Considering the Effects of Cannabinoids and Exercise on the Brain: A Narrative Review
Abstract
1. Introduction
2. Literature Search Methods
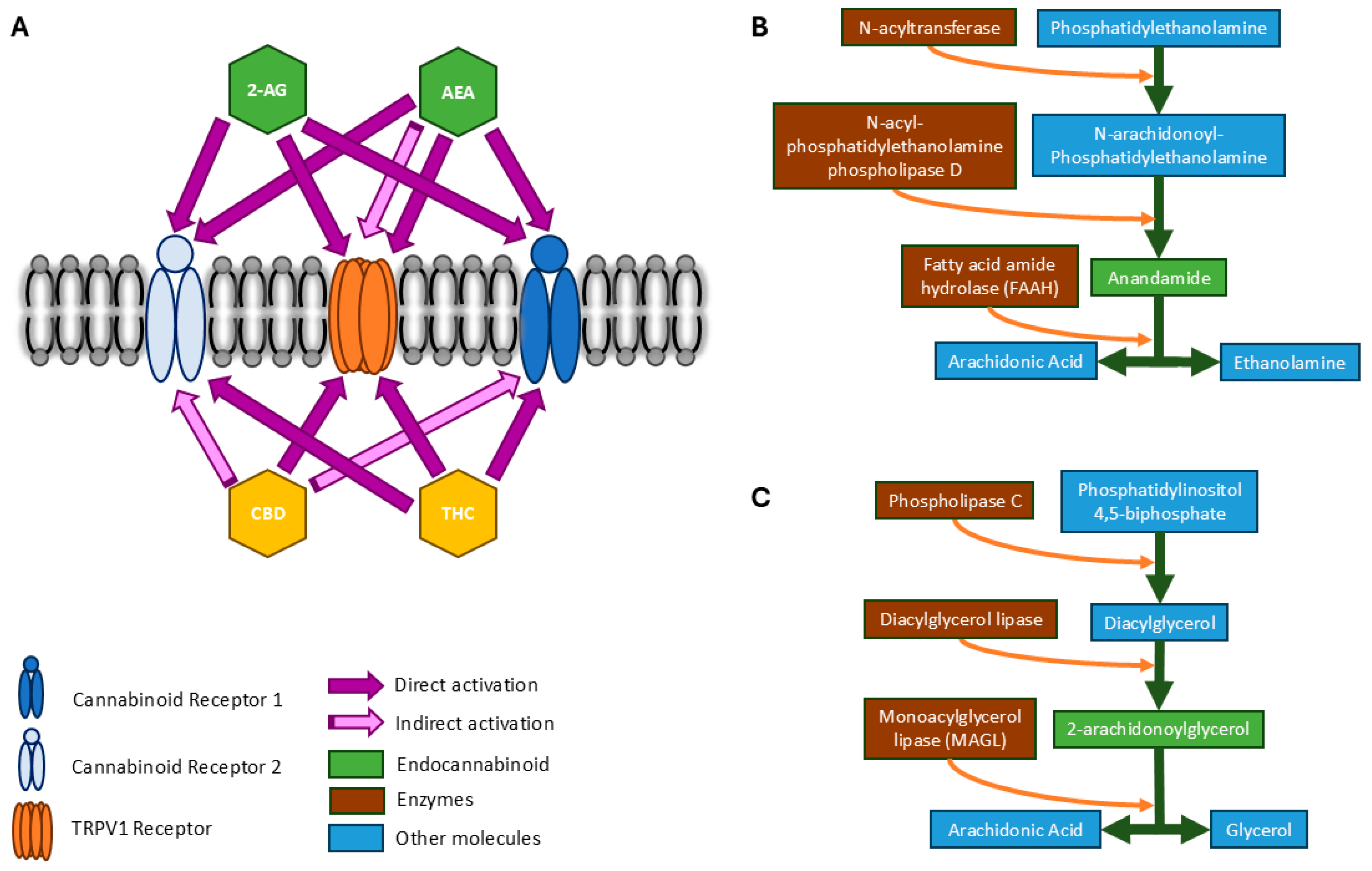
3. Overview of the Endocannabinoid System
4. Targeting the Endocannabinoid System to Alter Brain Function
5. Regulating Inflammation
6. Regulating Vascular Function
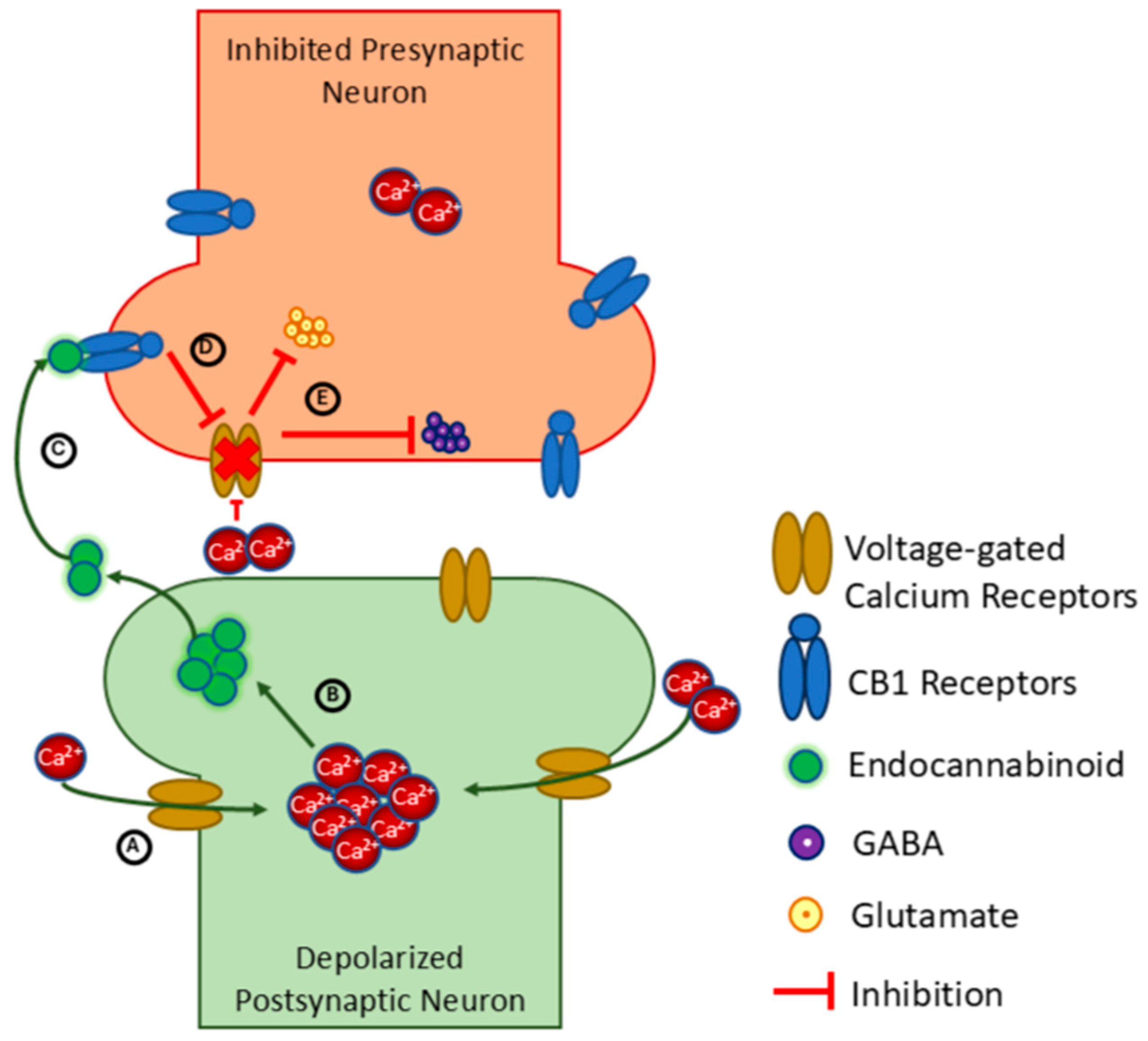
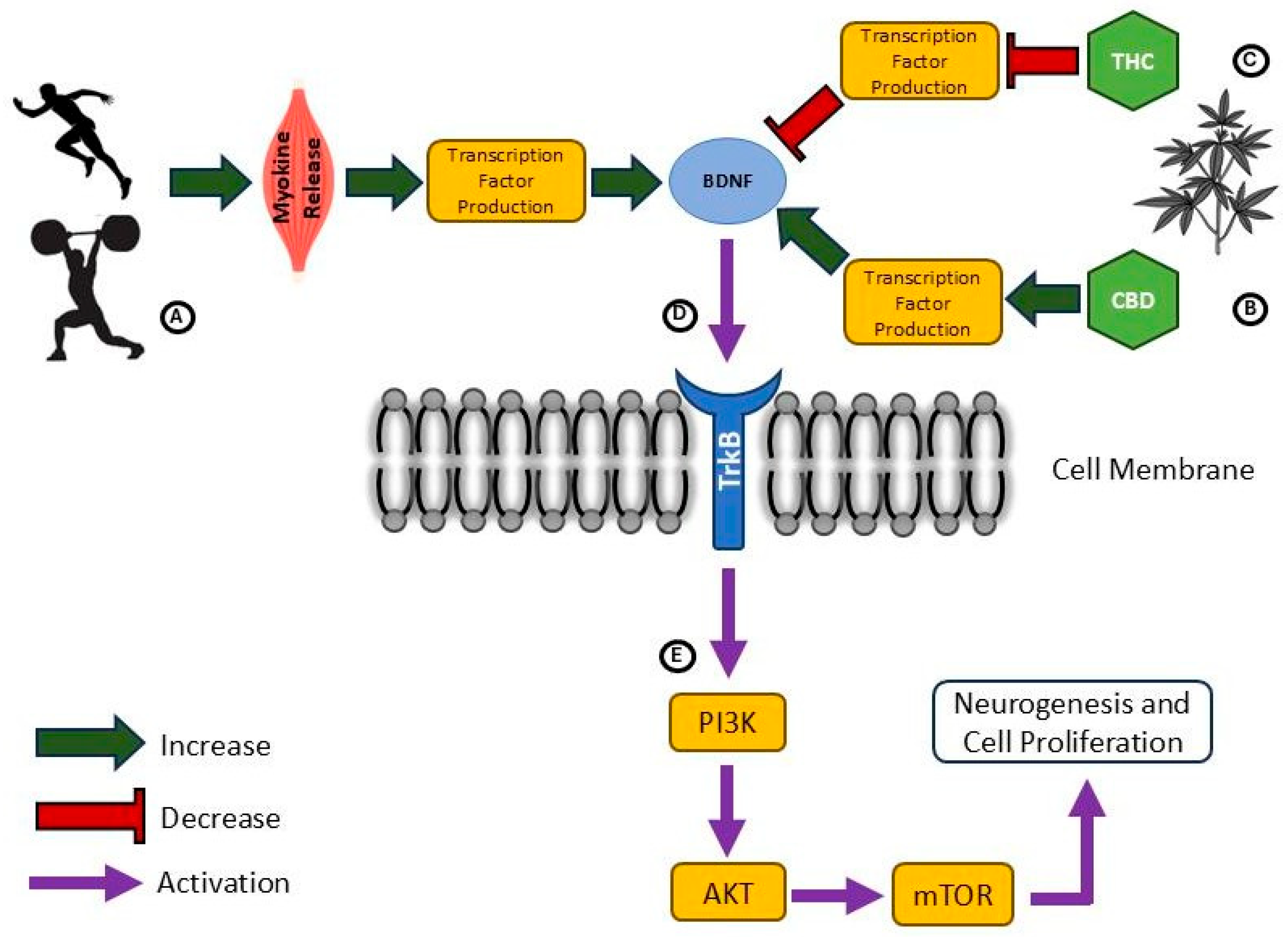
7. Promoting Neuroplasticity
8. Implications and Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farrelly, K.N.; Wardell, J.D.; Marsden, E.; Scarfe, M.L.; Najdzionek, P.; Turna, J.; MacKillop, J. The Impact of Recreational Cannabis Legalization on Cannabis Use and Associated Outcomes: A Systematic Review. Subst. Abus. 2023, 17, 23–55. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Ameri, A. The Effects of Cannabinoids on the Brain. Prog. Neurobiol. 1999, 58, 315–348. [Google Scholar] [CrossRef]
- Chye, Y.; Kirkham, R.; Lorenzetti, V.; McTavish, E.; Solowij, N.; Yücel, M. Cannabis, Cannabinoids, and Brain Morphology: A Review of the Evidence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 627–635. [Google Scholar] [CrossRef]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and Immune Regulation. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2009, 60, 85. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Endocannabinoids and the Cardiovascular System in Health and Disease. In Endocannabinoids; Pertwee, R.G., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 231, pp. 393–422. ISBN 978-3-319-20825-1. [Google Scholar]
- Kreitzer, A.C. Neurotransmission: Emerging Roles of Endocannabinoids. Curr. Biol. 2005, 15, R549–R551. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, C.; Panlilio, L.V.; Aliczki, M.; Goldberg, S.R.; Haller, J.; Yasar, S. Effects of Endocannabinoid System Modulation on Cognitive and Emotional Behavior. Front. Behav. Neurosci. 2011, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G.; Rosenfeld, M.J. The Endocannabinoid System, Cannabinoids, and Pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Thompson, E.S.; Alcorn, J.; Neary, J.P. Cannabinoid Therapy in Athletics: A Review of Current Cannabis Research to Evaluate Potential Real-World Cannabinoid Applications in Sport. Sports Med. 2024, 54, 2743–2769. [Google Scholar] [CrossRef]
- Young, R.J. The Effect of Regular Exercise on Cognitive Functioning and Personality. Br. J. Sports Med. 1979, 13, 110–117. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training After Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabilit. Neural Repair 2018, 32, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef]
- Nay, K.; Smiles, W.J.; Kaiser, J.; McAloon, L.M.; Loh, K.; Galic, S.; Oakhill, J.S.; Gundlach, A.L.; Scott, J.W. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4052. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and Inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef]
- Won, J.; Callow, D.D.; Pena, G.S.; Gogniat, M.A.; Kommula, Y.; Arnold-Nedimala, N.A.; Jordan, L.S.; Smith, J.C. Evidence for Exercise-Related Plasticity in Functional and Structural Neural Network Connectivity. Neurosci. Biobehav. Rev. 2021, 131, 923–940. [Google Scholar] [CrossRef]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and Exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef] [PubMed]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise Activates the Endocannabinoid System. NeuroReport 2003, 14, 2209. [Google Scholar] [CrossRef] [PubMed]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing Narrative Literature Reviews for Peer-Reviewed Journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef]
- Agarwal, S.; Charlesworth, M.; Elrakhawy, M. How to Write a Narrative Review. Anaesthesia 2023, 78, 1162–1166. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Li, X.; Shen, L.; Hua, T.; Liu, Z.-J. Structural and Functional Insights into Cannabinoid Receptors. Trends Pharmacol. Sci. 2020, 41, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Kaba, A.; Ray, S.D. Cannabinoids. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2024; pp. 461–471. ISBN 978-0-323-85434-4. [Google Scholar]
- Chiurchiù, V. Endocannabinoids and Immunity. Cannabis Cannabinoid Res. 2016, 1, 59–66. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The Synthetic Cannabinoids Phenomenon: From Structure to Toxicological Properties. A Review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Drug Synthetic Cannabinoids|National Institute on Drug Abuse (NIDA). Available online: https://nida.nih.gov/research-topics/synthetic-cannabinoids (accessed on 10 October 2024).
- de Almeida, D.L.; Devi, L.A. Diversity of Molecular Targets and Signaling Pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.F.; Nappe, T.M. Cannabinoid Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Teixeira, H.M. Phytocanabinoids and Synthetic Cannabinoids: From Recreational Consumption to Potential Therapeutic Use—A Review. Front. Toxicol. 2025, 6, 1495547. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Zádor, F.; Joca, S.; Nagy-Grócz, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Benyhe, S.; Vécsei, L. Pro-Inflammatory Cytokines: Potential Links between the Endocannabinoid System and the Kynurenine Pathway in Depression. Int. J. Mol. Sci. 2021, 22, 5903. [Google Scholar] [CrossRef]
- Ward, S.J.; Tuma, R.F. Endocannabinoids. Encycl. Neurol. Sci. 2014, 42–47. [Google Scholar] [CrossRef]
- Forteza, F.; Giorgini, G.; Raymond, F. Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome. Cells 2021, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 Channels Are Critical Brain Inflammation Detectors and Neuropathic Pain Biomarkers in Mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef] [PubMed]
- El Manira, A.; Kyriakatos, A. The Role of Endocannabinoid Signaling in Motor Control. Physiology 2010, 25, 230–238. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Shosaku, T.; Kano, M. Endocannabinoid-Mediated Retrograde Modulation of Synaptic Transmission. Curr. Opin. Neurobiol. 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-Induced Endocannabinoid Signaling Is Modulated by Intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef]
- Singh, J.; Ellingson, C.J.; Shafiq, M.A.; Alcorn, J.; Neary, J.P. Cannabidiol and Cognition: A Literature Review of Human Randomized Controlled Trials. Behav. Pharmacol. 2025, 36, 203–216. [Google Scholar] [CrossRef]
- Shahbazi-Raz, F.; Meister, D.; Mohammadzadeh, A.; Trant, J.F. How THC Works: Explaining Ligand Affinity for, and Partial Agonism of, Cannabinoid Receptor 1. iScience 2025, 28, 112706. [Google Scholar] [CrossRef]
- Ketcherside, A.; Noble, L.J.; McIntyre, C.K.; Filbey, F.M. Cannabinoid Receptor 1 Gene by Cannabis Use Interaction on CB1 Receptor Density. Cannabis Cannabinoid Res. 2017, 2, 202–209. [Google Scholar] [CrossRef]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules 2023, 28, 5980. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Basu, S.; Maresz, K.; Bifulco, M.; Dittel, B.N. What We Know and Do Not Know about the Cannabinoid Receptor 2 (CB2). Semin. Immunol. 2014, 26, 369–379. [Google Scholar] [CrossRef]
