Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 Super Duplex Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
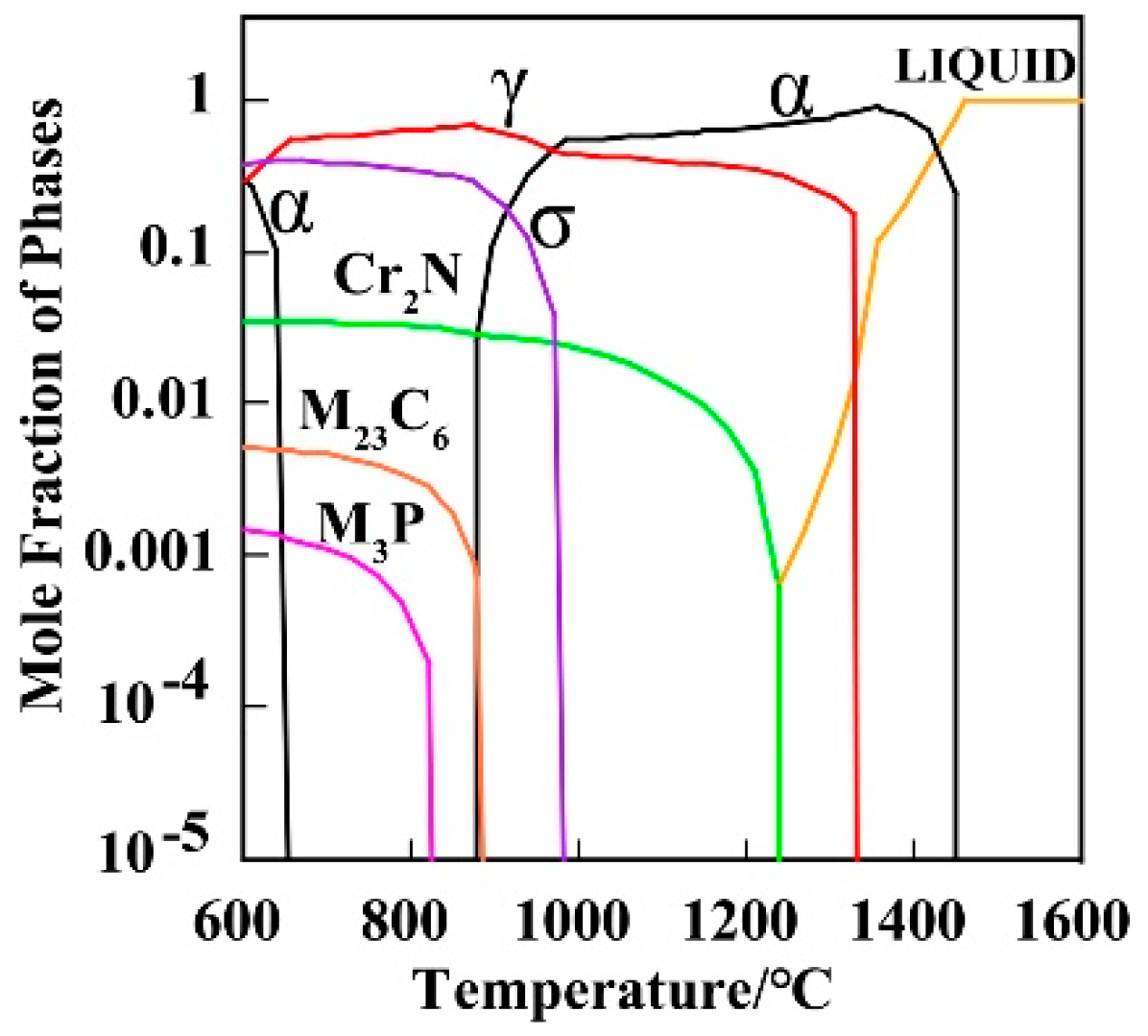
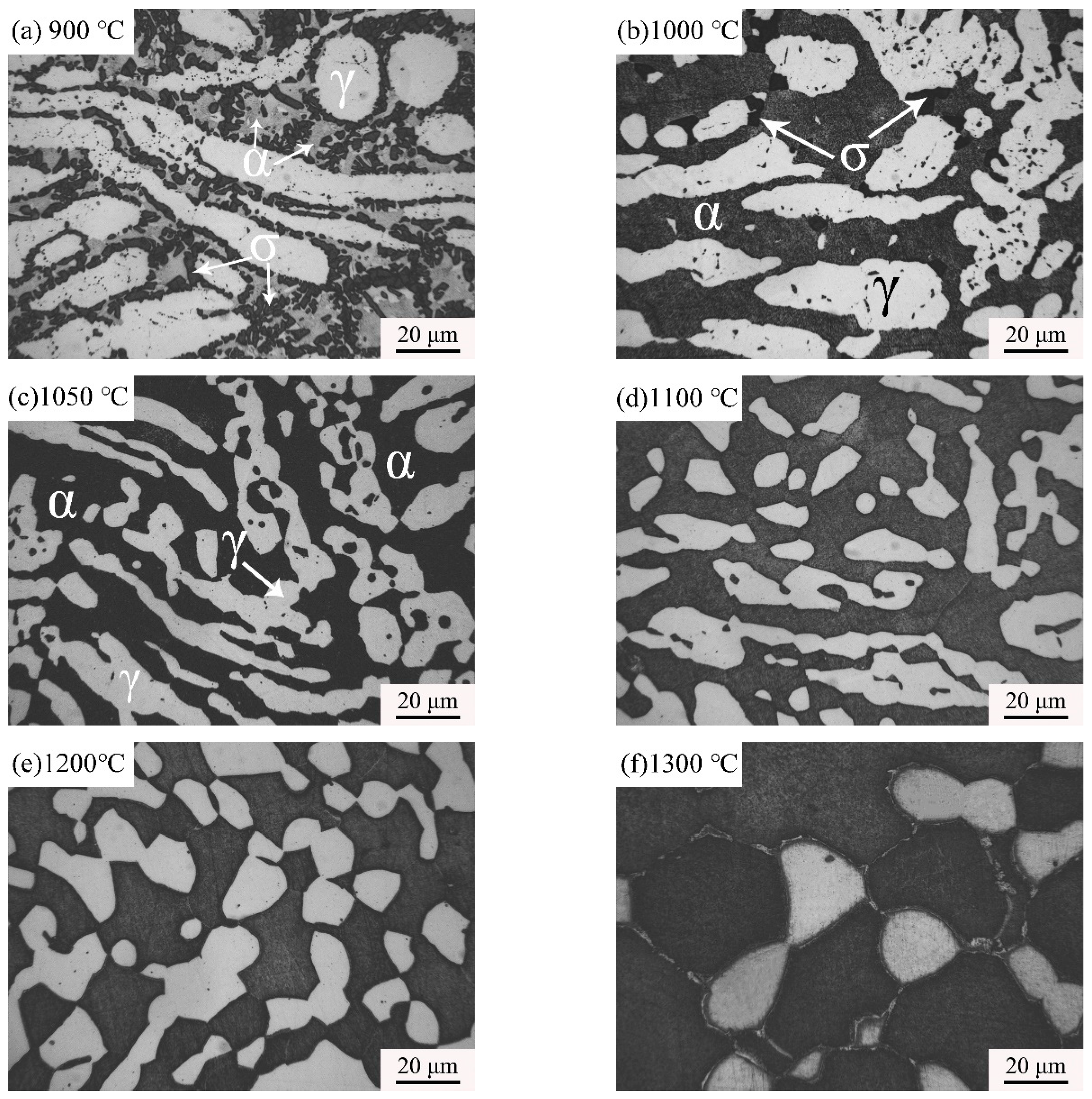
3.1. Effect of Solution Treatment Temperature on Microstructural Evolution
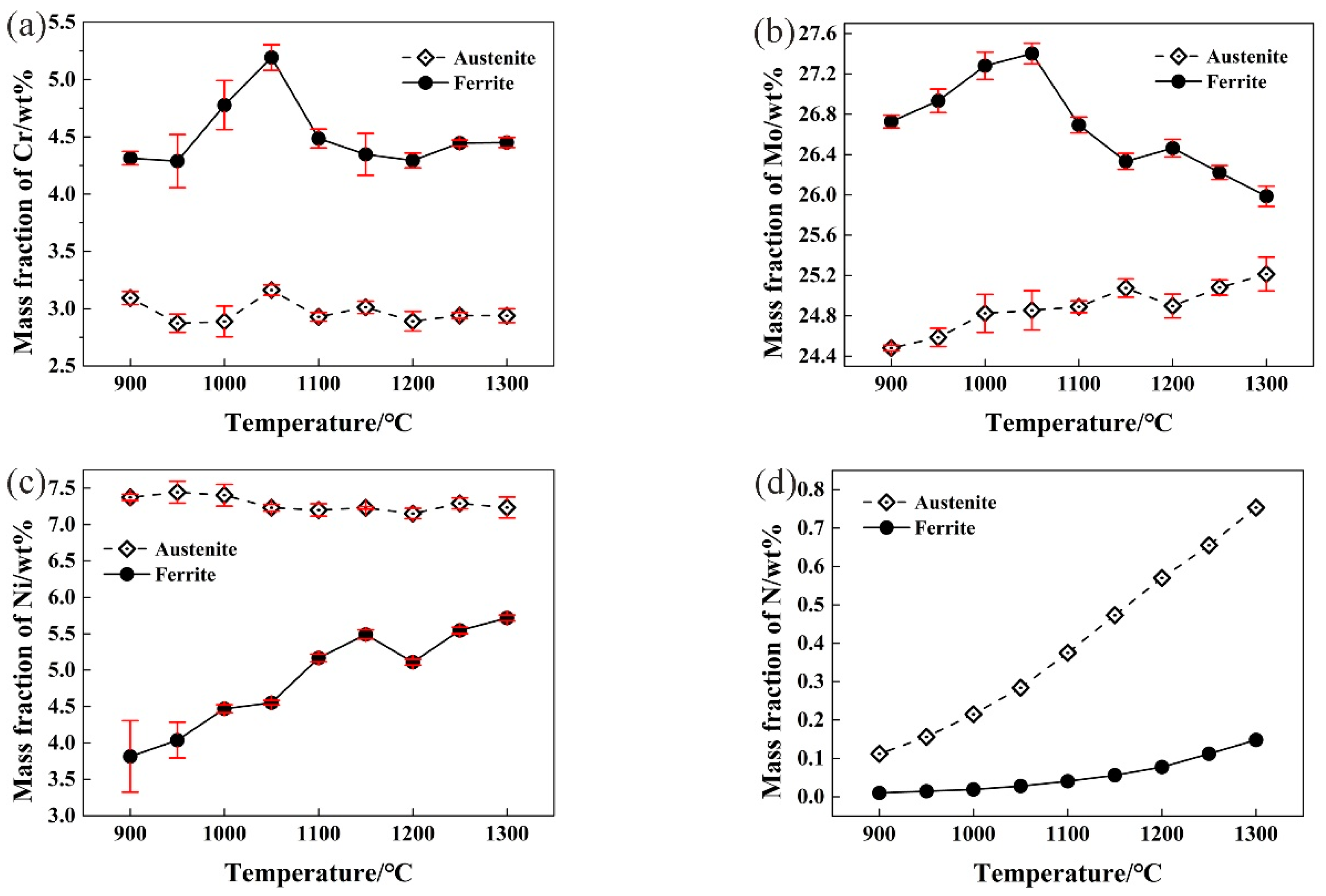
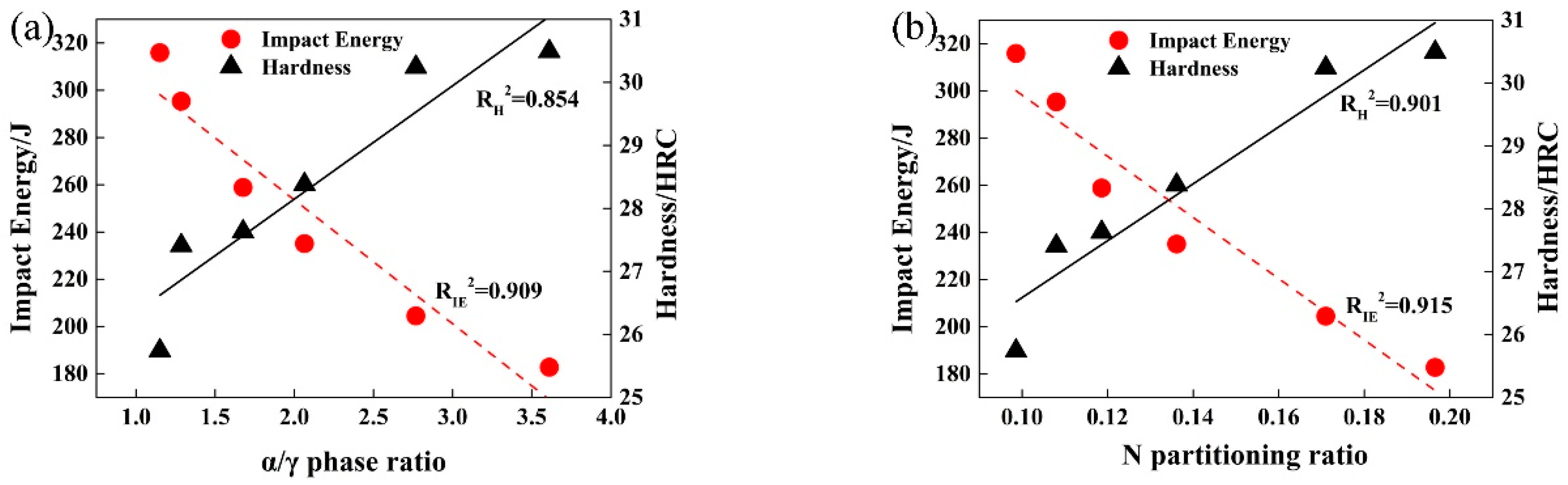
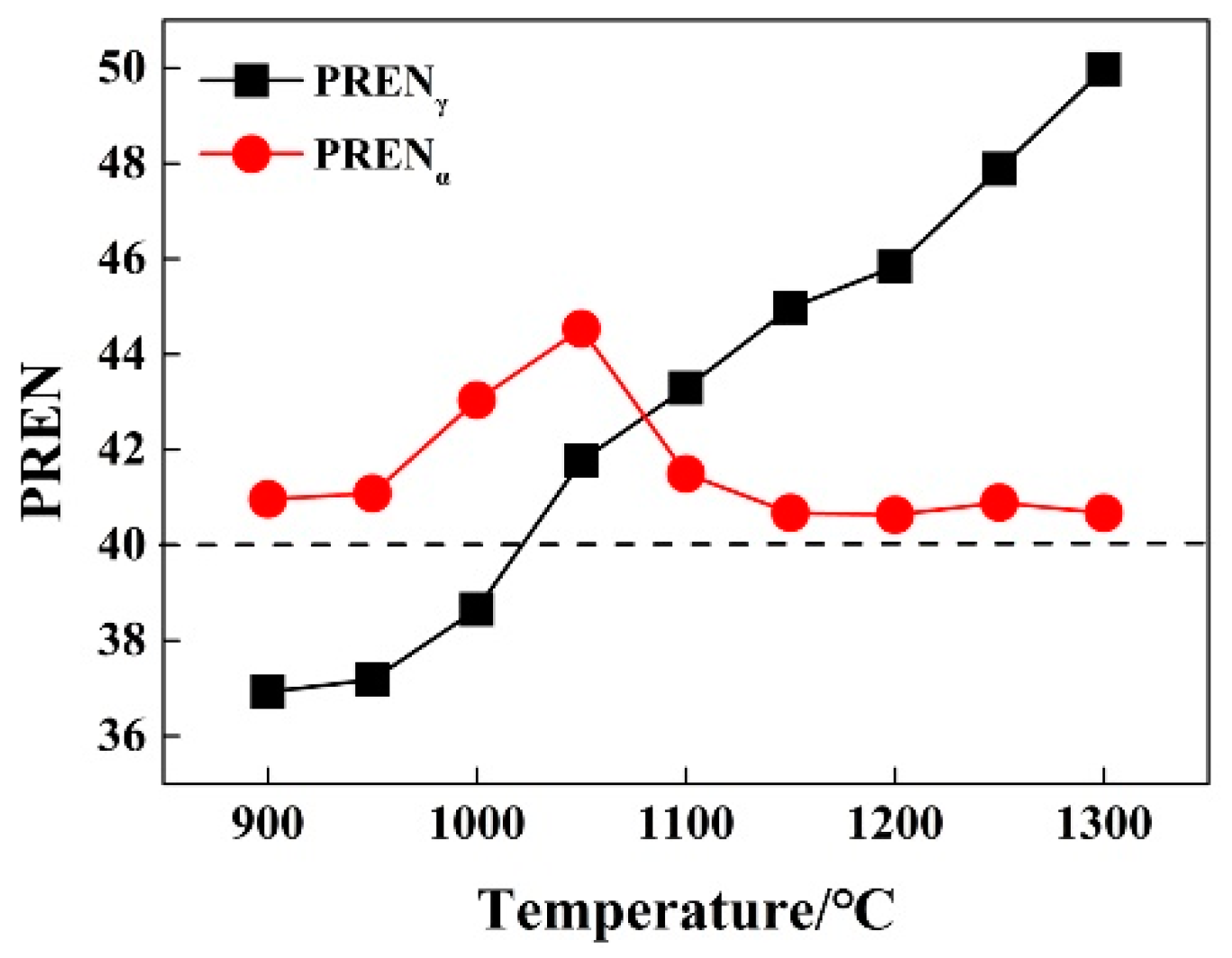
3.2. Effect of Solution Treatment Temperature on Alloying Elements Distribution in Two-Phase Structures
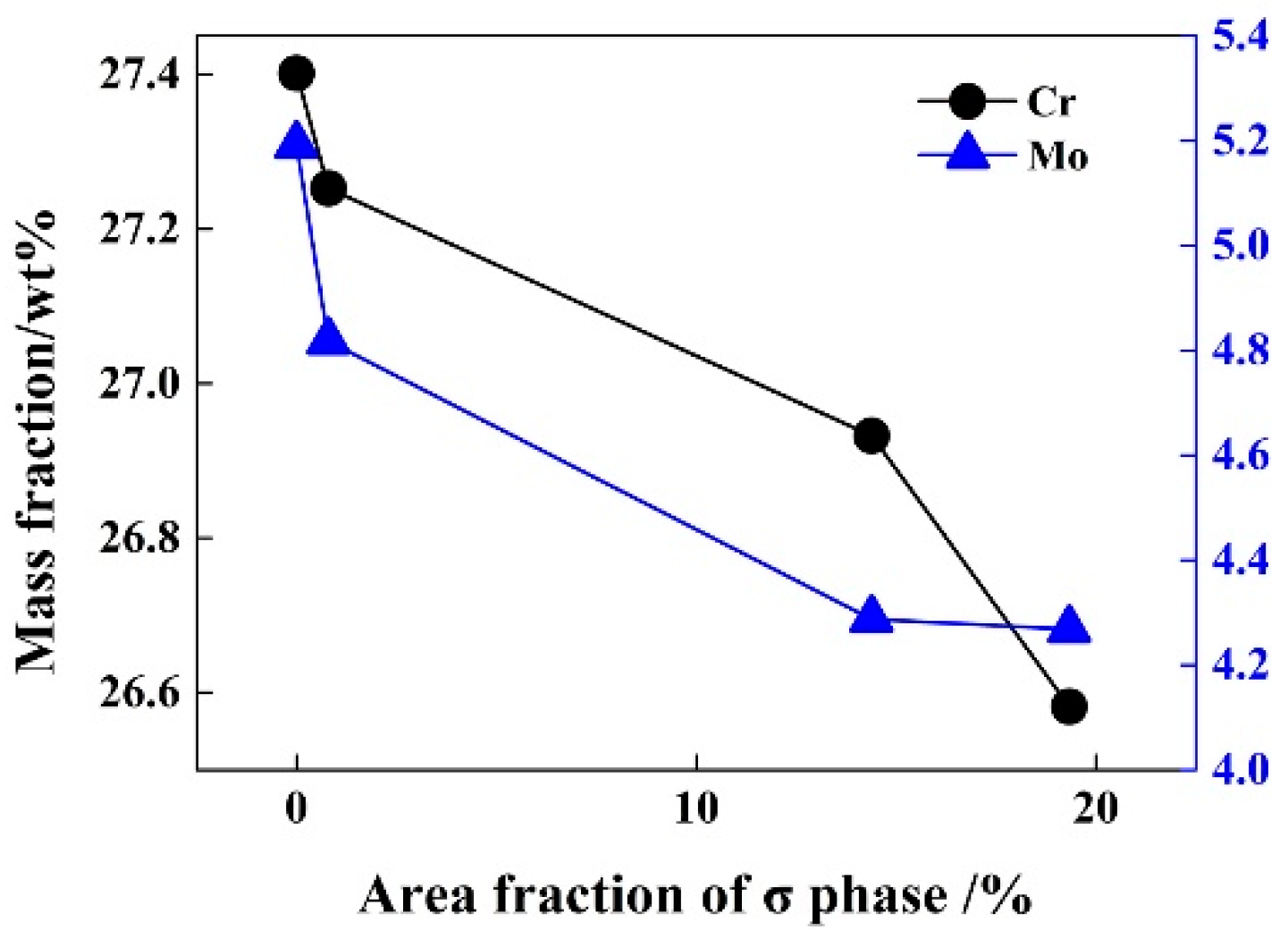
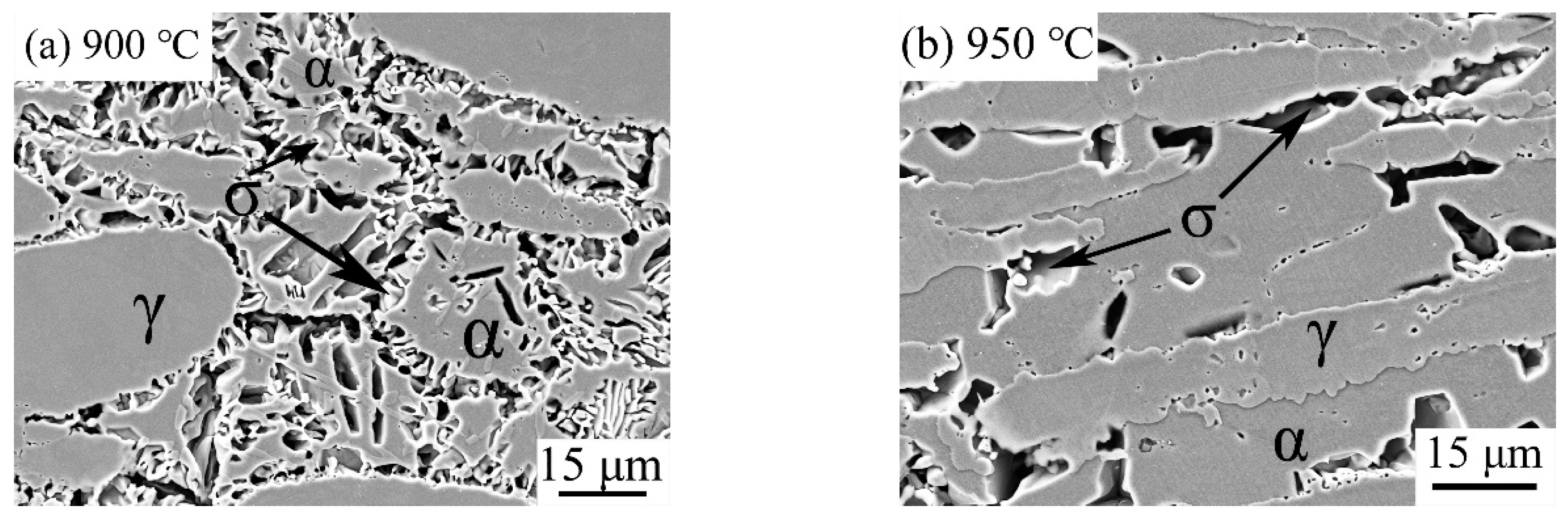
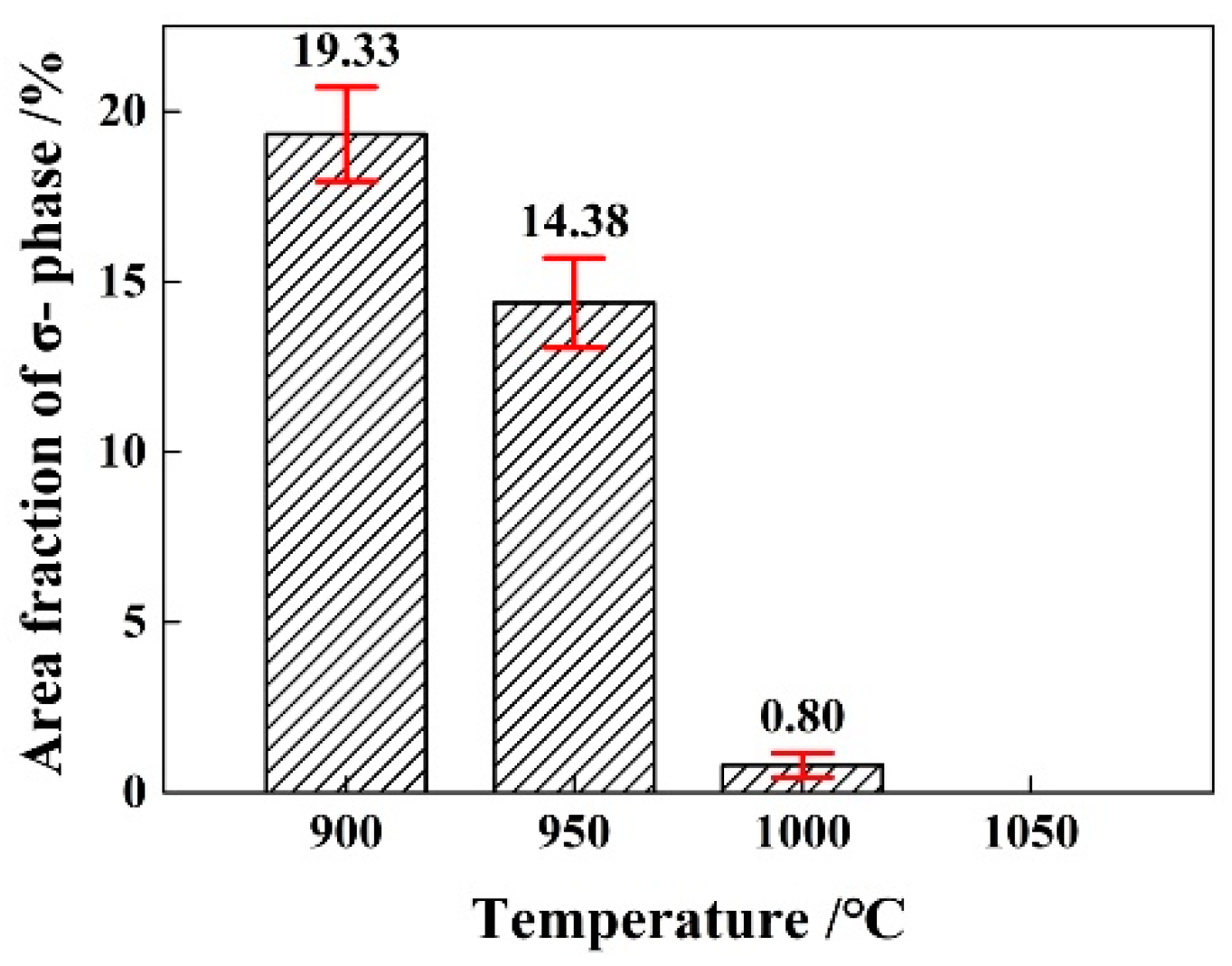
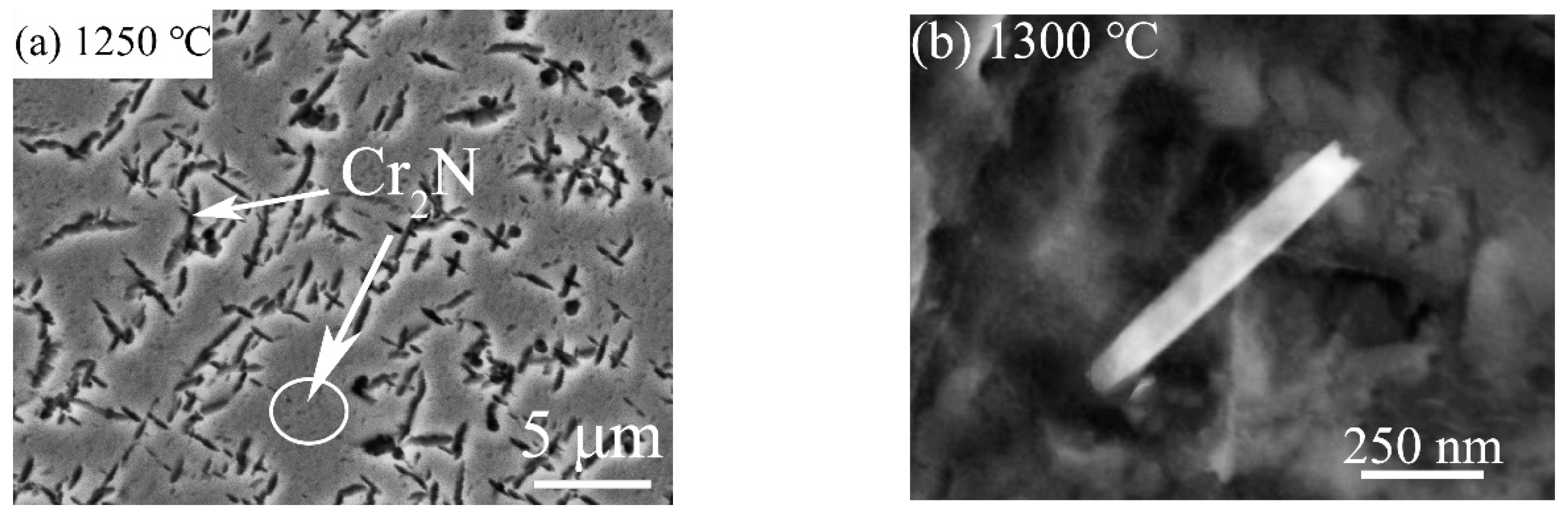
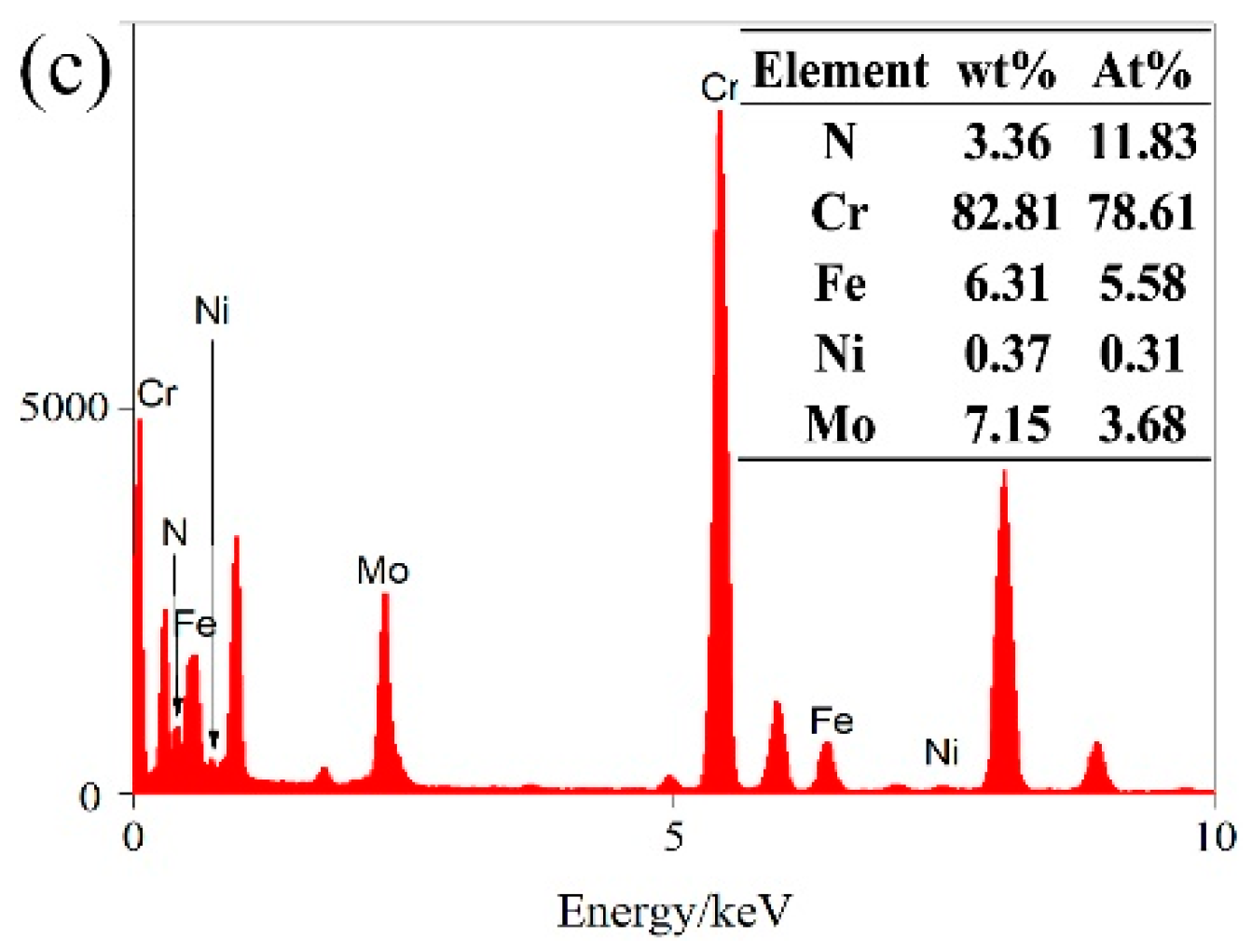
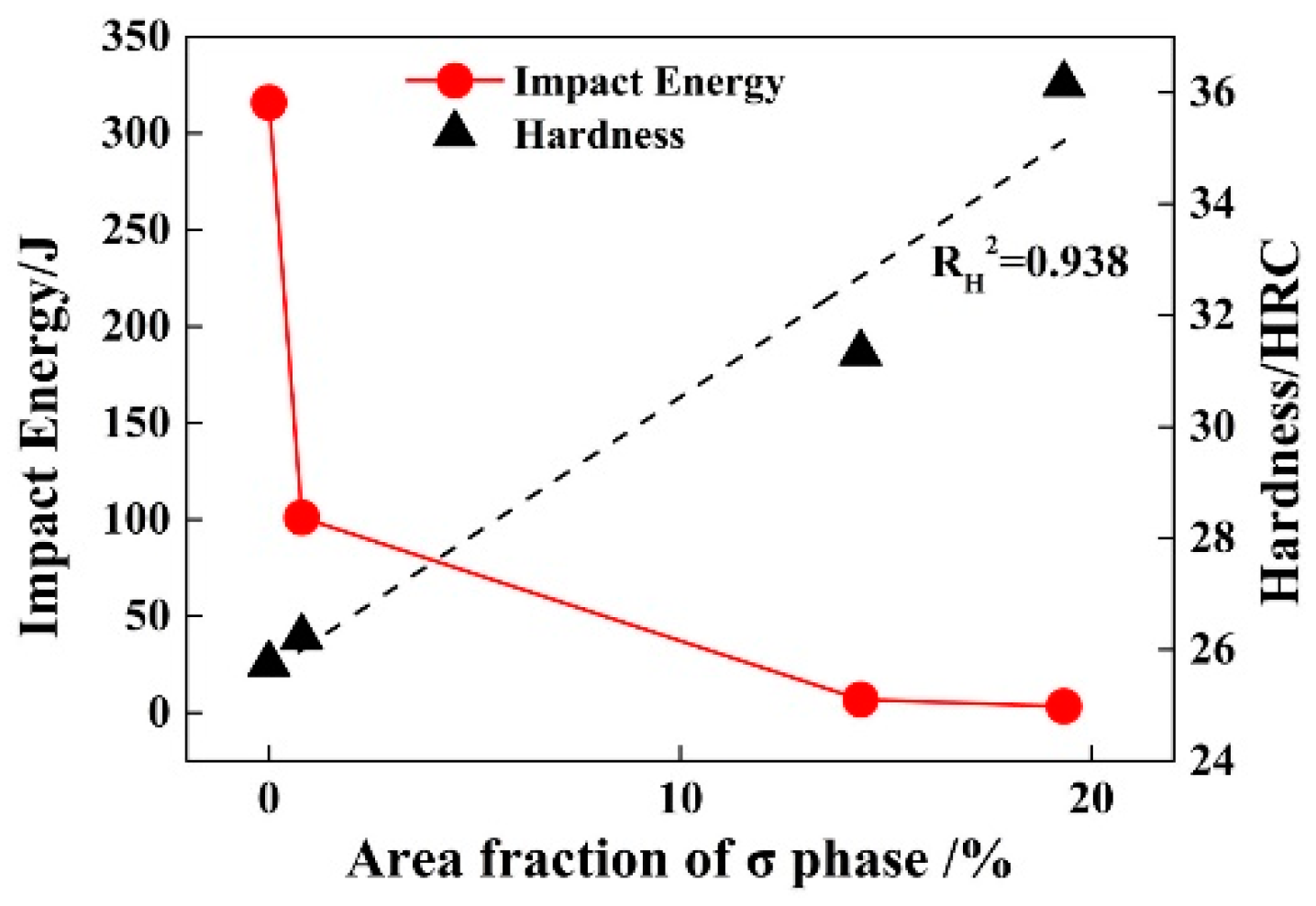
3.3. Effect of Solution Treatment Temperature on the Precipitation Phase
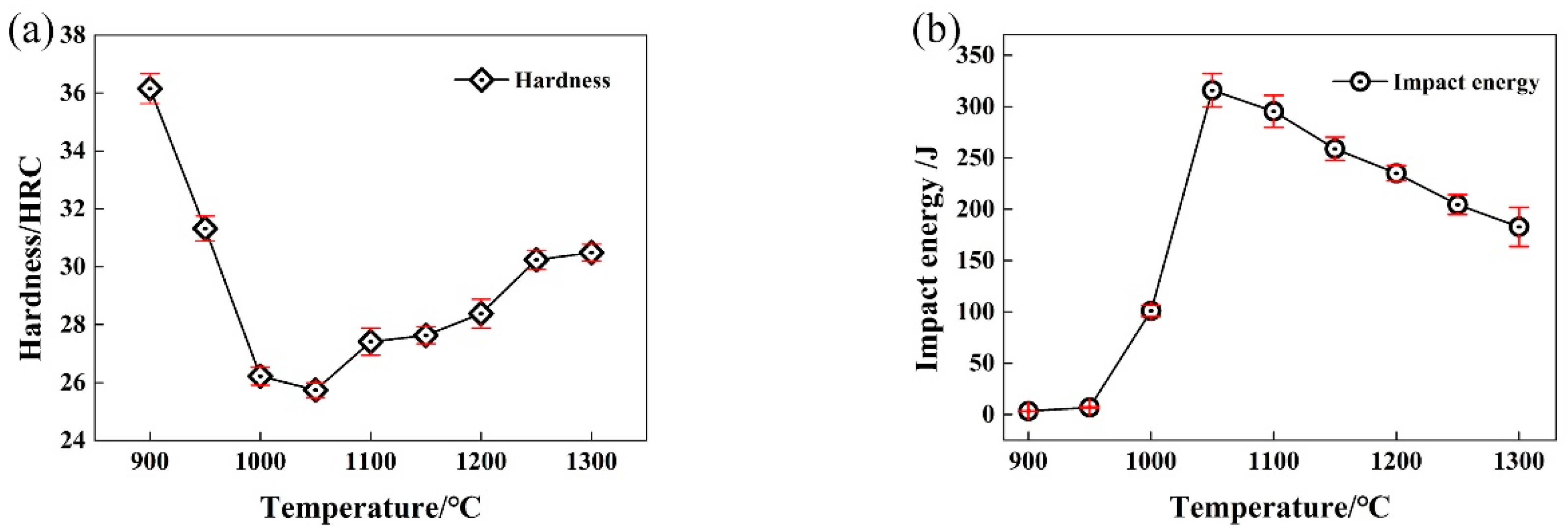
3.4. Effect of Solution Treatment Temperature on Mechanical Properties
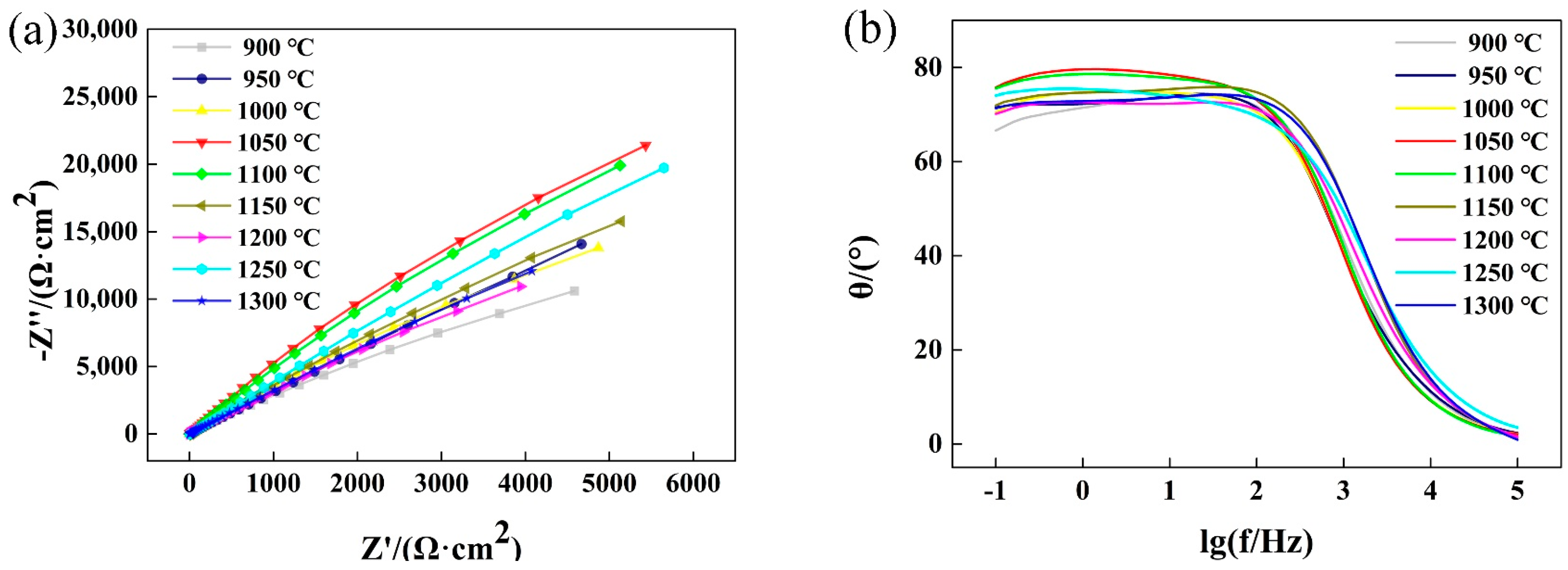
3.5. Effect of Solution Treatment Temperature on the Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nilsson, J.O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Adhe, K.M.; Kain, V.; Madangopal, K.; Gadiyar, H.S. Influence of Sigma-Phase Formation on The Localized Corrosion Behavior of A Duplex Stainless Steel. J. Mater. Eng. Perform. 1996, 5, 500–506. [Google Scholar] [CrossRef]
