Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals
Abstract
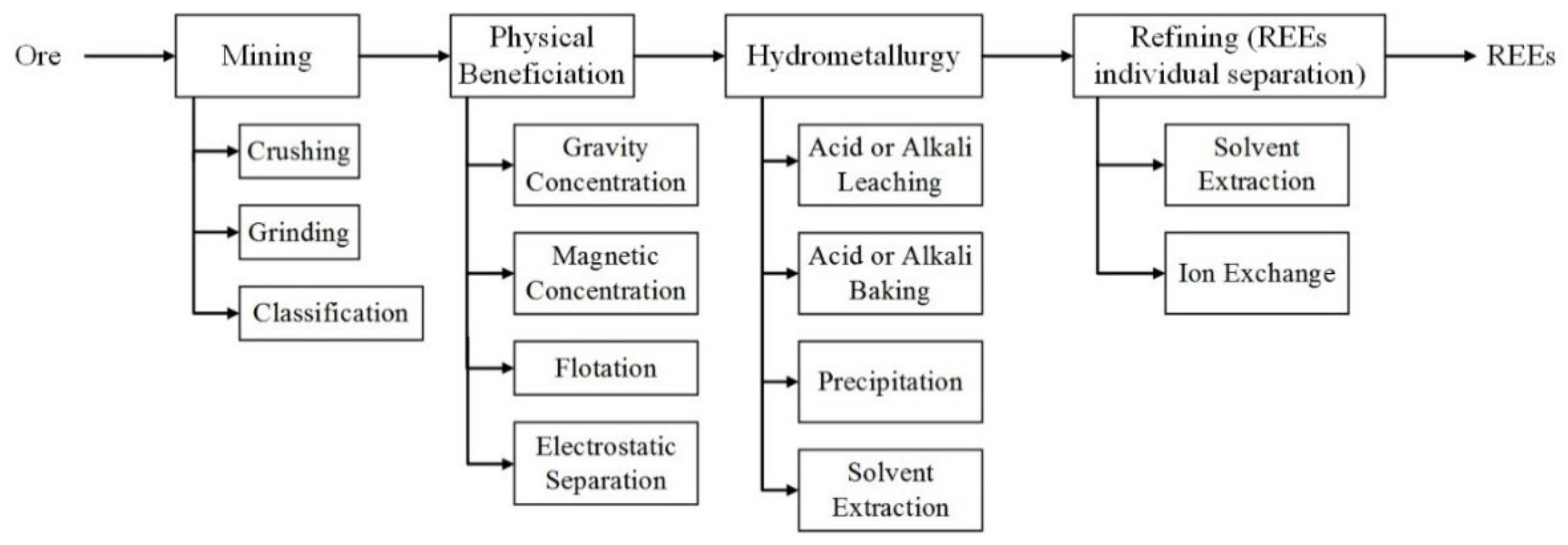
1. Introduction
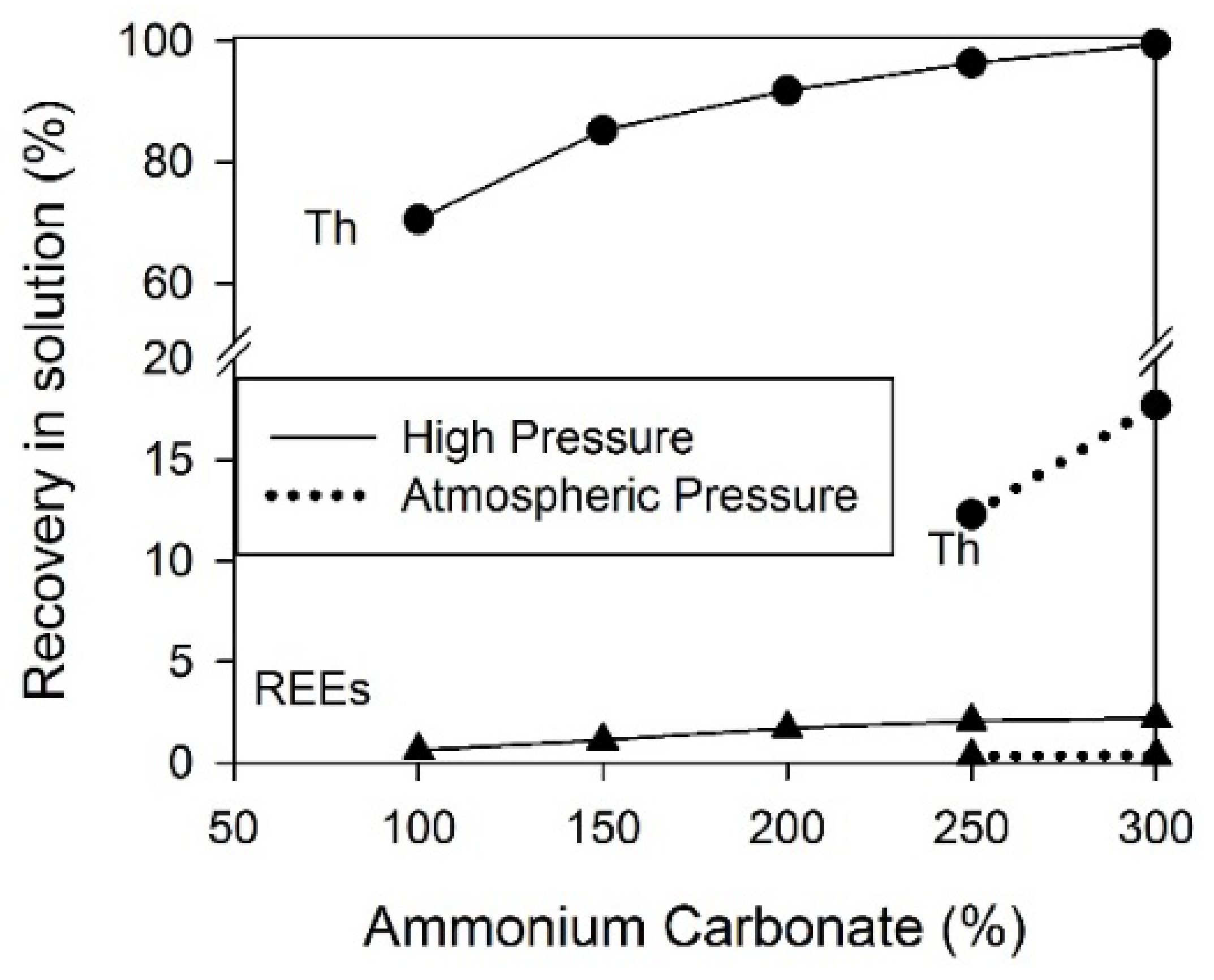
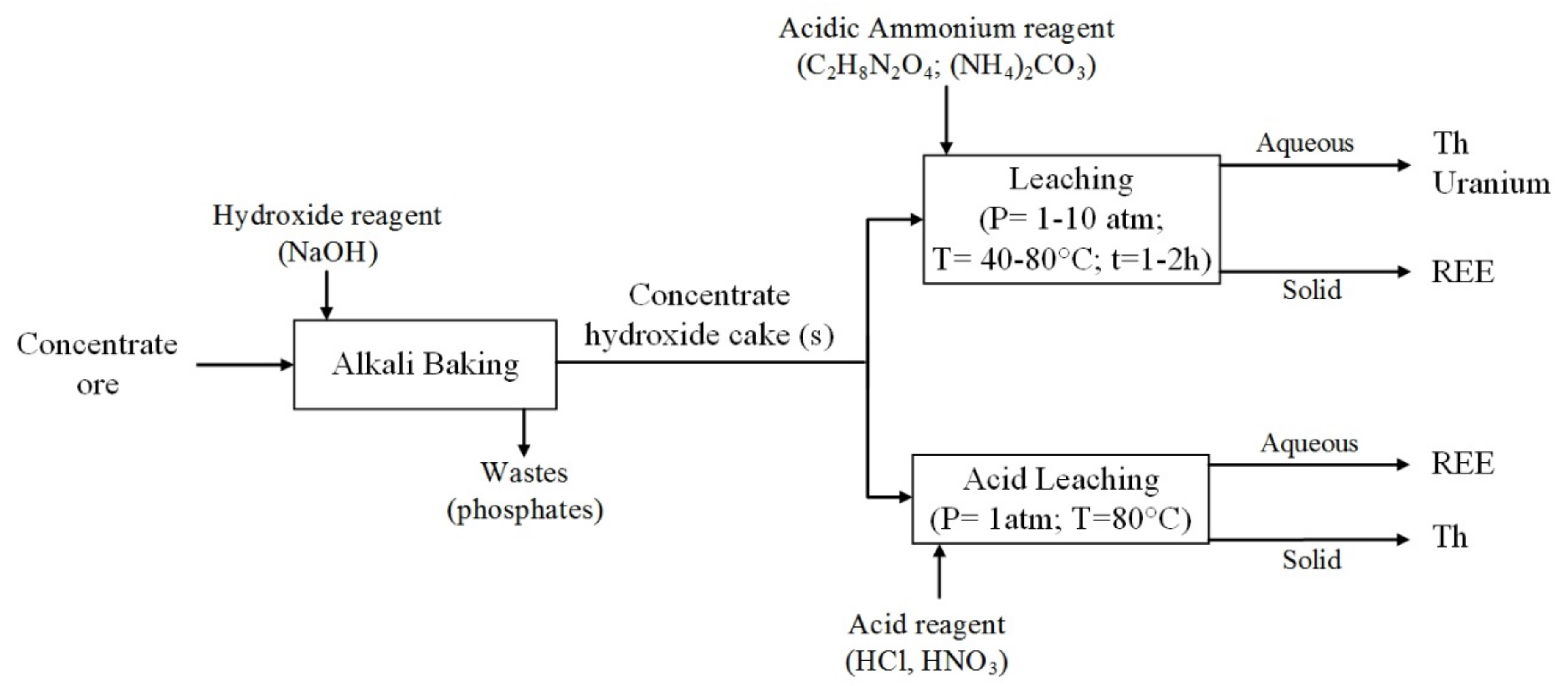
2. Separation by Leaching
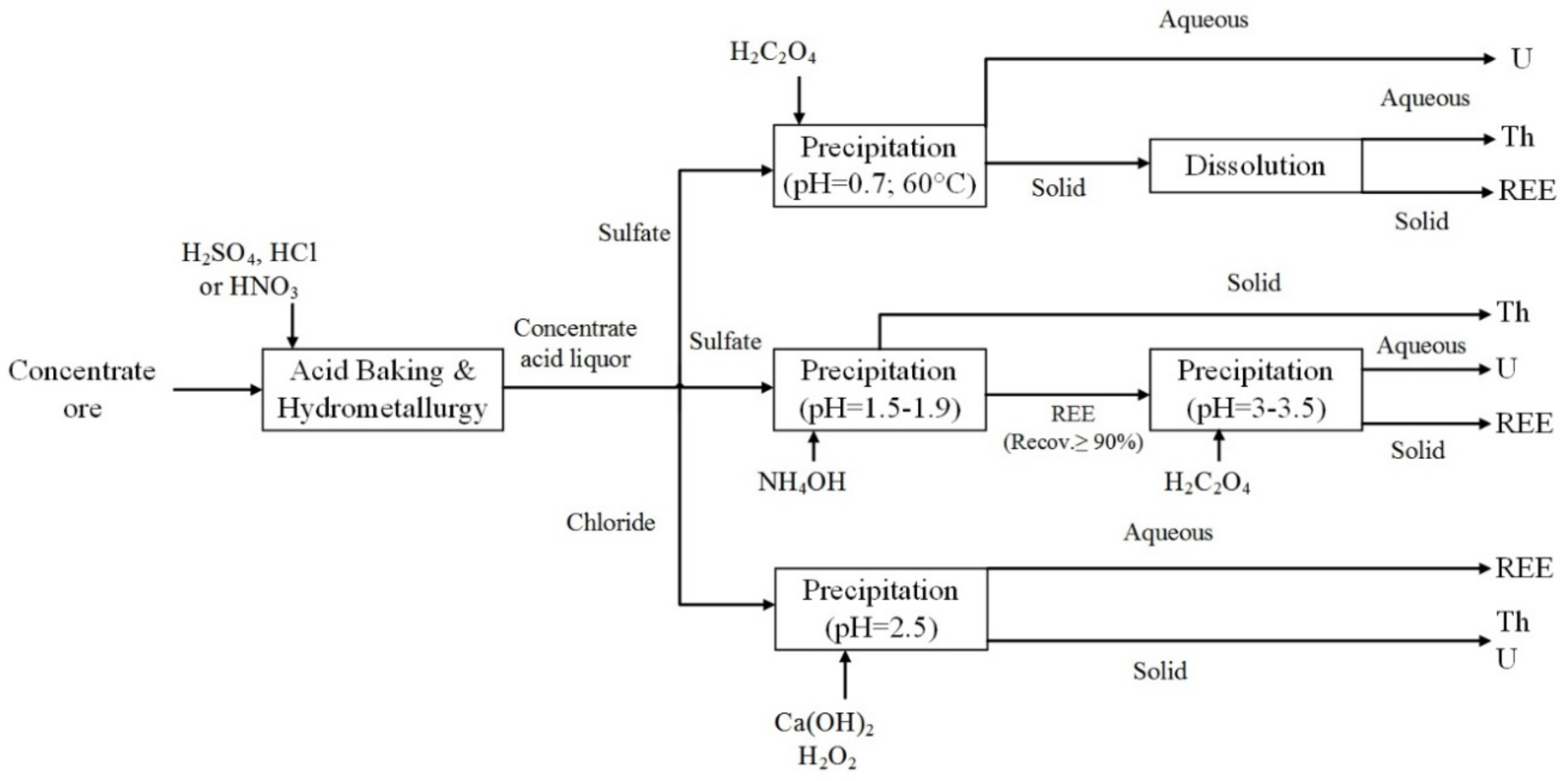
3. Separation by Precipitation
3.1. Types of the Liquor and Reagent
3.2. Multi- or Single-Step Precipitation
4. Separation by Solvent Extraction
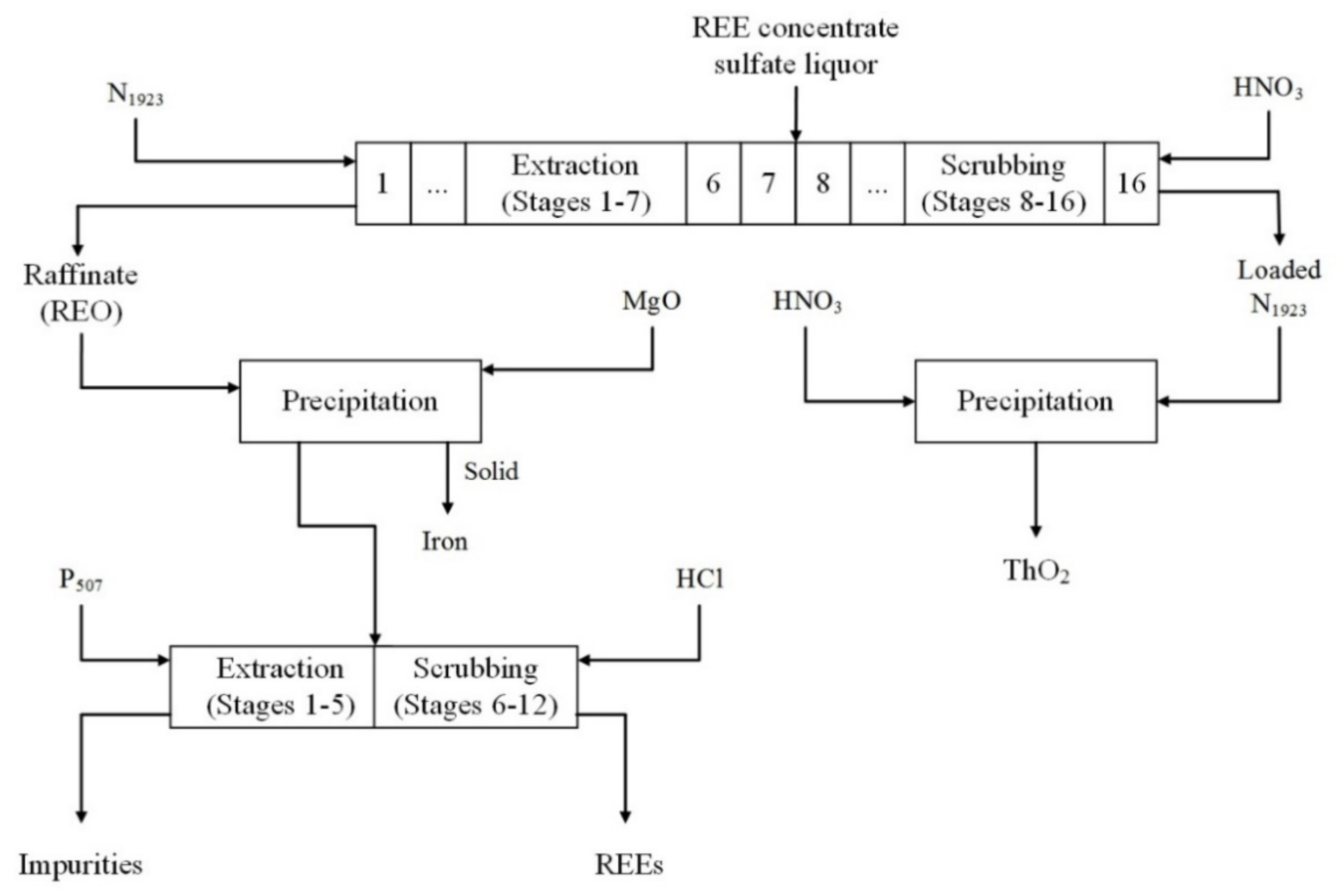
4.1. Solvent Extraction with Amine Extractants
4.2. Solvent Extraction with Phosphorous Extractants
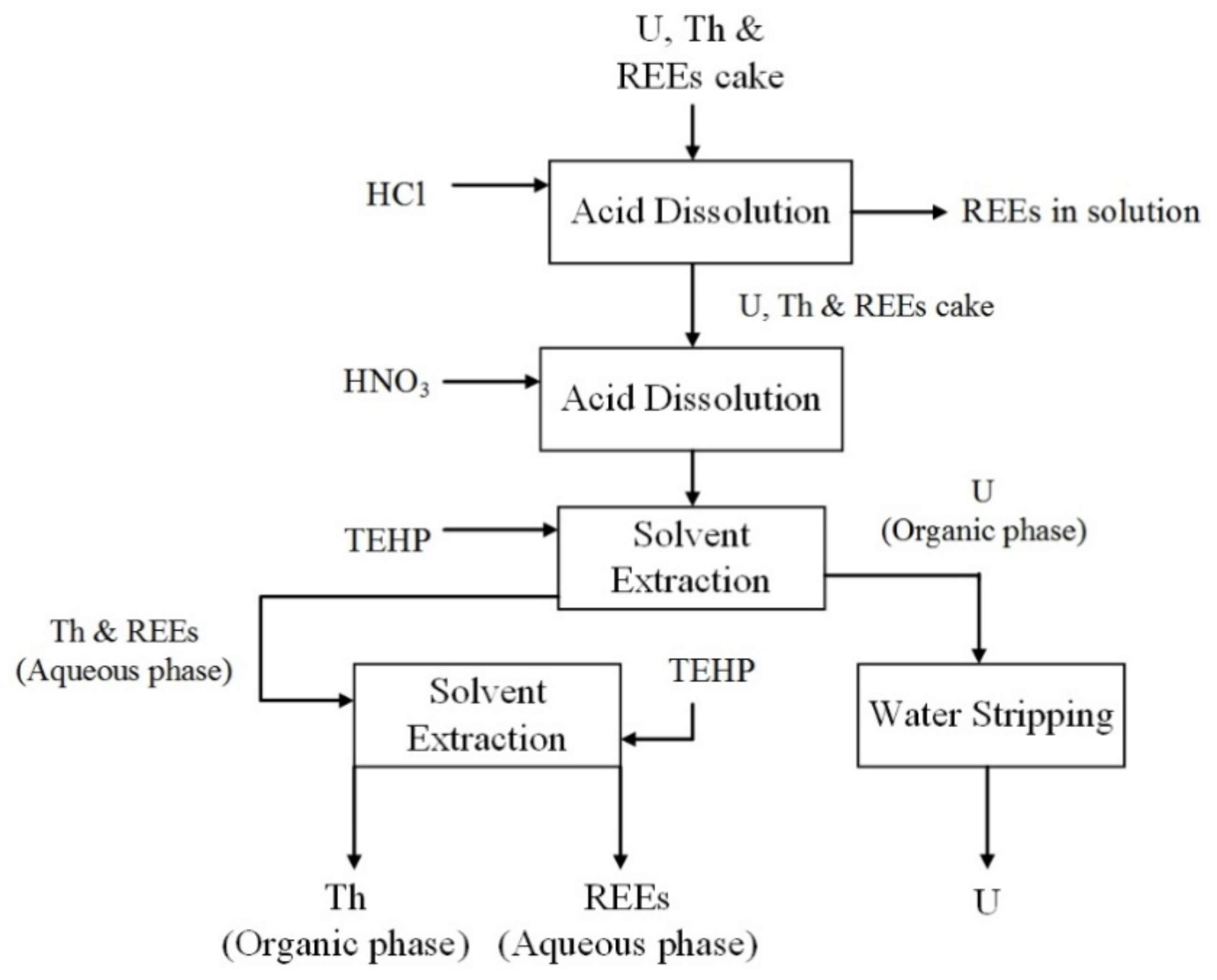
4.2.1. Acid Organophosphorus Extractants
4.2.2. Neutral Organophosphorus Extractants
4.2.3. Improvement of Solvent Extraction Process
5. Separation by Ion Chromatography
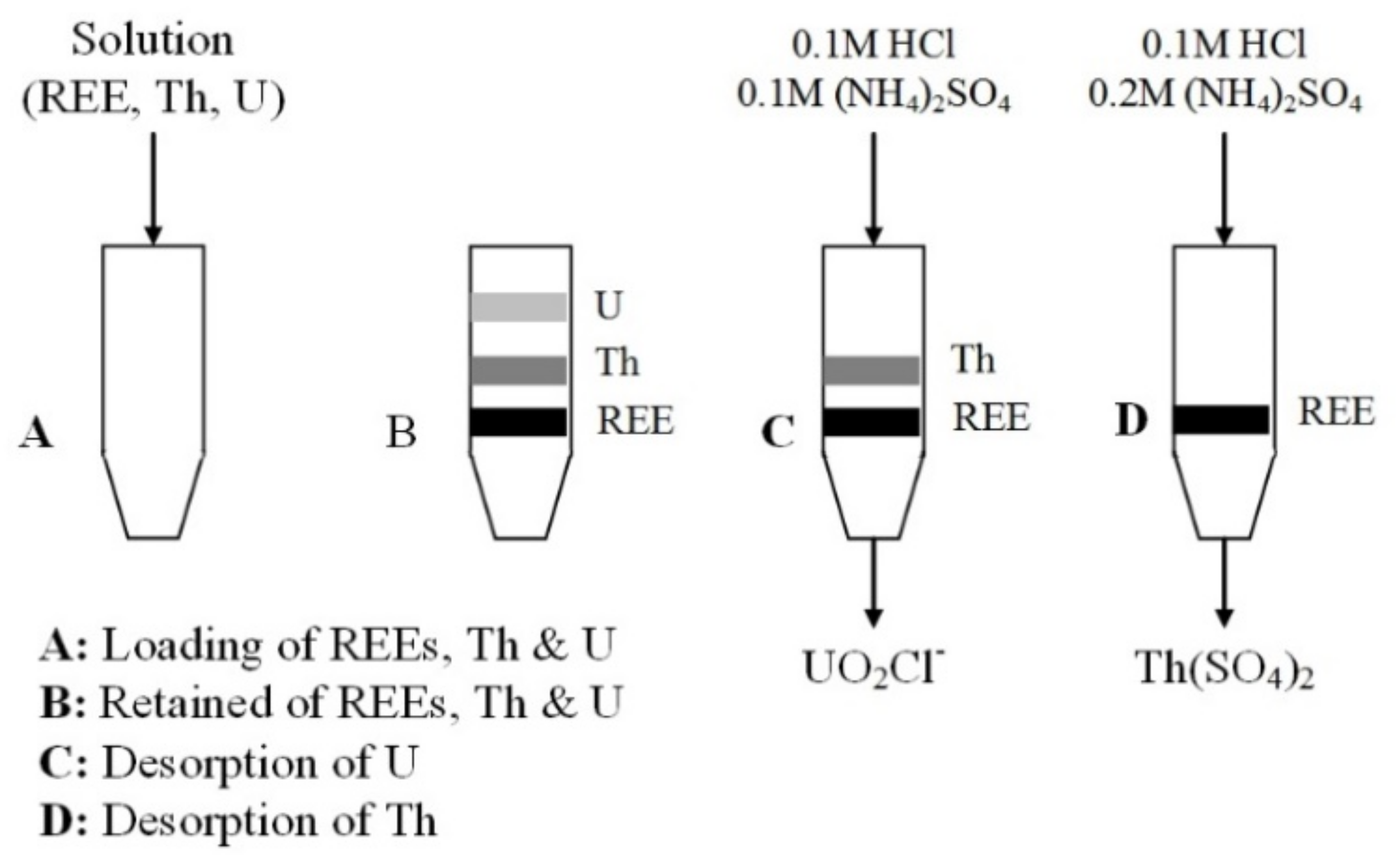
5.1. Cation Exchange Resin
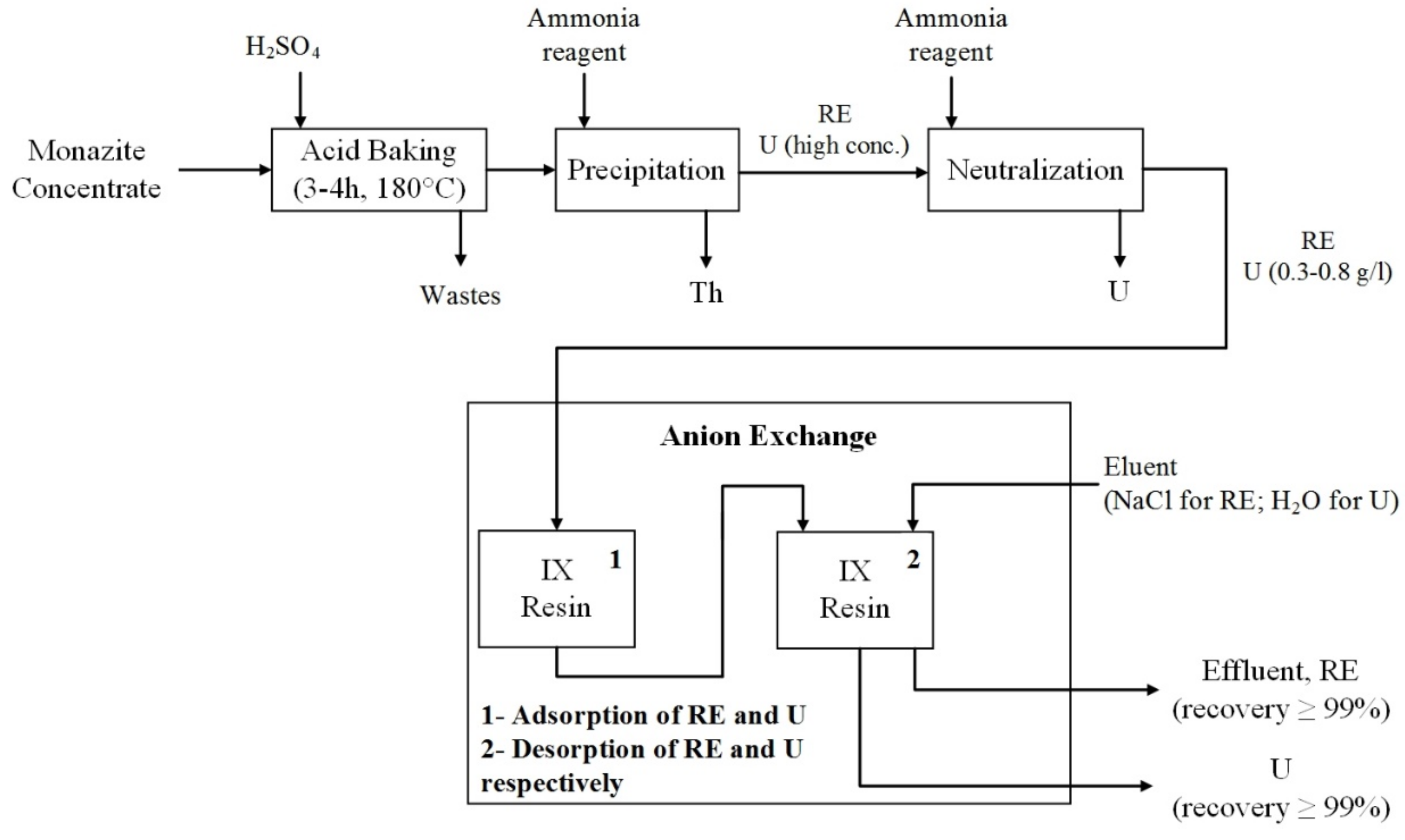
5.2. Anion Exchange Resin
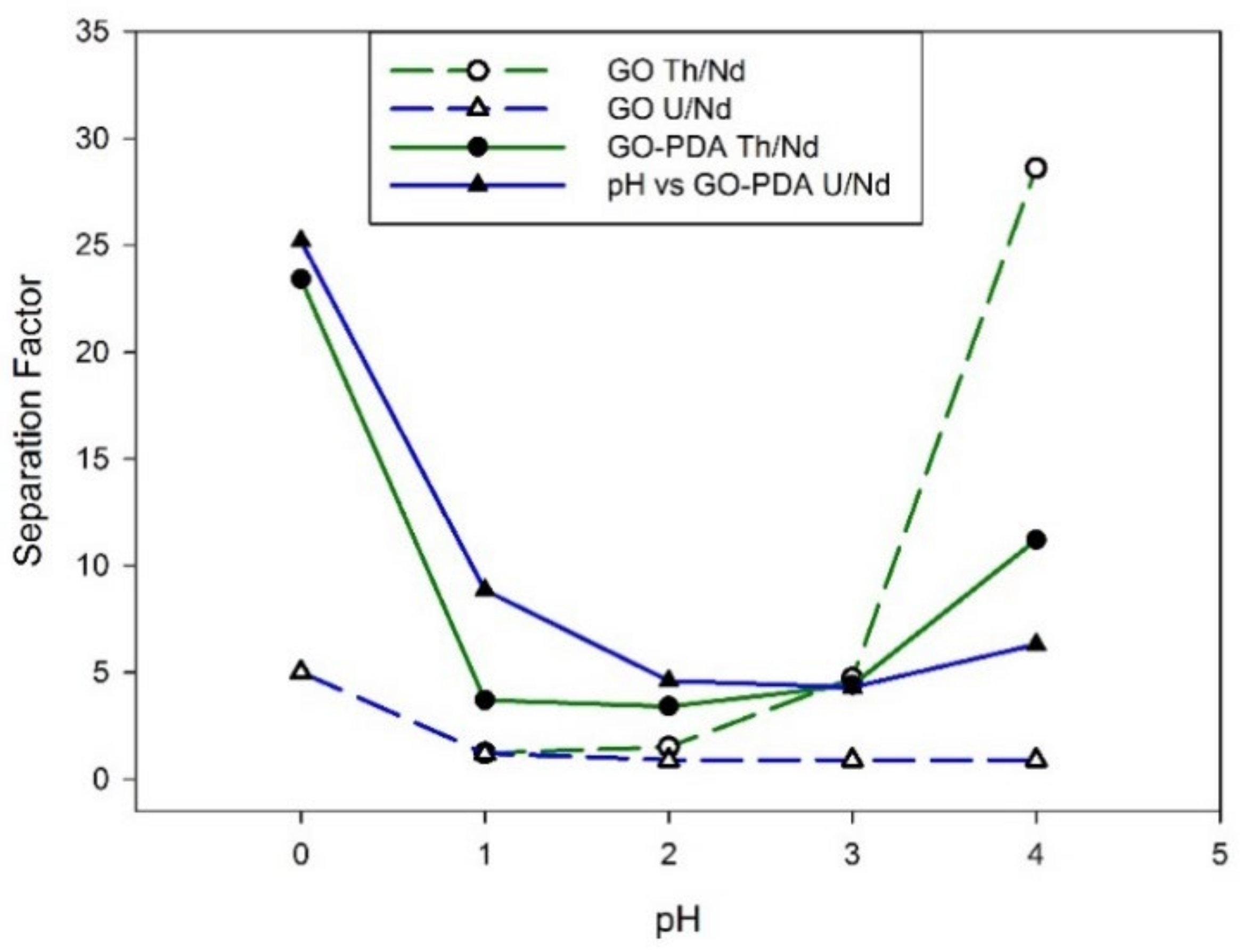
6. Separation by Membranes
7. Selection of a Separation Process and Potential Waste Management Approaches
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alami, D. Environmental Applications of Rare-Earth Manganites as Catalysts: A Comparative Study. Environ. Eng. Res. 2013, 18, 211–219. [Google Scholar] [CrossRef]
- Bouzigues, C.; Gacoin, T.; Alexandrou, A. Biological applications of rare-earth based nanoparticles. ACS Nano 2011, 5, 8488–8505. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Si, Z.C.; Wu, X.D.; Weng, D.; Ran, R.; Yu, J. Rare earth containing catalysts for selective catalytic reduction of NOx with ammonia: A Review. J. Rare Earths 2014, 32, 907–917. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Hu, T.; Deng, R.D.; Yang, X.F. Applications and challenges of rare earth resources in China. In Proceedings of the 2013 3rd International Conference on Information Science, Automation and Material System, ISAM 2013, Guangzhou, China, 13–14 April 2013; pp. 159–162. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Ram, R.; Pownceby, M.; Grocott, S.; Ring, B.; Tardio, J.; Jones, L. A review of acid leaching of uraninite. Hydrometallurgy 2015, 151, 10–24. [Google Scholar] [CrossRef]
- Jaireth, S.; Hoatson, D.M.; Miezitis, Y. Geological setting and resources of the major rare-earth-element deposits in Australia. Ore Geol. Rev. 2014, 62, 72–128. [Google Scholar] [CrossRef]
- Clow, G.; Salmon, B.; Lavigne, M.; McDonough, B.; Pelletier, P.; Vallières, D. Technical Report on Expansion Options at the Niobec Mine, Québec, Canada; IAMGOLD Corporation: Toronto, ON, Canada, 2011. [Google Scholar]
- Bosserman, P.J. Recovery of Cerium; Google Patents: Mountain View, CA, USA, 1995. [Google Scholar]
- Zou, D.; Chen, J.; Li, D.Q. Separation chemistry and clean technique of cerium(IV): A review. J. Rare Earths 2014, 32, 681–685. [Google Scholar] [CrossRef]
- Simandl, G.J. Geology and economic sifnificance of current and future rare earth element sources. In Proceedings of the 51st Annual Conference of Metallurgists, Niagara Falls, ON, Canada, 30 September–3 October 2012. [Google Scholar]
- Mowafy, A.M. Biological leaching of rare earth elements. World J. Microbiol. Biotechnol. 2020, 36, 61. [Google Scholar] [CrossRef]
- Driscoll, M.O. An Overview of Rare Earth Minerals Supply and Applications. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 1991; pp. 409–420. [Google Scholar]
- Jones, A.P.; Wall, F.; Williams, C.T. Rare Earth Minerals: Chemistry, Origin and Deposits; Springer: London, UK, 1996. [Google Scholar]
- Zhang, J.; Edwards, C. A Review of Rare Earth Mineral Processing Technology. In Proceedings of the 44th Annual Canadian Mineral Processors Operators Conference, Ottawa, ON, Canada, 19–21 January 2010. [Google Scholar]
- Chambers, D.B.; Lowe, L.M.; Feasby, D.G. Radiological aspects of naturally occuring radioactive materials (NORM) in the ore processing and production of rare earth element concentrates. In Proceedings of the 51st Annual Conference of Metallurgists, COM, Niagara Falls, ON, Canada, 30 September–3 October 2012. [Google Scholar]
