The Effect of Consuming Caffeine Before Late Afternoon/Evening Training or Competition on Sleep: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection Criteria
2.2. Search Strategy
2.3. Data Extraction and Quality Assessment
3. Results
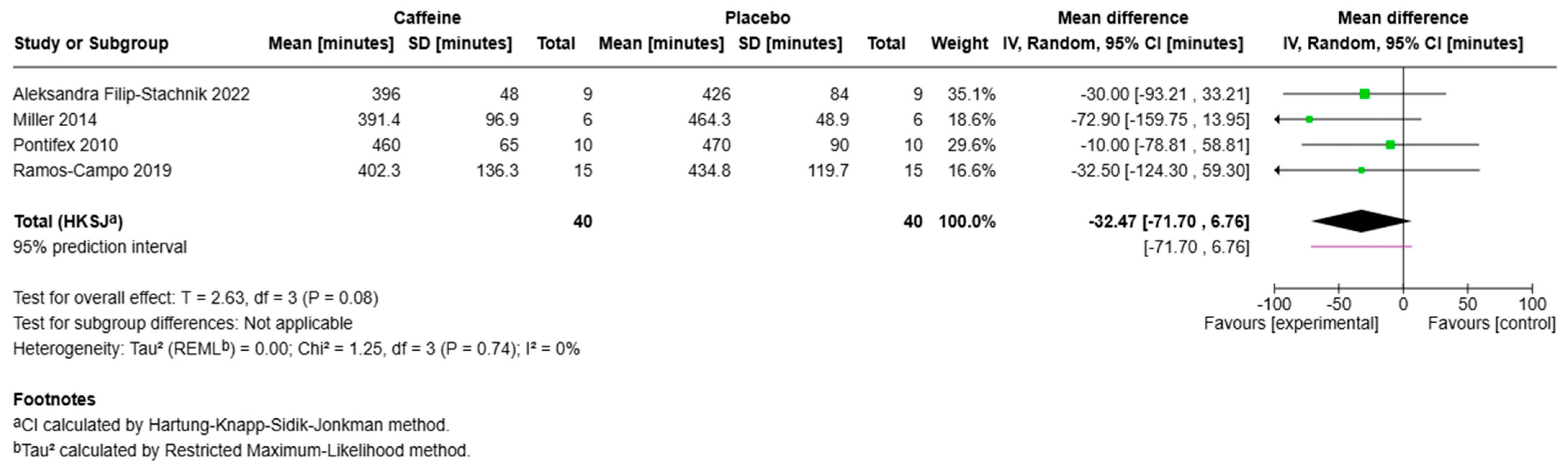
3.1. Total Sleep Time
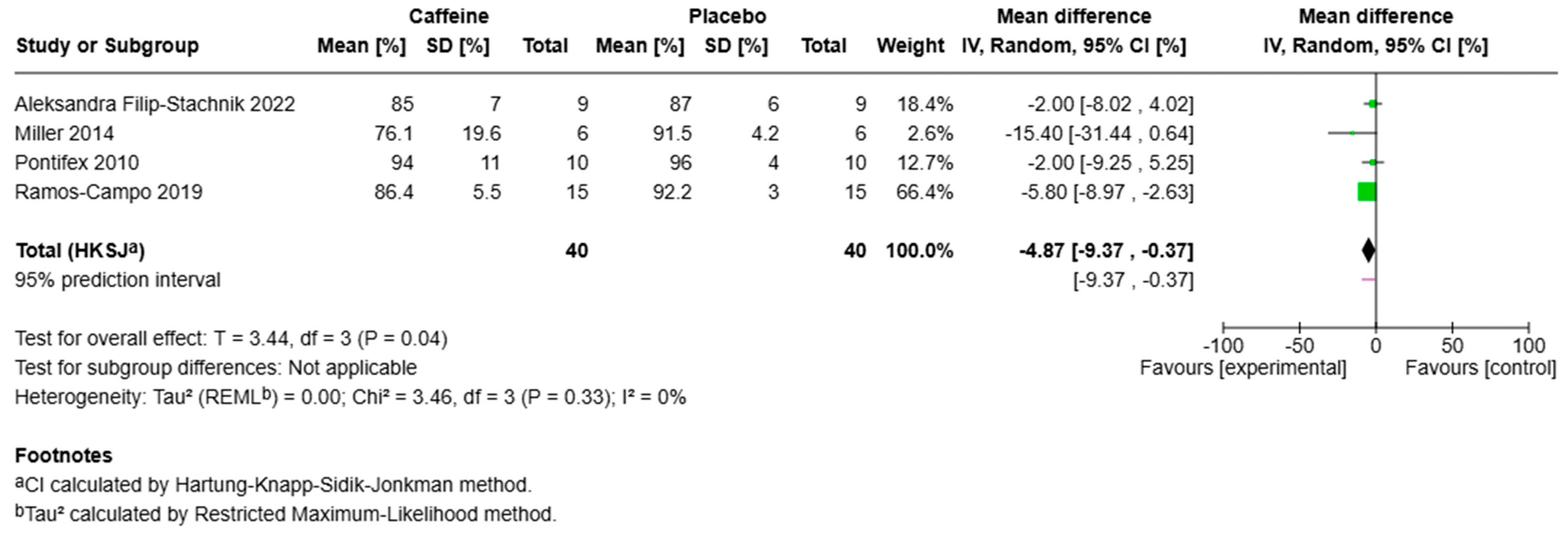
3.2. Sleep Efficiency
3.3. Wake After Sleep Onset
3.4. Sleep Onset Latency
3.5. Number of Awakenings
3.6. Subjective Sleep
4. Discussion
4.1. Subjective–Objective Disconnect
4.2. Sleep Outcomes and Meta-Analysis Findings
4.3. Neurophysiological Mechanisms
4.4. Individual Variability and Metabolism
4.5. Real-World Competition Context
4.6. Methodological Considerations and Timing Effects
4.7. Caffeine Interactions with Concurrent Supplements
4.8. Practical Implications
4.9. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carley, D.W.; Farabi, S.S. Physiology of Sleep. Diabetes Spectr. 2016, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Lastella, M.; Roach, G.D.; Halson, S.L.; Sargent, C. Sleep/Wake Behaviours of Elite Athletes from Individual and Team Sports. Eur. J. Sport Sci. 2015, 15, 94–100. [Google Scholar] [CrossRef]
- Sargent, C.; Lastella, M.; Halson, S.L.; Roach, G.D. The Impact of Training Schedules on the Sleep and Fatigue of Elite Athletes. Chronobiol. Int. 2014, 31, 1160–1168. [Google Scholar] [CrossRef]
- Clark, I.; Landolt, H.P. Coffee, Caffeine, and Sleep: A Systematic Review of Epidemiological Studies and Randomized Controlled Trials. Sleep Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef]
- Fabbri, M.; Beracci, A.; Martoni, M.; Meneo, D.; Tonetti, L.; Natale, V. Measuring Subjective Sleep Quality: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Gervais, B.M.; Adomeit, J.L.; Greenlund, I.M. Subjective and Objective Sleep Differ in Male and Female Collegiate Athletes. Sleep Health 2020, 6, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, M.J.; Deuchrass, R.W.; Olsen, P.D.; Choukri, M.A.; Marshall, H.C.; Lizamore, C.A.; Leong, C.; Elliot, C.A. The Effect of Sleep Quality and Quantity on Athlete’s Health and Perceived Training Quality. Front. Sports Act. Living 2021, 3, 705650. [Google Scholar] [CrossRef]
- Hayat, Z.; Sharma, S.; Minhaj, T. Efficacy of Caffeine on Athletic Performance: A Systematic Review and Meta-Analysis. Sci. Sports 2022, 37, 333–353. [Google Scholar] [CrossRef]
- Blanchard, J.; Sawers, S. The Absolute Bioavailability of Caffeine in Man. Eur. J. Clin. Pharmacol. 1983, 24, 93–98. [Google Scholar] [CrossRef]
- Ruggiero, M.; Ferrante, L.; Tafuri, D.; Meccariello, R.; Mazzeo, F. Trends in Antidepressant, Anxiolytic, and Cannabinoid Use among Italian Elite Athletes (2011–2023): A Longitudinal Anti-Doping Analysis. Sports 2025, 13, 233. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T. Adenosine in Sleep and Wakefulness. Ann. Med. 1999, 31, 125–129. [Google Scholar] [CrossRef]