- Momeni, A.; Dehghani, K. Effect of Hot Working on Secondary Phase Formation in 2205 Duplex Stainless Steel. J. Mater. Sci. Technol. 2010, 26, 851–857. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ochi, R.; Yasuda, K.; Aramaki, M.; Munetoh, S.; Furukimi, O. Effect of γ-Phase Stability on Local Deformation Energy of α-γ Duplex Stainless Steel. Mater. Trans. 2017, 58, 1379–1385. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of Intermetallic Precipitations on The Properties of Duplex Stainless Steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Ziying, Z.; Zhao, H.; Zhang, H.; Hu, J.; Jin, J. Microstructure Evolution and Pitting Corrosion Behavior of UNS S32750 Super Duplex Stainless Steel Welds After Short-Time Heat Treatment. Corros. Sci. 2017, 121, 22–31. [Google Scholar]
- Jeon, S.H.; Hur, D.H.; Kim, H.J.; Park, Y.S. Effect of Ce Addition on The Precipitation of Deleterious Phases and The Associated Intergranular Corrosion Resistance of 27Cr–7Ni Hyper Duplex Stainless Steels. Corros. Sci. 2015, 90, 313–322. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Răducanu, D.; Angelescu, M.L.; Vintil, A.N.; Serban, N.; Dan, I.; Cojocaru, E.M.; Cinca, I. Influence of Solution Treatment Duration on Microstructural Features of An Industrial Forged UNS S32750/1.4410/F53 Super Duplex Stainless Steel (SDSS) Alloy. JOM 2017, 69, 1439–1445. [Google Scholar] [CrossRef]
- Jang, M.H.; Moon, J.; Lee, T.H.; Park, S.J.; Han, H.N. Effect of Nitrogen Partitioning on Yield Strength in Nitrogen-Alloyed Duplex Stainless Steel During Annealing. Metall. Mater. Trans. A 2014, 45, 1653–1658. [Google Scholar] [CrossRef]