- Feasby, D.G.; Chambers, D.B.; Lowe, L.M. Assesment and management of radioactivity in rare earth elements production. In Proceedings of the Rare Earth Elements (COM 2013), West Westmount, QC, Canada, 27–31 October 2013. [Google Scholar]
- Park, B.T. Management of thorium and uranium in mining and processing of rare earth minerals. In Proceedings of the 51st Annual Conference of Metallurgists, Niagara, ON, Canada, 30 September–3 October 2012; pp. 171–184. [Google Scholar]
- Cheng, J.; Hou, Y.; Che, L. Flotation separation on rare earth minerals and gangues. J. Rare Earths 2007, 25, 62–66. [Google Scholar]
- Fang, J.; Zhao, D. Separation of rare earth from tails of magnetite separation in Bao Steel’s concentrator. Met. Mine 2003, 321, 47–49. [Google Scholar]
- Goode, J.R. Thorium and rare earth recovery in Canada: The first 30 years. Can. Metall. Q. 2013, 52, 234–242. [Google Scholar] [CrossRef]
- Kogel, J.E.; Trivedi, N.C.; Barker, J.M.; Krukowsky, S.T. Industrial Minerals & Rocks: Commodities, Markets and Uses, 7th ed.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2006. [Google Scholar]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Islam, S.Z.; Bhave, R.R. Selective Recovery of Rare Earth Elements from a Wide Range of E-Waste and Process Scalability of Membrane Solvent Extraction. Environ. Sci. Technol. 2020, 54, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric Mining of Rare Earth Elements from Bauxite Residue (Red Mud): Process Optimization, Kinetic Investigation, and Microwave Pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Tuan, L.Q.; Thenepalli, T.; Chilakala, R.; Vu, H.H.; Ahn, J.W.; Kim, J. Leaching Characteristics of Low Concentration Rare Earth Elements in Korean (Samcheok) CFBC Bottom Ash Samples. Sustainability 2019, 11, 2562. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.M. An innovative method for processing Egyptian monazite. Hydrometallurgy 2002, 67, 9–17. [Google Scholar] [CrossRef]
- El-Nadi, Y.A.; Daoud, J.A.; Aly, H.F. Modified leaching and extraction of uranium from hydrous oxide cake of Egyptian monazite. Int. J. Miner. Process. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- Lapidus, G.T.; Doyle, F.M. Selective thorium and uranium extraction from monazite: I. Single-stage oxalate leaching. Hydrometallurgy 2015, 154, 102–110. [Google Scholar] [CrossRef]
- Lapidus, G.T.; Doyle, F.M. Selective thorium and uranium extraction from monazite: II. Approaches to enhance the removal of radioactive contaminants. Hydrometallurgy 2015, 155, 161–167. [Google Scholar] [CrossRef]
- Alex, P.; Hubli, R.C.; Suri, A.K. Processing of rare earth concentrates. Rare Met. 2005, 24, 210–215. [Google Scholar]
- Eyal, Y.; Olander, D.R. Leaching of uranium and thorium from monazite: I. Initial leaching. Geochim. Et Cosmochim. Acta 1990, 54, 1867–1877. [Google Scholar] [CrossRef]
- Panda, R.; Kumari, A.; Jha, M.K.; Hait, J.; Kumar, V.; Kumar, J.R.; Lee, J.Y. Leaching of rare earth metals (REMs) from Korean monazite concentrate. J. Ind. Eng. Chem. 2014, 20, 2035–2042. [Google Scholar] [CrossRef]
- Shaw, K.G. A Process for Separating Thorium Compounds from Monazite Sands; Iowa State University: Ames, IA, USA, 1953. [Google Scholar]
- Amer, T.E.; Abdella, W.M.; Wahab, G.M.A.; El-Sheikh, E.M. A suggested alternative procedure for processing of monazite mineral concentrate. Int. J. Miner. Process. 2013, 125, 106–111. [Google Scholar] [CrossRef]
- Chi, R.; Xu, Z. A solution chemistry approach to the study of rare earth element precipitation by oxalic acid. Met. Mater Trans B 1999, 30, 189–195. [Google Scholar] [CrossRef]
- Kul, M.; Topkaya, Y.; Karakaya, I. Rare earth double sulfates from pre-concentrated bastnasite. Hydrometallurgy 2008, 93, 129–135. [Google Scholar] [CrossRef]
- Fourest, B.; Lagarde, G.; Perrone, J.; Brandel, V.; Dacheux, N.; Genet, M. Solubility of thorium phosphate-diphosphate. New J. Chem. 1999, 23, 645–649. [Google Scholar] [CrossRef]
- Borai, E.H.; Abd El-Ghany, M.S.; Ahmed, I.M.; Hamed, M.M.; Shahr El-Din, A.M.; Aly, H.F. Modi fi ed acidic leaching for selective separation of thorium, phosphate and rare earth concentrates from Egyptian crude monazite. Int. J. Miner. Process. 2016, 149. [Google Scholar] [CrossRef]
