Repeated Menthol Mouth Swilling Affects Neither Strength nor Power Performance
Abstract
1. Introduction
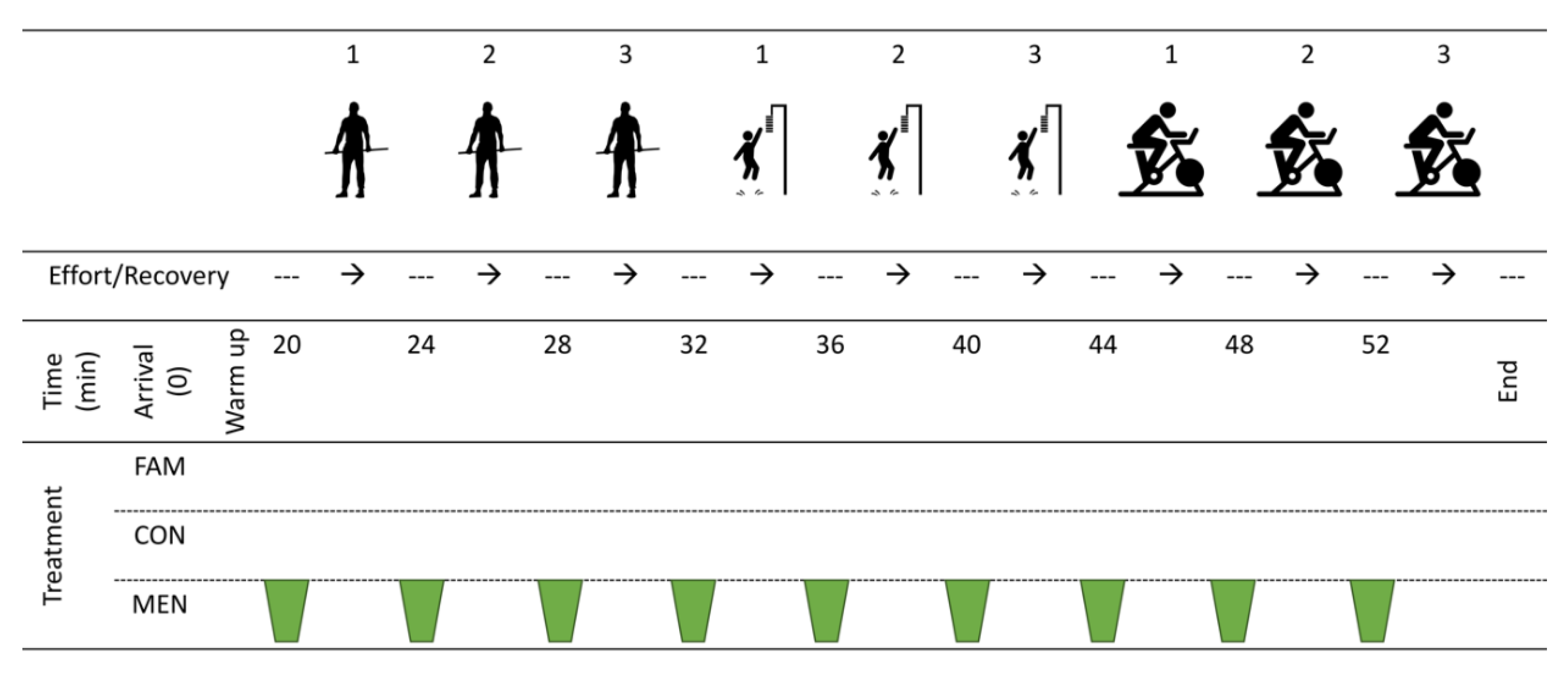
2. Materials and Methods
2.1. Participants
2.2. Menthol Solution and Swilling
2.3. Isometric Mid-Thigh Pull Protocol
2.4. Vertical Jump Protocol
2.5. Repeated Six Second Peak Power Protocol
2.6. Statistical Analyses
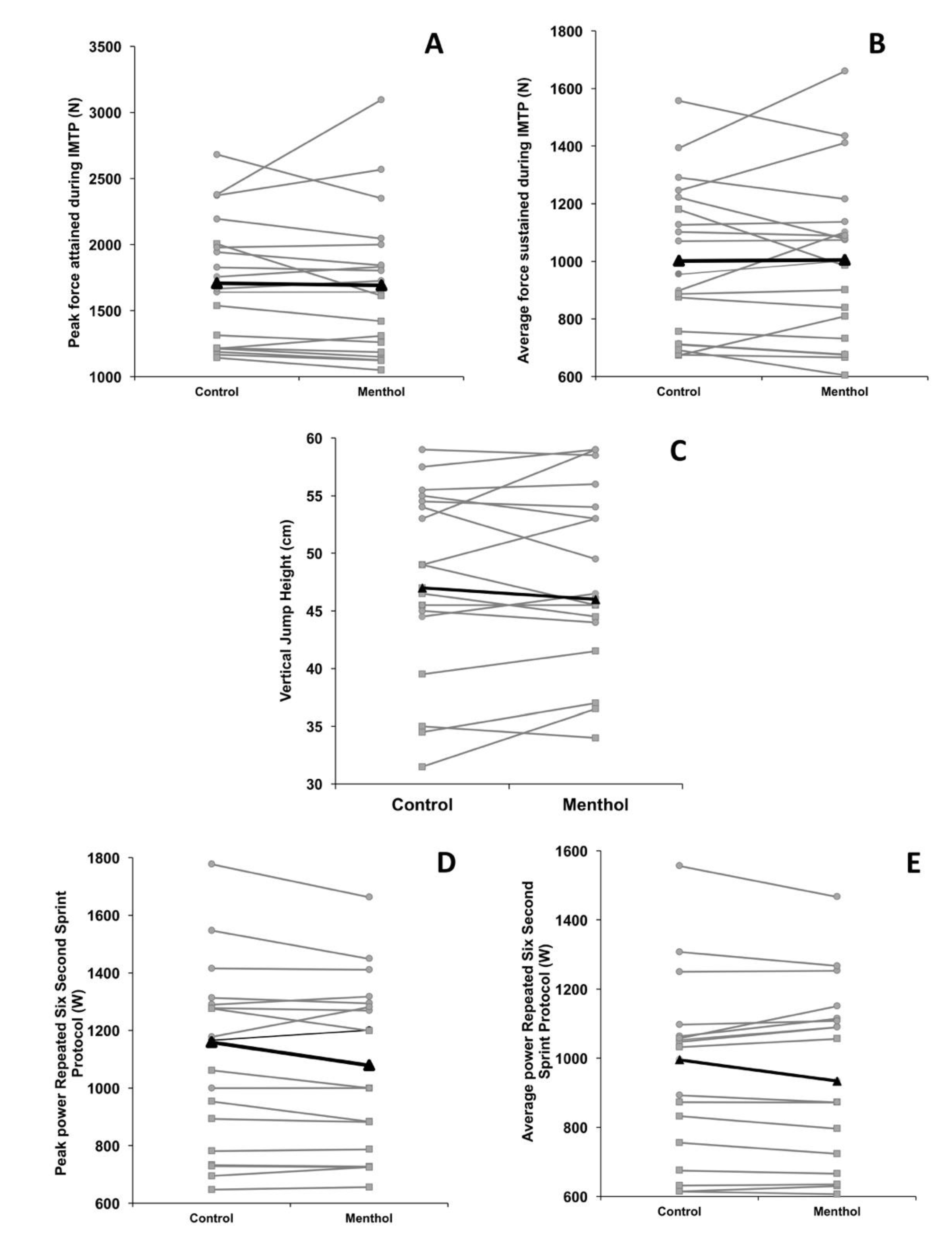
3. Results
3.1. Familiarisation and Experimental Trials
3.2. Experimental Trials
3.3. Differences between and within Participant Sexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Best, R.; Spears, I.R.; Hurst, P.; Berger, N.J.A. The development of a menthol solution for use during sport and exercise. Beverages 2018, 4, 44. [Google Scholar] [CrossRef]
- Meamarbashi, A.; Rajabi, A. The effects of peppermint on exercise performance. J. Int. Soc. Sports Nutr. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, K.; Peart, D.J. Aerobic capacity is not improved following 10-day supplementation with peppermint essential oil. Appl. Physiol. Nutr. Metab. 2017, 42, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Boden, C. Effects of chewing menthol gum on the alertness of healthy volunteers and those with an upper respiratory tract illness. Stress Health 2012, 29, 138–142. [Google Scholar] [CrossRef]
- Mahachandra, M.; Garnaby, E.D. The Effectiveness of in-vehicle peppermint fragrance to maintain car driver’s alertness. Procedia Manuf. 2015, 4, 471–477. [Google Scholar] [CrossRef]
- Stevens, C.J.; Best, R. Menthol: A fresh ergogenic aid for athletic performance. Sports Med. 2016, 47, 1035–1042. [Google Scholar] [CrossRef]
- Jeffries, O.; Waldron, M. The effects of menthol on exercise performance and thermal sensation: A meta-analysis. J. Sci. Med. Sport 2019, 22, 707–715. [Google Scholar] [CrossRef]
- Gibson, O.; Wrightson, J.G.; Hayes, M. Intermittent sprint performance in the heat is not altered by augmenting thermal perception via L-menthol or capsaicin mouth rinses. Eur. J. Appl. Physiol. 2018, 119, 653–664. [Google Scholar] [CrossRef]
- Pritchard, H.J.; Stannard, S.; Barnes, M.J. Ammonia inhalant and stimulant use among powerlifters: Results from an international survey. J. Aust. Strength Cond. 2014, 22, 52–54. [Google Scholar]
- Bartolomei, S.; Nigro, F.; Gubellini, L.; Semprini, G.; Ciacci, S.; Hoffman, J.R.; Merni, F.; Luca, G.; Gabriele, S. Acute effects of ammonia inhalants on strength and power performance in trained men. J. Strength Cond. Res. 2018, 32, 244–247. [Google Scholar] [CrossRef]