- Alfulaij, N.; Meiners, F.; Michalek, J.; Small-Howard, A.L.; Turner, H.C.; Stokes, A.J. Cannabinoids, the Heart of the Matter. J. Am. Heart Assoc. 2018, 7, e009099. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Claasen, J.-H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation Is Detrimental for Neurogenesis in Adult Brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Kohman, R.A.; Rhodes, J.S. Neurogenesis, Inflammation and Behavior. Brain Behav. Immun. 2013, 27, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Venters, H.D.; Broussard, S.R.; Zhou, J.-H.; Bluthé, R.-M.; Freund, G.G.; Johnson, R.W.; Dantzer, R.; Kelley, K.W. Tumor Necrosis Factorα and Insulin-like Growth Factor-I in the Brain: Is the Whole Greater than the Sum of Its Parts? J. Neuroimmunol. 2001, 119, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Balazs, R.; Soiampornkul, R.; Thangnipon, W.; Cotman, C.W. Interleukin-1β Impairs Brain Derived Neurotrophic Factor-Induced Signal Transduction. Neurobiol. Aging 2008, 29, 1380–1393. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS Inflammation and Neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; van Veluw, S.J.; et al. The Neurovasculome: Key Roles in Brain Health and Cognitive Impairment: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke 2023, 54, e251–e271. [Google Scholar] [CrossRef]
- Lovinger, D.M. Communication Networks in the Brain. Alcohol Res. Health 2008, 31, 196–214. [Google Scholar]
- Antonelli, M.; Kushner, I. It’s Time to Redefine Inflammation. FASEB J. 2017, 31, 1787–1791. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- De Paula, G.C.; Simões, R.F.; Garcia-Serrano, A.M.; Duarte, J.M.N. High-Fat and High-Sucrose Diet-induced Hypothalamic Inflammation Shows Sex Specific Features in Mice. Neurochem. Res. 2024, 49, 3356–3366. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, B.; Chen, K. The Impact of Physical Exercise on Neuroinflammation Mechanism in Alzheimer’s Disease. Front. Aging Neurosci. 2024, 16, 1444716. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation during Skeletal Muscle Regeneration and Tissue Remodeling: Application to Exercise-Induced Muscle Damage Management. Immunol. Cell Biol. 2016, 94, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 Signaling in Acute Exercise and Chronic Training: Potential Consequences for Health and Athletic Performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Walter, L.; Stella, N. Cannabinoids and Neuroinflammation. Br. J. Pharmacol. 2004, 141, 775. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-Induced Apoptosis in Immune Cells as a Pathway to Immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Shohami, E.; Cohen-Yeshurun, A.; Magid, L.; Algali, M.; Mechoulam, R. Endocannabinoids and Traumatic Brain Injury. Br. J. Pharmacol. 2011, 163, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Rapino, C.; Talamonti, E.; Leuti, A.; Lanuti, M.; Gueniche, A.; Jourdain, R.; Breton, L.; Maccarrone, M. Anandamide Suppresses Proinflammatory T Cell Responses In Vitro through Type-1 Cannabinoid Receptor-Mediated mTOR Inhibition in Human Keratinocytes. J. Immunol. 2016, 197, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Pestonjamasp, V.K.; Burstein, S.H. Anandamide Synthesis Is Induced by Arachidonate Mobilizing Agonists in Cells of the Immune System. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1998, 1394, 249–260. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Arai, S.; Waku, K.; Sugiura, T. Activation by 2-Arachidonoylglycerol, an Endogenous Cannabinoid Receptor Ligand, of P42/44 Mitogen-Activated Protein Kinase in HL-60 Cells1. J. Biochem. 2001, 129, 665–669. [Google Scholar] [CrossRef]
- Leonard, B.E.; Aricioglu, F. Cannabinoids and Neuroinflammation: Therapeutic Implications. J. Affect. Disord. Rep. 2023, 12, 100463. [Google Scholar] [CrossRef]
- Rahaman, O.; Ganguly, D. Endocannabinoids in Immune Regulation and Immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Production of Endocannabinoids by Activated T Cells and B Cells Modulates Inflammation Associated with Delayed-Type Hypersensitivity. Eur. J. Immunol. 2016, 46, 1472–1479. [Google Scholar] [CrossRef]
- Ruhl, T.; Corsten, C.; Beier, J.P.; Kim, B.-S. The Immunosuppressive Effect of the Endocannabinoid System on the Inflammatory Phenotypes of Macrophages and Mesenchymal Stromal Cells: A Comparative Study. Pharmacol. Rep. 2021, 73, 143–153. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Moreno-Martet, M.; Rodríguez-Cueto, C.; Palomo-Garo, C.; Gómez-Cañas, M.; Valdeolivas, S.; Guaza, C.; Romero, J.; Guzmán, M.; Mechoulam, R.; et al. Prospects for Cannabinoid Therapies in Basal Ganglia Disorders. Br. J. Pharmacol. 2011, 163, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Crossland, B.W.; Rigby, B.R.; Duplanty, A.A.; King, G.A.; Juma, S.; Levine, N.A.; Clark, C.E.; Ramirez, K.P.; Varone, N.L. Acute Supplementation with Cannabidiol Does Not Attenuate Inflammation or Improve Measures of Performance Following Strenuous Exercise. Healthcare 2022, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Sahinovic, A.; Irwin, C.; Doohan, P.T.; Kevin, R.C.; Cox, A.J.; Lau, N.S.; Desbrow, B.; Johnson, N.A.; Sabag, A.; Hislop, M.; et al. Effects of Cannabidiol on Exercise Physiology and Bioenergetics: A Randomised Controlled Pilot Trial. Sports Med. Open 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.; Tolusso, D.; Pacheco, G.; Brgoch, S.; Nguyen, V. A Pilot Study on Cannabidiol (CBD) and Eccentric Exercise: Impact on Inflammation, Performance, and Pain. Int. J. Exerc. Sci. 2023, 16, 109–117. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Fallas-Campos, A. Cannabidiol in Sports: Insights on How CBD Could Improve Performance and Recovery. Front. Pharmacol. 2023, 14, 1210202. [Google Scholar] [CrossRef]
- Aloufi, N.; Namkung, Y.; Traboulsi, H.; Wilson, E.T.; Laporte, S.A.; Kaplan, B.L.F.; Ross, M.K.; Nair, P.; Eidelman, D.H.; Baglole, C.J. Standardized Cannabis Smoke Extract Induces Inflammation in Human Lung Fibroblasts. Front. Pharmacol. 2022, 13, 852029. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Prini, P.; Rubino, T.; Parolaro, D. Cortical Neuroinflammation Contributes to Long-Term Cognitive Dysfunctions Following Adolescent Delta-9-Tetrahydrocannabinol Treatment in Female Rats. Eur. Neuropsychopharmacol. 2015, 25, 2404–2415. [Google Scholar] [CrossRef]
- Wong, A.; Gunasekaran, N.; Hancock, D.P.; Denyer, G.S.; Meng, L.; Radford, J.L.; McGregor, I.S.; Arnold, J.C. The Major Plant-Derived Cannabinoid Δ(9)-Tetrahydrocannabinol Promotes Hypertrophy and Macrophage Infiltration in Adipose Tissue. Horm. Metab. Res. Thieme Connect. 2012, 44, 105–113. [Google Scholar] [CrossRef]
- Cutando, L.; Busquets-Garcia, A.; Puighermanal, E.; Gomis-González, M.; Delgado-García, J.M.; Gruart, A.; Maldonado, R.; Ozaita, A. Microglial Activation Underlies Cerebellar Deficits Produced by Repeated Cannabis Exposure. J. Clin. Investig. 2013, 123, 2816–2831. [Google Scholar] [CrossRef]