- Krebs, D.G.I.; Furfaro, D. The Kvanefjeld process. In Proceedings of the Alta 2013 Uranium-REE Conference, Perth, Australia, 25 May–1 June 2013. [Google Scholar]
- Pawlik, C. Recovery of rare earth elements from complex and low grade deposits. In Proceedings of the ALTA 2013 Uranium-REE Conference, Perth, Australia, 25 May–1 June 2013. [Google Scholar]
- Vijayalakshmi, R.; Mishra, S.L.; Singh, H.; Gupta, C.K. Processing of xenotime concentrate by sulphuric acid digestion and selective thorium precipitation for separation of rare earths. Hydrometallurgy 2001, 61, 75–80. [Google Scholar] [CrossRef]
- Bearse, A.E.; Calkins, G.D.; Clegg, J.W.; Filbert, J.R.B. Thorium and rare earths from monazite. Chem. Eng. Prog. 1954, 50, 235–239. [Google Scholar]
- Mackowski, S.J.; Raiter, R.; Soldenhoff, K.H.; Ho, E.M. Recovery of Rare Earth Elements. U.S. Patent 7,993,612 B2, 9 August 2011. [Google Scholar]
- Yu, B.; Verbaan, N.; Pearse, G.; Britt, S. Beneficiation and extraction of REE from GEOMEGA resources’ Montviel project. In Proceedings of the Rare Earth Elements (COM 2013), West Westmount, QC, Canada, 30 September–3 October 2013. [Google Scholar]
- Grimaldi, F.S. The analytical chemistry of uranium and thorium. In Proceedings of the United Nations International Conference on Peaceful Uses of Atomic Energy, Geneva, Switzerland, 8 August 1995; pp. 605–617. [Google Scholar]
- Tomazic, B.; Branica, M. Separation of uranium(VI) from rare earths(III) by hydrolytic precipitation. Inorg. Nucl. Chem. Lett. 1968, 4, 377–380. [Google Scholar] [CrossRef]
- Kang, M.J.; Han, B.E.; Hahn, P.S. Precipitation and adsorption of uranium (VI) under various aqueous conditions. Environ. Eng. Res. 2002, 7, 149–157. [Google Scholar]
- Abreu, R.D.; Morais, C.A. Purification of rare earth elements from monazite sulphuric acid leach liquor and the production of high-purity ceric oxide. Miner. Eng. 2010, 23, 536–540. [Google Scholar] [CrossRef]
- Carter, G.; Everest, D.A.; Wells, R.A. Selective oxalate precipitation of thorium from sulfate leach solutions derived from monazite sands. J. Appl. Chem. 1960, 10, 149–155. [Google Scholar] [CrossRef]
- Sozanski, A. Separation of Trace Quantities of Thorium from Lanthanide Solutions by the Method of Coprecipitation. Przem. Chem. 1978, 57, 360–362. [Google Scholar]
- Chi, R.; Xu, Z.; Zhang, Z.; He, Z.; Ruan, Y. Review on separation and treatment of thorium resources. In Proceedings of the Conference of Metallurgists Proceedings, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Crouse, D.J.; Brown, K.B. The Amex Process for Extracting Thorium Ores with Alkyl Amines. Ind. Eng. Chem. 1959, 51, 1461–1464. [Google Scholar] [CrossRef]
- Amaral, J.C.B.S.; Morais, C.A. Thorium and uranium extraction from rare earth elements in monazite sulfuric acid liquor through solvent extraction. Miner. Eng. 2010, 23, 498–503. [Google Scholar] [CrossRef]
- Borai, E.H.; Shahr El-Din, A.M.; El-Sofany, E.A.; Sakr, A.A.; El-Sayed, G.O. Extraction and Separation of Some Naturally Occurring Radionuclides from Rare Earth Elements by Different Amines. Arab J. Nucl. Sci. Appl. 2014, 47, 48–60. [Google Scholar]
- Li, D.Q.; Zuo, Y.; Meng, S.L. Separation of thorium(IV) and extracting rare earths from sulfuric and phosphoric acid solutions by solvent extraction method. J. Alloy. Compd. 2004, 374, 431–433. [Google Scholar] [CrossRef]
- Crouse, D.J.; Brown, K.B. Recovery of Thorium, Uranium and Rare Earths from Monazite Sulfate Liquors by the Amine Extraction (AMEX) Process; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1959. [Google Scholar]
- Cox, J.J.; Ciuculescu, T.; Altman, K.; Hwozdyk, L. Technical Report on the Eco Ridge Mine Project, Elliot Lake Area, Ontario, Canada; Roscoe Postle Associates Inc.: Toronto, ON, Canada, 2012. [Google Scholar]
- Chunhua, Y.; Jiangtao, J.; Chunsheng, L.; Sheng, W.; Guangxia, X. Rare Earth Separation in China. Tsingshua Sci. Technol. 2006, 11, 241–247. [Google Scholar]
- Wang, Y.L.; Li, Y.L.; Li, D.Q.; Liao, W.P. Kinetics of thorium extraction with di-(2-ethylhexyl) 2-ethylhexyl phosphonate from nitric acid medium. Hydrometallurgy 2013, 140, 66–70. [Google Scholar] [CrossRef]
