Effect of Level and Downhill Running on Breathing Efficiency
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Participants
2.2. Study Design
2.3. Maximal Voluntary Contraction
2.4. Maximal Oxygen Consumption
2.5. Level and Downhill Running
2.6. Statistical Analysis
3. Results
3.1. Isometric Force of Knee Extensors
3.2. Level and Downhill Running Comparison
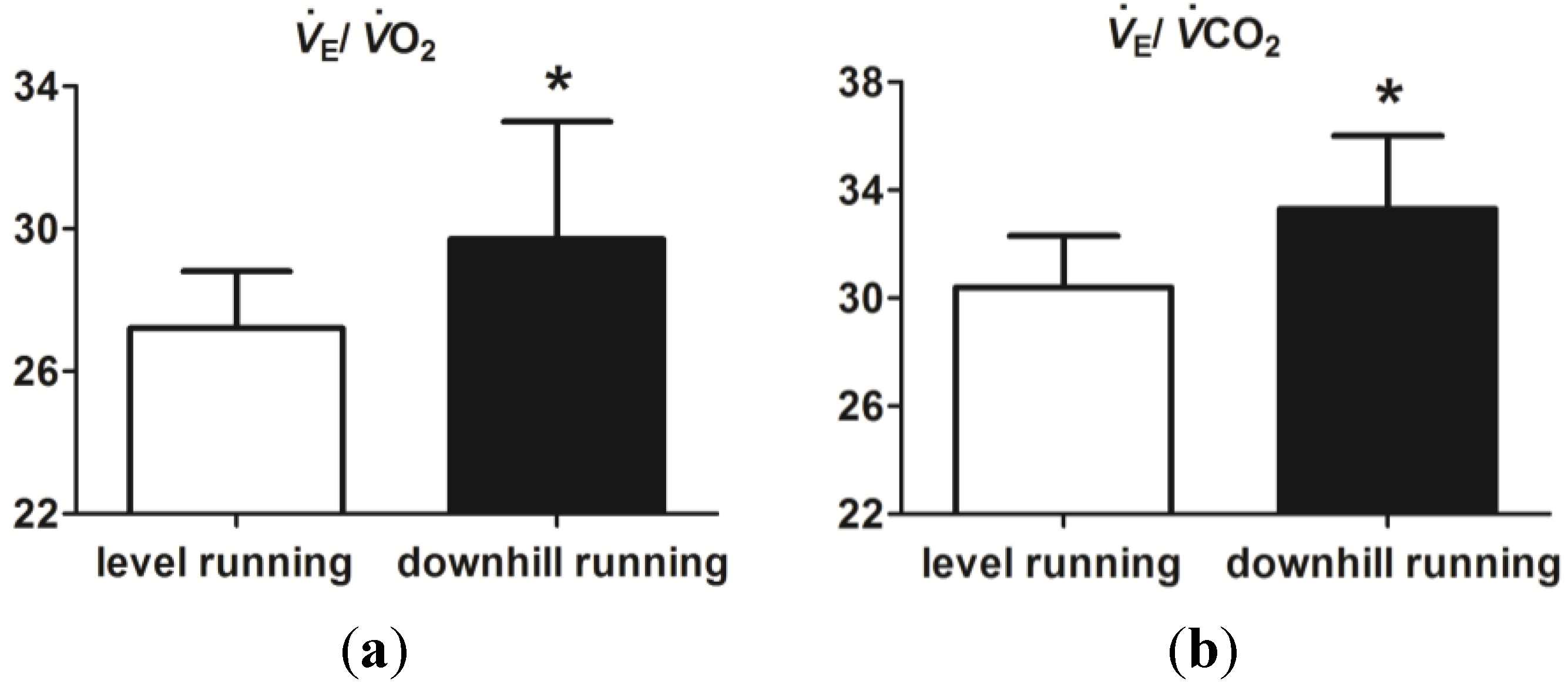
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Cooper, C.B.; Storer, T.W. Exercise and Testing and Interpretation: A Practical Guide; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Ofir, D.; Laveneziana, P.; Webb, K.A.; Lam, Y.M.; O’Donnell, D.E. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J. Appl. Physiol. 2007, 104, 1583–1593. [Google Scholar] [CrossRef]
- Rasmussen, B.; Klausen, K.; Clausen, J.P.; Trap-Jensen, J. Pulmonary ventilation, blood gases, and blood pH after training of the arms or the legs. J. Appl. Physiol. 1975, 38, 250–256. [Google Scholar] [PubMed]
- Armstrong, N.; Davies, B. An ergometric analysis of age group swimmers. Br. J. Sports Med. 1981, 15, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Laursen, P.B.; Rhodes, E.C.; Langill, R.H.; Taunton, J.E.; McKenzie, D.C. Exercise-induced arterial hypoxemia is not different during cycling and running in triathletes. Scand. J. Med. Sci. Sports 2005, 15, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Gavin, T.P.; Stager, J.M. The effect of exercise modality on exercise-induced hypoxemia. Respir. Physiol. 1999, 115, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Minetti, A.E.; Moia, C.; Roi, G.S.; Susta, D.; Ferretti, G. Energy cost of walking and running at extreme uphill and downhill slopes. J. Appl. Physiol. 2002, 93, 1039–1046. [Google Scholar] [PubMed]
- Bigland-Ritchie, B.; Woods, J.J. Integrated electromyogram and oxygen uptake during positive and negative work. J. Physiol. 1976, 260, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Twist, C.; Eston, R.G. The effect of exercise-induced muscle damage on perceived exertion and cycling endurance performance. Eur. J. Appl. Physiol. 2009, 105, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.C.; Eston, R.G.; Poole, D.C.; Rowlands, A.V.; DiMenna, F.; Wilkerson, D.P.; Twist, C.; Jones, A.M. Effect of eccentric exercise-induced muscle damage on the dynamics of muscle oxygenation and pulmonary oxygen uptake. J. Appl. Physiol. 2008, 105, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [PubMed]
- Jones, A.M.; Doust, J.H. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J. Sport Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef]
- Robergs, R.A.; Wagner, D.R.; Skemp, K.M. Oxygen consumption and energy expenditure of level versus downhill running. J. Sports Med. Phys. Fitness 1997, 37, 168–174. [Google Scholar] [PubMed]
