Changes in Pain and Nutritional Intake Modulate Ultra-Running Performance: A Case Report
Abstract
1. Introduction
2. Materials and Methods
3. Results
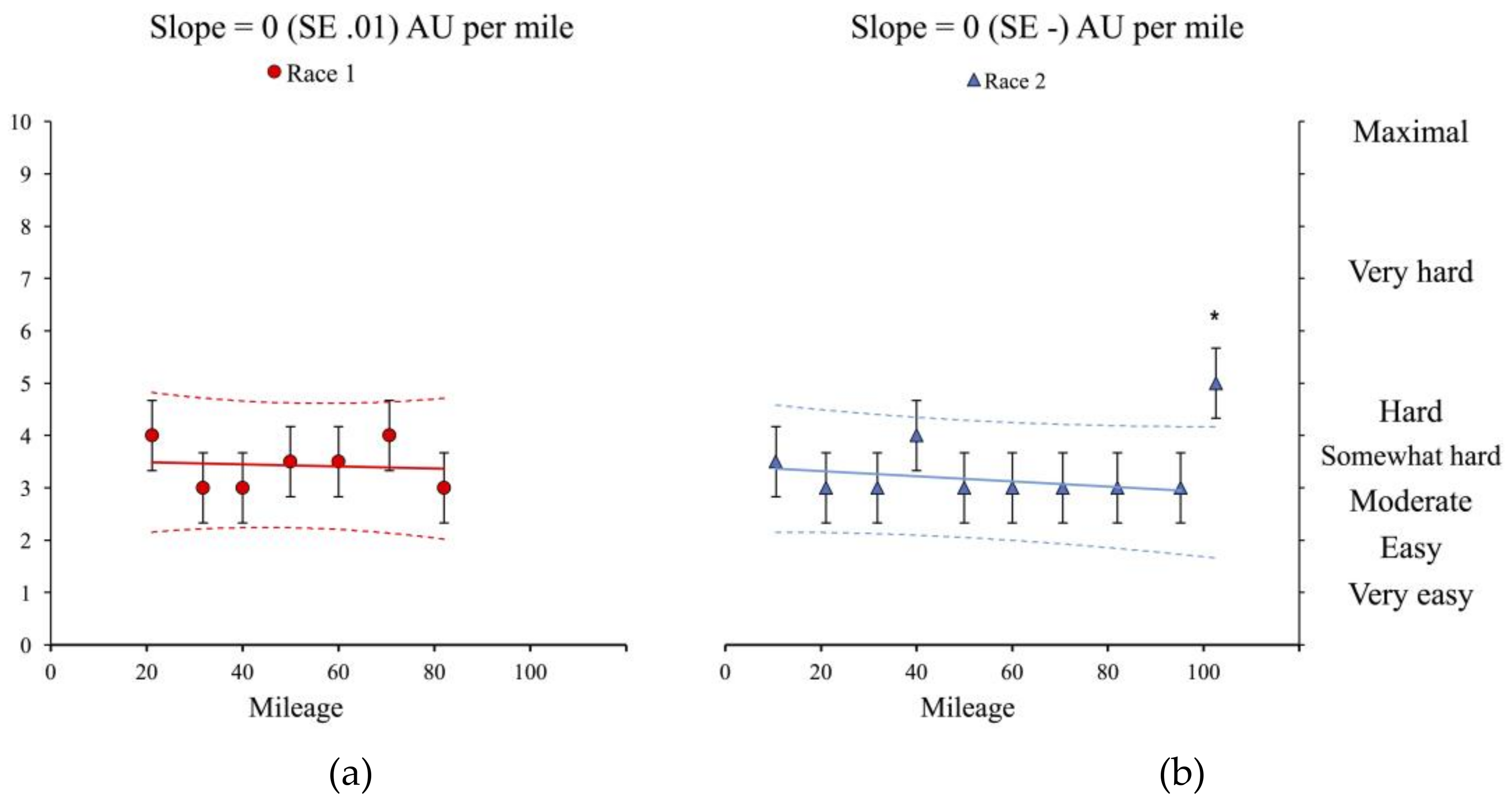
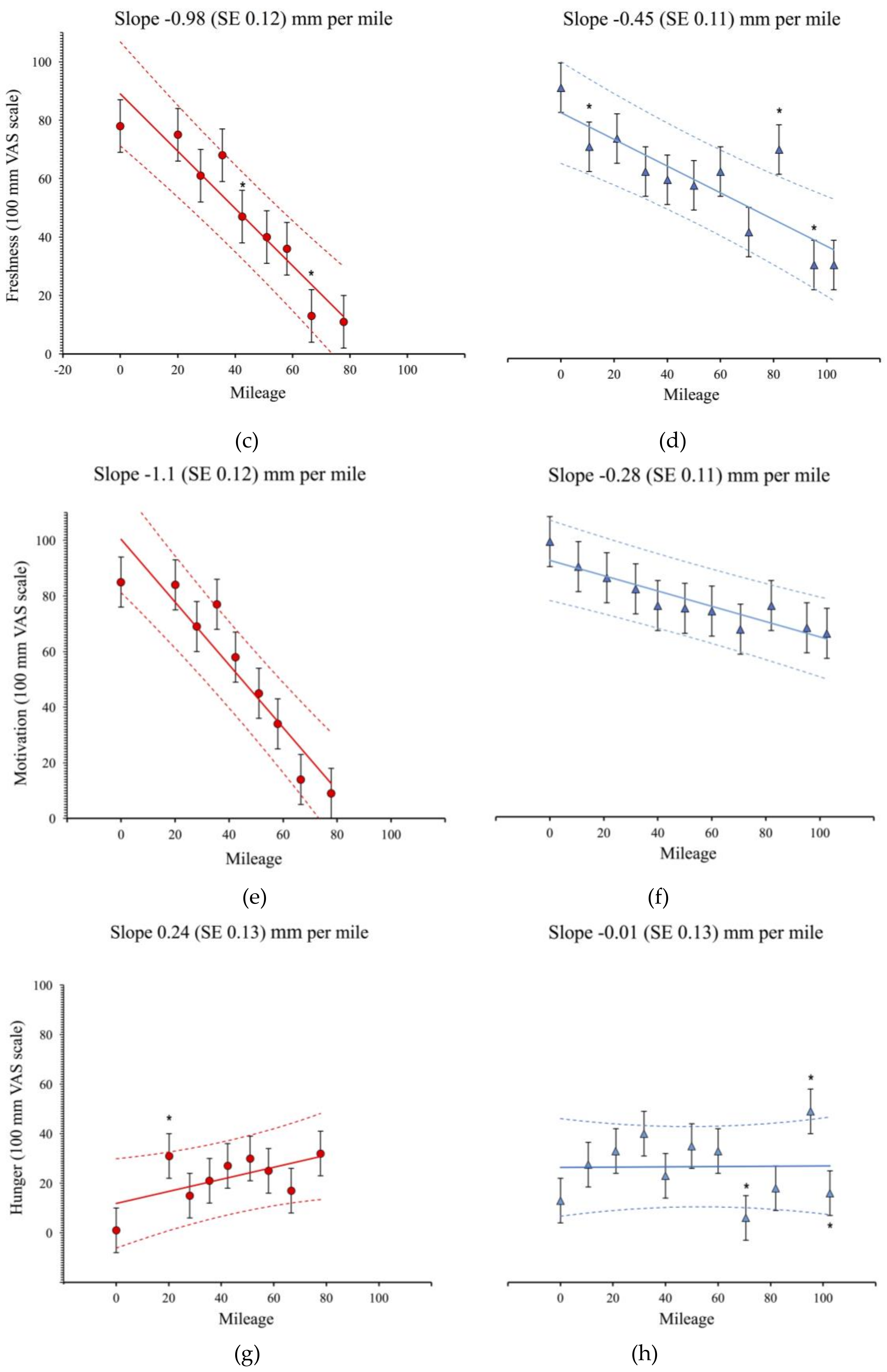
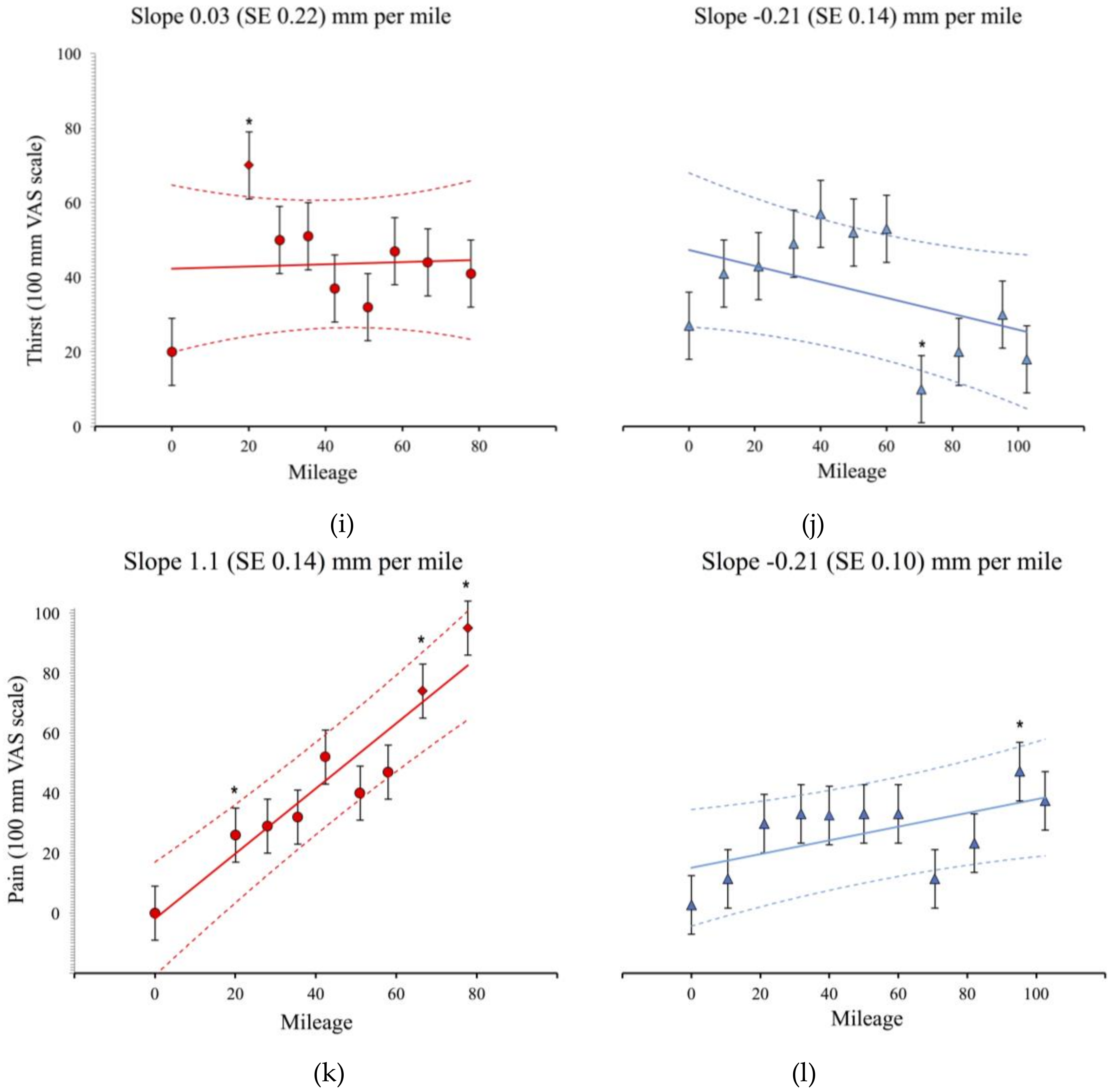