- Zhao, J.M.; Zuo, Y.; Li, D.Q.; Liu, S.Z. Extraction and separation of cerium(IV) from nitric acid solutions containing thorium(IV) and rare earths(III) by DEHEHP. J. Alloy. Compd. 2004, 374, 438–441. [Google Scholar] [CrossRef]
- Liu, J.J.; Wang, W.W.; Li, D.Q. Interfacial behavior of primary amine N1923 and the kinetics of thorium(IV) extraction in sulfate media. Colloid Surf. A 2007, 311, 124–130. [Google Scholar] [CrossRef]
- Cianetti, C.; Danesi, P.R. Kinectics and mechanism in metal solvent extraction by some organophosphorous extractants. In Proceedings of the International Solvent Extraction Conference, ISEC, Denver, CO, USA, 26 August–2 September 1983. [Google Scholar]
- Biswas, S.; Pathak, P.N.; Singh, D.K.; Roy, S.B. Comparative Evaluation of Tri-n-butyl Phosphate (TBP) and Tris(2-ethylhexyl) Phosphate (TEHP) for the Recovery of Uranium from Monazite Leach Solution. Sep. Sci. Technol. 2013, 48, 2013–2019. [Google Scholar] [CrossRef]
- Nasab, M.E.; Sam, A.; Milani, S.A. Determination of optimum process conditions for the separation of thorium and rare earth elements by solvent extraction. Hydrometallurgy 2011, 106, 141–147. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare-Earths. Int. Mater. Rev. 1992, 37, 197–248. [Google Scholar] [CrossRef]
- Lu, Y.; Bi, Y.; Bai, Y.; Liao, W. Extraction and separation of thorium and rare earths from nitrate medium withp-phosphorylated calixarene. J. Chem. Technol. Biotechnol. 2013, 88, 1836–1840. [Google Scholar] [CrossRef]
- Rabie, K.A.; Abdel-Wahaab, S.M.; Mahmoud, K.F.; Hussein, A.E.M.; Abd El-Fatah, A.I. Monazite- Uranium Separation and Purification Applying Oxalic- Nitrate-TBP extraction. Arab J. Nucl. Sci. Appl. 2013, 46, 30–42. [Google Scholar]
- Zhang, Y.Q.; Xu, Y.; Huang, X.W.; Long, Z.Q.; Cui, D.L.; Hu, F. Study on thorium recovery from bastnaesite treatment process. J. Rare Earths 2012, 30, 374–377. [Google Scholar] [CrossRef]
- Rakesh, K.B.; Suresh, A.; Rao, P.R.V. Separation of U(VI) and Th(IV) from Nd(III) by Cross-Current Solvent Extraction Mode Using Tri-iso-amyl Phosphate as the Extractant. Solvent Extr. Ion Exch. 2015, 33, 448–461. [Google Scholar] [CrossRef]
- Li, Y.L.; Lu, Y.C.; Bai, Y.; Liao, W.P. Extraction and separation of thorium and rare earths with 5,11,17,23-tetra (diethoxyphosphoryl)-25,26,27,28-tetraacetoxycalix[4]arene. J. Rare Earths 2012, 30, 1142–1145. [Google Scholar] [CrossRef]
- Ali, A.M.I.; El-Nadi, Y.A.; Daoud, J.A.; Aly, H.F. Recovery of thorium (IV) from leached monazite solutions using counter-current extraction. Int. J. Miner. Process. 2007, 81, 217–223. [Google Scholar] [CrossRef]
- He, L.T.; Jiang, Q.; Jia, Y.M.; Fang, Y.Y.; Zou, S.L.; Yang, Y.Y.; Liao, J.L.; Liu, N.; Feng, W.; Luo, S.Z.; et al. Solvent extraction of thorium(IV) and rare earth elements with novel polyaramide extractant containing preorganized chelating groups. J. Chem. Technol. Biot. 2013, 88, 1930–1936. [Google Scholar] [CrossRef]
- Sato, T. The extraction of uranium (VI) from sulphuric acid solutions by di-(2-ethyl hexyl)-phosphoric acid. J. Inorg. Nucl. Chem. 1962, 24, 699–706. [Google Scholar] [CrossRef]
- Sato, T. The Extraction of Thorium from Hydrochloric Acid Solutions by di-(2-ethylhexyl)-phosphoric acid. Z. Für Anorg. Und Allg. Chem. 1968, 358, 296–304. [Google Scholar] [CrossRef]
- Sato, T. Liquid-Liquid Extraction of Rare-Earth Elements from Aqueous Acid Solutions by Acid Organophosphorus Compounds. Hydrometallurgy 1989, 22, 121–140. [Google Scholar] [CrossRef]
- Gupta, B.; Malik, P.; Deep, A. Extraction of uranium, thorium and lanthanides using Cyanex-923: Their separations and recovery from monazite. J. Radioanal. Nucl. Chem. 2002, 251, 451–456. [Google Scholar] [CrossRef]
- Karve, M.; Gaur, C. Extraction of U(VI) with Cyanex 302. J. Radioanal. Nucl. Chem. 2007, 273, 405–409. [Google Scholar] [CrossRef]
- Nasab, M.E.; Milani, S.A.; Sam, A. Extractive separation of Th(IV), U(VI), Ti(IV), La(III) and Fe(III) from Zarigan ore. J. Radioanal. Nucl. Chem. 2011, 288, 677–683. [Google Scholar] [CrossRef]