- Kong, K.H.; Jeon, S.H.; Kim, S.T.; Kim, D.H.; Kim, B.J.; Guim, H.U.; Moon, M.B.; Park, Y.S. Effects of Cu Addition on the Microstructure and Localized Corrosion Resistance of Hyper Duplex Stainless Steels Aged at 748 K. Mater. Trans. 2015, 56, 749–754. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, C.Y. Effect of R Phase Formation on the Mechanical Properties of 25Cr-7Ni-2Mo-4W Super Duplex Stainless Steel. Korean J. Mater. Res. 2014, 24, 401–406. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, H.; Zhang, Z.; Wang, Z.; Jiang, Y.; Jiang, L.; Li, J. Effect of Annealing Temperature on the Pitting Corrosion Behavior of UNS S82441 Duplex Stainless Steel. Corrosion 2013, 69, 167–173. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Chen, Y.; Wang, Y. Effect of Dual-Phase Structure on The Microstructure and Deformation Inhomogeneity of UNS S32750 Duplex Stainless Steel. Ironmak. Steelmak. 2020, 1–9. [Google Scholar]
- Han, Y.; Zou, D.N.; Zhang, W.; Yu, J.H.; Qiao, Y.Y. Influence of Sigma Phase Precipitation on Pitting Corrosion of UNS S32750 Super-Duplex Stainless Steel. Mater. Sci. Forum 2010, 658, 380–383. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Yuan, Y.F.; Guo, S.Y. Effect of Microstructure and Passive Film on Corrosion Resistance of UNS S32750 Super Duplex Stainless Steel Prepared by Different Cooling Methods in Simulated Marine Environment. Int. J. Miner. Metall. Mater. 