corrosion 2013, 69, 744–751. [Google Scholar] [CrossRef]
- Nam, N.D.; Mathesh, M.; Forsyth, M.; Jo, D.S. Effect of manganese additions on the corrosion behavior of an extruded Mg–5Al based alloy. J. Alloys Compd. 2012, 542, 199–206. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Tang, Y.; Zhao, M.-C.; Liu, C.; Liu, L.; Gao, C.-D.; Shuai, C.; Atrens, A. Study on Fe-xGO Composites Prepared by Selective Laser Melting: Microstructure, Hardness, Biodegradation and Cytocompatibility. JOM 2020, 72, 1163–1174. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, M.-C.; Zhao, Y.-C.; Liu, L.; Liu, C.; Gao, C.; Shuai, C.; Atrens, A. Improved biodegradation resistance by grain refinement of novel antibacterial ZK30-Cu alloys produced via selective laser melting. Mater. Lett. 2019, 237, 253–257. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, L.; Ni, D.; Chen, J.; Zhao, Y.-C.; Liu, L.; Shuai, C.; Yang, K.; Atrens, A.; Zhao, M.-C. Effect of grain refinement and crystallographic texture produced by friction stir processing on the biodegradation behavior of a Mg-Nd-Zn alloy. J. Mater. Sci. Technol. 2019, 35, 777–783. [Google Scholar] [CrossRef]
- Díaz, I.; Pacha-Olivenza, M.Á.; Tejero, R.; Anitua, E.; González-Martín, M.L.; Escudero, M.L.; García-Alonso, M.C. Corrosion behavior of surface modifications on titanium dental implant. In situ bacteria monitoring by electrochemical techniques. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 997–1009. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Li, X.-T.; Zeng, R.-C.; Li, S.-Q.; Han, E.-H.; Song, L. In vitro corrosion of Mg–Ca alloy—The influence of glucose content. Front. Mater. Sci. 2017, 11, 284–295. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Farahany, S.; Staiger, M.P. The Mechanical Properties and Corrosion Behavior of Quaternary Mg-6Zn-0.8Mn-xCa Alloys. J. Mater. Eng. Perform. 2015, 24, 598–608. [Google Scholar] [CrossRef]
