Abstract
The chemical and mineral composition of the red mud from the Ural Aluminum Plant were studied by XRF, XRD, and Mössbauer spectroscopy. Experiments on reductive smelting of red mud were carried out in a range of temperatures (1650–1750 °C) to recover iron from the aluminum production waste with maximum efficiency. It was found that it is possible to obtain pig iron with a high content of titanium, phosphorus, and vanadium, and low sulfur content. The efficiency of iron recovery at 1750 °C was found to be around 98%. Thermodynamic calculations were carried out to assist in finding the optimal conditions for the process (e.g., carbon content, furnace temperature, slag liquidus temperature). It was also found that the pig iron phase obtained at 1650 to 1700 °C is not separated from the slag phase into ingot compared with the sample obtained at 1750 °C. Pig iron obtained at 1750 °C can be used to produce molds for the steel-casting equipment.
1. Introduction
The accumulation and storage of the alumina production waste, red mud (RM), represents one of the most critical ecological problems for the aluminum industry. Depending on the composition of the initial bauxite ore and its processing technology, 1.0 to 1.4 tons of RM can be formed from the production of 1 ton of alumina [1,2]. At the moment, RM in Russia is barely utilized but stored in slurry storages. Currently, about 600 million tons of RM are already accumulated at aluminum plants in Russia [3]. The construction and operation of slurry storages require significant costs that leads to an increase of the cost value of alumina and, consequently, of metallic aluminum. There is also a high risk of ecological disasters: Dams breaks and weathering process make this problem even more severe [4].
RM contains up to 60% iron compounds [5], which implies the practicality of their processing using reductive smelting with extraction of iron into a separate phase and reception of slag suitable for further extraction of Al, Ti, and rare metals by hydrometallurgical methods [6,7,8]. A high content of alkali in RM (up to 12% Na2O [9]) leads to furnace lining destruction caused by high RM reactivity during the melting process. Despite this fact, most studies are devoted to the pyrometallurgical processing of the initial RM [10,11,12], because the alkali present in RM significantly reduces its melting point and improves the separation of slag and metal [13,14]. However, from our point of view, it would be more practical to regenerate the alkali for recirculation by autoclave leaching of bauxite [15,16], since during the smelting of red mud with high sodium content, the alkali goes into the gas phase, and its further regeneration is very difficult [17].
The most valuable components in slag obtained after RM reductive smelting are aluminum, titanium, and scandium [18,19]. Because of the high content of silicon dioxide (10–15 wt. %) in the slag, it is impractical to process it by alkaline technology, as part of the alkali will be lost due to a chemical reaction with silica. The most perspective slag treatment is a leaching process by hydrochloric acid to obtain aluminum chloride solutions [20,21]. Then, aluminum chloride hexahydrate (AlCl3·H2O) and scandium (Sc) compounds may selectively be extracted from these solutions [22,23,24,25,26,27]. Silicon dioxides and titanium oxides will concentrate in a solid residue. The residue can be leached with sulfuric acid to extract titanium into the solution [28,29]. Thus, the proposed way of RM processing consists of reductive smelting of neutralized RM to obtain pig iron and further hydrometallurgical treatment of the produced slag by hydrochloric acid to extract valuable products: Sandy grade alumina (Al2O3), scandium concentrate, titanium concentrate, and wollastonite (CaSiO3), as shown in Figure 1.
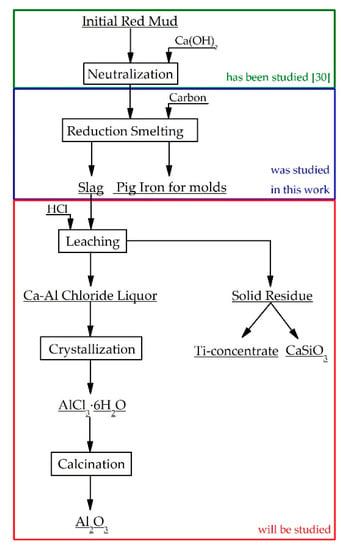
Figure 1.
A flowsheet of the recycling treatment of red mud.
In the present paper, the second stage of the abovementioned treatment, reductive smelting of the neutralized RM, was investigated to obtain: 1) The maximum amount of the pig iron, 2) complete separation of the metal and slag, and 3) analysis of the resulting pig iron with a description of potential applications.
2. Materials and Methods
2.1. Raw Materials
Table 1 shows the chemical composition of the neutralized RM from Ural Aluminum Plant (56.304530, 61.980334; Kamensk-Uralsky, Russia). Sodium was removed from RM on the plant by leaching with a lime slurry at 90 °C for a duration of 3 h [30], which can be described by the following equation [31,32]:
Na8Al6(SiO4)3(OH)14·nH2O(s) + 9Ca(OH)2(l) = 3Ca3Al2(SiO4)(OH)8(s) + 8NaOH(l) + nH2O(l).

Table 1.
The average chemical composition (wt %) of RM from Ural Aluminum Plant (Kamensk-Uralsky, Russia).
2.2. Thermodynamic Modeling
Liquidus projections were calculated using FactSage software (version 7.1) [33]. The amount of reducing agent for complete reduction of iron-containing phases was calculated using HSC Chemistry software (version 6.00) [34].
2.3. Reductive Smelting of Red Mud
Reductive smelting of the neutralized RM was carried out in a vertical resistance tube (Tammann) furnace, as shown in Figure 2.
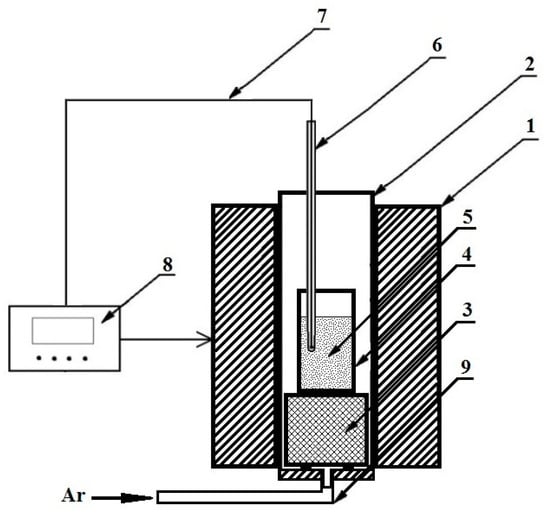
Figure 2.
Schematic of the Tamman resistance furnace (1—furnace; 2—graphite heater; 3—graphite stands; 4—crucible; 5—mixture of RM with carbon; 6—thermocouple shield; 7—W-Re thermocouple; 8—automatic temperature controller; 9—gas inlet).
