Individual Responses for Muscle Activation, Repetitions, and Volume during Three Sets to Failure of High- (80% 1RM) versus Low-Load (30% 1RM) Forearm Flexion Resistance Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. One Repetition Maximum
2.4. Resistance Exercise
2.5. Electromyography
2.6. Signal Processing
2.7. Statistics
| Group | Subject | Repetitions Completed | Individual Volume | Mean Volume | ||
|---|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 3 | All sets | |||
| 80% 1RM | 1 | 11 | 9 | 6 | 339.7 | 350.8 ± 72.8 |
| 5 | 12 | 8 | 6 | 294.8 | ||
| 6 | 7 | 7 | 6 | 344.7 | ||
| 9 | 10 | 7 | 4 | 190.5 | ||
| 10 | 10 | 6 | 2 | 367.4 | ||
| 13 | 12 | 11 | 8 | 492.2 | ||
| 14 | 15 | 10 | 8 | 411.6 | ||
| 18 | 12 | 8 | 3 | 365.1 | ||
| 30% 1RM | 2 | 58 | 24 | 26 | 269.4 | 382.8 ± 101.4 |
| 3 | 37 | 24 | 14 | 323.2 | ||
| 4 | 39 | 20 | 20 | 308.2 | ||
| 7 | 47 | 16 | 15 | 398.0 | ||
| 11 | 54 | 14 | 14 | 390.5 | ||
| 12 | 37 | 20 | 20 | 384.2 | ||
| 15 | 51 | 28 | 20 | 606.2 | ||
3. Results
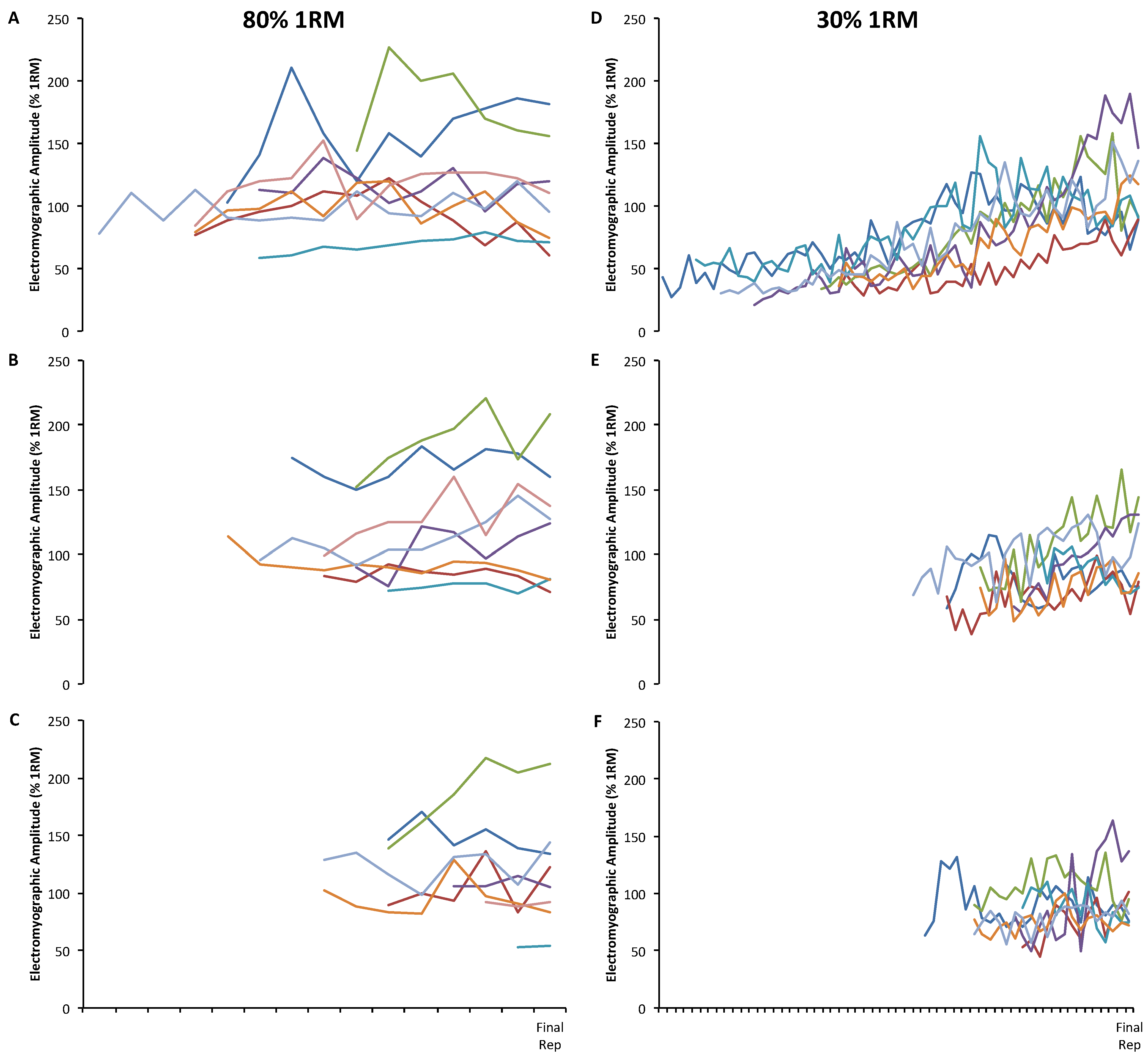
| Group | Subject | Set 1 | Set 2 | Set 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | r2 | SEE | p-value | r | r2 | SEE | p-value | r | r2 | SEE | p-value | ||
| 80% 1RM | 1 | 0.50 | 0.25 | 28.36 | 0.11 | 0.24 | 0.06 | 12.11 | 0.54 | 0.58 | 0.34 | 12.13 | 0.23 |
| 5 | 0.31 | 0.10 | 18.10 | 0.32 | 0.34 | 0.12 | 6.60 | 0.41 | 0.41 | 0.17 | 21.36 | 0.42 | |
| 6 | 0.32 | 0.10 | 31.90 | 0.48 | 0.66 | 0.44 | 19.08 | 0.11 | 0.90 | 0.82 | 14.95 | 0.01 * | |
| 9 | 0.10 | 0.01 | 13.04 | 0.79 | 0.66 | 0.43 | 15.29 | 0.11 | 0.16 | 0.03 | 5.31 | 0.84 | |
| 10 | 0.84 | 0.71 | 3.56 | <0.01 * | 0.40 | 0.16 | 4.58 | 0.44 | 1.00 | - | - | - | |
| 13 | 0.08 | 0.01 | 15.80 | 0.80 | 0.59 | 0.35 | 6.95 | 0.05 | 0.07 | <0.01 | 16.81 | 0.88 | |
| 14 | 0.33 | 0.11 | 11.80 | 0.23 | 0.77 | 0.59 | 11.09 | <0.01 * | 0.11 | 0.01 | 16.86 | 0.79 | |
| 18 | 0.28 | 0.08 | 17.90 | 0.37 | 0.66 | 0.43 | 16.71 | 0.08 | 0.06 | <0.01 | 3.18 | 0.96 | |
| 30% 1RM | 2 | 0.76 | 0.58 | 16.70 | <0.01 * | 0.18 | 0.03 | 16.18 | 0.39 | 0.13 | 0.02 | 18.76 | 0.52 |
| 3 | 0.85 | 0.73 | 9.12 | <0.01 * | 0.52 | 0.27 | 12.89 | <0.01 * | 0.66 | 0.44 | 13.35 | 0.01 * | |