- Belova, V.V.; Egorova, N.S.; Voshkin, A.A.; Khol’kin, A.I. Extraction of rare earth metals, uranium, and thorium from nitrate solutions by binary extractants. Theor. Found. Chem. Eng. 2015, 49, 545–549. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, S.L.; Vijayalakshmi, R. Uranium recovery from phosphoric acid by solvent extraction using a synergistic mixture of di-nonyl phenyl phosphoric acid and tri-n-butyl phosphate. Hydrometallurgy 2004, 73, 63–70. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhami, P.S.; Tripathi, S.C.; Dakshinamoorthy, A. Studies on the recovery of uranium from phosphoric acid medium using synergistic mixture of (2-Ethyl hexyl) Phosphonic acid, mono (2-ethyl hexyl) ester (PC88A) and Tri-n-butyl phosphate (TBP). Hydrometallurgy 2009, 95, 170–174. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Suresh, A.; Sivaraman, N.; Vasudeva Rao, P.R. Solvent extraction studies with some fission product elements from nitric acid media employing tri-iso-amyl phosphate and tri-n-butyl phosphate as extractants. J. Radioanal. Nucl. Chem. 2014, 303, 2165–2172. [Google Scholar] [CrossRef]
- Jain, V.K.; Pandya, R.A.; Pillai, S.G.; Shrivastav, P.S. Simultaneous preconcentration of uranium(VI) and thorium(IV) from aqueous solutions using a chelating calix[4]arene anchored chloromethylated polystyrene solid phase. Talanta 2006, 70, 257–266. [Google Scholar] [CrossRef]
- Patil, N.N.; Shinde, V.M. Extraction study of uranium(VI) and thorium(IV) salicylates with triphenylarsine oxide. J. Radioanal. Nucl. Chem. 1997, 222, 21–24. [Google Scholar] [CrossRef]
- Singh, H.; Gupta, C.K. Solvent Extraction in Production and Processing of Uranium and Thorium. Miner. Process. Extr. Metall. Rev. 2000, 21, 307–349. [Google Scholar] [CrossRef]
- Borai, E.H.; Mady, A.S. Separation and quantification of 238U, 232Th and rare earths in monazite samples by ion chromatography coupled with on-line flow scintillation detector. Appl. Radiat. Isot. Incl. Datainstrumentation Methods Use Agric. Ind. Med. 2002, 57, 463–469. [Google Scholar] [CrossRef]
- Jeyakumar, S.; Mishra, V.G.; Das, M.K.; Raut, V.V.; Sawant, R.M.; Ramakumar, K.L. Separation behavior of U(VI) and Th(IV) on a cation exchange column using 2,6-pyridine dicarboxylic acid as a complexing agent and its application for the rapid separation and determination of U and Th by ion chromatography. J. Sep. Sci. 2011, 34, 609–616. [Google Scholar] [CrossRef]
- Pin, C.; Zalduegui, J.F.S. Sequential separation of light rare-earth elements, thorium and uranium by miniaturized extraction chromatography: Application to isotopic analyses of silicate rocks. Anal. Chem. Acta 1997, 339, 79–89. [Google Scholar] [CrossRef]
- Soran, M.L.; Curtui, M.; Marutoiu, C. Separation of U(VI) and Th(IV) from some rare earths by thin layer chromatography with di-(2-ethylhexyl)-dithiophosphoric acid on silica gel. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2515–2524. [Google Scholar] [CrossRef]
- Sivaraman, N.; Kumar, R.; Subramaniam, S.; Rao, P.R.V. Separation of lanthanides using ion-interaction chromatography with HDEHP coated columns. J. Radioanal. Nucl. Chem. 2002, 252, 491–495. [Google Scholar] [CrossRef]
- Ostapenko, V.; Vasiliev, A.; Lapshina, E.; Ermolaev, S.; Aliev, R.; Totskiy, Y.; Zhuikov, B.; Kalmykov, S. Extraction chromatographic behavior of actinium and REE on DGA, Ln and TRU resins in nitric acid solutions. J. Radioanal. Nucl. Chem. 2015, 306, 707–711. [Google Scholar] [CrossRef]
- Ling, L.; Wang, N.H. Ligand-assisted elution chromatography for separation of lanthanides. J. Chromatogr. A 2015, 1389, 28–38. [Google Scholar] [CrossRef]
- Soran, M.-L.; Hodişan, T.; Curtui, M.; Casoni, D. TLC separation of rare earths using di(2-ethylhexyl)dithiophosphoric acid as complexing reagent. J. Planar Chromatogr. Mod. Tlc 2005, 18, 160–163. [Google Scholar] [CrossRef]
- Korkisch, J.; Hazan, I. Anion-exchange behaviour of uranium and other elements in the presence of aliphatic di- and tricarboxylic acids. Talanta 1964, 11, 523–530. [Google Scholar] [CrossRef]
- Dev, K.; Pathak, R.; Rao, G.N. Sorption behaviour of lanthanum(III), neodymium(III), terbium(III), thorium(IV) and uranium(VI) on Amberlite XAD-4 resin functionalized with bicine ligands. Talanta 1999, 48, 579–584. [Google Scholar] [CrossRef]