A RM and carbon mixture (5) was filled into carbon crucibles (4), which were placed into a Tammann furnace (1) with a graphite heater (2) on a graphite platform (3). The furnace dimensions (height/diameter/tube diameter) are 360/300/74 mm. The furnace temperature was controlled by an automatic PID-controller (8) and by a W-Re thermocouple (type: WR20 with ±∆T = 0.005T) (7) in an Al2O3 shield (6), which was lowered into the graphite crucible (4). To reduce the oxygen potential in the gas phase and to avoid erosion of the carbon heater due to burning, Ar gas was introduced into the tube with a 0.5 L/min flow rate through a gas inlet (9) in the lower part of the furnace.
The mixture with 27.6 g of RM and 2.4 g of carbon graphite was used for reductive smelting. The furnace was heated up to 1300 °C with a maximal rate during 1 h, then a required temperature within the range 1650–1750 °C was reached with the 10 °C/min rate and kept for 10 min. After that, the furnace was switched off and the crucibles were cooled down with the furnace.
The slag chemical composition was analyzed using an X-ray fluorescence spectrometer (XRF) ZXS Primus II (Rigaku, Tokyo, Japan). The mineralogical composition of the samples was determined by X-ray diffraction analysis (XRD) on a MiniFlex 600 (Rigaku, Tokyo, Japan). Quantitative analysis of phase fractions was performed by the Rietveld method using PDXL-2 program (Rigaku). The elemental composition of pig iron was determined on an atomic emission spectrometer GDS-850A (LECO Corporation, St. Joseph, MI, USA) while the carbon and sulfur content were analyzed on a CS-600 (LECO Corporation, St. Joseph, MI, USA). Samples (~1 g) were placed in ceramic crucibles and then loaded into an induction furnace. The C and S concentrations were determined by infrared absorption of gaseous CO2 and SO2 during sample combustion in oxygen atmosphere.
The Mössbauer analysis of RM and pig iron were obtained using a spectrometer MC1104EM (KORDON, Rostov-on-Don, Russia) at temperatures of 296 ± 3 and 77.5 ± 0.5 K in a vacuum cryostat. The 57Co nuclei with 47 mCi activity in a Rh matrix (RITVERC JSC, St-Petersburg, Russia) was used as the γ-radiation source. The Mössbauer spectra were analyzed using SpectrRelax 2.4 software (MSU, Moscow, Russia). The values of chemical shifts are presented relative to α-Fe.
Surface visualization and chemical element distribution in arbitrarily selected segments of the slag and metal samples were performed by a scanning electron microscope (SEM) Vega III (Tescan, Brno, Czech Republic).
Microindentation hardness testing was carried out using an optical microscope METAM RV-23M with a microhardness tester PMT-3 (all equipment from LOMO, St. Petersburg, Russia). The pig iron sample was etched with a solution of 3% nitric acid (HNO3) in ethanol (C2H5OH) to reveal the microstructure and determine the phase composition. The force applied to the diamond indenter was 0.49 N, with a duration of 10 s.
The Vickers hardness number was calculated using the following equation by ISO 6507-1-2007: Metals and alloys. Vickers hardness test. Part 1. Test method:
where F is the force applied to the diamond indenter, N; and d is the average length of the diagonal left by the indenter, mm.
HV = 0.189 × (F/d2),
3. Results
3.1. Composition of the Neutralized Red Mud
Figure 3 shows the results of the XRD analysis of RM collected from Ural Aluminum Plant. It can be seen that the RM consists mainly of hematite (α-Fe2O3), katoite (Ca3Al2SiO4(OH)8), calcite (CaCO3), and quartz (SiO2). It also contains small amounts of portlandite (Ca(OH)2) and goethite (α-FeOOH). Titanium is distributed between titanate (CaTiSiO5) and di-iron titanate (Fe2TiO5).
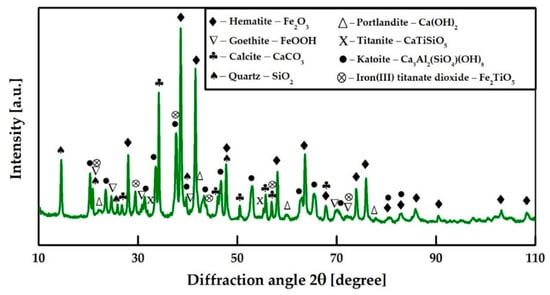
Figure 3.
XRD analysis of the neutralized RM from Ural Aluminum Plant (Kamensk-Uralsky, Sverdlovsk region, Russia).
The Mössbauer spectrum of this RM sample was analyzed in a previous work [35]. In this study, a more complete and correct description of the RM sample from Ural Aluminum Plant was obtained using the complex Mössbauer analysis.
The RM Mössbauer spectra obtained at 296 and 77.5 K (Figure 4) have a similar nature and contain a sextet distorted by broadening into the interior of the spectrum, two additional resonance lines asymmetric in intensity in the interior of the spectrum, with a width comparable to sextet lines, and a relatively wide resonance line in the center of the spectrum.
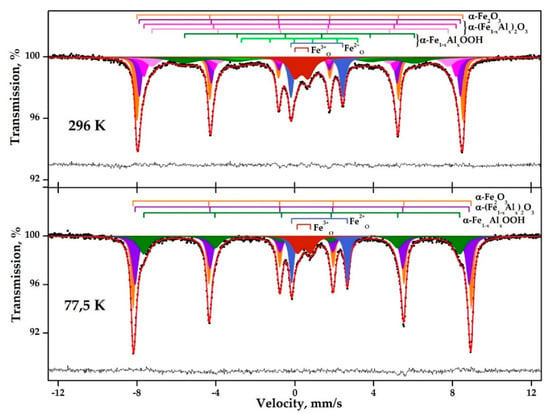
Figure 4.
Mössbauer spectra of the RM sample obtained at 296 and 77.5 K.
The difference between the low-temperature and high-temperature spectra is as follows: The presence of sharp shoulders on lines 1 and 6 of the sextet (see #1–6, Table 2); the absence of absorption between 1-2-3 and 4-5-6 resonant sextet lines; and a noticeable increase in the intensity of the resonance lines of the sextet. At the same time, the intensity of the paramagnetic part of the spectrum (an asymmetric doublet and a wide resonance line in the center) has not changed. This makes it possible to exclude the occurrence of superparamagnetism in this sample, a phenomenon that is characteristic of nano-scale iron-containing particles. Therefore, the asymmetry of the resonance lines at room temperature is only due to the locally inhomogeneous environment of the Fe atoms in at least one of the present phases.

Table 2.
Mössbauer spectra parameters of red RM obtained at 296 and 77.5 K.
The shoulders in the low-temperature spectra and the resonance lines reaching the baseline indicate a superposition of the spectra of at least two magnetically ordered phases, and one of them at room temperature was obviously responsible for the distortion of the background line in the central part of the spectrum.