- Vigil, J.N.; Sabatini, P.L.; Hill, L.C.; Swain, D.P.; Branch, J.D. Ammonia inhalation does not increase deadlift 1-repetition maximum in college-aged male and female weight lifters. J. Strength Cond. Res. 2018, 32, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, M.C.; Cholewa, J.; Freire, R.V.; Carmo, B.A.; Bottan, J.; Bratfich, M.; Della Bandeira, M.P.; Goncalves, D.; Caperuto, E.; De Lira, F.S.; et al. Acute capsaicin supplementation improves resistance training performance in trained men. J. Strength Cond. Res. 2018, 32, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.R.; Hems, R.; Rowsell, D.G.; Spring, D.J. New compounds with the menthol cooling effect. J. Soc. Cosmet. Chem. 1978, 29, 185–200. [Google Scholar]
- Mahieu, F.; Owsianik, G.; Verbert, L.; Janssens, A.; De Smedt, H.; Nilius, B.; Voets, T. TRPM8-independent Menthol-induced Ca2+ Release from Endoplasmic Reticulum and Golgi. J. Boil. Chem. 2006, 282, 3325–3336. [Google Scholar] [CrossRef]
- Meamarbashi, A. Instant effects of peppermint essential oil on the physiological parameters and exercise performance. Avicenna J. Phytomed. 2014, 4, 72–78. [Google Scholar]
- Perry, B.G.; Pritchard, H.; Barnes, M.J. Cerebrovascular, cardiovascular and strength responses to acute ammonia inhalation. Eur. J. Appl. Physiol. 2015, 116, 583–592. [Google Scholar] [CrossRef]
- De Freitas, M.C.; Cholewa, J.; Gobbo, L.A.; De Oliveira, J.V.; De Lira, F.S.; Rossi, F.E.; De Oliveira, J.V.N.S. Acute capsaicin supplementation improves 1500-m running time-trial performance and rate of perceived exertion in physically active adults. J. Strength Cond. Res. 2018, 32, 572–577. [Google Scholar] [CrossRef]
- Lotteau, S.; Ducreux, S.; Romestaing, C.; Legrand, C.; Coppenolle, F. Characterization of functional TRPV1 channels in the sarcoplasmic reticulum of mouse skeletal muscle. PLoS ONE 2013, 8, e58673. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.; Lamont, H.S.; Moir, G.L. Understanding vertical jump potentiation: A deterministic model. Sports Med. 2015, 46, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.B.; Haff, G.G. Factors modulating post-activation potentiation of jump, sprint, throw, and upper-body ballistic performances: A systematic review with meta-analysis. Sports Med. 2015, 46, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Beaven, C.M.; Maulder, P.; Pooley, A.; Kilduff, L.; Cook, C. Effects of caffeine and carbohydrate mouth rinses on repeated sprint performance. Appl. Physiol. Nutr. Metab. 