- Jamontt, J.; Molleman, A.; Pertwee, R.; Parsons, M. The Effects of Δ9-Tetrahydrocannabinol and Cannabidiol Alone and in Combination on Damage, Inflammation and in Vitro Motility Disturbances in Rat Colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef]
- Gaffal, E.; Cron, M.; Glodde, N.; Tüting, T. Anti-Inflammatory Activity of Topical THC in DNFB-Mediated Mouse Allergic Contact Dermatitis Independent of CB1 and CB2 Receptors. Allergy 2013, 68, 994–1000. [Google Scholar] [CrossRef]
- Ismail, M.; Hasan, H.; El-Orfali, Y.; Ismail, H.; Khawaja, G. Anti-Inflammatory, Antioxidative, and Hepatoprotective Effects of Trans Δ9-Tetrahydrocannabinol/Sesame Oil on Adjuvant-Induced Arthritis in Rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 9365464. [Google Scholar] [CrossRef]
- Sellami, M.; Bragazzi, N.L.; Aboghaba, B.; Elrayess, M.A. The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Front. Immunol. 2021, 12, 631873. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Koppo, K. Cannabinoid Receptor 1 Expression Is Higher in Muscle of Old vs. Young Males, and Increases upon Resistance Exercise in Older Adults. Sci. Rep. 2021, 11, 18349. [Google Scholar] [CrossRef]
- Valencia-Sánchez, S.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Prospéro-García, O.; Morales-Montor, J.; Drucker-Colín, R. Chronic Exercise Modulates the Cellular Immunity and Its Cannabinoid Receptors Expression. PLoS ONE 2019, 14, e0220542. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 6301. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Inflammatory Neuroprotection Following Traumatic Brain Injury. Science 2016, 353, 783–785. [Google Scholar] [CrossRef]
- de Vries, H.E.; Blom-Roosemalen, M.C.M.; van Oosten, M.; de Boer, A.G.; van Berkel, T.J.C.; Breimer, D.D.; Kuiper, J. The Influence of Cytokines on the Integrity of the Blood-Brain Barrier in Vitro. J. Neuroimmunol. 1996, 64, 37–43. [Google Scholar] [CrossRef]
- Laflamme, N.; Lacroix, S.; Rivest, S. An Essential Role of Interleukin-1β in Mediating NF-κB Activity and COX-2 Transcription in Cells of the Blood–Brain Barrier in Response to a Systemic and Localized Inflammation But Not During Endotoxemia. J. Neurosci. 1999, 19, 10923–10930. [Google Scholar] [CrossRef]
- Cunningham, A.J.; Murray, C.A.; O’Neill, L.A.J.; Lynch, M.A.; O’Connor, J.J. Interleukin-1β (IL-1β) and Tumour Necrosis Factor (TNF) Inhibit Long-Term Potentiation in the Rat Dentate Gyrus in Vitro. Neurosci. Lett. 1996, 203, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-Like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.-J. Mitochondrial Dysfunction and Cell Death in Neurodegenerative Diseases through Nitroxidative Stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef]
- Carson, M.J.; Cameron Thrash, J.; Walter, B. The Cellular Response in Neuroinflammation: The Role of Leukocytes, Microglia and Astrocytes in Neuronal Death and Survival. Clin. Neurosci. Res. 2006, 6, 237–245. [Google Scholar] [CrossRef]
- Yekhtin, Z.; Petukhov, D.; Khuja, I.; Kogan, N.M.; Or, R.; Almogi-Hazan, O. Differential Metabolic Pathways Underlie THC- and CBD-Mediated Inhibition of B-Cell Activation in Both Young and Aged Mice. Front. Immunol. 2025, 16, 1605474. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Ruiz-Iglesias, P.; Périz, M.; Franch, À.; Pérez-Cano, F.J.; Camps-Bossacoma, M.; Castell, M. Changes in Lymphocyte Composition and Functionality After Intensive Training and Exhausting Exercise in Rats. Front. Physiol. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L. Emerging Role of the CB2 Cannabinoid Receptor in Immune Regulation and Therapeutic Prospects. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef]
- Nielsen, H.B. Lymphocyte Responses to Maximal Exercise: A Physiological Perspective. Sports Med. 2003, 33, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Voskoboynik, Y.; McCulloch, A.D.; Sahoo, D. Macrophages on the Run: Exercise Balances Macrophage Polarization for Improved Health. Mol. Metab. 2024, 90, 102058. [Google Scholar] [CrossRef]
- Lesnak, J.B.; Berardi, G.; Sluka, K.A. Influence of Routine Exercise on the Peripheral Immune System to Prevent and Alleviate Pain. Neurobiol. Pain 2023, 13, 100126. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.L.F. The Role of CB1 in Immune Modulation by Cannabinoids. Pharmacol. Ther. 2013, 137, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cepero, M.; Friedman, M.; Klein, T.; Friedman, H. Tetrahydrocannabinol-Induced Suppression of Macrophage Spreading and Phagocytic Activity In Vitro. J. Leukoc. Biol. 1986, 39, 679–686. [Google Scholar] [CrossRef]
- Llorca-Bofí, V.; Mur, M.; Font, M.; Palacios-Garrán, R.; Sellart, M.; del Agua-Martínez, E.; Bioque, M.; Arteaga-Henríquez, G. Differences in Total and Differential White Blood Cell Counts and in Inflammatory Parameters between Psychiatric Inpatients with and without Recent Consumption of Cannabinoids, Opioids, or Cocaine: A Retrospective Single-Center Study. Brain Behav. Immun. Health 2024, 42, 100898. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, T.Y.; Woo, S.W.; Moon, H.Y. Effect of Exercise-Induced Neutrophil Maturation on Skeletal Muscle Repair In Vitro. Biochem. Biophys. Rep. 2024, 38, 101699. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, L.; Fan, X.; Zhao, X.; Chang, N.; Yang, L.; Li, L. Neutrophil Chemotaxis and NETosis in Murine Chronic Liver Injury via Cannabinoid Receptor 1/ Gαi/o/ ROS/ P38 MAPK Signaling Pathway. Cells 2020, 9, 373. [Google Scholar] [CrossRef]
- Wang, F.; Bashiri Dezfouli, A.; Multhoff, G. The Immunomodulatory Effects of Cannabidiol on Hsp70-Activated NK Cells and Tumor Target Cells. Mol. Immunol. 2024, 174, 1–10. [Google Scholar] [CrossRef]
- Quintana-Mendias, E.; Rodríguez-Villalobos, J.M.; Gastelum-Arellanez, A.; Cervantes, N.; Carrasco-Legleu, C.E.; Espino-Solis, G.P. The Effect of Acute Physical Exercise on Natural Killer Cells Populations and Cytokine Levels in Healthy Women. Sports 2023, 11, 189. [Google Scholar] [CrossRef]
- Specter, S.C.; Klein, T.W.; Newton, C.; Mondragon, M.; Widen, R.; Friedman, H. Marijuana Effects on Immunity: Suppression of Human Natural Killer Cell Activity by Delta-9-Tetrahydrocannabinol. Int. J. Immunopharmacol. 1986, 8, 741–745. [Google Scholar] [CrossRef]
- Benyó, Z.; Ruisanchez, É.; Leszl-Ishiguro, M.; Sándor, P.; Pacher, P. Endocannabinoids in Cerebrovascular Regulation. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H785–H801. [Google Scholar] [CrossRef]
- Hiley, C.R. Endocannabinoids and the Heart. J. Cardiovasc. Pharmacol. 2009, 53, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Puhl, S.-L. Cannabinoid-Sensitive Receptors in Cardiac Physiology and Ischaemia. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2020, 1867, 118462. [Google Scholar] [CrossRef]
- Hillard, C.J. Endocannabinoids and Vascular Function. J. Pharmacol. Exp. Ther. 2000, 294, 27. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, M.L.; Thewke, D.P. The Endocannabinoid System and Heart Disease: The Role of Cannabinoid Receptor Type 2. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 34–51. [Google Scholar] [CrossRef]