- Grgic, J.; Mikulic, P.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. The Influence of Caffeine Supplementation on Resistance Exercise: A Review. Sports Med. 2019, 49, 17–30. [Google Scholar] [CrossRef]
- Burke, L.M. Practical Issues in Evidence-Based Use of Performance Supplements: Supplement Interactions, Repeated Use and Individual Responses. Sports Med. 2017, 47, 79–100. [Google Scholar] [CrossRef]
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and Athletic Performance: The Effects of Sleep Loss on Exercise Performance, and Physiological and Cognitive Responses to Exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef]
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J. Recovery and Performance in Sport: Consensus Statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lastella, M.; Vincent, G.E.; Duffield, R.; Roach, G.D.; Halson, S.L.; Heales, L.J.; Sargent, C. Can Sleep Be Used as an Indicator of Overreaching and Overtraining in Athletes? Front. Physiol. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Weakley, J.; Burke, L.M.; Roach, G.D.; Sargent, C.; Maniar, N.; Townshend, A.; Halson, S.L. The Effect of Caffeine on Subsequent Sleep: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2023, 69, 101764. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J.; Roberts, S.; Lastella, M.; Aisbett, B.; Condo, D. The Impact of Dietary Factors on the Sleep of Athletically Trained Populations: A Systematic Review. Nutrients 2022, 14, 3271. [Google Scholar] [CrossRef]
- Silva, H.; Del Coso, J.; Pickering, C. Caffeine and Sports Performance: The Conflict between Caffeine Intake to Enhance Performance and Avoiding Caffeine to Ensure Sleep Quality. Sports Med. 2025, 55, 1579–1592. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. Rob 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, i4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. Robins-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4898. [Google Scholar] [CrossRef]
- Filip-Stachnik, A. Does Acute Caffeine Intake before Evening Training Sessions Impact Sleep Quality and Recovery-Stress State? Preliminary Results from a Study on Highly Trained Judo Athletes. Appl. Sci. 2022, 12, 9957. [Google Scholar] [CrossRef]
- Miller, B.; O’Connor, H.; Orr, R.; Ruell, P.; Cheng, H.L.; Chow, C.M. Combined Caffeine and Carbohydrate Ingestion: Effects on Nocturnal Sleep and Exercise Performance in Athletes. Eur. J. Appl. Physiol. 2014, 114, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, K.; Wallman, K.; Dawson, B.; Goodman, C. Effects of Caffeine on Repeated Sprint Ability, Reactive Agility Time, Sleep and Next Day Performance. J. Sports Med. Phys. Fit. 2010, 50, 455–464. [Google Scholar]