2020, 27, 1100–1114. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, K.T.; Lee, Y.D.; Lee, C.S. Effect of Heat Treatment on Mechanical Properties of Super Duplex Stainless Steel. Adv. Mater. Res. 2010, 89–91, 290–294. [Google Scholar] [CrossRef]
- Han, Y.; Zou, D.N.; Yao, H.H.; Zhang, W.; Yu, J.H. Microstructural Evolutions and its Influence on Properties of Super-Duplex Stainless Steel. Adv. Mater. Res. 2010, 97–101, 656–659. [Google Scholar] [CrossRef]
- Ferro, P. A Dissolution Kinetics Model and Its Application to Duplex Stainless Steels. Acta Mater. 2013, 61, 3141–3147. [Google Scholar] [CrossRef]
- Byun, S.-H.; Kang, N.; Lee, T.-H.; Ahn, S.-K.; Lee, H.; Chang, W.-S.; Cho, K.-M. Kinetics of Cr/Mo-rich Precipitates Formation for 25Cr-6.9Ni-3.8Mo-0.3N Super Duplex Stainless Steel. Met. Mater. Int. 2012, 18, 201–207. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, Y.; Gao, J.; Li, J. Effect of Annealing Treatment on Microstructure Evolution and The Associated Corrosion Behavior of A Super-Duplex Stainless Steel. J. Alloys Compd. 2010, 493, 461–464. [Google Scholar] [CrossRef]
- Jin, L. Effect of Aging Treatment on Corrosion Resistance of S32750 Super Duplex Stainless Steel in Simulated Seawater at Low Temperature. Int. J. Electrochem. Sci. 2019, 14, 3651–3662. [Google Scholar] [CrossRef]