2013, 38, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Spanidis, Y.; Stagos, D.; Papanikolaou, C.; Karatza, K.; Theodosi, A.; Veskoukis, A.S.; Deli, C.K.; Poulios, A.; Koulocheri, S.D.; Jamurtas, A.Z.; et al. Resistance-trained individuals are less susceptible to oxidative damage after eccentric exercise. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. Role of cold receptors and menthol in thirst, the drive to breathe and arousal. Appetite 2000, 34, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Standing, R.; Best, R. Strength and reaction time capabilities of New Zealand polo players and their association with polo playing handicap. J. Funct. Morphol. Kinesiol. 2019, 4, 48. [Google Scholar] [CrossRef]
- De Witt, J.K.; English, K.L.; Crowell, J.B.; Kalogera, K.L.; Guilliams, M.E.; Nieschwitz, B.E.; Hanson, A.M.; Ploutz-Snyder, L.L. Isometric midthigh pull reliability and relationship to deadlift one repetition maximum. J. Strength Cond. Res. 2018, 32, 528–533. [Google Scholar] [CrossRef]
- James, L.P.; Roberts, L.A.; Haff, G.G.; Kelly, V.G.; Beckman, E. Validity and reliability of a portable isometric mid-thigh clean pull. J. Strength Cond. Res. 2017, 31, 1378–1386. [Google Scholar] [CrossRef]
- Nuzzo, J.L.; Anning, J.H.; Scharfenberg, J.M. The reliability of three devices used for measuring vertical jump height. J. Strength Cond. Res. 2011, 25, 2580–2590. [Google Scholar] [CrossRef]
- Gonzalez-Tablas, A.; Martin-Santana, E.; Torres, M. Designing a cost-effective power profile test for talent identification programs. J. Sci. Cycl. 2016, 5, 27–28. [Google Scholar]
- Herbert, P.; Sculthorpe, N.; Baker, J.S.; Grace, F.; Sculthorpe, N. Validation of a six second cycle test for the determination of peak power output. Res. Sports Med. 2015, 23, 115–125. [Google Scholar] [CrossRef]
- Standing, R.J.; Maulder, P.S. The biomechanics of standing start and initial acceleration: Reliability of the key determining kinematics. J. Sports Sci. Med. 2017, 16, 154–162. [Google Scholar]
- Bradshaw, E.; Maulder, P.S.; Keogh, J.W.L. Biological movement variability during the sprint start: Performance enhancement or hindrance? Sports Biomech. 2007, 6, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Best, R.; Standing, R. All things being equal: Spatiotemporal differences between Open and Women’s 16-goal Polo. Int. J. Perform. Anal. Sport 2019, 19, 919–929. [Google Scholar] [CrossRef]