- Ramos-Campo, D.J.; Pérez, A.; Ávila-Gandía, V.; Pérez-Piñero, S.; Rubio-Arias, J.Á. Impact of Caffeine Intake on 800-M Running Performance and Sleep Quality in Trained Runners. Nutrients 2019, 11, 2040. [Google Scholar] [CrossRef]
- Ali, A.; O’Donnell, J.; Starck, C.; Rutherfurd-Markwick, K. The Effect of Caffeine Ingestion during Evening Exercise on Subsequent Sleep Quality in Females. Int. J. Sports Med. 2015, 36, 433–439. [Google Scholar] [CrossRef] [PubMed]
- López-Samanes, Á.; Moreno-Pérez, V.; Travassos, B.; Del Coso, J. Effects of Acute Caffeine Ingestion on Futsal Performance in Sub-Elite Players. Eur. J. Nutr. 2021, 60, 4531–4540. [Google Scholar] [CrossRef]
- Newbury, J.W.; Saunders, B.; Gough, L.A. Evening Caffeine Did Not Improve 100-M Swimming Time Trials Performed 60 Min Post-Ingestion or the Next Morning after Sleep. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 453–461. [Google Scholar] [CrossRef]
- Raya-González, J.; Scanlan, A.T.; Soto-Célix, M.; Rodríguez-Fernández, A.; Castillo, D. Caffeine Ingestion Improves Performance during Fitness Tests but Does Not Alter Activity during Simulated Games in Professional Basketball Players. Int. J. Sports Physiol. Perform. 2021, 16, 387–394. [Google Scholar] [CrossRef]
- Caia, J.; Halson, S.L.; Holmberg, P.M.; Kelly, V.G. Does Caffeine Consumption Influence Postcompetition Sleep in Professional Rugby League Athletes? A Case Study. Int. J. Sports Physiol. Perform. 2022, 17, 126–129. [Google Scholar] [CrossRef]
- Dunican, I.C.; Higgins, C.C.; Jones, M.J.; Clarke, M.W.; Murray, K.; Dawson, B.; Caldwell, J.A.; Halson, S.L.; Eastwood, P.R. Caffeine Use in a Super Rugby Game and Its Relationship to Post-Game Sleep. Eur. J. Sport Sci. 2018, 18, 513–523. [Google Scholar] [CrossRef]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2021, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Kahale, L.A.; Khamis, A.M.; Diab, B.; Chang, Y.; Lopes, L.C.; Agarwal, A.; Li, L.; Mustafa, R.A.; Koujanian, S.; Waziry, R. Potential Impact of Missing Outcome Data on Treatment Effects in Systematic Reviews: Imputation Study. BMJ 2020, 370, m2898. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Vandermeer, B.W.; Shamliyan, T.A.; O’Neil, M.E.; Yazdi, F.; Fox, S.H.; Morton, S.C. Handling Continuous Outcomes in Quantitative Synthesis. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013. [Google Scholar]
- Cumming, G. The New Statistics: Why and How. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- von Hippel, P.T. The Heterogeneity Statistic I 2 Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. Grade: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L.; Study of Osteoporotic Fractures Research Group. Comparison of Sleep Parameters from Actigraphy and Polysomnography in Older Women: The Sof Study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Patsopoulos, N.A.; Evangelou, E. Uncertainty in Heterogeneity Estimates in Meta-Analyses. BMJ 2007, 335, 914–916. [Google Scholar] [CrossRef]