- Fujii, M.; Sherchan, P.; Krafft, P.R.; Rolland, W.B.; Soejima, Y.; Zhang, J.H. Cannabinoid Type 2 Receptor Stimulation Attenuates Brain Edema by Reducing Cerebral Leukocyte Infiltration Following Subarachnoid Hemorrhage in Rats. J. Neurol. Sci. 2014, 342, 101–106. [Google Scholar] [CrossRef]
- Fujii, M.; Sherchan, P.; Soejima, Y.; Hasegawa, Y.; Flores, J.; Doycheva, D.; Zhang, J.H. Cannabinoid Receptor Type 2 Agonist Attenuates Apoptosis by Activation of Phosphorylated CREB–Bcl-2 Pathway after Subarachnoid Hemorrhage in Rats. Exp. Neurol. 2014, 261, 396–403. [Google Scholar] [CrossRef]
- Stein, E.A.; Fuller, S.A.; Edgemond, W.S.; Campbell, W.B. Selective Effects of the Endogenous Cannabinoid Arachidonylethanolamide (Anandamide) on Regional Cerebral Blood Flow in the Rat. Neuropsychopharmacol 1998, 19, 481–491. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Osei-Hyiaman, D.; Offertáler, L.; Liu, J.; Harvey-White, J.; Brassai, A.; Járai, Z.; Cravatt, B.F.; Kunos, G. Hemodynamic Profile, Responsiveness to Anandamide, and Baroreflex Sensitivity of Mice Lacking Fatty Acid Amide Hydrolase. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H533–H541. [Google Scholar] [CrossRef]
- Mathew, R.J.; Wilson, W.H.; Humphreys, D.F.; Lowe, J.V.; Wiethe, K.E. Regional Cerebral Blood Flow after Marijuana Smoking. J. Cereb. Blood Flow Metab. 1992, 12, 750–758. [Google Scholar] [CrossRef]
- Mathew, R.J.; Wilson, W.H.; Chiu, N.Y.; Turkington, T.G.; Degrado, T.R.; Coleman, R.E. Regional Cerebral Blood Flow and Depersonalization after Tetrahydrocannabinol Administration. Acta Psychiatr. Scand. 1999, 100, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ogunbiyi, M.O.; Hindocha, C.; Freeman, T.P.; Bloomfield, M.A.P. Acute and Chronic Effects of Δ9-Tetrahydrocannabinol (THC) on Cerebral Blood Flow: A Systematic Review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 101, 109900. [Google Scholar] [CrossRef]
- Wagner, J.A.; Járai, Z.; Bátkai, S.; Kunos, G. Hemodynamic Effects of Cannabinoids: Coronary and Cerebral Vasodilation Mediated by Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 2001, 423, 203–210. [Google Scholar] [CrossRef]
- Mathew, R.J.; Wilson, W.H. The Effects of Marijuana on Cerebral Blood Flow and Metabolism. In Marijuana/Cannabinoids; CRC Press: Boca Raton, FL, USA, 1992; ISBN 978-0-429-27627-9. [Google Scholar]
- Bloom, A.S.; Tershner, S.; Fuller, S.A.; Stein, E.A. Cannabinoid-Induced Alterations in Regional Cerebral Blood Flow in the Rat. Pharmacol. Biochem. Behav. 1997, 57, 625–631. [Google Scholar] [CrossRef]
- Cheung, C.P.; Coates, A.M.; Baker, R.E.; Burr, J.F. Acute Effects of Cannabis Inhalation on Arterial Stiffness, Vascular Endothelial Function, and Cardiac Function. J. Am. Heart Assoc. 2024, 13, e037731. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Hwang, Y.J.; Elton-Marshall, T.; Rehm, J.; Imtiaz, S. The Longitudinal Relationship between Cannabis Use and Hypertension. Drug Alcohol Rev. 2021, 40, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Grant, I.; Bradley, R.; Allison, M.A. Trends in Cannabis Use, Blood Pressure, and Hypertension in Middle-Aged Adults: Findings From NHANES, 2009–2018. Am. J. Hypertens. 2023, 36, 651–659. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.H.; Ghali, J.K. Role of Cannabis in Cardiovascular Disorders. J. Thorac. Dis. 2017, 9, 2079–2092. [Google Scholar] [CrossRef]
- Nardone, M.; Cheung, C.P.; Baker, R.E.; Pfundt, K.; Lee, J.B.; Burr, J.F.; Millar, P.J. Inhalation of THC-Containing Cannabis Selectively Diminishes Cardiac Autonomic Function in Humans. Clin. Auton. Res. 2023, 33, 919–922. [Google Scholar] [CrossRef]
- Hirvonen, J.; Goodwin, R.S.; Li, C.-T.; Terry, G.E.; Zoghbi, S.S.; Morse, C.; Pike, V.W.; Volkow, N.D.; Huestis, M.A.; Innis, R.B. Reversible and Regionally Selective Downregulation of Brain Cannabinoid CB1 Receptors in Chronic Daily Cannabis Smokers. Mol. Psychiatry 2012, 17, 642–649. [Google Scholar] [CrossRef]
- Piscura, M.K.; Henderson-Redmond, A.N.; Barnes, R.C.; Mitra, S.; Guindon, J.; Morgan, D.J. Mechanisms of Cannabinoid Tolerance. Biochem. Pharmacol. 2023, 214, 115665. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Bhagaloo, L.; Piskorski, J.; Neary, J.P. Effects of Phytocannabinoids on Heart Rate Variability and Blood Pressure Variability in Female Post-Concussion Syndrome Patients: Case Series. Can. J. Physiol. Pharmacol. 2022, 100, 192–196. [Google Scholar] [CrossRef]
- Clausen, M.; Pendergast, D.R.; Willer, B.; Leddy, J. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. J. Head Trauma Rehabil. 2016, 31, 215. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxidative Med. Cell. Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef] [PubMed]
- Arbab-Zadeh, A.; Perhonen, M.; Howden, E.; Peshock, R.M.; Zhang, R.; Adams-Huet, B.; Haykowsky, M.J.; Levine, B.D. Cardiac Remodeling in Response to 1 Year of Intensive Endurance Training. Circulation 2014, 130, 2152–2161. [Google Scholar] [CrossRef]
- Bloor, C.M. Angiogenesis during Exercise and Training. Angiogenesis 2005, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P.; Adam, T.C.; Liu, T.T. Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise. Nutrients 2018, 10, 530. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise Training for Blood Pressure: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Lopes, S.; Mesquita-Bastos, J.; Garcia, C.; Bertoquini, S.; Ribau, V.; Teixeira, M.; Ribeiro, I.P.; Melo, J.B.; Oliveira, J.; Figueiredo, D.; et al. Effect of Exercise Training on Ambulatory Blood Pressure Among Patients With Resistant Hypertension: A Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 1317–1323. [Google Scholar] [CrossRef]
- Fagard, R.H.; Cornelissen, V.A. Effect of Exercise on Blood Pressure Control in Hypertensive Patients. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 12–17. [Google Scholar] [CrossRef]
- Alpsoy, Ş. Exercise and Hypertension. In Physical Exercise for Human Health; Xiao, J., Ed.; Springer Nature: Singapore, 2020; pp. 153–167. ISBN 978-981-15-1792-1. [Google Scholar]
- Murrell, C.J.; Cotter, J.D.; Thomas, K.N.; Lucas, S.J.E.; Williams, M.J.A.; Ainslie, P.N. Cerebral Blood Flow and Cerebrovascular Reactivity at Rest and during Sub-Maximal Exercise: Effect of Age and 12-Week Exercise Training. Age 2013, 35, 905–920. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.A.; Atkinson, G. Elevation in Cerebral Blood Flow Velocity with Aerobic Fitness throughout Healthy Human Ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef]
- Steventon, J.J.; Hansen, A.B.; Whittaker, J.R.; Wildfong, K.W.; Nowak-Flück, D.; Tymko, M.M.; Murphy, K.; Ainslie, P.N. Cerebrovascular Function in the Large Arteries Is Maintained Following Moderate Intensity Exercise. Front. Physiol. 2018, 9, 1657. [Google Scholar] [CrossRef]
- Viboolvorakul, S.; Patumraj, S. Exercise Training Could Improve Age-Related Changes in Cerebral Blood Flow and Capillary Vascularity through the Upregulation of VEGF and eNOS. BioMed Res. Int. 2014, 2014, 230791. [Google Scholar] [CrossRef]