3.1. Physiological and Subjective Measures
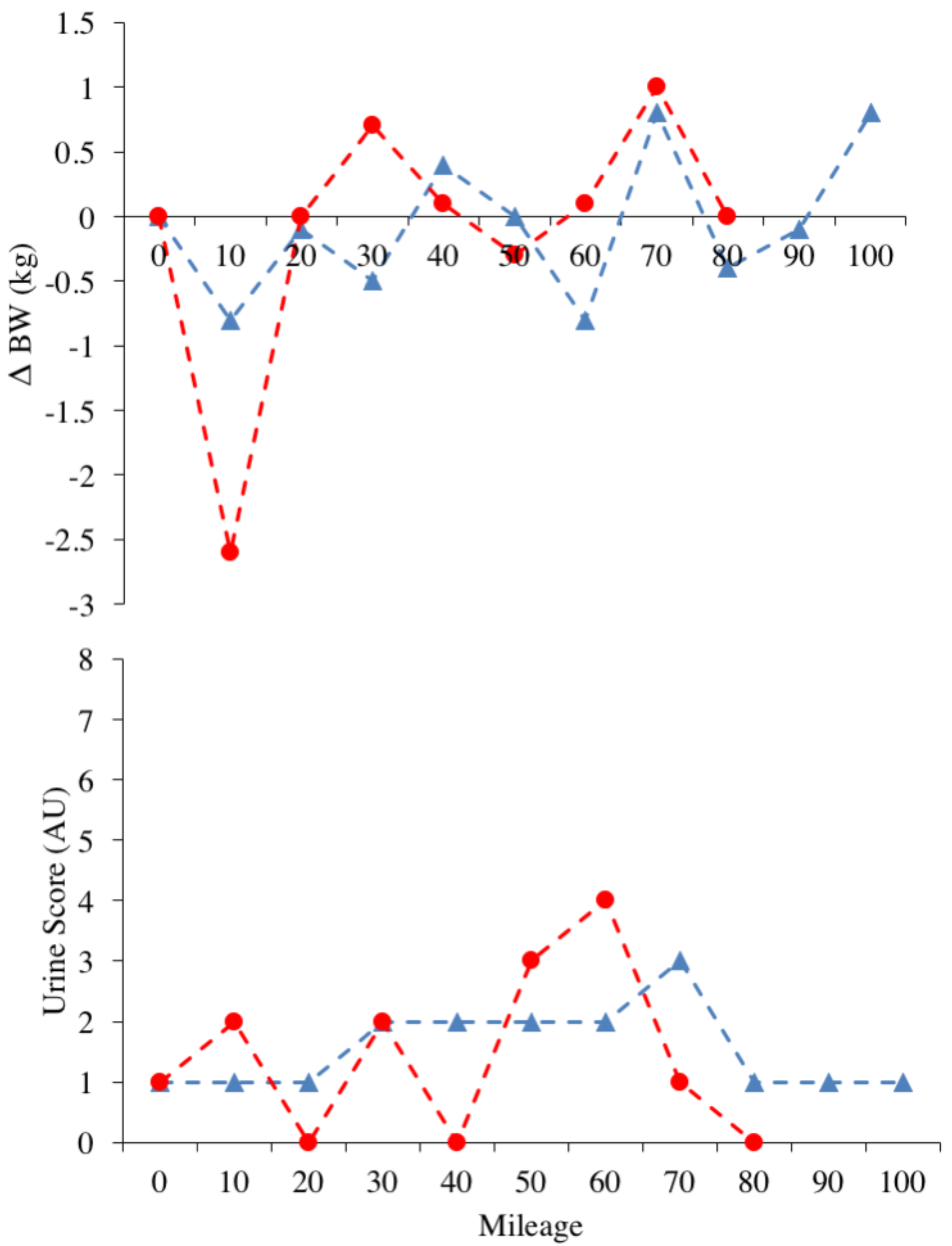
3.2. Nutritional Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IAAF Ultra-Running. Available online: http://www.iaaf.org/disciplines/ultra-running/ultra-running (accessed on 9 August 2018).