- Karve, M.; Rajgor, R.V. Amberlite XAD-2 impregnated organophosphinic acid extractant for separation of uranium(VI) from rare earth elements. Desalination 2008, 232, 191–197. [Google Scholar] [CrossRef]
- Merdivan, M.; Duz, M.Z.; Hamamci, C. Sorption behaviour of uranium(VI) with N,N-dibutyl-N’-benzoylthiourea Impregnated in Amberlite XAD-16. Talanta 2001, 55, 639–645. [Google Scholar] [CrossRef]
- Metilda, P.; Sanghamitra, K.; Mary Gladis, J.; Naidu, G.R.; Prasada Rao, T. Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium(VI). Talanta 2005, 65, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Metwally, E.; Saleh, A.S.; El-Naggar, H.A. Extraction and Separation of Uranium (VI) and Thorium (IV) Using Tri-n-dodecylamine impregnated resins. J. Nucl. Radiochem. Sci. 2005, 6, 119–126. [Google Scholar] [CrossRef][Green Version]
- Prabhakaran, D.; Subramanian, M.S. Extraction of U(VI), Th(IV), and La(III) from acidic streams and geological samples using AXAD-16-POPDE polymer. Anal. Bioanal. Chem. 2004, 380, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Rabie, K.A.; AbdElMoneam, Y.K.; Abdelfattah, A.I.; Demerdahs, M.; Salem, A.R. Adaptation of anion exchange process to decontaminate monazite rare earth group from its uranium content. Int. J. Res. Eng. Technol. 2014, 3, 374–382. [Google Scholar]
- Singh, B.N.; Maiti, B. Separation and preconcentration of U(VI) on XAD-4 modified with 8-hydroxy quinoline. Talanta 2006, 69, 393–396. [Google Scholar] [CrossRef]
- Ang, K.L.; Li, D.; Nikoloski, A.N. The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 1. Anionic and cationic resins. Hydrometallurgy 2017, 174, 147–155. [Google Scholar] [CrossRef]
- Li, Z.; Chen, F.; Yuan, L.; Liu, Y.; Zhao, Y.; Chai, Z.; Shi, W. Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem. Eng. J. 2012, 210, 539–546. [Google Scholar] [CrossRef]
- Li, F.; Yang, Z.; Weng, H.; Chen, G.; Lin, M.; Zhao, C. High efficient separation of U(VI) and Th(IV) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide. Chem. Eng. J. 2018, 332, 340–350. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.; Wang, Q.; Shao, D.; Wang, X. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys. Chem. Chem. Phys. 2015, 17, 398–406. [Google Scholar] [CrossRef]
- Sadeghi, S.; Sheikhzadeh, E. Solid phase extraction using silica gel modified with murexide for preconcentration of uranium (VI) ions from water samples. J. Hazard Mater. 2009, 163, 861–868. [Google Scholar] [CrossRef]
- Xu, H.; Li, G.; Li, J.; Chen, C.; Ren, X. Interaction of Th(IV) with graphene oxides: Batch experiments, XPS investigation, and modeling. J. Mol. Liq. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, M.; Yang, Z.; Sun, Y.; Ding, C. The efficient enrichment of U(vi) by graphene oxide-supported chitosan. Rsc Adv. 2014, 4, 61919–61926. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.L.; Liu, C.L. Synthesis and Th(IV) sorption characteristics of functionalised graphene oxide. J. Radioanal. Nucl. Chem. 2014, 302, 489–496. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Wang, X.; Chen, C.; Wang, X. High performance of phosphate-functionalized graphene oxide for the selective adsorption of U(VI) from acidic solution. J. Nucl. Mater. 2015, 466, 56–64. [Google Scholar] [CrossRef]
- Pan, N.; Li, L.; Ding, J.; Li, S.; Wang, R.; Jin, Y.; Wang, X.; Xia, C. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J. Hazard Mater. 2016, 309, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Hou, G.; Li, J.; Wen, T.; Ren, X.; Wang, X. PANI/GO as a super adsorbent for the selective adsorption of uranium(VI). Chem. Eng. J. 2014, 255, 604–612. [Google Scholar] [CrossRef]
- Sun, Y.; Shao, D.; Chen, C.; Yang, S.; Wang, X. Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ. Sci. Technol. 2013, 47, 9904–9910. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, L.; Huang, X. Functionalization Methods for Membrane Surfaces. Surf. Eng. Polym. Membr. 2009, 64–79. [Google Scholar] [CrossRef]