- Bolduc, V.; Thorin-Trescases, N.; Thorin, E. Endothelium-Dependent Control of Cerebrovascular Functions Through Age: Exercise for Healthy Cerebrovascular Aging. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H620–H633. [Google Scholar] [CrossRef]
- Lisano, J.K.; Flores, V.A.; Kisiolek, J.N.; Stewart, L.K. Regular Use of Cannabis in Female Athletes Is Associated With a Reduction in Early Anaerobic Power Production. J. Strength Cond. Res. 2023, 37, 616. [Google Scholar] [CrossRef] [PubMed]
- García-Rivas, G.; Lozano, O.; Ramos, M.; Silva-Platas, C.; Bernal-Ramirez, J.; Alves-Figueiredo, H.; Garza, E.V.; Rubio-Infante, N.; Torre, G. Cannabidiol Therapy For Chronic Heart Failure Prevents Cardiac Hypertrophy And Contractile Dysfunction By Reducing Mitochondrial Ros Generation In A Murine Model. J. Card. Fail. 2022, 28, S103. [Google Scholar] [CrossRef]
- Tao, L.; Bei, Y.; Zhang, H.; Xiao, J.; Li, X. Exercise for the Heart: Signaling Pathways. Oncotarget 2015, 6, 20773–20784. [Google Scholar] [CrossRef]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L.A. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell Metab. 2017, 25, 1012–1026. [Google Scholar] [CrossRef]
- Goeder, D.; Kröpfl, J.M.; Angst, T.; Hanssen, H.; Hauser, C.; Infanger, D.; Maurer, D.; Oberhoffer-Fritz, R.; Schmidt-Trucksäss, A.; Königstein, K. VascuFit: Aerobic Exercise Improves Endothelial Function Independent of Cardiovascular Risk: A Randomized-Controlled Trial. Atherosclerosis 2024, 399, 118631. [Google Scholar] [CrossRef] [PubMed]
- More, S.A.; Deore, R.S.; Pawar, H.D.; Sharma, C.; Nakhate, K.T.; Rathod, S.S.; Ojha, S.; Goyal, S.N. CB2 Cannabinoid Receptor as a Potential Target in Myocardial Infarction: Exploration of Molecular Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 1683. [Google Scholar] [CrossRef]
- Jehle, J.; Eich, L.; Danisch, M.; Bagheri, S.; Avraamidou, E.; Pfeifer, P.; Tiyerili, V.; Bindila, L.; Lutz, B.; Nickenig, G. The Endocannabinoid 2-Arachidonoylglycerol Inhibits Endothelial Function and Repair. Int. J. Cardiol. 2021, 323, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. The Endocannabinoid Anandamide Causes Endothelium-Dependent Vasorelaxation in Human Mesenteric Arteries. Pharmacol. Res. 2016, 113, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Haskó, J.; Fazakas, C.; Molnár, J.; Nyúl-Tóth, Á.; Herman, H.; Hermenean, A.; Wilhelm, I.; Persidsky, Y.; Krizbai, I.A. CB2 Receptor Activation Inhibits Melanoma Cell Transmigration through the Blood-Brain Barrier. Int. J. Mol. Sci. 2014, 15, 8063–8074. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Ramos-González, M.; Lozano, O.; Jerjes-Sánchez, C.; García-Rivas, G. Therapeutic Applications of Cannabinoids in Cardiomyopathy and Heart Failure. Oxidative Med. Cell. Longev. 2020, 2020, 4587024. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Huntsman, H.D.; Valero, C.; Skelton, J.T.; Adams, J.T.; Mahmassani, Z.; Boppart, M.D. Pericytes Contribute to Exercise-Induced Skeletal Muscle Hypertrophy. FASEB J. 2012, 26, 856.3. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, R.; Deng, J.; Guo, W. Research Progress in the Management of Vascular Disease with Cannabidiol: A review. J. Cardiothorac. Surg. 2024, 19, 6. [Google Scholar] [CrossRef]
- Dziemitko, S.; Harasim-Symbor, E.; Chabowski, A. How Do Phytocannabinoids Affect Cardiovascular Health? An Update on the Most Common Cardiovascular Diseases. Ther. Adv. Chronic Dis. 2023, 14, 20406223221143239. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Chen, T.; Yang, C. Does Exercise Training Improve the Function of Vascular Smooth Muscle? A Systematic Review and Meta-Analysis. Res. Sports Med. 2022, 30, 577–592. [Google Scholar] [CrossRef]
- De Chiara, V.; Errico, F.; Musella, A.; Rossi, S.; Mataluni, G.; Sacchetti, L.; Siracusano, A.; Castelli, M.; Cavasinni, F.; Bernardi, G.; et al. Voluntary Exercise and Sucrose Consumption Enhance Cannabinoid CB1 Receptor Sensitivity in the Striatum. Neuropsychopharmacol 2010, 35, 374–387. [Google Scholar] [CrossRef][Green Version]
- From, M.; Crosby, K.M. Endocannabinoid and Nitric Oxide Interactions in the Brain. Neuroscience 2025, 569, 267–276. [Google Scholar] [CrossRef]
- Arefirad, T.; Seif, E.; Sepidarkish, M.; Mohammadian Khonsari, N.; Mousavifar, S.A.; Yazdani, S.; Rahimi, F.; Einollahi, F.; Heshmati, J.; Qorbani, M. Effect of Exercise Training on Nitric Oxide and Nitrate/Nitrite (NOx) Production: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 953912. [Google Scholar] [CrossRef]
- Taylor, J.M.; Montgomery, M.H.; Gregory, E.J.; Berman, N.E.J. Exercise Preconditioning Improves Traumatic Brain Injury Outcomes. Brain Res. 2015, 1622, 414–429. [Google Scholar] [CrossRef]
- Fuchs, E.; Flügge, G. Adult Neuroplasticity: More Than 40 Years of Research. Neural Plast. 2014, 2014, 541870. [Google Scholar] [CrossRef]
- Chen, C. Homeostatic Regulation of Brain Functions by Endocannabinoid Signaling. Neural Regen. Res. 2015, 10, 691. [Google Scholar] [CrossRef]
- Lunardi, P.; de Souza, L.W.; dos Santos, B.; Popik, B.; de Oliveira Alvares, L. Effect of the Endocannabinoid System in Memory Updating and Forgetting. Neuroscience 2020, 444, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. The Role of the Endocannabinoid System in Pain. In Pain Control; Schaible, H.-G., Ed.; Springer: Berlin, Heidelberg, 2015; pp. 119–143. ISBN 978-3-662-46450-2. [Google Scholar]
- Ashton, C.H.; Moore, P.B. Endocannabinoid System Dysfunction in Mood and Related Disorders. Acta Psychiatr. Scand. 2011, 124, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Sun, M.; Kang, J.; Zhao, C. Transient Receptor Potential Vanilloid1 (TRPV1) Channel Opens Sesame of T Cell Responses and T Cell-Mediated Inflammatory Diseases. Front. Immunol. 2022, 13, 870952. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endogenous Cannabinoids Mediate Retrograde Signalling at Hippocampal Synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef]
- Diana, M.A.; Marty, A. Endocannabinoid-Mediated Short-Term Synaptic Plasticity: Depolarization-Induced Suppression of Inhibition (DSI) and Depolarization-Induced Suppression of Excitation (DSE). Br. J. Pharmacol. 2004, 142, 9–19. [Google Scholar] [CrossRef]
- Mackie, K. Mechanisms of CB1 Receptor Signaling: Endocannabinoid Modulation of Synaptic Strength. Int. J. Obes. 2006, 30, S19–S23. [Google Scholar] [CrossRef]
- Dudok, B.; Fan, L.Z.; Farrell, J.S.; Malhotra, S.; Homidan, J.; Kim, D.K.; Wenardy, C.; Ramakrishnan, C.; Li, Y.; Deisseroth, K.; et al. Retrograde Endocannabinoid Signaling at Inhibitory Synapses In Vivo. Science 2024, 383, 967–970. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Regehr, W.G. Retrograde Inhibition of Presynaptic Calcium Influx by Endogenous Cannabinoids at Excitatory Synapses onto Purkinje Cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Shosaku, T.; Maejima, T.; Kano, M. Endogenous Cannabinoids Mediate Retrograde Signals from Depolarized Postsynaptic Neurons to Presynaptic Terminals. Neuron 2001, 29, 729–738. [Google Scholar] [CrossRef]