- Peat, J.; Barton, B. Medical Statistics: A Guide to Data Analysis and Critical Appraisal; John Wiley & Sons: Malden, MI, USA, 2008; pp. 28–43. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Batterham, A.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Boil. 2015, 13, e1002128. [Google Scholar] [CrossRef]
- Gelal, A.; Jacob, P.; Yu, L.; Benowitz, N.L. Disposition kinetics and effects of menthol. Clin. Pharmacol. Ther. 1999, 66, 128–135. [Google Scholar] [CrossRef]
- Best, R.; McDonald, K.; Hurst, P.; Pickering, C. Can taste be ergogenic? Eur. J. Nutr. 2020, 1–10. [Google Scholar] [CrossRef]
- Stevens, C.J.; Thoseby, B.; Sculley, D.V.; Callister, R.; Taylor, L.; Dascombe, B.J. Running performance and thermal sensation in the heat are improved with menthol mouth rinse but not ice slurry ingestion. Scand. J. Med. Sci. Sports 2015, 26, 1209–1216. [Google Scholar] [CrossRef]
- Haeseler, G.; Maue, D.; Grosskreutz, J.; Bufler, J.; Nentwig, B.; Piepenbrock, S.; Dengler, R.; Leuwer, M. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur. J. Anaesthesiol. 2002, 19, 571–579. [Google Scholar] [CrossRef]
- Topp, R.; Ledford, E.R.; Jacks, D.E. Topical menthol, ice, peripheral blood flow, and perceived discomfort. J. Athl. Train. 2013, 48, 220–225. [Google Scholar] [CrossRef]
- Topp, R.; Winchester, L.J.; Schilero, J.; Jacks, D. Effect of topical menthol on ipsilateral and contralateral superficial blood flow following a bout of maximum voluntary muscle contraction. Int. J. Sports Phys. Ther. 2011, 6, 83–91. [Google Scholar] [PubMed]
- Gillis, D.J.; Vellante, A.; Gallo, J.A.; D’amico, A.P. Influence of menthol on recovery from exercise-induced muscle damage. J. Strength Cond. Res. 2020, 34, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Opheim, M.N.; Rankin, J.W. Effect of capsaicin supplementation on repeated sprinting performance. J. Strength Cond. Res. 2012, 26, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Cliff, M.A.; Green, B.G. Sensitization and desensitization to capsaicin and menthol in the oral cavity: Interactions and individual differences. Physiol. Behav. 1996, 59, 487–494. [Google Scholar] [CrossRef]
- Labbe, D.; Martin, N.; Le Coutre, J.; Hudry, J. Impact of refreshing perception on mood, cognitive performance and brain oscillations: An exploratory study. Food Qual. Prefer. 2011, 22, 92–100. [Google Scholar] [CrossRef]