In the model description, the Mössbauer spectrum obtained at room temperature can be fully characterized by a superposition of six sextets, divided into two groups of four and two sub-spectra, and two doublets (see Table 2). The low-temperature spectrum is described by a superposition of three sextets (grouped as two plus one) and two doublets.
The intensity and appearance of the paramagnetic part of the spectrum practically do not change in the studied temperature range. One of the doublets, characterized by high values of isomeric shift and quadrupole splitting (#7, Table 2), corresponds to Fe2+ ions in a high-spin state in an octahedral oxygen environment [36]. Obviously, these iron atoms correspond to a layered alumosilicate mineral. The second doublet (# 8, Table 2) corresponds to Fe3+ ions in a high-spin state in an octahedral oxygen environment [36]. It has a large line width, which may indicate a lower degree of crystallinity of the phase containing it (as compared, for example, with #7, Table 2). The absence of temperature dependence for its intensity does not allow us to interpret this subspectrum as being related to iron (III) hydroxide [37]. Since its quadrupole splitting value does not change with temperature, and the line width decreases, the assignment of this doublet to superparamagnetic particles [38] is also excluded. Obviously, this broadened doublet may contain subspectra of Fe2TiO5 with paramagnetic properties up to 55 K [39,40].
The magnetically split part of the spectrum can be described by two groups of sextets related to compounds of a different nature. The relatively narrow and asymmetric resonance lines of the high-temperature spectrum, representing a distinctly broadened sextet, can be described by a superposition of four sextets, which differ mainly in the magnitude of the magnetic splitting and line width (#1–4, Table 2). The parameters of the external sextet of this group (#1, Table 2) are in very good agreement with the parameters of hematite—α-Fe2O3 [42,43]. Internal sextets, which differ mainly in larger line widths, their lower intensity, and lower magnitude of magnetic splitting, obviously belong to defective forms of hematite, differing in various degrees of substitution of iron atoms by atoms of another nature (#2–4, Table 2). Given the origin of the sample, it is possible to assume, with a high degree of certainty, isomorphic substitution of a part of iron atoms by aluminum atoms [44]. The substitution of a part of atoms in the iron sublattice by aluminum atoms leads to a proportional impurity content and to a decrease in the magnitude of the magnetic splitting in proportion to the impurity content [45]. As the sample temperature decreases to 77.5 K, the discussed part of the spectrum is described by two sextets of different widths. In this case, the magnetic field of the outer sextet of a smaller width does not reach the value characteristic of hematite [43], which confirms the version of isomorphic substitution of part of the iron atoms. In the studied temperature range, the sample does not undergo the Morin transition [46], which confirms the distortion of the hematite structure by the impurity atoms present in the iron sublattice. We also note that an increase in the content of hydroxyl groups in the oxygen positions of aluminum-hematite can also leads to a decrease in the Morin transition temperature [47]. In this compound, a strong magnetic dipole and electric quadrupole interactions are observed (obviously caused by distortion of the crystal lattice of aluminum-hematite by impurity atoms), which required this part of the spectrum to be described by taking into account the terms second-order of quadrupole perturbation [41] (a+, a−; see Table 2).
3.2. Thermodynamic Calculations
It can be seen from Table 1 that Fe2O3, CaO, Al2O3, SiO2, and TiO2 are the major components of the RM. After reductive smelting, the total iron is reduced to a metallic phase while the other oxides occur in a slag phase. Thermodynamic modeling of the slag phase was carried out for complete (no Fe in slag) and partial (2.5 mass % of FeO added to slag) reduction of iron.
Liquidus projections for the CaO-Al2O3-SiO2-TiO2 system calculated in the temperature range of 1300 to 1800 °C are shown in Figure 5a. The slag chemical composition obtained after reductive smelting of the neutralized RM [35] was recalculated to four components (26.04 mass % Al2O3, 20.46 SiO2, 45.21 CaO, 8.29 TiO2) and is also shown in Figure 5a. It was assumed that all iron-containing phases are completely reduced and the Fe content in the slag is zero.
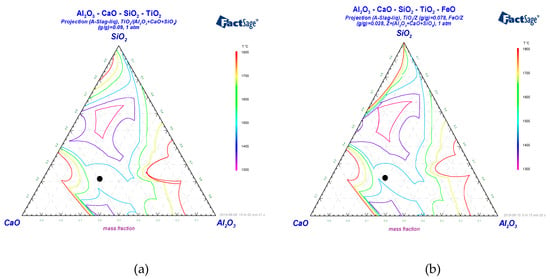
Figure 5.
Liquidus projections for (a) the CaO-Al2O3-SiO2-8.21 wt % TiO2 and (b) the CaO-Al2O3-SiO2-7.5 wt % TiO2-2.5 wt % FeO slag systems (the slag chemical composition is marked by a black dot).
Figure 5a indicates that the RM melting point under complete reduction of iron is about 1600 °C while the presence of iron oxide in slag under reducing conditions shifts the liquidus towards lower temperatures (Figure 5b).
Figure 5b shows the same liquidus projections calculated for the CaO-Al2O3-SiO2-FeO-TiO2 system with 2.5 mass% of FeO and 7.5 mass% of TiO2. This five-component system represents a more realistic situation of incomplete reduction of iron due to non-equilibrium conditions.
The iron oxide reduction process may be represented as the following reactions:
3Fe2O3(s) + C(s) = 2Fe3O4(s) + CO(g),
Fe3O4(s) + C(s) = 3FeO(s) + CO(g),
FeO(s) + C(s) = Fe(s) + CO(g),
3Fe(s) + C(s) = Fe3C(s).
At the same time, at temperatures >700 °C, carbon monoxide is regenerated by the Boudouard–Bell reaction, and Equation (5) is actually a total reaction of:
FeO(s) + CO(g) = Fe(s) + CO2(g),
C(s) + CO2(g) = 2CO(g).
Silicon oxides can be reduced by solid carbon only at temperatures above 1400 °C by the following reactions:
SiO2(s) + C(s) = SiO(g) + CO(g),
SiO(g) + C(s) = Si(l) + CO(g),
SiO2(g) + 2C(s) = Si(l) + 2CO(g),
SiO2(s) + 2C(s) + Fe(s, l) = FeSi(l) + 2CO(g),
Fe(l) + Si(l) = FeSi(l).
Titanium and phosphorus oxides can be reduced by the reactions:
TiO2(s) + 3C(c) = TiC(c) + 2CO(g),
P2O5(s) + 3CaO(c) = Ca3(PO4)2(s),
Ca3(PO4)2(s) + 5C(s) = 3CaO(s) + 2P(g) + 5CO(g),
Fe(l) + P(g) = Fe3P.