- Liu, J.M.; Liu, J.; Fan, G.W.; Du, D.F.; Li, G.P.; Chai, C.J. Effect of Solution Treatment on Microstructure and Properties of the UNS S32750 Super Duplex Stainless Steel. Mater. Sci. Forum 2012, 724, 3–6. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, Y.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of Annealing Temperature on The Pitting Corrosion Resistance of Super Duplex Stainless Steel UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Sato, Y.S.; Kokawa, H. Preferential Precipitation Site of Sigma Phase in Duplex Stainless Steel Weld Metal. Scr. Mater. 1999, 40, 659–663. [Google Scholar] [CrossRef]
- Pettersson, N.H.; Lindell, D.; Lindberg, F.; Borgenstam, A. Formation of Chromium Nitride and Intragranular Austenite in a Super Duplex Stainless Steel. Metall. Mater. Trans. A 2019, 50, 5594–5601. [Google Scholar] [CrossRef]
- Ramirez, A.J.; Lippold, J.C.; Brandi, S.D. The Relationship between Chromium Nitride and Secondary Austenite Precipitation in Duplex Stainless Steels. Metall. Mater. Trans. A 2003, 34, 1575–1597. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson RF, A.; Wessman, S. Precipitation of Chromium Nitrides in The Super Duplex Stainless Steel UNS S32750. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Weber, L.; Uggowitzer, P.J. Partitioning of Chromium and Molybdenum in Super Duplex Stainless Steels with Respect to Nitrogen and Nickel Content. Mater. Sci. Eng. A 1998, 242, 222–229. [Google Scholar] [CrossRef]