- Landolt, H.P.; Rétey, J.V.; Tönz, K.; Gottselig, J.M.; Khatami, R.; Buckelmüller, I.; Achermann, P. Caffeine Attenuates Waking and Sleep Electroencephalographic Markers of Sleep Homeostasis in Humans. Neuropsychopharmacology 2004, 29, 1933–1939. [Google Scholar] [CrossRef]
- Fiani, B.; Zhu, L.; Musch, B.L.; Briceno, S.; Andel, R.; Sadeq, N.; Ansari, A.Z.; Briceno, S.A. The Neurophysiology of Caffeine as a Central Nervous System Stimulant and the Resultant Effects on Cognitive Function. Cureus 2021, 13, e15032. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A.; Kabagambe, E.K.; Campos, H. Coffee, Cyp1a2 Genotype, and Risk of Myocardial Infarction. JAMA 2006, 295, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.V.; Desmond, P.V.; Johnson, R.F.; Schenker, S. Impaired Elimination of Caffeine by Oral Contraceptive Steroids. J. Lab. Clin. Med. 1980, 95, 603–608. [Google Scholar]
- Yang, A.; Palmer, A.A.; De Wit, H. Genetics of Caffeine Consumption and Responses to Caffeine. Psychopharmacology 2010, 211, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.E.; Leota, J.; Mascaro, L.; Hoffman, D.; Facer-Childs, E.R. Sleep Patterns before and after Competition: A Real-World Examination of Elite Athletes. J. Sports Sci. 2023, 41, 2014–2026. [Google Scholar] [CrossRef]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [CrossRef]
- Kemp, S.; Spence, A.L.; Keller, B.S.; Ducker, K.J.; Gucciardi, D.F. Intraindividual Variability in Sleep among Athletes: A Systematic Review of Definitions, Operationalizations, and Key Correlates. Scand. J. Med. Sci. Sports 2023, 33, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Bulman, A.; D’Cunha, N.M.; Marx, W.; Turner, M.; Mckune, A.; Naumovski, N. The Effects of L-Theanine Consumption on Sleep Outcomes: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2025, 81, 102076. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Ostojic, S.M.; Prokopidis, K.; Stock, M.S.; Harmon, K.K.; Faulkner, P. “Heads up” for Creatine Supplementation and Its Potential Applications for Brain Health and Function. Sports Med. 2023, 53, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.R.; Harty, P.S.; Camic, C.L. Common Ingredient Profiles of Multi-Ingredient Pre-Workout Supplements. Nutrients 2019, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Kaida, K.; Takahashi, M.; Åkerstedt, T.; Nakata, A.; Otsuka, Y.; Haratani, T.; Fukasawa, K. Validation of the Karolinska Sleepiness Scale against Performance and Eeg Variables. Clin. Neurophysiol. 2006, 117, 1574–1581. [Google Scholar] [CrossRef] [PubMed]



| Paper (Author, Year, Country) | Participants (Number, Age, Sex) | Sport (Type, Athlete Calibre) [2] | Study Design | Intervention (Dose and Timing of Caffeine or Biomarker) | Comparator | Scenario (Training or Competition, Time) | Sleep Measures | Effect of Caffeine vs. Comparator |