So, reduced silicon, titanium, and phosphorus can pass into metal according reactions, as shown in Equations (12)–(14) and (17).
Equilibrium calculations of complete iron reduction while considering the silicon dioxide, phosphorus, and titanium oxides reduction were performed by a Gibbs energy minimization method using the HSC Chemistry program. The normalized composition of the neutralized RM from Table 1 was used for calculations. The proportions of iron-containing phases calculated as a function of the carbon amount are shown in Figure 6. Calculation was performed for 100 kg of RM.
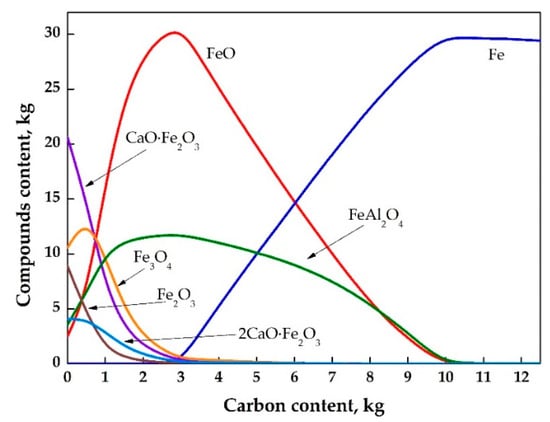
Figure 6.
The equilibrium compositions of iron-containing phases as a function of the added carbon in the system at 1600 °C.
Figure 6 shows that as the amount of carbon in the system increases from 3 to 10 kg (per 100 kg of RM), the content of pure iron increases from 0% to 100%, and the concentrations of the other iron-containing phases decreases to zero. With a lack of carbon in the system, the formation of wustite (FeO) and hercynite (FeAl2O4) occurs in the system. It can be seen that at least 10 kg of carbon per 100 kg of neutralized RM is necessary for complete recovery of iron compounds.
3.3. Reductive Smelting of the Neutralized Red Mud
A series of experiments were carried out at different temperatures in the range 1650–1750 °C to study the effect of the furnace temperature on metal extraction and the metal/slag separation process. Figure 7 shows the change in the iron content in the slag at different furnace temperatures. At 1650 °C, the slag phase contains ~8 wt % of iron, which is not separated into ingot (see Figure 8a). By increasing the temperature up to 1700 °C, the metal ingot is formed at the bottom of the crucible, but a part of the iron remains in the slag (Figure 8b). A further increase of temperature by 50 °C leads to an almost complete iron reduction and formation of large metal ingots on the bottom of the crucible (Figure 8c).
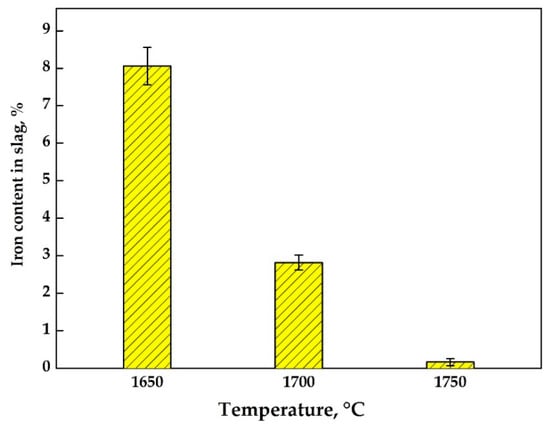
Figure 7.
The iron content (wt %) in slag after reductive smelting of RM at different temperatures.
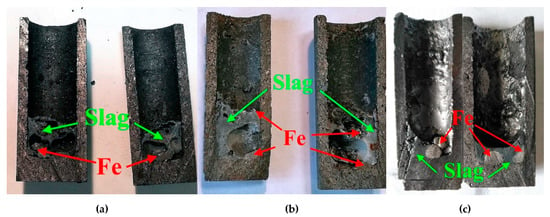
Figure 8.
Samples of metal and slag obtained after reduction smelting of RM at (a) 1650 °C, (b) 1700 °C, and (c) 1750 °C.
An increase of the temperature from 1650 to 1750 °C results in increasing the CaO and Al2O3 content in slag (Table 3). At the same time, the SiO2 content in slag decreases, due to silicon reduction by Equations (10)–(13). The TiO2 content in slag increases at temperatures of 1650 to 1700 °C, which indicates that the formed of titanium carbide remains in slag phase. At 1750 °C, TiO2 reduces to titanium carbide, which further dissolves in a metallic phase.

Table 3.
The slag chemical composition after reductive smelting of the RM (wt %).
Figure 9 represents the phases found in slag by XRD analysis. The main slag phase present at 1650 °C is gehlenite (Ca2Al2SiO7). It also contains minor amounts of larnite (Ca2SiO4), third and fifth calcium aluminates (Ca3Al2O6 and Ca5Al6O14), perovskite (Ca2TiO3), and hatrurite (Ca3SiO5). At 1700 °C, as the SiO2 content in slag decreases due to silicon reduction into the metal, and the slag composition shifts from the gehlenite primary phase field towards the larnite primary phase field, which leads to an increase of its content in the slag. Gehlenite and perovskite are not present in slag at 1750 °C, and the main slag phase is di-calcium silicate (γ-Ca2SiO4). This may be explained by the shifting of the slag composition to the larnite primary phase field [48].
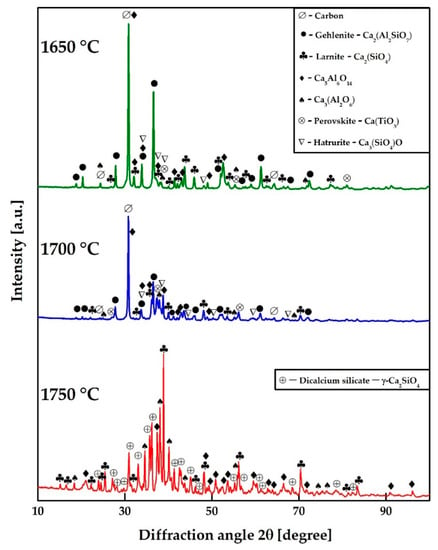
Figure 9.
XRD analysis of slag after reductive smelting of RM at 1650, 1700, and 1750 °C.
It can be concluded on the basis of XRD analysis that titanium carbide is likely formed as a result of the following reactions between perovskite and carbon:
CaO(s) + TiO2(s) = CaTiO3(s),
CaO·TiO2(s) + 3C(s) = CaO(s) + TiC(s) + 2CO(g).
Table 4 provides values of the Gibbs free energy calculated for Equation (18) at different temperatures. It can be seen that the TiC formation is likely to occur in slag at temperatures higher than 1600 °C.

Table 4.
Thermodynamic calculations for Equation (19).