- Seagard, J.L.; Dean, C.; Patel, S.; Rademacher, D.J.; Hopp, F.A.; Schmeling, W.T.; Hillard, C.J. Anandamide Content and Interaction of Endocannabinoid/GABA Modulatory Effects in the NTS on Baroreflex-Evoked Sympathoinhibition. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H992–H1000. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ledri, M.; Tóth, B.; Marchionni, I.; Henstridge, C.M.; Dudok, B.; Kenesei, K.; Barna, L.; Szabó, S.I.; Renkecz, T.; et al. Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release. J. Neurosci. 2015, 35, 10039–10057. [Google Scholar] [CrossRef]
- Carlson, G.; Wang, Y.; Alger, B.E. Endocannabinoids Facilitate the Induction of LTP in the Hippocampus. Nat. Neurosci. 2002, 5, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santisteban, R.; Lillo, A.; Lillo, J.; Rebassa, J.-B.; Contestí, J.S.; Saura, C.A.; Franco, R.; Navarro, G. N-Methyl-D-aspartate (NMDA) and Cannabinoid CB2 Receptors Form Functional Complexes in Cells of the Central Nervous System: Insights into the Therapeutic Potential of Neuronal and Microglial NMDA Receptors. Alzheimer’s Res. Ther. 2021, 13, 184. [Google Scholar] [CrossRef]
- Jennings, E.A.; Vaughan, C.W.; Christie, M.J. Cannabinoid Actions on Rat Superficial Medullary Dorsal Horn Neurons In Vitro. J. Physiol. 2001, 534, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Gifford, A.N.; Ashby, C.R. Electrically Evoked Acetylcholine Release from Hippocampal Slices Is Inhibited by the Cannabinoid Receptor Agonist, WIN 55212-2, and Is Potentiated by the Cannabinoid Antagonist, SR 141716A. J. Pharmacol. Exp. Ther. 1996, 277, 1431–1436. [Google Scholar] [CrossRef]
- Cadogan, A.K.; Alexander, S.P.; Boyd, E.A.; Kendall, D.A. Influence of Cannabinoids on Electrically Evoked Dopamine Release and Cyclic AMP Generation in the Rat Striatum. J. Neurochem. 1997, 69, 1131–1137. [Google Scholar] [CrossRef]
- Ishac, E.J.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of Exocytotic Noradrenaline Release by Presynaptic Cannabinoid CB1 Receptors on Peripheral Sympathetic Nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef]
- Nakazi, M.; Bauer, U.; Nickel, T.; Kathmann, M.; Schlicker, E. Inhibition of Serotonin Release in the Mouse Brain via Presynaptic Cannabinoid CB1 Receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 361, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cheer, J.F.; Wassum, K.M.; Sombers, L.A.; Heien, M.L.A.V.; Ariansen, J.L.; Aragona, B.J.; Phillips, P.E.M.; Wightman, R.M. Phasic Dopamine Release Evoked by Abused Substances Requires Cannabinoid Receptor Activation. J. Neurosci. 2007, 27, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, K.U.; Holden, M.E.; Wiley, M.T.; Suppiramaniam, V.; Reed, M.N. Effects of Cannabis on Glutamatergic Neurotransmission: The Interplay between Cannabinoids and Glutamate. Cells 2024, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Testai, F.D.; Gorelick, P.B.; Aparicio, H.J.; Filbey, F.M.; Gonzalez, R.; Gottesman, R.F.; Melis, M.; Piano, M.R.; Rubino, T.; Song, S.Y.; et al. Use of Marijuana: Effect on Brain Health: A Scientific Statement From the American Heart Association. Stroke 2022, 53, e176–e187. [Google Scholar] [CrossRef]
- Pretzsch, C.M.; Freyberg, J.; Voinescu, B.; Lythgoe, D.; Horder, J.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; et al. Effects of Cannabidiol on Brain Excitation and Inhibition Systems; a Randomised Placebo-Controlled Single Dose Trial during Magnetic Resonance Spectroscopy in Adults with and without Autism Spectrum Disorder. Neuropsychopharmacology 2019, 44, 1398–1405. [Google Scholar] [CrossRef]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R.L. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef]
- Mottarlini, F.; Fumagalli, M.; Castillo-Díaz, F.; Piazza, S.; Targa, G.; Sangiovanni, E.; Pacchetti, B.; Sodergren, M.H.; Dell’Agli, M.; Fumagalli, F.; et al. Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network. Biomedicines 2022, 10, 1853. [Google Scholar] [CrossRef]
- Umegaki, H.; Sakurai, T.; Arai, H. Active Life for Brain Health: A Narrative Review of the Mechanism Underlying the Protective Effects of Physical Activity on the Brain. Front. Aging Neurosci. 2021, 13, 761674. [Google Scholar] [CrossRef]
- Silva, N.C.B.S.; Barha, C.K.; Erickson, K.I.; Kramer, A.F.; Liu-Ambrose, T. Physical Exercise, Cognition, and Brain Health in Aging. Trends Neurosci. 2024, 47, 402–417. [Google Scholar] [CrossRef]
- TONG, L.; Shen, H.; Perreau, V.M.; Balazs, R.; Cotman, C.W. Effects of Exercise on Gene-Expression Profile in the Rat Hippocampus. Neurobiol. Dis. 2001, 8, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, H.; Zeng, Y.; Qu, Y.; Liu, Q.; Zhao, F.; Duan, J.; Jiang, Y.; Li, S.; Ying, J.; et al. Physical Exercise Promotes Brain Remodeling by Regulating Epigenetics, Neuroplasticity and Neurotrophins. Rev. Neurosci. 2021, 32, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A Meta-Analytic Review of the Effects of Exercise on Brain-Derived Neurotrophic Factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Jung, M.; Hillman, C.H.; Kang, M.; Loprinzi, P.D. Interrelationships between Exercise, Functional Connectivity, and Cognition among Healthy Adults: A Systematic Review. Psychophysiology 2022, 59, e14014. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, C.; Chen, A. A Systematic Review and Meta-Analysis of the Effects of Physical Exercise on White Matter Integrity and Cognitive Function in Older Adults. Geroscience 2024, 46, 2641–2651. [Google Scholar] [CrossRef]
- Aghjayan, S.L.; Lesnovskaya, A.; Esteban-Cornejo, I.; Peven, J.C.; Stillman, C.M.; Erickson, K.I. Aerobic Exercise, Cardiorespiratory Fitness, and the Human Hippocampus. Hippocampus 2021, 31, 817–844. [Google Scholar] [CrossRef]
- Wilckens, K.A.; Stillman, C.M.; Waiwood, A.M.; Kang, C.; Leckie, R.L.; Peven, J.C.; Foust, J.E.; Fraundorf, S.H.; Erickson, K.I. Exercise Interventions Preserve Hippocampal Volume: A Meta-Analysis. Hippocampus 2021, 31, 335–347. [Google Scholar] [CrossRef]
- Hendrikse, J.; Chye, Y.; Thompson, S.; Rogasch, N.C.; Suo, C.; Coxon, J.P.; Yücel, M. Regular Aerobic Exercise Is Positively Associated with Hippocampal Structure and Function in Young and Middle-Aged Adults. Hippocampus 2022, 32, 137–152. [Google Scholar] [CrossRef]
- Cirillo, J.; Lavender, A.P.; Ridding, M.C.; Semmler, J.G. Motor Cortex Plasticity Induced by Paired Associative Stimulation Is Enhanced in Physically Active Individuals. J. Physiol. 2009, 587, 5831–5842. [Google Scholar] [CrossRef]
- Hayek, L.E.; Khalifeh, M.; Zibara, V.; Assaad, R.A.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Smith, A.E.; Goldsworthy, M.R.; Garside, T.; Wood, F.M.; Ridding, M.C. The Influence of a Single Bout of Aerobic Exercise on Short-Interval Intracortical Excitability. Exp. Brain Res. 2014, 232, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Staines, W.R. The Effects of Acute Aerobic Exercise on the Primary Motor Cortex. J. Mot. Behav. 2015, 47, 328–339. [Google Scholar] [CrossRef]
- Mellow, M.L.; Goldsworthy, M.R.; Coussens, S.; Smith, A.E. Acute Aerobic Exercise and Neuroplasticity of the Motor Cortex: A Systematic Review. J. Sci. Med. Sport 2020, 23, 408–414. [Google Scholar] [CrossRef]