| 4 | 0.88 | 0.77 | 16.84 | <0.01 * | 0.80 | 0.64 | 16.91 | <0.01 * | 0.12 | 0.02 | 17.09 | 0.60 | |
| 7 | 0.90 | 0.80 | 21.50 | <0.01 * | 0.98 | 0.95 | 5.85 | <0.01 * | 0.77 | 0.59 | 26.43 | <0.01 * | |
| 11 | 0.76 | 0.58 | 19.26 | <0.01 * | 0.53 | 0.28 | 12.49 | 0.05 | 0.61 | 0.37 | 13.42 | 0.02 * | |
| 12 | 0.92 | 0.84 | 10.26 | <0.01 * | 0.48 | 0.23 | 14.22 | 0.03 * | 0.22 | 0.05 | 9.90 | 0.35 | |
| 15 | 0.93 | 0.86 | 12.91 | <0.01 * | 0.51 | 0.26 | 16.01 | <0.01 * | 0.50 | 0.25 | 9.72 | 0.03 * | |
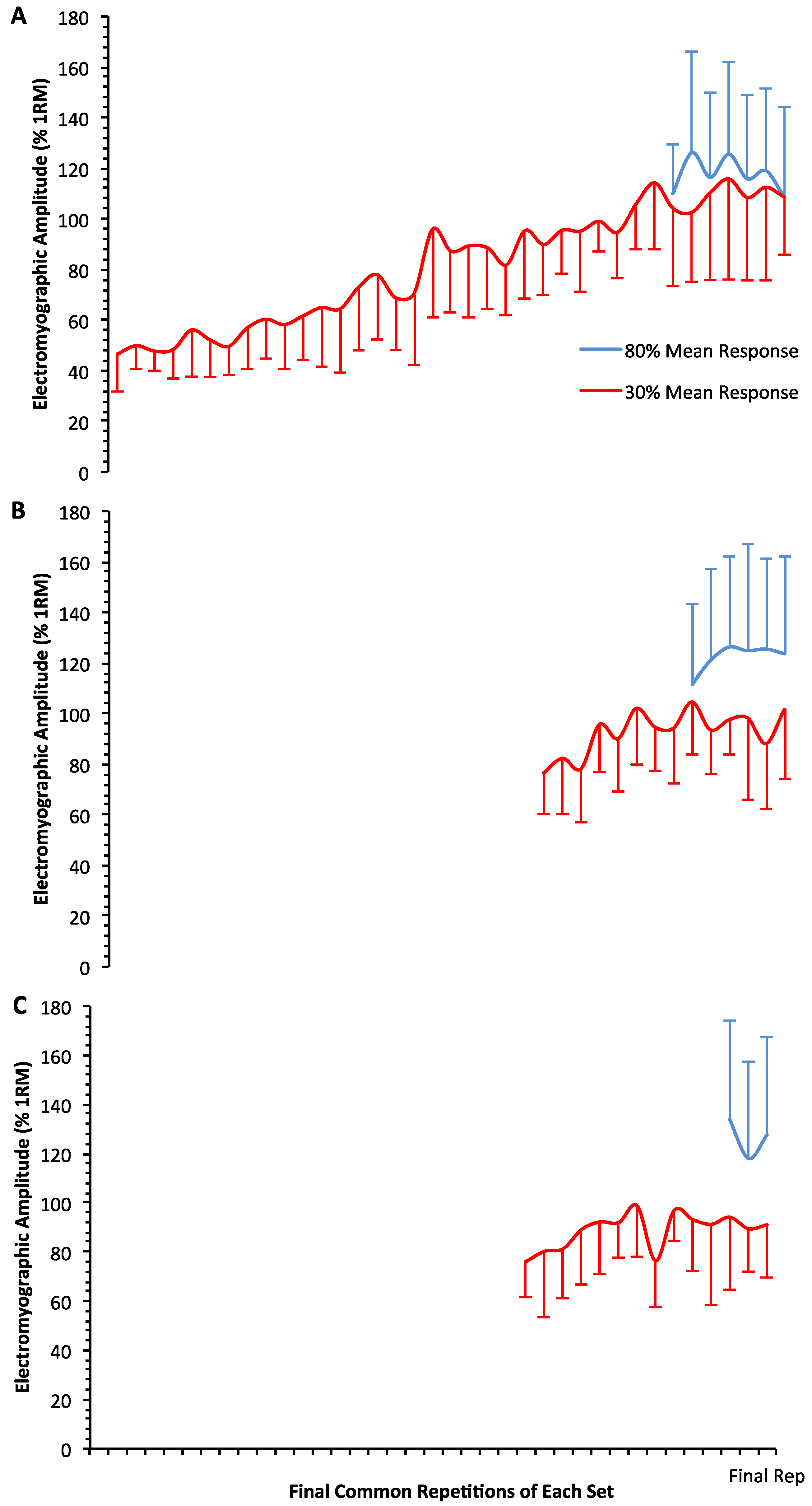
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- National Strength and Conditioning Association. Essentials of Strength Training and Conditioning, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Burd, N.A.; West, D.W.; Staples, A.W.; Atherton, P.J.; Baker, J.M.; Moore, D.R.; Holwerda, A.M.; Parise, G.; Rennie, M.J.; Baker, S.K.; et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, R.; Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. Int. J. Clin. Med. 2013, 4, 114–121. [Google Scholar] [CrossRef]
- Burd, N.A.; Moore, D.R.; Mitchell, C.J.; Phillips, S.M. Big claims for big weights but with little evidence. Eur. J. Appl. Physiol. 2013, 113, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Schuenke, M.D.; Herman, J.; Staron, R.S. Preponderance of evidence proves “big” weights optimize hypertrophic and strength adaptations. Eur. J. Appl. Physiol. 2013, 113, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Carpinelli, R.N. The size principle and a critical analysis of the unsubstantiated heavier-is-better recommendation for resistance training. J. Exerc. Sci. Fit. 2008, 6, 67–86. [Google Scholar]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Functional significance of cell size in spinal motoneurons. J. Neurophysiol. 1965, 28, 560–580. [Google Scholar] [PubMed]