To explain the behavior of titanium in the reduction smelting process of the RM, the slag and metal samples obtained at 1650 and 1750 °C were analyzed by SEM (Figure 10). Figure 10a indicates that titanium is distributed over the whole slag volume, but the majority is concentrated around the molten metal drops. In this case, titanium is not dissolved in the metallic phase and it may prevent the coagulation of metal drops into one large ingot. With the temperature increasing to 1750 °C, most of the titanium is present in the metal phase as carbide (Figure 10b,c).

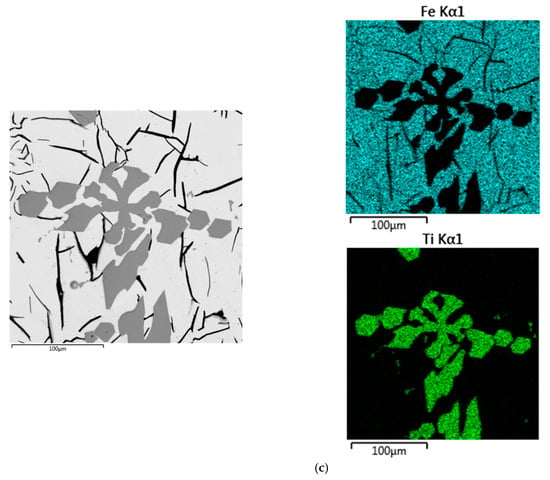
Figure 10.
SEM images of the slag and pig iron samples: reduction smelting at 1650 °C (a), reduction smelting at 1750 °C (b), titanium carbide in pig iron (c) (the mapping shows distribution of Fe and Ti).
3.4. Composition of Pig Iron
The chemical composition of the metallic phase obtained in reductive smelting of the RM at 1700 and 1750 °C are presented in Table 5. The chemical composition can be attributed to the pig iron having a higher content of titanium, phosphorus, and manganese, and a low sulfur content. It can be seen that the metallic phase obtained at 1700 °C contains much lower phosphorous and titanium as well as other elements.

Table 5.
Chemical composition of pig iron after reductive smelting of the RM at 1700 and 1750 °C (wt %).
Figure 11 represents the phase composition of pig iron obtained at 1750 °C. The XRD phase analysis showed that graphite is present in pig iron in both a free and bound form, and titanium occurs in the form of titanium carbide.
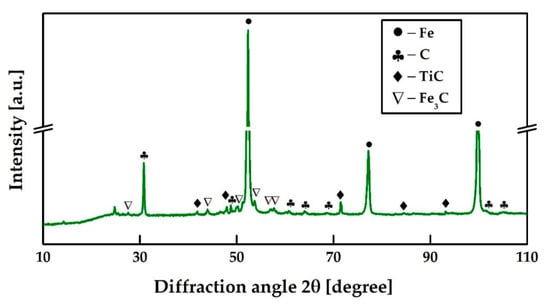
Figure 11.
XRD analysis of the pig iron after reductive smelting of the RM at 1750 °C.
The Mössbauer spectrum of a pig iron sample obtained at room temperature is a distorted broadened sextet (Figure 12). The asymmetric broadening of the resonance lines to the center of the spectrum is observed for one, two, and six lines, as three, four, and especially five lines are relatively symmetrical. One, two, and six sextet lines are clearly split into at least two, and on the inner side of the first resonance line, an additional shoulder is observed. The intensity of the internal lines is comparable with the intensity of the external lines of the sextet, and the intensities of lines two and five even exceed them. All this data indicates that the sample spectrum is a superposition of several subspectra of sextets describing the iron atoms in the alloy, surrounded by a different number of impurity atoms. The Mössbauer spectrum of the pig iron sample can be described by a superposition of seven narrow subspectra of sextets with related parameters, a sextet with a small splitting, but wide lines, and one low-intensity doublet (Table 6).
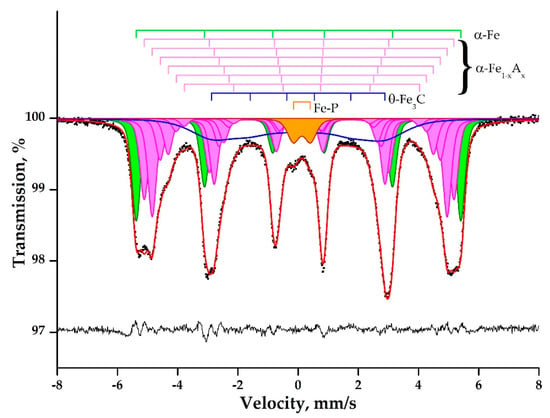
Figure 12.
Mössbauer spectra of pig iron sample obtained at 296 K.

Table 6.
Mössbauer spectra parameters of pig iron obtained at 296 K.
The internal sextet (#8, Table 6) includes a combination of subspectrum with very close hyperfine parameters for compounds of iron with carbon: θ-Fe3C [49,50], and/or phosphorus: (Fe1−xAx)2P, where A is a transition metal [51]. The doublet (#9, Table 6) is characterized by a small isomeric shift, for example, characteristic of iron compounds in a tetrahedral or pyramidal environment with phosphorus [52,53] or for complex solutions of the type (Fe1−xMnx)2P [54].
The parameters of the external narrow sextet (#1, Table 6) correspond to α-Fe [55], and were the starting parameters for calculating the parameters of the satellite subspectra. The following six satellite subspectra correspond to the iron atoms in the "alloy" lattice, α-Fe1-xAx, which are surrounded by one, two, or more atoms of a different nature. Their parameters are similar to those of subspectrum #1, with the only difference being that their chemical shift and effective magnetic field are successively different from the original ones by n*dδ and −n*dH, where n is the serial number of the satellite, and dF is the change in the Mössbauer parameter (see Table 6). Taking into account that the obtained step, dH, is approximately two times smaller than that usually observed for iron alloys with various metals and nonmetals, it can be assumed that the observed satellite subspectra describe the changes inmagnetic fields of iron atoms under the action of impurity atoms from at least two nearest coordination spheres [55,56].
3.5. Pig Iron Application
At the steel mill EVRAZ ZSMK (53.878267, 87.269946; Novokuznetsk, Russia), the steel-casting equipment (bottom pallets for through molds and molds for forging ingots) is made from pig iron. Titanium and vanadium alloying is normally used to increase the durability of the molds at the plant [57,58]. It has previously been shown [59] that the titanium carbide particles enhance wear resistance of the steel composite. The influence of the content of titanium and vanadium in pig iron on the tensile strength can be described by the equations:
where σ is the tensile strength of pig iron, kg/mm2; V, Ti is the element content in pig iron, %.