- Ha, H.Y.; Kwon, H.S. Effects of Cr2N on The Pitting Corrosion of High Nitrogen Stainless Steels. Electrochim. Acta 2007, 52, 2175–2180. [Google Scholar] [CrossRef]
- Shin, B.H.; Kim, D.; Park, S.; Hwang, M.; Park, J.; Chung, W. Precipitation Condition and Effect of Volume Fraction on Corrosion Properties of Secondary Phase on Casted Super-Duplex Stainless Steel UNS S32750. Anti-Corros. Methods Mater. 2018, 66, 61–66. [Google Scholar] [CrossRef]
- Ha, H.Y.; Jang, M.H.; Lee, T.H.; Moon, J. Interpretation of The Relation Between Ferrite Fraction and Pitting Corrosion Resistance of Commercial 2205 Duplex Stainless Steel. Corros. Sci. 2014, 89, 154–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Jiang, Y.; Tan, H.; Han, D.; Guo, Y.; Li, J. Effect of Post-Weld Heat Treatment on Microstructure Evolution and Pitting Corrosion Behavior of UNS S31803 Duplex Stainless Steel Welds. Corros. Sci. 2012, 62, 42–50. [Google Scholar] [CrossRef]
- Kim, S.T.; Lee, I.S.; Kim, J.S.; Jang, S.H.; Park, Y.S.; Kim, K.T.; Kim, Y.S. Investigation of The Localized Corrosion Associated with Phase Transformation of Tube—To–Tube Sheet Welds of Hyper Duplex Stainless Steel in Acidified Chloride Environments. Corros. Sci. 2012, 64, 164–173. [Google Scholar] [CrossRef]
- Lee, T.-H.; Ha, H.-Y.; Kang, J.-Y.; Hwang, B.; Woo, W.; Shin, E. In Situ and Ex Situ Neutron Diffraction Study on Deformation Behavior of High-Nitrogen, Ni-Free Duplex Stainless Steel. Scr. Mater. 2012, 67, 141–144. [Google Scholar] [CrossRef]