- Maccarrone, M.; Fiori, A.; Bari, M.; Granata, F.; Gasperi, V.; De Stefano, M.E.; Finazzi-Agrò, A.; Strom, R. Regulation by Cannabinoid Receptors of Anandamide Transport across the Blood-Brain Barrier and through Other Endothelial Cells. Thromb. Haemost. 2006, 95, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical Activity and the Endocannabinoid System: An Overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.-X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense Exercise Increases Circulating Endocannabinoid and BDNF Levels in Humans—Possible Implications for Reward and Depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef]
- Mang, C.S.; Snow, N.J.; Campbell, K.L.; Ross, C.J.D.; Boyd, L.A. A Single Bout of High-Intensity Aerobic Exercise Facilitates Response to Paired Associative Stimulation and Promotes Sequence-Specific Implicit Motor Learning. J. Appl. Physiol. 2014, 117, 1325–1336. [Google Scholar] [CrossRef]
- Mang, C.S.; Brown, K.E.; Neva, J.L.; Snow, N.J.; Campbell, K.L.; Boyd, L.A. Promoting Motor Cortical Plasticity with Acute Aerobic Exercise: A Role for Cerebellar Circuits. Neural Plast. 2016, 2016, 6797928. [Google Scholar] [CrossRef]
- Cooper, M.A.; Kluding, P.M.; Wright, D.E. Emerging Relationships between Exercise, Sensory Nerves, and Neuropathic Pain. Front. Neurosci. 2016, 10, 372. [Google Scholar] [CrossRef]
- Walter, C.; Oertel, B.G.; Felden, L.; Kell, C.A.; Nöth, U.; Vermehren, J.; Kaiser, J.; Deichmann, R.; Lötsch, J. Brain Mapping-Based Model of Δ9-Tetrahydrocannabinol Effects on Connectivity in the Pain Matrix. Neuropsychopharmacology 2016, 41, 1659–1669. [Google Scholar] [CrossRef]
- Haynes, E.M.K.; Kalmar, J.M.; Vis-Dunbar, M.; Crosby, K.M.; Mriduraj, A.; Jakobi, J.M. A Systematic Review of How Cannabinoids Affect Motoneuron Output. J. Neurophysiol. 2023, 130, 247–263. [Google Scholar] [CrossRef]
- Gardiner, P.; Dai, Y.; Heckman, C.J. Effects of Exercise Training on Alpha-Motoneurons. J. Appl. Physiol. (1985) 2006, 101, 1228–1236. [Google Scholar] [CrossRef]
- Eo, S.-J.; Leem, Y.-H. Effects of Exercise Intensity on the Reactive Astrocyte Polarization in the Medial Prefrontal Cortex. Phys. Act. Nutr. 2023, 27, 19–24. [Google Scholar] [CrossRef]
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid Signaling in Astrocytes. Glia 2023, 71, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Agata, V.; Musumeci, G. Role of Exercise in the Brain: Focus on Oligodendrocytes and Remyelination. Neural Regen. Res. 2023, 18, 2645–2646. [Google Scholar] [CrossRef]
- Huerga-Gómez, A.; Aguado, T.; Sánchez-de la Torre, A.; Bernal-Chico, A.; Matute, C.; Mato, S.; Guzmán, M.; Galve-Roperh, I.; Palazuelos, J. Δ9-Tetrahydrocannabinol Promotes Oligodendrocyte Development and CNS Myelination In Vivo. Glia 2021, 69, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Michaels, N.J.; Ilyntskyy, S.; Keough, M.B.; Kovalchuk, O.; Yong, V.W. Multimodal Enhancement of Remyelination by Exercise with a Pivotal Role for Oligodendroglial PGC1α. Cell Rep. 2018, 24, 3167–3179. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, E.; Vela, J.M.; Arévalo-Martín, A.; Almazán, G.; Molina-Holgado, F.; Borrell, J.; Guaza, C. Cannabinoids Promote Oligodendrocyte Progenitor Survival: Involvement of Cannabinoid Receptors and Phosphatidylinositol-3 Kinase/Akt Signaling. J. Neurosci. 2002, 22, 9742–9753. [Google Scholar] [CrossRef]
- Strohm, A.O.; Majewska, A.K. Physical Exercise Regulates Microglia in Health and Disease. Front. Neurosci. 2024, 18, 1420322. [Google Scholar] [CrossRef]
- Duffy, S.S.; Hayes, J.P.; Fiore, N.T.; Moalem-Taylor, G. The Cannabinoid System and Microglia in Health and Disease. Neuropharmacology 2021, 190, 108555. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol Prevents LPS-Induced Microglial Inflammation by Inhibiting ROS/NF-κB-Dependent Signaling and Glucose Consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Hainline, B.; Turner, J.A.; Caneiro, J.P.; Stewart, M.; Moseley, G.L. Pain in Elite Athletes—Neurophysiological, Biomechanical and Psychosocial Considerations: A Narrative Review. Br. J. Sports Med. 2017, 51, 1259–1264. [Google Scholar] [CrossRef]
- Zideman, D.A.; Derman, W.; Hainline, B.; Moseley, G.L.; Orchard, J.; Pluim, B.M.; Siebert, C.H.; Turner, J.A. Management of Pain in Elite Athletes: Identified Gaps in Knowledge and Future Research Directions. Clin. J. Sport Med. 2018, 28, 485. [Google Scholar] [CrossRef]
- Überall, M.A. A Review of Scientific Evidence for THC:CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J. Pain Res. 2020, 13, 399–410. [Google Scholar] [CrossRef]
- Richardson, J.D. Cannabinoids Modulate Pain by Multiple Mechanisms of Action. J. Pain 2000, 1, 2–14. [Google Scholar] [CrossRef]
- Starowicz, K.; Finn, D.P. Chapter Thirteen—Cannabinoids and Pain: Sites and Mechanisms of Action. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Cannabinoid Pharmacology; Academic Press: New York, NY, USA, 2017; Volume 80, pp. 437–475. [Google Scholar]
- Üeberall, M.A.; Essner, U.; Mueller-Schwefe, G.H. Effectiveness and Tolerability of THC:CBD Oromucosal Spray as Add-on Measure in Patients with Severe Chronic Pain: Analysis of 12-Week Open-Label Real-World Data Provided by the German Pain e-Registry. J. Pain Res. 2019, 12, 1577–1604. [Google Scholar] [CrossRef] [PubMed]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Singh, J.; Neary, J.P. Neuroprotection Following Concussion: The Potential Role for Cannabidiol. Can. J. Neurol. Sci. 2020, 47, 289–300. [Google Scholar] [CrossRef]
- Voormolen, D.C.; Haagsma, J.A.; Polinder, S.; Maas, A.I.R.; Steyerberg, E.W.; Vuleković, P.; Sewalt, C.A.; Gravesteijn, B.Y.; Covic, A.; Andelic, N.; et al. Post-Concussion Symptoms in Complicated vs. Uncomplicated Mild Traumatic Brain Injury Patients at Three and Six Months Post-Injury: Results from the CENTER-TBI Study. J. Clin. Med. 2019, 8, 1921. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75 (Suppl. S4), S24–S33. [Google Scholar] [CrossRef]
- Kroll, H.R. Exercise Therapy for Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.; Hinds, A.; Sirica, D.; Willer, B. The Role of Controlled Exercise in Concussion Management. PM&R 2016, 8, S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Kolakowsky-Hayner, S.A.; Ghajar, J.; Balamane, M. The Effect of Physical Exercise After a Concussion: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2018, 46, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Haider, M.N.; Ellis, M.J.; Mannix, R.; Darling, S.R.; Freitas, M.S.; Suffoletto, H.N.; Leiter, J.; Cordingley, D.M.; Willer, B. Early Subthreshold Aerobic Exercise for Sport-Related Concussion: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 319–325. [Google Scholar] [CrossRef]
| Model | Dose Intake | Tissue | Outcome | Source |
|---|---|---|---|---|
| Adult Mice | 1–20 mg/kg, twice daily | Cerebellar microglia | Dysregulation of CB1 and microglia activation | [83] |
| Adolescent Rats | 2.5–10 mg/kg, twice daily | Prefrontal cortex | Increase in TNF-α and IL-10 levels | [81] |
| Adult Rats | 10 mg/kg injected intraperitoneally | Adipose tissue | Increase in macrophage infiltration, TNF-α levels and adipocyte hypertrophy | [82] |