σ = −3.71 + 291.7 × V,
σ = −5.00 + 325.0 × Ti,
An increase in the strength properties of cast iron leads to a decrease in the erosion of molds by a falling stream of metal. The alloying process used in the EVRAZ ZSMK increases the heat resistance of molds by 7% to 12% [60]. However, a high content of vanadium and titanium in pig iron leads to low fluidity. In EVRAZ ZSMK, ferrophosphorus (P ~17 wt %) is used to increase the fluidity (in the ratio of 800 kg flux per 90-ton ladle). Industrial tests have shown that shrinkage effects and micropores are practically absent in castings doped with phosphorus due to better filling of the molds [61]. This effect was observed when the phosphorus content in pig iron was above 0.21 wt % [62].
The pig iron obtained in our study contains high amounts of Ti, V, and P (Table 5). The SEM analysis of the pig iron microstructure showed triple phosphide eutectic (Fe3P) in addition to titanium carbide (Figure 13).
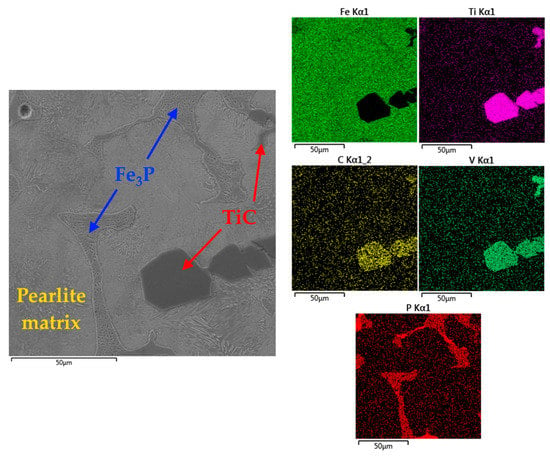
Figure 13.
SEM images of the pig iron sample obtained at 1750 °C (the mapping shows distribution of Fe, Ti, C, V, and P).
Analysis of the main phases of pig iron included titanium carbide, and phosphide eutectic and pearlite matrix was used in microindentation hardness testing (Figure 14). After measuring the diagonals of the indenter prints, the average size for each of the phases was determined. Using Equation (2), the value of the Vickers hardness was calculated (Table 7). TiC has an extremely high hardness index (5130HV50/10). Fe3P has a Vickers hardness almost three times higher (934HV50/10) compared to the pearlite matrix (336HV50/10). Thus, this pig iron has all the characteristics necessary to increase the mold lifecycle: High strength and good fluidity due to the presence of phosphide eutectic. Since, nowadays, expensive ligatures containing titanium, vanadium, and ferroalloys are used to produce pig iron with similar properties to EVRAZ ZSMK, the use of RM as a raw material will reduce the costs of the production of molds.
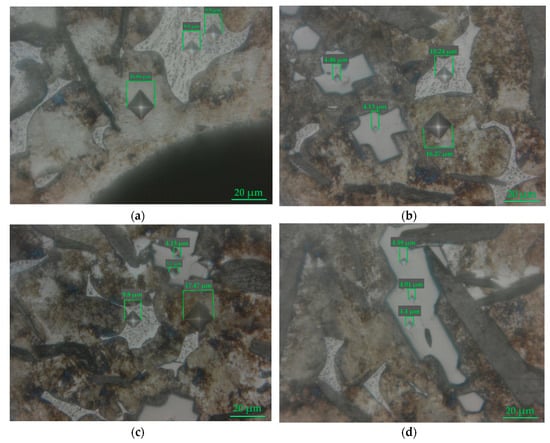
Figure 14.
Micrographs of microindentation hardness testing of the pig iron sample obtained (a–d) at 1750 °C (0.49 H, τ = 10 s).

Table 7.
Microindentation hardness data of pig iron phase.
4. Conclusions
It was experimentally proven that it is possible to obtain pig iron using reductive smelting of the neutralized RM at a temperature of 1750 °C. The metallic phase (pig iron) contains high amounts of titanium, phosphorus, and vanadium and a low amount of sulfur. The iron extraction optimum at 1750 °C was found to be more than 98%. The obtained pig iron can successfully be used at EVRAZ ZSMK as a raw material to produce molds with higher wear resistance.
The research will be continued to determine optimal parameters for better slag–metal separation. A perspective way is to adjust the slag composition by adding a flux containing Al2O3, which can be made economically feasible if aluminum dross is used. The second part of the research will be focused on hydrometallurgical processing of slag to extract alumina, wollastonite, and titanium concentrate.
Author Contributions
Mössbauer spectroscopy, D.P.; Funding acquisition, D.V.; Investigation D.V., D.Z. and D.P.; Methodology, D.Z. and D.P.; Project administration, D.V.; Resources, D.V. and D.Z.; Software, D.Z. and A.K.; Visualization, D.V. and D.P.; Writing—original draft, D.V., D.Z., A.K., D.L. and D.P.; Writing—review and editing, D.V., D.Z., A.K. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was funded by Program of Basic research of the Presidium of Russian Academy of Science №39 «Fundamentals and energy-efficient, resource-saving, innovative technologies of mineral processing, utilization of industrial and household waste» (Grant No. AAA-A18-118031690039-9). Alex Kondratiev is financially supported by the Ministry of Education and Science of the Russian Federation in the framework of the basic part of the state program ‘Organisation of the Research Work’ for higher educational institutions in 2017–2019 no. 11.7971.2017/6.7.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, S.; Ma, S.; Zhang, Y. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J. Hazard. Mater. 2011, 189, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A. The Comprehensive Utilisation of Red Mud Utilisation in Blast Furnace. In Recovery and Utilization of Metallurgical Solid Waste; Zhang, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-102-1. [Google Scholar]
- Tabereaux, A.T. Hungarian Red Mud Disaster: Addressing Environmental Liabilities of Alumina Residue Storage & Disposal. Light Met. Age 2010, 68, 22–24. [Google Scholar]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Vind, J.; Alexandri, A.; Vassiliadou, V.; Panias, D.; Vind, J.; Alexandri, A.; Vassiliadou, V.; Panias, D. Distribution of Selected Trace Elements in the Bayer Process. Metals 2018, 8, 327. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.; Tkaczyk, A.; Vassiliadou, V.; Panias, D.; Vind, J.; Malfliet, A.; Blanpain, B.; et al. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Meshram, P. Overview on Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Kaußen, F.; Friedrich, B. Reductive Smelting of Red Mud for Iron Recovery. Chem. Ing. Tech. 2015, 87, 1535–1542. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Kaußen, F.M.; Friedrich, B. Phase characterization and thermochemical simulation of (landfilled) bauxite residue (“red mud”) in different alkaline processes optimized for aluminum recovery. Hydrometallurgy 2018, 176, 49–61. [Google Scholar] [CrossRef]