- Da Fonseca, G.S.; De Oliveira, P.M.; Diniz, M.G.; Bubnoff, D.V.; De Castro, J.A. Sigma Phase in Superduplex Stainless Steel: Formation, Kinetics and Microstructural Path. Mater. Res. 2017, 20, 249–255. [Google Scholar] [CrossRef]
- Wei, Z.; Laizhu, J.; Jincheng, H.; Hongmei, S. Effect of Ageing on Precipitation and Impact Energy of 2101 Economical Duplex Stainless Steel. Mater. Charact. 2009, 60, 50–55. [Google Scholar] [CrossRef]
- Cabral, A.M.; Trabelsi, W.; Serra, R.; Montemor, M.F.; Zheludkevich, M.L.; Ferreira MG, S. The Corrosion Resistance of Hot Dip Galvanised Steel and AA2024-T3 Pre-Treated with bis-[triethoxysilylpropyl] tetrasulfide solutions doped with Ce(NO3)3. Corros. Sci. 2006, 48, 3740–3758. [Google Scholar] [CrossRef]
- Cervo, R.; Ferro, P.; Tiziani, A. Annealing Temperature Effects on Super Duplex Stainless Steel UNS s32750 Welded Joints. I: Microstructure and Partitioning of Elements. J. Mater. Sci. 2010, 45, 4369–4377. [Google Scholar] [CrossRef]
- Cervo, R.; Ferro, P.; Tiziani, A.; Zucchi, F. Annealing Temperature Effects on Superduplex Stainless Steel UNS S32750 Welded Joints. II: Pitting Corrosion Resistance Evaluation. J. Mater. Sci. 2010, 45, 4378–4389. [Google Scholar] [CrossRef]




| C | Si | Mn | P | S | Cr | Ni | Mo | N | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.024 | 0.39 | 0.67 | 0.022 | 0.002 | 25.49 | 6.18 | 3.47 | 0.288 | Bal. |