- Knoth, C.; Knechtle, B.; Rüst, C.A.; Rosemann, T.; Lepers, R. Participation and performance trends in multistage ultramarathons-the “Marathon des Sables” 2003–2012. Extrem. Physiol. Med. 2012, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Cona, G.; Cavazzana, A.; Paoli, A.; Marcolin, G.; Grainer, A.; Bisiacchi, P.S. It’s a Matter of Mind! Cognitive Functioning Predicts the Athletic Performance in Ultra-Marathon Runners. PLoS ONE 2015, 10, e0132943. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Millet, G.Y. Ultramarathon is an outstanding model for the study of adaptive responses to extreme load and stress. BMC Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Staiano, W.; Menaspà, P.; Hennessey, T.; Marcora, S.; Keegan, R.; Thompson, K.G.; Martin, D.; Halson, S.; Rattray, B. Superior Inhibitory Control and Resistance to Mental Fatigue in Professional Road Cyclists. PLoS ONE 2016, 11, e0159907. [Google Scholar] [CrossRef] [PubMed]
- Freund, W.; Weber, F.; Billich, C.; Birklein, F.; Breimhorst, M.; Schuetz, U.H. Ultra-Marathon Runners Are Different: Investigations into Pain Tolerance and Personality Traits of Participants of the TransEurope FootRace 2009. Pain Pract. 2013, 13, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.Y. Can Neuromuscular Fatigue Explain Running Strategies and Performance in Ultra-Marathons? Sports Med. 2011, 41, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Glace, B.; Murphy, C.; McHugh, M. Food and fluid intake and disturbances in gastrointestinal and mental function during an ultramarathon. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Glace, B.W.; Murphy, C.A.; McHugh, M.P. Food Intake and Electrolyte Status of Ultramarathoners Competing in Extreme Heat. J. Am. Coll. Nutr. 2013, 21, 553–559. [Google Scholar] [CrossRef]
- Knechtle, B.; Knechtle, P.; Rosemann, T.; Senn, O. What is associated with race performance in male 100-km ultra-marathoners–anthropometry, training or marathon best time? J. Sports Sci. 2011, 29, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.Y.; Hoffman, M.D.; Morin, J.B. Sacrificing economy to improve running performance--a reality in the ultramarathon? J. Appl. Physiol. 2012, 113, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Lazzer, S.; Salvadego, D.; Rejc, E.; Buglione, A.; Antonutto, G.; di Prampero, P.E. The energetics of ultra-endurance running. Eur. J. Appl. Physiol. 2012, 112, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Freund, W.; Weber, F.; Billich, C.; Schuetz, U.H. The foot in multistage ultra-marathon runners: Experience in a cohort study of 22 participants of the Trans Europe Footrace Project with mobile MRI. BMJ Open 2012, 2, e001118. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.; Levers, K.; Galvan, E.; Joubert, D.; Rasmussen, C.; Greenwood, M.; Kreider, R.B. Effects of an Ultra-Endurance Event on Body Composition, Exercise Performance and Markers of Clinical Health: A Case Study. Bioenergetics 2014, 3. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Gill, S.K.; Hankey, J.; Wright, A.; Marczak, S. Perturbed energy balance and hydration status in ultra-endurance runners during a 24 h ultra-marathon. Br. J. Nutr. 2014, 112, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.; Camões-Costa, V.; Scheer, V.; Murray, A. The Impact of Gastrointestinal Symptoms and Dermatological Injuries on Nutritional Intake and Hydration Status During Ultramarathon Events. Sports Med. Open 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T. Competition Nutrition Practices of Elite Ultramarathon Runners. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Is Drinking to Thirst Optimum? Ann. Nutr. Metab. 2010, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the Third International Exercise-Associated Hyponatremia Consensus Development Conference, Carlsbad, California, 2015. Clin. J. Sports Med. 2015, 25, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Feeding ultra-endurance athletes: An interview with Dr. Helen O’Connor and Gregory Cox. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 490–494. [Google Scholar] [CrossRef]