|---|---|---|---|---|---|---|---|---|
| Filip-Stachnik, 2022, Poland [23] | 5 males Age: 24 ± 5 yrs 4 females Age: 20 ± 1 yrs | Judo Tier 3 (Highly Trained/National) | RCT Double-blind Cross-over | Caffeine 3 mg/kg BM Consumed 18:00 | Placebo | Training Session 19:00 | Actigraphy (Activeinsight’s GENEActiv watch) Karolinska Sleep Questionnaire (KSQ) | ↔ No significant differences in actigraphy sleep measures KSQ: ↓ Sleep Quality (KSQ) 3.9 ± 0.6 vs. 3.0 ± 1.0 (p = 0.03) |
| Miller et al. 2014, Australia [24] | 6 males Age: 28 ± 7 yrs | Cycling Triathlon Tier 2 (Trained) | RCT Double-blind Cross-over | Caffeine 2 × 3 mg/kg BM Consumed 16:00 and 17:40 | Placebo | Training Session 17:00–19:00 | Polysomnography | ↑ Sleep latency 51.1 ± 34.7 vs. 10.2 ± 4.6 min (p = 0.028) ↓ REM sleep 62.1 ± 19.6 vs. 85.8 ± 24.7 min (p = 0.028) ↓ Total sleep time 391.4 ± 96.9 vs. 464.3 ± 48.9 min (p = 0.028) ↑ WASO 75.1 ± 86.6 vs. 31.9 ± 17.0 min (p = 0.046) ↓ Sleep efficiency 76.1 ± 19.6% vs. 91.5 ± 4.2% (p = 0.028) |
| Pontifex et al. 2010, Australia [25] | 10 males Age: 18 ± 1 yrs | Australian Rules Football Soccer Field Hockey Recreational | RCT Single-blind Cross-over | Caffeine 6 mg/kg BM Consumed 16:00–19:00 | Placebo | Repeated sprint exercise trail 17:00–20:00 | Actigraphy | ↔ No significant differences in actigraphy sleep measures |
| Ramos-Campo et al. 2019, Spain [26] | 15 males Age: 24 ± 8 yrs | Middle Distance Runners Tier 3 (Highly Trained/National) | RCT Single-blind Cross-over | Caffeine 6 mg/kg BM Consumed 19:45 | Placebo | 800 m Running Time Trial 20:00 | Actigraphy Karolinska Sleep Questionnaire (KSQ) | Actigraphy: ↓ Sleep Efficiency 86.4 ± 5.8% vs. 92.2 ± 3.9% (p = 0.003; ES = 0.71) ↓ Wake Time 52.1 ± 23.2 min vs. 29.2 ± 15.4 min (p = 0.001; ES = −1.18) ↑ No. Wake Times 18.85 ± 7.50 vs. 13.62 ± 7.05 (p = 0.005; ES = −0.96) KSQ: ↓ Sleep Quality 2.21 ± 0.98 vs. 3.36 ± 0.75 (p = 0.005; ES = 1.11) ↓ Calm Sleep 2.56 ± 1.15 vs. 3.50 ± 1.09 (p = 0.005; ES = 1.11) ↓ Ease of Falling Asleep 1.57 ± 0.85 vs. 3.43 ± 1.22 (p = 0.003; ES = 1.38) ↓ Feeling Refreshed After Awakening 1.50 ± 0.65 vs. 2.07 ± 0.73 (p = 0.006; ES = 1.11) |
| Ali et al. 2015, New Zealand [27] | 10 females Age: 24 ± 4 yrs | Soccer Hockey Netball Recreational to International | RCT Double-blind Cross-over | Caffeine 6 mg/kg BM Consumed 17:15 | Placebo | Intermittent exercise protocol to simulate soccer match 18:00 | Leeds Sleep Evaluation Questionnaire | LSEQ: ↑ Sleep Latency 5.9 ± 3.2 cm vs. 3.1 ± 1.7 cm (p < 0.05) ↑ Time to Get to Sleep 5.9 ± 3.2 cm vs. 2.8 ± 1.5 cm (p < 0.05) ↑ Restless Sleep 7.1 ± 2.5 cm vs. 3.8 ± 2.3 (p < 0.05) |
| López-Samanes et al. 2021, Portugal [28] | 16 males Age: 28 ± 4 yrs | Futsal Tier 2 (Trained) | RCT Double-blind Cross-over | Caffeine 3 mg/kg BM Consumed 17:00 | Placebo | Intermittent exercise protocol to simulate futsal match 17:00–19:00 | Side Effects Questionnaire | ↔ No significant differences in insomnia |