- Kim, W.-C.; Kim, J.-G.; Lee, J.-Y.; Seok, H.-K. Influence of Ca on the corrosion properties of magnesium for biomaterials. Mater. Lett. 2008, 62, 4146–4148. [Google Scholar] [CrossRef]
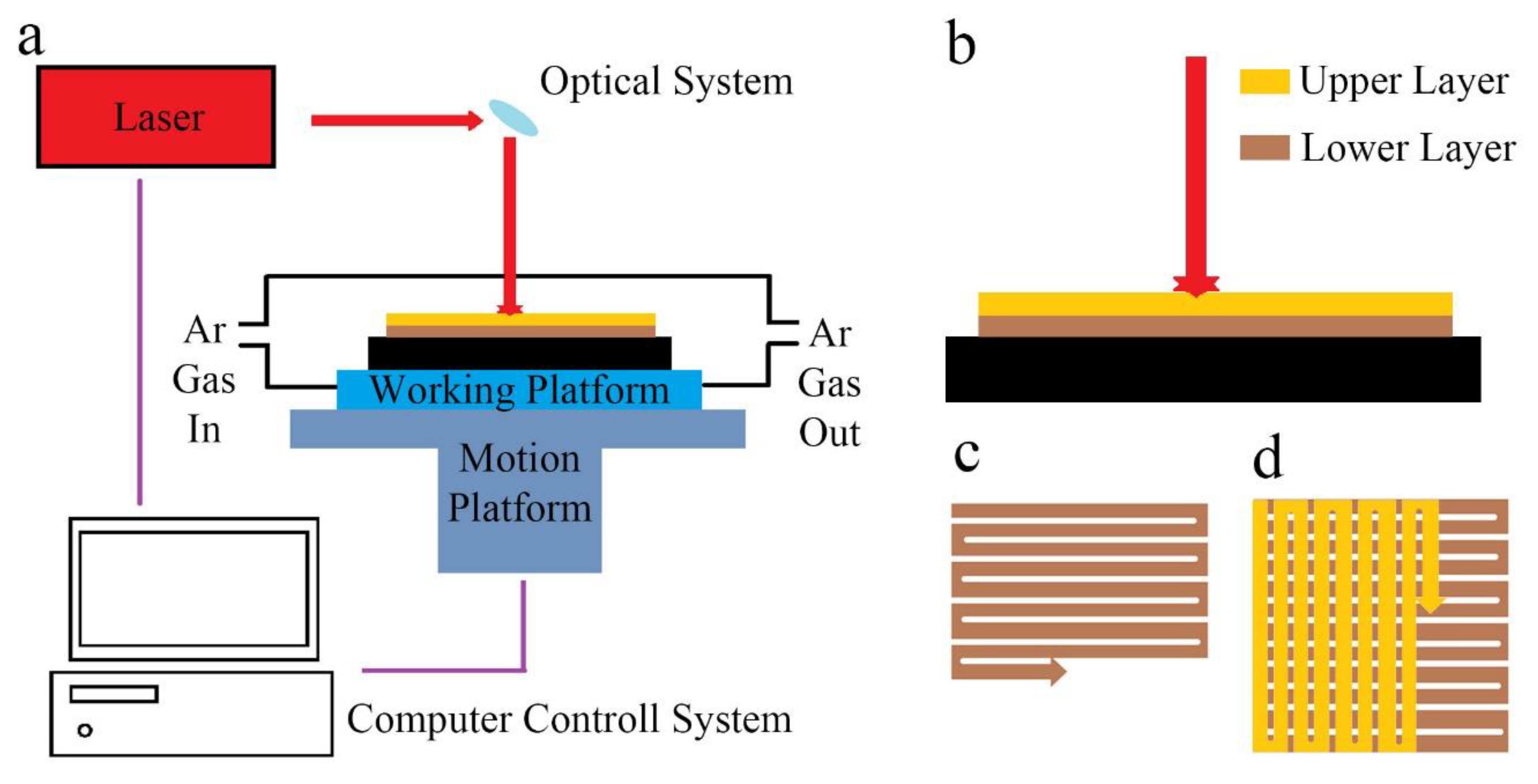
| Laser Power | Scaning Speed | Laser Spot | Layer Thickness | Scanning Pitch |
|---|---|---|---|---|
| 75 W | 15 mm/s | 150 μm | 50 μm | 70 μm |
| Materials | Mg | Mg-0.4Mn | Mg-0.8Mn | Mg-1.2Mn | Mg-1.6Mn |
|---|---|---|---|---|---|
| icorr (μA cm−2) | 29.21 | 22.08 | 10.18 | 28.25 | 37.82 |
| Pi (mm year−1) | 0.67 | 0.50 | 0.23 | 0.65 | 0.86 |
| ΔW (mg (cm2 day)−1) | 0.99 ± 0.04 | 0.88 ± 0.03 | 0.74 ± 0.03 | 1.04 ± 0.02 | 1.31 ± 0.03 |
| Pw (mm year−1) | 2.09 ± 0.08 | 1.84 ± 0.06 | 1.56 ± 0.07 | 2.19 ± 0.05 | 2.75 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, B.; Zhao, M.-C.; Zhao, Y.-C.; Tian, Y.; Yin, D.; Gao, C.; Shuai, C.; Atrens, A. Effect of Alloying Mn by Selective Laser Melting on the Microstructure and Biodegradation Properties of Pure Mg. Metals 2020, 10, 1527. https://doi.org/10.3390/met10111527
Xie B, Zhao M-C, Zhao Y-C, Tian Y, Yin D, Gao C, Shuai C, Atrens A. Effect of Alloying Mn by Selective Laser Melting on the Microstructure and Biodegradation Properties of Pure Mg. Metals. 2020; 10(11):1527. https://doi.org/10.3390/met10111527
Chicago/Turabian StyleXie, Bin, Ming-Chun Zhao, Ying-Chao Zhao, Yan Tian, Dengfeng Yin, Chengde Gao, Cijun Shuai, and Andrej Atrens. 2020. "Effect of Alloying Mn by Selective Laser Melting on the Microstructure and Biodegradation Properties of Pure Mg" Metals 10, no. 11: 1527. https://doi.org/10.3390/met10111527
APA StyleXie, B., Zhao, M.-C., Zhao, Y.-C., Tian, Y., Yin, D., Gao, C., Shuai, C., & Atrens, A. (2020). Effect of Alloying Mn by Selective Laser Melting on the Microstructure and Biodegradation Properties of Pure Mg. Metals, 10(11), 1527. https://doi.org/10.3390/met10111527