| Temperature/°C | Icoor/A∙cm−2 | Ecoor vs. SCE/V | Ep vs. SCE/V | (Ep-Ecoor)/V |
|---|---|---|---|---|
| 900 | 9.8877 × 10−8 | −0.2686 | 0.8460 | 1.1146 |
| 950 | 7.4826 × 10−8 | −0.2831 | 0.8772 | 1.1603 |
| 1000 | 6.1035 × 10−8 | −0.2956 | 0.9634 | 1.2590 |
| 1050 | 4.9744 × 10−8 | −0.2812 | 1.0019 | 1.2831 |
| 1100 | 5.7373 × 10−8 | −0.2928 | 0.9451 | 1.2380 |
| 1150 | 5.1575 × 10−8 | −0.2898 | 0.9694 | 1.2592 |
| 1200 | 5.8594 × 10−8 | −0.2884 | 0.9616 | 1.2500 |
| 1250 | 5.8948 × 10−8 | −0.2719 | 0.9425 | 1.2144 |
| 1300 | 6.1156 × 10−8 | −0.2634 | 0.9317 | 1.1951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Shen, W.; Lin, P.; Wang, F.; Yang, Z. Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 Super Duplex Stainless Steel. Metals 2020, 10, 1481. https://doi.org/10.3390/met10111481
Li J, Shen W, Lin P, Wang F, Yang Z. Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 Super Duplex Stainless Steel. Metals. 2020; 10(11):1481. https://doi.org/10.3390/met10111481
Chicago/Turabian StyleLi, Junhe, Wei Shen, Ping Lin, Fuming Wang, and Zhanbing Yang. 2020. "Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 Super Duplex Stainless Steel" Metals 10, no. 11: 1481. https://doi.org/10.3390/met10111481
APA StyleLi, J., Shen, W., Lin, P., Wang, F., & Yang, Z. (2020). Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 Super Duplex Stainless Steel. Metals, 10(11), 1481. https://doi.org/10.3390/met10111481