| Newbury et al. 2022, United Kingdom [29] | 5 males 3 females Age: 18 ± 1 yrs | Swimming Tier 3 (Highly Trained) | RCT Double-blind Cross-over | Caffeine 3 mg/kg BM Consumed 16:30 | Placebo | Training Session 17:30–20:30 | Core Consensus Sleep Diary | ↔ No significant differences in subjective sleep measures |
| Raya-González et al. 2021, Spain [30] | 14 males Age: 21 ± 2 yrs | Basketball Tier 4 (Elite) | RCT Double-blind Counter-balanced Cross-over | Caffeine 6 mg/kg BM Consumed 18:30 | Placebo | Intermittent exercise protocol to simulate basketball match 19:30–21:00 | Side Effects Questionnaire | ↑ Insomnia 57% vs. 14% (p < 0.05) |
| Caia et al. 2022, Australia [31] | 15 males Age: 23 ± 4 yrs | Rugby League Tier 4 (Elite) | Quasi-experimental | High Salivary Caffeine Post Match | Low Salivary Caffeine Post Match | Ad libitum caffeine consumption prior to and during evening (19:00–21:00) game Salivary caffeine measured 90 min post-game | Actigraphy (Phillip’s Respironics Actiwatch) night prior, night of, night after match Sleep Diary | ↔ No significant correlation between post-competition salivary caffeine and sleep parameters |
| Dunican et al. 2018, Australia [32] | 20 males Age: 26 ± 3 yrs | Rugby Union Tier 4 (Elite) | Quasi-experimental | High Salivary Caffeine Post Match | Low Salivary Caffeine Post Match | Ad libitum caffeine consumption prior to and during evening (19:00–21:00) game Salivary caffeine measured before (17:00) and after game (21:30) | Actigraphy (Fatigue Science’s Readiband) | ↑ Sleep Latency ↓ Sleep Efficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocak, A.; Georgousopoulou, E.; Knight-Agarwal, C.R.; Matthews, R.; Minehan, M. The Effect of Consuming Caffeine Before Late Afternoon/Evening Training or Competition on Sleep: A Systematic Review with Meta-Analysis. Sports 2025, 13, 317. https://doi.org/10.3390/sports13090317
Kocak A, Georgousopoulou E, Knight-Agarwal CR, Matthews R, Minehan M. The Effect of Consuming Caffeine Before Late Afternoon/Evening Training or Competition on Sleep: A Systematic Review with Meta-Analysis. Sports. 2025; 13(9):317. https://doi.org/10.3390/sports13090317
Chicago/Turabian StyleKocak, Adem, Ekavi Georgousopoulou, Catherine R. Knight-Agarwal, Raymond Matthews, and Michelle Minehan. 2025. "The Effect of Consuming Caffeine Before Late Afternoon/Evening Training or Competition on Sleep: A Systematic Review with Meta-Analysis" Sports 13, no. 9: 317. https://doi.org/10.3390/sports13090317
APA StyleKocak, A., Georgousopoulou, E., Knight-Agarwal, C. R., Matthews, R., & Minehan, M. (2025). The Effect of Consuming Caffeine Before Late Afternoon/Evening Training or Competition on Sleep: A Systematic Review with Meta-Analysis. Sports, 13(9), 317. https://doi.org/10.3390/sports13090317