- Wardenaar, F.C.; Dijkhuizen, R.; Ceelen, I.J.M.; Jonk, E.; de Vries, J.H.M.; Witkamp, R.F.; Mensink, M. Nutrient Intake by Ultramarathon Runners: Can They Meet Recommendations? Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A. A Step Towards Personalized Sports Nutrition: Carbohydrate Intake During Exercise. Sports Med. 2014, 44, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints During Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. Assessing Hydration Status: The Elusive Gold Standard. J. Am. Coll. Nutr. 2013, 26, 575S–584S. [Google Scholar] [CrossRef]
- Pageaux, B. Perception of effort in Exercise Science: Definition, measurement and perspectives. Eur. J. Sport Sci. 2016, 16, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sport Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. A Spreadsheet for Monitoring an Individual’s Changes and Trend. Sportscience 2017, 21, 5–9. [Google Scholar]
- Hopkins, W.G. A Spreadsheet for Combining Outcomes from Several Subject Groups. Sport Sci. 2006, 10, 51–53. [Google Scholar]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.K.; Slattery, K.M.; Impellizzeri, F.M.; Coutts, A.J. Establishing the criterion validity and reliability of common methods for quantifying training load. J. Strength Cond. Res. 2014, 28, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg. Med. J. 2001, 18, 18–205. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.J.; Motl, R.W.; Broglio, S.P.; Ely, M.R. Dose-dependent effect of caffeine on reducing leg muscle pain during cycling exercise is unrelated to systolic blood pressure. Pain 2004, 109, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.; Kaizer, S.; Warren, R. Psychotropic effects of caffeine in man. II. Alertness, psychomotor coordination, and mood. J. Pharmacol. Exp. Ther. 1965, 150, 146–151. [Google Scholar] [PubMed]
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the “Nail in the Coffin” Too Soon? Sports Med. 2015, 45, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. New Insights into the Interaction of Carbohydrate and Fat Metabolism During Exercise. Sports Med. 2014, 44, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am. J. Physiol. Endocrinol. Metab. 2005, 290, E380–E388. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.K.; Carey, A.L.; Burke, L.; Spriet, L.L.; Hawley, J.A. Fat adaptation in well-trained athletes: Effects on cell metabolism. Appl. Physiol. Nutr. Metab. 2011, 36, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J. Protein metabolism and endurance exercise. Sports Med. 2007, 37, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Gualano, A.B.; Bozza, T.; Lopes, D. Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J. Sports Med. Phys. Fit. 2011, 51, 82–88. [Google Scholar]
- McKenzie, A.L.; Munoz, C.X.; Armstrong, L.E. Accuracy of Urine Color to Detect Equal to or Greater Than 2% Body Mass Loss in Men. J. Athl. Train 2015, 50, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Stellingwerff, T.; Costa, R.J.S. Considerations for ultra-endurance activities: Part 2–hydration. Res. Sports Med. 2018, 00, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite Control and Energy Balance: Impact of Exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wasse, L.K.; Sunderland, C.; King, J.A.; Batterham, R.L.; Stensel, D.J. Influence of rest and exercise at a simulated altitude of 4000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J. Appl. Physiol. 2012, 112, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Sasaki, H.; Tsuchiya, Y.; Goto, K. The influence of environmental temperature on appetite-related hormonal responses. J. Physiol. Anthropol. 2015, 34. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Esser, K.A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med. Sci. Sport Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, H.K.J.; Rutters, F.; Mazuy, C.; Martens, E.A.P.; Adam, T.C.; Westerterp-Plantenga, M.S. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am. J. Clin. Nutr. 2012, 96, 689–697. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value |
|---|---|
| Age | 41 years |
| Height | 168 cm |
| Weight | 67.1 kg |
| Running Experience | 5.5 years |
| Current coaching period | 2 years |
| 10 km PB 1 | 39:42 |