- Jeffries, O.; Goldsmith, M.; Waldron, M. L-menthol mouth rinse or ice slurry ingestion during the latter stages of exercise in the heat provide a novel stimulus to enhance performance despite elevation in mean body temperature. Eur. J. Appl. Physiol. 2018, 118, 2435–2442. [Google Scholar] [CrossRef]
- Benedetti, F. Placebo effects: From the neurobiological paradigm to translational implications. Neuron 2014, 84, 623–637. [Google Scholar] [CrossRef]
- Beedie, C.; Foad, A.; Hurst, P. Capitalizing on the placebo component of treatments. Curr. Sports Med. Rep. 2015, 14, 284–287. [Google Scholar] [CrossRef]
- Saint-Eve, A.; Déléris, I.; Feron, G.; Ibarra, D.; Guichard, E.; Souchon, I. How trigeminal, taste and aroma perceptions are affected in mint-flavored carbonated beverages. Food Qual. Prefer. 2010, 21, 1026–1033. [Google Scholar] [CrossRef]
- Burrows, M.; Peters, C.E. The influence of oral contraceptives on athletic performance in female athletes. Sports Med. 2007, 37, 557–574. [Google Scholar] [CrossRef]
- Redman, L.M.; Weatherby, R.P. Measuring performance during the menstrual cycle: A model using oral contraceptives. Med. Sci. Sports Exerc. 2004, 36, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Giacomoni, M.; Bernard, T.; Gavarry, O.; Altare, S.; Falgairette, G. Influence of the menstrual cycle phase and menstrual symptoms on maximal anaerobic performance. Med. Sci. Sports Exerc. 2000, 32, 486. [Google Scholar] [CrossRef] [PubMed]
- Tsampoukos, A.; Peckham, E.A.; James, R.; Nevill, M.E. Effect of menstrual cycle phase on sprinting performance. Eur. J. Appl. Physiol. 2010, 109, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Masterson, G. The impact of menstrual phases on anaerobic power performance in collegiate women. JSCR 1999, 13, 325–329. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Best, R.; Temm, D.; Hucker, H.; McDonald, K. Repeated Menthol Mouth Swilling Affects Neither Strength nor Power Performance. Sports 2020, 8, 90. https://doi.org/10.3390/sports8060090
Best R, Temm D, Hucker H, McDonald K. Repeated Menthol Mouth Swilling Affects Neither Strength nor Power Performance. Sports. 2020; 8(6):90. https://doi.org/10.3390/sports8060090
Chicago/Turabian StyleBest, Russ, Dani Temm, Holly Hucker, and Kerin McDonald. 2020. "Repeated Menthol Mouth Swilling Affects Neither Strength nor Power Performance" Sports 8, no. 6: 90. https://doi.org/10.3390/sports8060090
APA StyleBest, R., Temm, D., Hucker, H., & McDonald, K. (2020). Repeated Menthol Mouth Swilling Affects Neither Strength nor Power Performance. Sports, 8(6), 90. https://doi.org/10.3390/sports8060090