- Eston, R.G.; Lemmey, A.B.; McHugh, P.; Byrne, C.; Walsh, S.E. Effect of stride length on symptoms of exercise-induced muscle damage during a repeated bout of downhill running. Scand. J. Med. Sci. Sports 2000, 10, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Nosaka, K.; Tu, J.H. Changes in running economy following downhill running. J. Sports Sci. 2007, 25, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Doyle, J.A.; Ingalls, C.P.; Benardot, D.; Rupp, J.C.; Corona, B.T. Adaptation of insulin-resistance indicators to a repeated bout of eccentric exercise in human skeletal muscle. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 181–190. [Google Scholar] [PubMed]
- Kaufman, M.P.; Hayes, S.G.; Adreani, C.M.; Pickar, J.G. Discharge properties of group III and IV muscle afferents. Adv. Exp. Med. Biol. 2002, 508, 25–32. [Google Scholar] [PubMed]
- Kaufman, M.P.; Longhurst, J.C.; Rybicki, K.J.; Wallach, J.H.; Mitchell, J.H. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 105–112. [Google Scholar] [PubMed]
- Haouzi, P. Tracking pulmonary gas exchange by breathing control during exercise: Role of muscle blood flow muscle blood flow. J. Physiol. 2014, 592, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Padilla, D.J.; Behnke, B.J.; Hageman, K.S.; Musch, T.I.; Poole, D.C. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J. Appl. Physiol. 2005, 99, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Chenuel, B.; Huszczuk, A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: Neurophysiological basis and implication for respiratory control. J. Appl. Physiol. 2004, 96, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010, 109, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Dousset, E.; Laurin, J.; Gondin, J.; Gautier, M.; Decherchi, P. Group III and IV muscle afferent discharge patterns after repeated lengthening and shortening actions. Muscle Nerve 2009, 40, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Duranti, R.; Pantaleo, T.; Bellini, F.; Bongianni, F.; Scano, G. Respiratory responses induced by the activation of somatic nociceptive afferents in humans. J. Appl. Physiol. 1991, 71, 2440–2448. [Google Scholar] [PubMed]
- Gleeson, M.; Blannin, A.K.; Walsh, N.P.; Field, C.N.; Pritchard, J.C. Effect of exercise-induced muscle damage on the blood lactate response to incremental exercise in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Mense, S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain 1993, 54, 241–289. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, M.D.; Myers, S.D.; Kelly, J.S.M.; Willems, M.E.T. Effect of Level and Downhill Running on Breathing Efficiency. Sports 2015, 3, 12-20. https://doi.org/10.3390/sports3010012
Cook MD, Myers SD, Kelly JSM, Willems MET. Effect of Level and Downhill Running on Breathing Efficiency. Sports. 2015; 3(1):12-20. https://doi.org/10.3390/sports3010012
Chicago/Turabian StyleCook, Matthew D., Stephen D. Myers, John S. M. Kelly, and Mark E. T. Willems. 2015. "Effect of Level and Downhill Running on Breathing Efficiency" Sports 3, no. 1: 12-20. https://doi.org/10.3390/sports3010012
APA StyleCook, M. D., Myers, S. D., Kelly, J. S. M., & Willems, M. E. T. (2015). Effect of Level and Downhill Running on Breathing Efficiency. Sports, 3(1), 12-20. https://doi.org/10.3390/sports3010012