| Half Marathon PB | 1:26:10 |
| Marathon PB | 2:56:49 |
| Ultra-Marathon Experience | Previous DNC 2 |
| RACE 1 (DNC) | Freshness | Motivation | Hunger | Thirst | Pain |
|---|---|---|---|---|---|
| Freshness | 1.00 | Near Perfect (0.99, 0.96 to 1.0) | Moderate (0.40, −0.24 to 0.80) | Small (0.14, −0.49 to 0.67) | Near perfect (0.93, 0.76 to 0.98) |
| Motivation | – | 1.00 | Moderate (0.38, −0.26 to 0.79) | Small (0.14, −0.49 to 0.67) | Near perfect (0.91, 0.69 to 0.98) |
| Hunger | – | – | 1.00 | Small (0.48, −1.5 to 0.83) | Large (0.56, −0.04 to 0.86) |
| Thirst | – | – | – | 1.00 | Trivial (0.08, −0.53 to 0.64) |
| Pain | – | – | – | – | 1.00 |
| RACE 2 | Freshness | Motivation | Hunger | Thirst | Pain |
| Freshness | 1.00 | Near Perfect (0.90, 0.74 to 0.97) | Trivial (0.15, −0.38 to 0.6) | Moderate (0.31, −0.22 to 0.7) | Large (0.62, 0.17 to 0.85) |
| Motivation | – | 1.00 | Trivial (0.06, −0.45 to 0.54) | Small (0.24, −0.29 to 0.66) | Large (0.63, 0.19 to 0.86) |
| Hunger | – | – | 1.00 | Large (0.6, 0.14 to 0.85) | Very large (0.7, 0.3 to 0.89) |
| Thirst | – | – | – | 1.00 | Moderate (0.33, −0.2 to 0.71) |
| Pain | – | – | – | – | 1.00 |
| Miles (x) | Increases in Perceived Pain per x Miles (mm, 90% CI) | Was the Athlete Experiencing a Substantial Increase in Pain? | Increases in Perceived Pain per x Miles (mm, 90% CI) | Was the Athlete Experiencing a Substantial Increase in Pain? | Difference in Increase of Perceived Pain in Race 2. (mm, 90% CI) |
|---|---|---|---|---|---|
| 10 | 9.3, 7.5 to 11 | Unlikely (6/94%) | 2.1, 0.5 to 3.7 | Most unlikely (100/0%) | Most likely, trivial (7.2, 4.9 to 9.5) |
| 20 | 22, 20 to 24 | Very likely (99/1%) | 4.2, 2.6 to 5.9 | Most unlikely (100/0%) | Most likely, less painful (18, 15 to 20) |
| 40 | 43, 42 to 45 | Almost certainly (100/0%) | 8.4, 6.8 to 10 | Unlikely (19/81%) | Most likely, less painful (35, 33 to 37) |
| 60 | 65, 63 to 67 | Almost certainly (100/0%) | 13, 11 to 14 | Possibly (54/46%) | Most likely, less painful (52.5, 50.2 to 54.8) |
| 80 | 87, 85 to 89 | Almost certainly (100/0%) | 17, 15 to 19 | Possibly (73/27%) | Most likely, less painful (70, 68 to 72) |
| Miles (x) | Change in Hunger per x Miles (mm, 90% CI) | Was the Athlete Experiencing a Substantial Increase in hunger? | Increases in Perceived Hunger per x Miles (mm, 90% CI) | Was the Athlete Experiencing a Substantial Increase in Hunger? | Difference in Increase of Perceived Hunger in Race 2. (mm, 90% CI) |
|---|---|---|---|---|---|
| 10 | 2.4, 0.3 to 4.6 | Most unlikely (0/100/0%) | 0.1, −2.1 to 2.2 | Most unlikely (0/100/0%) | Most likely, trivial (2.4, -0.5 to 5.3) |
| 20 | 4.9, 2.7 to 7.0 | Very unlikely (1/99/0%) | 0.1, −2.0 to 2.3 | Most unlikely (0/100/0%) | Most likely, trivial (4.8, 1.9 to 7.6) |
| 40 | 9.7, 7.6 to 12 | Possibly (33/67/0%) | 0.2, −1.9 to 2.4 | Very unlikely (2/96/2%) | Likely trivial (9.5, 6.6 to 12) |
| 60 | 15, 13 to 17 | Possibly (63/36/0%) | 0.4, −1.8 to 2.5 | Unlikely (8/86/7%) | Likely, lower ↑ hunger (14, 11 to 17) |
| 80 | 20, 17 to 22 | Likely (76/23/1%) | 0.5, −1.7 to 2.6 | Unlikely (14/74/12%) | Most likely, lower ↑ hunger (19, 16 to -22) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Best, R.; Barwick, B.; Best, A.; Berger, N.; Harrison, C.; Wright, M.; Sparrow, J. Changes in Pain and Nutritional Intake Modulate Ultra-Running Performance: A Case Report. Sports 2018, 6, 111. https://doi.org/10.3390/sports6040111
Best R, Barwick B, Best A, Berger N, Harrison C, Wright M, Sparrow J. Changes in Pain and Nutritional Intake Modulate Ultra-Running Performance: A Case Report. Sports. 2018; 6(4):111. https://doi.org/10.3390/sports6040111
Chicago/Turabian StyleBest, Russ, Benjamin Barwick, Alice Best, Nicolas Berger, Claire Harrison, Matthew Wright, and Julie Sparrow. 2018. "Changes in Pain and Nutritional Intake Modulate Ultra-Running Performance: A Case Report" Sports 6, no. 4: 111. https://doi.org/10.3390/sports6040111
APA StyleBest, R., Barwick, B., Best, A., Berger, N., Harrison, C., Wright, M., & Sparrow, J. (2018). Changes in Pain and Nutritional Intake Modulate Ultra-Running Performance: A Case Report. Sports, 6(4), 111. https://doi.org/10.3390/sports6040111