- Conwit, R.A.; Stashuk, D.; Suzuki, H.; Lynch, N.; Schrager, M.; Metter, E.J. Fatigue effects on motor unit activity during submaximal contractions. Arch. Phys. Med. Rehabil. 2000, 81, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Mitchell, C.J.; Churchward-Venne, T.A.; Phillips, S.M. Bigger weights may not beget bigger muscles: Evidence from acute muscle protein synthetic responses after resistance exercise. Appl. Physiol. Nutr. Metab. 2012, 37, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.D.; Housh, T.J.; Bergstrom, H.C.; Cochrane, K.C.; Hill, E.C.; Smith, C.M.; Johnson, G.O.; Schmidt, R.J.; Cramer, J.T. Muscle activation during three sets to failure at 80% vs. 30% 1rm resistance exercise. Eur. J. Appl. Physiol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Akima, H.; Saito, A. Activation of quadriceps femoris including vastus intermedius during fatiguing dynamic knee extensions. Eur. J. Appl. Physiol. 2013, 113, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Murphy, B.G.; Labarbera, K.E. Neuromuscular function after a bout of low-load blood flow-restricted exercise. Med. Sci. Sports Exerc. 2013, 45, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hagg, G. Seniam 8: European Recommendations for Surface Electromyography; Roessngh Research and Development: Enschede, The Netherlands, 1999. [Google Scholar]
- Beck, T.W.; Housh, T.J. Use of electromyography in studying human movement. In Routledge Handbook of Biomechanics and Human Movement; Hong, Y., Bartlett, R., Eds.; Routledge: New York, NY, USA, 2008; pp. 214–230. [Google Scholar]
- Schoenfeld, B.J.; Contreras, B.; Willardson, J.M.; Fontana, F.; Tiryaki-Sonmez, G. Muscle activation during low- versus high-load resistance training in well-trained men. Eur. J. Appl. Physiol. 2014, 114, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Samanek, M.; Goetzova, J.; Fiserova, J.; Skovranek, J. Differences in muscle blood flow in upper and lower extremities of patients after correction of coarctation of the aorta. Circulation 1976, 54, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Sundaraj, K.; Badlishah Ahmad, R.; Ahamed, N.U.; Islam, A.; Sundaraj, S. Muscle fatigue in the three heads of the triceps brachii during a controlled forceful hand grip task with full elbow extension using surface electromyography. J. Hum. Kinet. 2015, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Harwood, B.; Dalton, B.H.; Power, G.A.; Rice, C.L. Motor unit properties from three synergistic muscles during ramp isometric elbow extensions. Exp. Brain Res. 2013, 231, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface emg. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W.; Housh, T.J.; Cramer, J.T.; Weir, J.P.; Johnson, G.O.; Coburn, J.W.; Malek, M.H.; Mielke, M. Mechanomyographic amplitude and frequency responses during dynamic muscle actions: A comprehensive review. Biomed. Eng. Online 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.; Holbourn, A.H. The sounds from single motor units in a contracting muscle. J. Physiol. 1948, 107, 456–464. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkins, N.D.M.; Housh, T.J.; Buckner, S.L.; Bergstrom, H.C.; Cochrane, K.C.; Smith, C.M.; Hill, E.C.; Schmidt, R.J.; Cramer, J.T. Individual Responses for Muscle Activation, Repetitions, and Volume during Three Sets to Failure of High- (80% 1RM) versus Low-Load (30% 1RM) Forearm Flexion Resistance Exercise. Sports 2015, 3, 269-280. https://doi.org/10.3390/sports3040269
Jenkins NDM, Housh TJ, Buckner SL, Bergstrom HC, Cochrane KC, Smith CM, Hill EC, Schmidt RJ, Cramer JT. Individual Responses for Muscle Activation, Repetitions, and Volume during Three Sets to Failure of High- (80% 1RM) versus Low-Load (30% 1RM) Forearm Flexion Resistance Exercise. Sports. 2015; 3(4):269-280. https://doi.org/10.3390/sports3040269
Chicago/Turabian StyleJenkins, Nathaniel D. M., Terry J. Housh, Samuel L. Buckner, Haley C. Bergstrom, Kristen C. Cochrane, Cory M. Smith, Ethan C. Hill, Richard J. Schmidt, and Joel T. Cramer. 2015. "Individual Responses for Muscle Activation, Repetitions, and Volume during Three Sets to Failure of High- (80% 1RM) versus Low-Load (30% 1RM) Forearm Flexion Resistance Exercise" Sports 3, no. 4: 269-280. https://doi.org/10.3390/sports3040269
APA StyleJenkins, N. D. M., Housh, T. J., Buckner, S. L., Bergstrom, H. C., Cochrane, K. C., Smith, C. M., Hill, E. C., Schmidt, R. J., & Cramer, J. T. (2015). Individual Responses for Muscle Activation, Repetitions, and Volume during Three Sets to Failure of High- (80% 1RM) versus Low-Load (30% 1RM) Forearm Flexion Resistance Exercise. Sports, 3(4), 269-280. https://doi.org/10.3390/sports3040269