- Anisonyan, K.G.; Kopyev, D.Y.; Goncharov, K.V.; Sadykhov, G.B. An investigation of a single-stage red mud reducing roasting process with the cast iron and aluminate slag production. Non-Ferr. Met. 2018, 44, 18–23. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M.; Ning, G.; Zhang, B.; Liu, C.; Li, S.; et al. Large-Scale Consumption and Zero-Waste Recycling Method of Red Mud in Steel Making Process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Luo, M.; Qi, X.; Zhang, Y.; Ren, Y.; Tong, J.; Chen, Z.; Hou, Y.; Yeerkebai, N.; Wang, H.; Feng, S.; et al. Study on dealkalization and settling performance of red mud. Environ. Sci. Pollut. Res. 2017, 24, 1794–1802. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Lyu, G.; Guo, F.; Zhang, W.; Zhang, Y. Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. J. Clean. Prod. 2018, 188, 456–465. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Kurunov, I. Modern Blast Furnace Ironmaking: An Introduction, 3rd ed.; IOS Press: Amsterdam, The Netherlands, 2015; ISBN 9781614994992. [Google Scholar]
- Deng, B.; Li, G.; Luo, J.; Ye, Q.; Liu, M.; Peng, Z.; Jiang, T. Enrichment of Sc2O3 and TiO2 from bauxite ore residues. J. Hazard. Mater. 2017, 331, 71–80. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Yuan, M.; Qiao, X.; Yu, J. Phase equilibria of AlCl3 + FeCl3 + H2O, AlCl3 + CaCl2 + H2O, and FeCl3 + CaCl2 + H2O at 298.15 K. J. Chem. Eng. Data 2016, 61, 1749–1755. [Google Scholar] [CrossRef]
- Wang, J.; Petit, C.; Zhang, X.; Cui, S. Phase Equilibrium Study of the AlCl3-CaCl2-H2O System for the Production of Aluminum Chloride Hexahydrate from Ca-Rich Flue Ash. J. Chem. Eng. Data 2016, 61, 359–369. [Google Scholar] [CrossRef]
- Cui, L.; Cheng, F.; Zhou, J. Preparation of high purity AlCl3·6H2O crystals from coal mining waste based on iron (III) removal using undiluted ionic liquids. Sep. Purif. Technol. 2016, 167, 45–54. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.; Cui, H.; Cheng, F.; Yang, F. Crystallization behavior of AlCl3·6H2O in hydrochloric system. Huagong Xuebao/CIESC J. 2014, 65, 3960–3967. [Google Scholar] [CrossRef]
- Bonomi, C.; Alexandri, A.; Vind, J.; Panagiotopoulou, A.; Tsakiridis, P.; Panias, D.; Bonomi, C.; Alexandri, A.; Vind, J.; Panagiotopoulou, A.; et al. Scandium and Titanium Recovery from Bauxite Residue by Direct Leaching with a Brønsted Acidic Ionic Liquid. Metals 2018, 8, 834. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Dittrich, C.; Friedrich, B. Effect of aqueous media on the recovery of scandium by selective precipitation. Metals 2018, 8, 314. [Google Scholar] [CrossRef]
- Onghena, B.; Borra, C.R.; Van Gerven, T.; Binnemans, K. Recovery of scandium from sulfation-roasted leachates of bauxite residue by solvent extraction with the ionic liquid betainium bis (trifluoromethylsulfonyl) imide. Sep. Purif. Technol. 2017, 176, 208–219. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Tang, S.; Zeng, M.; Bai, P.; Chen, L. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere 2017, 175, 365–372. [Google Scholar] [CrossRef]
- Alkan, G.; Schier, C.; Gronen, L.; Stopic, S.; Friedrich, B. A Mineralogical Assessment on Residues after Acidic Leaching of Bauxite Residue (Red Mud) for Titanium Recovery. Metals 2017, 7, 458. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. Kinetics of titanium leaching with citric acid in sulfuric acid from red mud. Trans. Nonferr. Met. Soc. China 2015, 25, 3139–3145. [Google Scholar] [CrossRef]
- Shiryaeva, E.V.; Podgorodetskiy, G.S.; Malyscheva, T.Y.; Gorbunov, V.B.; Zavodyaniy, A.V.; Schapovalov, A.N. Influence of low alkaline red mud on the properties and microstructure of the agglomerates from the charge materials JSC “Ural steel. ” Izv. Vyss. Uchebnykh Zaved. Chernaya Metall. 2014, 57, 14–19. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Liu, Y.; Zhang, T. Approaches to improve alumina extraction based on the phase transformation mechanism of recovering alkali and extracting alumina by the calcification-carbonization method. Hydrometallurgy 2019, 189. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Zhang, T.-A.; Wang, Y.-X.; Lü, G.-Z.; Zhang, W.-G. Recovery of alkali and alumina from Bayer red mud by the calcification–carbonation method. Int. J. Miner. Metall. Mater. 2016, 23, 257–268. [Google Scholar] [CrossRef]
- Bale, C.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Ben Mahfoud, R.; Melancon, J.; Pelton, A.D.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Roine, A.; Kotiranta, T. Development of sustainable processes with new HSC Chemistry 6.0 software. In Proceedings of the European Metallurgical Conference, EMC 2007, Dusseldorf, Germany, 11–14 June 2007; Volume 4, pp. 1877–1888. [Google Scholar]
- Zinoveev, D.; Grudinsky, P.; Korneev, V.; Dyubanov, V.; Zheleznyi, M. Recycling red mud of JSC ural aluminum plant with the recovery of iron and construction materials. Key Eng. Mater. 2017, 743, 331–337. [Google Scholar] [CrossRef]
- Pankratov, D.A. Mössbauer study of oxo derivatives of iron in the Fe2O3-Na2O2 system. Inorg. Mater. 2014, 50, 82–89. [Google Scholar] [CrossRef]
- Raspopov, N.A.; Korneev, V.P.; Averin, V.V.; Lainer, Y.A.; Zinoveev, D.V.; Dyubanov, V.G. Reduction of iron oxides during the pyrometallurgical processing of red mud. Russ. Metall. 2013, 2013, 33–37. [Google Scholar] [CrossRef]