- Li, M.-P.; Zhang, X.; Zhang, H.; Liu, W.-L.; Huang, Z.-H.; Xie, F.; Ma, X.-H.; Xu, Z.-L. Hydrophilic yolk-shell ZIF-8 modified polyamide thin-film nanocomposite membrane with improved permeability and selectivity. Sep. Purif. Technol. 2020, 247. [Google Scholar] [CrossRef]
- Srinivasan, M.; Subba Rao, K.; Dingankar, M.V. The “Actinide Waste” Problem in Perspective. In Proceedings of the Indo-Japan Seminar on Thorium utilization, Bombay, India, 10–13 December 1990. [Google Scholar]
- Haridasan, P.P.; Pillai, P.B.M.; Tripathi, R.M.; Puranik, V.D. Operational radiation protection associated with thorium processing in India. In Proceedings of the Naturally Occurring Radioactive Material (NORM VI), Marrakesh, Morocco, 22–26 March 2010. [Google Scholar]
- Ault, T.; Krahn, S.; Croff, A. Assessment of the Potential of By-Product Recovery of Thorium to Satisfy Demands of a Future Thorium Fuel Cycle. Nucl. Technol. 2015, 189, 152–162. [Google Scholar] [CrossRef]
- Dekusar, V.M.; Kolesnikova, M.S.; Nikolaev, A.I.; Maiorov, V.G.; Zilberman, B.Y. Mineral reserves of naturally radioactive thorium-bearing raw materials. At. Energy 2012, 111, 185–194. [Google Scholar] [CrossRef]
- Humphries, M. Rare Earth Elements: The Global Supply Chain; Diane Publishing: Darby, PA, USA, 2010. [Google Scholar]
- Donati, G.; Paludetto, R. Scale up of chemical reactors. Catal. Today 1997, 34, 483–533. [Google Scholar] [CrossRef]
- Dudukovic, M.P. Challenges and Innovations in Reaction Engineering. Chem. Eng. Commun. 2008, 196, 252–266. [Google Scholar] [CrossRef]
- Forbes, L.K. The design of a full-scale industrial mineral leaching process. Appl. Math. Model. 2001, 25, 233–256. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C.; Bermejo, P. Demonstration Plant Equipment Design and Scale-Up from Pilot Plant of a Leaching and Solvent Extraction Process. Minerals 2015, 5, 298–313. [Google Scholar] [CrossRef]
- Bałdyga, J. Mixing and Fluid Dynamics Effects in Particle Precipitation Processes. Kona Powder Part. J. 2016, 33, 127–149. [Google Scholar] [CrossRef]
- Marchisio, D.L.; Rivautella, L.; Barresi, A.A. Design and scale-up of chemical reactors for nanoparticle precipitation. Aiche J. 2006, 52, 1877–1887. [Google Scholar] [CrossRef]
- De Santana, A.O.; Dantas, C.C. Scale up of the mixer of a mixer-settler model used in a uranium solvent extraction process. J. Radioanal. Nucl. Chem. 1995, 189, 257–268. [Google Scholar] [CrossRef]
- Geeting, M.W.; Brass, E.A.; Brown, S.J.; Campbell, S.G. Scale-up of Caustic-Side Solvent Extraction Process for Removal of Cesium at Savannah River Site. Sep. Sci. Technol. 2008, 43, 2786–2796. [Google Scholar] [CrossRef][Green Version]
- Kurniawansyah, F.; Mammucari, R.; Tandya, A.; Foster, N.R. Scale—Up and economic evaluation of the atomized rapid injection solvent extraction process. J. Supercrit. Fluids 2017, 127, 208–216. [Google Scholar] [CrossRef]
- Carta, G. Scale-Up and Optimization in Preparative Chromatography: Principles and Biopharmaceutical Applications. Chromatographic Science Series, Volume 88 Edited by Anurag, S. Rathore (Pharmacia Corporation, Chesterfield, MO) and Ajoy Velayudhan (Oregon State University). Marcel Dekker, Inc.: New York, Basel. 2002. xvi + 342 pp. $150.00. ISBN 0-8247-0826-1. J. Am. Chem. Soc. 2003, 125, 3398–3399. [Google Scholar] [CrossRef]
- Heuer, C.; Hugo, P.; Mann, G.; Seidel-Morgenstern, A. Scale up in preparative chromatography. J. Chromatogr. A 1996, 752, 19–29. [Google Scholar] [CrossRef]
- Rathore, A.S.; Velayudhan, A. Guidelines for optimization and scale up in preventive chromatography. Biopharm Int. 2003, 16, 1. [Google Scholar]
| Type | Mineral | Formula | Average Composition (wt.%) | Ref. | ||
|---|---|---|---|---|---|---|
| REE Oxide | ThO2 | UO2 | ||||
| Carbonate | Ancylite | Sr(Ce,La)(CO3)2OH·H2O | 46 | 0–0.4 | 0.1 | [6] |
| Bastnäsite | (Ce,La)CO3F | 74 | 0–0.3 | <0.9 | [6] | |
| Parisite | Ca(REE)2(CO3)3F2 | 59 | 0–0.5 | 0–0.3 | [6] | |
| Phosphate | Apatite | Ca5(PO4)3(F,Cl,OH) | 19 | - | - | [4,6] |
| Britholite | (REE,Ca)5(SiO4,PO4)3(F,OH) | 56 | 1.5 | - | [4,6] | |
| Monazite | (REE,Th)PO4 | 35–71 | 0–20 | 0–16 | [6,23] | |
| Xenotime | YPO4 | 61 | - | 0–5 | [4,6] | |