| Adult Rats | Separate 5, 10 and 20 mg/kg daily | Colon (colitis condition) | Decrease in TNF-α, IL-8 and neutrophil infiltration | [84] |
| Adult Mice | 30 µg in 20 µL of acetone applied topically | Ear tissue | Inhibition of interferon γ production, decrease in macrophage migration | [85] |
| Adjuvant-induced arthritic Rats | 2.5 mg/kg daily mixed in sesame oil | Hind paws | Reduction in the concentration levels of TNF-α, IL-1β, and IL-6. | [86] |
| Immune System Cell | Role(s) | Cannabinoid Influence | Exercise Influence | Source(s) |
|---|---|---|---|---|
| Lymphocyte B | -Produce antibodies -Keep “copy” of alien antigens | -CBD and THC mediate its inhibition -CB1 activation amplifies antibody production -CB2 activation reduces antibody production | -Acute exercise increases proliferation -Increase production of immunoglobulin | [100,101,102,103] |
| Macrophage | -Phagocytosis of aliens -Antigen detection | -CBD increases TNF-α secretion -THC reduces release of TNF-α and increases release of IL-1 | -Acute exercise is pro-inflammatory -Habitual exercise reduces overall inflammation -Released cytokines modulate macrophage activity | [104,105,106,107] |
| Neutrophil | -First responders -Phagocytosis of aliens -Tissue repair | -CB1 activation modulates chemotaxis -CB2 activation, CBD, and THC inhibit recruitment to sites of inflammation -THC reduces release of TNF-α and IL-6 | -Increased proliferation and mobilization | [108,109,110] |
| Natural Killer | -Kill harmful cells -Produce and release cytokines | -AEA and 2-AG induce migration and increase cytotoxicity -CBD inhibits its growth -THC reduces killing abilities | -Increased cytotoxicity -Increased proliferation and mobilization -Excessive exercise reduces its impact | [111,112,113] |
| Vascular System Cell | Role(s) | Cannabinoid Influence | Exercise Influence | Sources |
|---|---|---|---|---|
| Cardiomyocyte | -Generate contraction forces of heart -Secrete regulatory proteins (cardiokines) | -CBD prevents heart fibrosis and hypertrophy -CBD prevents Ca2+ overload to help with contraction -THC-activated CB1 increase contraction rate and blood pressure. -CB2 promotes survival | -Aerobic exercise increases mitochondrial activity and contraction rate -Promotes regeneration and growth of cardiomyocytes | [132,152,153,154] |
| Endothelial | -Vascular permeability -Release vasoconstriction and vasodilation factors -Release inflammatory modulators -Release thrombosis factors -Release insulin and lipid metabolism factors -Release vasculogenic and angiogenic factors | -CB2 activation attenuates inflammatory factors -2-AG inhibits endothelium repair and increases inflammation -AEA increases vasodilation -THC impairs endothelial functions including decrease in arterial flow-mediated dilation, and in VEGF-stimulated nitric oxide levels -CBD promotes vasorelaxation, and inhibits angiogenesis, and release of pro-inflammatory factors -THC-activated CB1 promotes release of pro-inflammatory factors, increases influx of Ca2+ | -Increase nitric oxide production which is responsible for vasodilation and vasorelaxation -Continuous aerobic exercise of various intensities optimizes flow-mediated dilation -Supports repair of tears and wall integrity -Lower circulation of inflammatory factors | [153,155,156,157,158,159] |
| Pericyte | -Modulate blood flow by modifying capillary diameter and blood–brain barrier passage -Support angiogenesis, myogenesis, and neurogenesis -Exterior protection of endothelium | -Influence blood flow and capillary diameters, especially during ischemic conditions -CBD lowers cellular damage and inhibits release of inflammatory factors in O2/glucose deprivation conditions -CB1 affects the vessel diameter and blood flow | -Increase growth rate leading to gains in skeletal muscle mass -Promotes muscle fiber regeneration and angiogenesis -Aerobic exercise enhances contraction force and oxygenation in blood flow | [14,17,160,161] |
| Vascular smooth muscle | -Regulating blood flow and pressure -Modulating dilation and contraction rates -Maintaining the overall structure by repairing tears and regulating thickness | -CBD inhibits muscle cell proliferation, reduces inflammatory responses and could modulate vasodilation -CB1-mediated THC decreases Ca2+ influx, increase vessel diameter | -Aerobic exercise improves contractility and vascular force -Intense exercise strengthens the vascularization wall | [17,162,163,164] |
| Nervous System Cell | Role(s) | Cannabinoid Influence | Exercise Influence | Source(s) |
|---|---|---|---|---|
| Sensory neuron | -Detect internal and external stimuli and transmit signals to CNS | -CB2 activation can regulate neuroinflammation -THC-activated CB1 inhibits neurotransmitter release | -Promotion of growth factor release -Enhanced neurogenesis and overall activity | [193,218,219] |
| Motor neuron | -Transmit signals from CNS to muscles and glands -Crucial in voluntary and involuntary movements | -Regulate excitatory and inhibitory synaptic transmissions | -Enhanced neuromuscular transmission -Promotion of protein production (e.g., myokines) essential for nerve maintenance | [220,221] |
| Astrocyte | -Regulate blood and ion flow -Neuroprotection and repair -Synaptic transmission and plasticity | -Activation of CB1 in astrocytes lead to the release gliotransmitters responsible for synaptic functions, and promotion of inflammatory factors | -May change morphology of the cells -Modulate inflammatory response and neuroplasticity | [222,223] |
| Oligodendrocyte | -Production and maintenance of myelin -Supporting role for nerves by supplying energy -Involvement in immunity | -CB1 and CB2 activation modulates cell differentiation -CBD promotes neuroprotection -THC enhances the myelination process | -Promotes growth factor production -Promotes cell proliferation -Promotes myelin synthesis and repair | [224,225,226,227] |
| Microglia | -Modulate immune response, remove debris, pathogens -Neurogenesis functions | -CB2 activation is anti-inflammatory -CBD tends to inhibit pro-inflammatory response | -Reduce inflammation -Promote microglia neurogenesis | [228,229,230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaei, A.Y.; Neary, J.P.; Thompson, E.S.; Singh, J.; Mang, C.S. Considering the Effects of Cannabinoids and Exercise on the Brain: A Narrative Review. Sports 2025, 13, 320. https://doi.org/10.3390/sports13090320
Rajaei AY, Neary JP, Thompson ES, Singh J, Mang CS. Considering the Effects of Cannabinoids and Exercise on the Brain: A Narrative Review. Sports. 2025; 13(9):320. https://doi.org/10.3390/sports13090320
Chicago/Turabian StyleRajaei, Amir Yahya, J. Patrick Neary, Elizabeth S. Thompson, Jyotpal Singh, and Cameron S. Mang. 2025. "Considering the Effects of Cannabinoids and Exercise on the Brain: A Narrative Review" Sports 13, no. 9: 320. https://doi.org/10.3390/sports13090320
APA StyleRajaei, A. Y., Neary, J. P., Thompson, E. S., Singh, J., & Mang, C. S. (2025). Considering the Effects of Cannabinoids and Exercise on the Brain: A Narrative Review. Sports, 13(9), 320. https://doi.org/10.3390/sports13090320