- Karimi, E.; Teixeira, I.F.; Ribeiro, L.P.; Gomez, A.; Lago, R.M.; Penner, G.; Kycia, S.W.; Schlaf, M. Ketonization and deoxygenation of alkanoic acids and conversion of levulinic acid to hydrocarbons using a Red Mud bauxite mining waste as the catalyst. Catal. Today 2012, 190, 73–88. [Google Scholar] [CrossRef]
- Seitz, G.; Penin, N.; Decoux, L.; Wattiaux, A.; Duttine, M.; Gaudon, M. Near the Ferric Pseudobrookite Composition (Fe2TiO5). Inorg. Chem. 2016, 55, 2499–2507. [Google Scholar] [CrossRef]
- Muranaka, S.; Shinjo, T.; Bando, Y.; Takada, T. Mossbauer Study of Fe2TiO5 and FeTi2O5. J. Phys. Soc. Jpn. 1971, 30, 890. [Google Scholar] [CrossRef]
- Onodera, H.; Fujita, A.; Yamamoto, H.; Sagawa, M.; Hirosawa, S. Mössbauer study of the intermetallic compound Nd2Fe14B. I. interpretation of complex spectrum. J. Magn. Magn. Mater. 1987, 68, 6–14. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Smirnov, V.V.; Tsodikov, M.V.; Bukhtenko, O.V.; Maksimov, Y.V.; Kiseleva, O.I.; Pankratov, D.A. Catalytic conversions of chloroolefins over iron oxide nanoparticles: 1. Isomerization of dichlorobutenes in the presence of iron oxide nanopaticles immobilized on silicas with different structures. Russ. Chem. Bull. 2005, 54, 1418–1424. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Yurev, A.I. Mössbauer diagnostics of the isomorphic substitution of iron for aluminum in triclinic iron vanadate. Bull. Russ. Acad. Sci. Phys. 2013, 77, 759–764. [Google Scholar] [CrossRef]
- Jónás, K.; Solymár, K.; Zöldi, J. Some applications of mössbauer spectroscopy for the quantitative analysis of minerals and mineral mixtures. J. Mol. Struct. 1980, 60, 449–452. [Google Scholar] [CrossRef]
- Morin, F.J. Magnetic susceptibility of α-Fe2O3 and α-Fe2O3 with added titanium. Phys. Rev. 1950, 78, 819–820. [Google Scholar] [CrossRef]
- Van San, E.; De Grave, E.; Vandenberghe, R.E.; Desseyn, H.O.; Datas, L.; Barrón, V.; Rousset, A. Study of Al-substituted hematites, prepared from thermal treatment of lepidocrocite. Phys. Chem. Miner. 2001, 28, 488–497. [Google Scholar] [CrossRef]
- Azof, F.I.; Kolbeinsen, L.; Safarian, J. Characteristics of Calcium-Aluminate Slags and Pig Iron Produced from Smelting-Reduction of Low-Grade Bauxites. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2018, 49, 2400–2420. [Google Scholar] [CrossRef]
- Sajitha, E.P.; Prasad, V.; Subramanyam, S.V.; Mishra, A.K.; Sarkar, S.; Bansal, C. Size-dependent magnetic properties of iron carbide nanoparticles embedded in a carbon matrix. J. Phys. Condens. Matter 2007, 19. [Google Scholar] [CrossRef]
- Yurkov, G.Y.; Shashkeev, K.A.; Kondrashov, S.V.; Popkov, O.V.; Shcherbakova, G.I.; Zhigalov, D.V.; Pankratov, D.A.; Ovchenkov, E.A.; Koksharov, Y.A. Synthesis and magnetic properties of cobalt ferrite nanoparticles in polycarbosilane ceramic matrix. J. Alloys Compd. 2016, 686, 421–430. [Google Scholar] [CrossRef]
- Fruchart, R.; Roger, A.; Senateur, J.P. Crystallographic and magnetic properties of solid solutions of the phosphides M2P, M = Cr, Mn, Fe, Co, and Ni. J. Appl. Phys. 1969, 40, 1250–1257. [Google Scholar] [CrossRef]
- Bailey, R.E.; Duncan, J.F. Moessbauer And Nuclear Magnetic Resonance Studies of Several Iron Phosphides. Inorg. Chem. 1967, 6, 1444–1447. [Google Scholar] [CrossRef]
- Presniakov, I.A.; Sobolev, A.V.; Chernyavskii, I.O.; Pankratov, D.A.; Morozov, I.V. Anisotropic magnetic hyperfine interactions in phosphide FeP. Bull. Russ. Acad. Sci. Phys. 2015, 79, 984–989. [Google Scholar] [CrossRef]
- Alleg, S.; Brahimi, A.; Azzaza, S.; Souilah, S.; Zergoug, M.; Suňol, J.J.; Greneche, J.M. X-ray diffraction, Mössbauer spectrometry and thermal studies of the mechanically alloyed (Fe1−xMnx)2P powders. Adv. Powder Technol. 2018, 29, 257–265. [Google Scholar] [CrossRef]
- Fionov, A.S.; Yurkov, G.Y.; Kolesov, V.V.; Pankratov, D.A.; Ovchenkov, E.A.; Koksharov, Y.A. Composite material based on iron containing nanoparticles for applications in the problems of electromagnetic compatibility. J. Commun. Technol. Electron. 2012, 57, 543–552. [Google Scholar] [CrossRef]
- Sauer, W.E.; Reynik, R.J. Electronic and Magnetic Structure of Dilute Iron-Base Alloys. J. Appl. Phys. 1971, 42, 1604–1605. [Google Scholar] [CrossRef]
- Lubyanoi, D.A.; Drobyshev, A.N.; Samsonov, Y.A.; Kaminskaya, I.A. Higher Efficiency of Manufacturing of Steel Casting Equipment from Pig Iron. Steel Transl. 1994, 6, 40–41. [Google Scholar]
- Andreev, V.V.; Lubyanoi, D.A.; Samsonov, Y.N.; Kaminskaya, I.A.; Lubyanaya, S.V. Development of Extra-Furnace Treatment Technology for Blast-Furnace Iron in Order to Manufacture Replacement Metallurgical Equipment with Improved Operating Life. Metallurgist 2014, 58, 492–495. [Google Scholar] [CrossRef]
- Berns, H.; Wewers, B. Development of an abrasion resistant steel composite with in situ TiC particles. Wear 2001, 251, 1386–1395. [Google Scholar] [CrossRef]
- Lubyanoi, D.A.; Sofroshenkov, A.F.; Sinyavskii, I.A.; Makarov, E.S.; Gorkavenko, V.V. Technology of neutralizing the harmful effect of phosphorus in iron of heat-resistant castings. Steel Transl. 1999, 29, 86–90. [Google Scholar]
- Lubyanoj, D.A.; Sinyavskij, I.A.; Selyanin, I.F.; Sofroshenkov, A.F.; Morozova, O.V. Influence of conditions of ladle treatment on structure, casting and mechanical properties of phosphorus-containing cast iron produced in blast furnaces. Izv. Ferr. Metall. 2001, 6, 53–55. [Google Scholar]
- Lubyanoi, D.A.; Gorkavenko, V.V.; Makarov, É.S.; Kaminskaya, I.A.; Frolov, A.G.; Yakovenko, N.A. Phosphorous cast iron for heat-resistant castings. Met. Sci. Heat Treat. 2002, 44, 452–453. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).