| Oxide | Brannerite | (U,REE,Ca)(Ti,Fe)2O6 | 6 | - | - | [6] |
| Perovskite | (Ca,REE)TiO3 | <37 | 0–2 | <0.05 | [6] | |
| Silicate | Allanite | (REE,Ca)2(Al,Fe)3(SiO4)3(OH) | 30 | 0.3 | - | [4,6] |
| Cheralite | (REE,Th,Ca)(P,Si)O4 | 5 | <30 | - | [4,6] | |
| REE-Bearing Mineral | Reagent | Operating Conditions | Overall Recovery in Leach Liquor (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Pressure (atm) | Time (h) | T (°C) | Th | U | REE | |||
| Xenotime | Nitric acid | 1 | - | 60 | <1 | - | >98 | [35] |
| Concentrated monazite (87 wt.% REE) | Ammonium carbonate | 6.5–10 | 1–2 | 80 | 99 | 95 | 2.5 | [31] |
| Monazite (18.5 wt.% REE) | Ammonium oxalate | 1 | <2 | 40 | 100 | >40 | <1 | [33] |
| Elements | Precipitation pH (Approx.) | |
| Chloride Liquor | Sulfate Liquor | |
| Th | 4.8–5.8 | 1–2 |
| U | 5.5–7 | 6 |
| REE | 6.8–8 | 3–5.5 |
| Original Ore | Upstream Liquor Feed | Precipitation Reagent | Final pH | Precipitation Recovery (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Th | U | REE | |||||
| Monazite | Sulfate | 1st step: Oxalic acid | 0.7 | 98 | - | 99 | [39] |
| 2nd step: Alkali leaching | - | >99 | - | <1 | |||
| Synthesized solution | Nitrate | KOH and NH4OH | 4.5 | - | 90 | low | [51] |
| Monazite, Apatite | Chloride | Hydrated lime and NH4OH | 2.5 | >99 | - | 5 | [48] |
| Monazite, REE carbonate | Chloride | Lime and H2O2 | - | 99 | >80 | <2 | [49] |
| Bastnäsite, Monazite | Hydroxide cake | HCl | 5.8 | >99 | >99 | 2.3 | [56] |
| Xenotime | Sulfate | 1st step: NH4OH | 1.5–1.9 | >99 | - | <6 | [46] |
| 2nd step: Oxalic acid | - | - | - | >98 | |||
| Amine | Experimental Conditions | Extraction (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Name | Liquor | Ore | pH | Time (min) | Thorium | Uranium | REE | |
| Primary | Primene JM-T | Sulfate | Monazite | - | 5 | 95.4 | 8.8 | 0.32 | [58] |
| N1923 | Sulfate | Bastnäsite | - | - | >97 | - | ECe: 3–8 | [60] | |
| Octylamine | Sulfate | Monazite | 4 | 15 | 70–80 | 50–60 | - | [59] | |
| Secondary | N-methylaniline | Sulfate | Monazite | 4 | 15 | 70–80 | 5–10 | 0 | [59] |
| Tertiary | Alamine 336 | Sulfate | Monazite | - | 5 | 3.1 | 82.4 | 0.02 | [58] |
| N,N-dimethylaniline | Sulfate | Monazite | 4 | 15 | 70 | 15–20 | ECe, Eu, Y: 0 | [59] | |
| 7 | 30 | 0 | 55 | ECe, Eu, Y: 0 | [59] | ||||
| Mixture | Primene JM-T and Alamine 336 | Sulfate | Monazite | - | 5 | 45.5 | 58.8 | 0.04 | [58] |
| Type of Extractant | Feed Liquor | Phosphorous Extractant | Extraction (%) | Ref. | ||
|---|---|---|---|---|---|---|
| Th | U | REE | ||||
| Acid | Acidic | Cyanex 272 | 83 | - | 12 | [69] |
| Nitrate | DEHEHP | 20 | Ce: 95 | [65] | ||
| Nitrate | Mixture of HEH and EHP in kerosene | 95 | - | Ce: 99 | [73] | |
| Neutral | Nitrate | TiAP (2 solvent extraction steps) | 99 | 95 | <2 | [74] |
| Nitrate | p-phosphorylated calixarene | 99 | - | 5 | [75] | |
| Nitrate | TEHP in n-paraffin | 50–77 | 2.5 | Y: 0.17 | [68] | |
| Other | Nitrate | Aliquat 336 | 97 | 54 | <3 | [32,76] |
| Nitrate | polyaramide | 90 | - | >48 | [77] | |
| Table | Advantages | Limitations | Recovery (%) | Scalability 4 |
|---|---|---|---|---|
| Leaching |
|
| Th: >98 3a U: 65–95 3a | +++ |
| Precipitation |
|
| Th ≥ 98 U > 80 3a Th > 98 U > 90 3b | ++ |
| Solvent Extraction |
|
| Th ≥ 70 U ≈ 55 3a Th > 95 U > 95 3b | +++ |
| Ion chromatography |
|
| Th: 90–99 U: 90–99 | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, A.C.; Latifi, M.; Amini, A.; Chaouki, J. Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals. Metals 2020, 10, 1524. https://doi.org/10.3390/met10111524
García AC, Latifi M, Amini A, Chaouki J. Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals. Metals. 2020; 10(11):1524. https://doi.org/10.3390/met10111524
Chicago/Turabian StyleGarcía, Adrián Carrillo, Mohammad Latifi, Ahmadreza Amini, and Jamal Chaouki. 2020. "Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals" Metals 10, no. 11: 1524. https://doi.org/10.3390/met10111524
APA StyleGarcía, A. C., Latifi, M., Amini, A., & Chaouki, J. (2020). Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals. Metals, 10(11), 1524. https://doi.org/10.3390/met10111524






