1. Introduction
The use of ERAS protocols has drastically changed the paradigm of surgical care in recent years. Originally developed in the late 1990s to reduce postoperative complications and shorten hospital stays, ERAS represents a shift from traditional perioperative management to a multimodal, evidence-based approach. These protocols are built upon key principles, including preoperative optimization, minimal invasiveness, multimodal analgesia, early mobilization, and nutritional support, all aimed at attenuating the stress response to surgery and promoting faster recovery. By optimizing perioperative care and addressing both physiological and psychological elements of recovery, ERAS strategies have consistently been shown to improve patient outcomes, resulting in reduced morbidity, shorter length of stay, and enhanced patient satisfaction. Prehabilitation, which enables patients to enhance their surgical preparedness through customized therapies—particularly Adapted Physical Exercise (APE)—is a cornerstone of this approach. APE consists of individualized exercise programs designed to increase muscle strength, cardiorespiratory fitness, and overall well-being, ultimately supporting patients in coping with the physical and psychological challenges associated with surgery. According to research, APE is generally associated with a reduction in hospital stay of approximately 1.5 days and with decreases in postoperative complications of up to 30% across various surgical procedures [
1]. These benefits align closely with the core objectives of ERAS protocols, which aim to optimize recovery through multimodal, evidence-based perioperative strategies. Within this context, prehabilitation—including structured APE programs—serves as a targeted approach to enhance patients’ physiological and psychological readiness for surgery. The ERAS framework has been progressively extended beyond its original application in colorectal surgery to a wide range of surgical specialties, including urological, gynecological, orthopedic, and cardiothoracic procedures [
2]. Although the core principles remain consistent—such as multimodal optimization of nutrition, pain management, mobilization, and early recovery—the implementation of ERAS protocols varies considerably between specialties, resulting in heterogeneous clinical pathways [
3]. This diversity highlights the need to critically synthesize the available evidence and to identify common benefits of prehabilitation interventions, particularly exercise-based strategies, in order to support the development of standardized and broadly applicable recommendations [
4]. APE may exert its beneficial effects in the perioperative setting through multiple biological and physiological mechanisms. Regular structured training has been shown to modulate systemic inflammatory responses, attenuating the release of pro-inflammatory cytokines and oxidative stress, while promoting a more balanced immune function. Furthermore, exercise improves skeletal muscle mass and strength, counteracting sarcopenia and enhancing metabolic efficiency, which are critical for postoperative recovery [
4]. Cardiopulmonary adaptations, including increased aerobic capacity and improved ventilatory function, further contribute to a greater functional reserve and resilience against surgical stress [
3]. Collectively, these mechanisms support the rationale for integrating prehabilitation programs within ERAS pathways, as they may translate into improved tolerance to surgery and enhanced recovery trajectories.
1.1. The ERAS Paradigm and Its Relevance in Modern Surgery
Since its introduction in the early 2000s, ERAS has revolutionized traditional surgical care by expediting recovery and minimizing complications [
5]. Recent updates from the ERAS Society in 2021 have provided comprehensive guidelines for preadmission, preoperative, intraoperative, and postoperative care, emphasizing the importance of patient education, lifestyle modifications, and tailored prehabilitation strategies.
1.2. The Role of Prehabilitation in Preparing Patients for Surgery
As core element of ERAS, prehabilitation optimizes patients’ physical and psychological readiness for surgery. APE is integral to this process, promoting cardiovascular fitness, muscle strength, and endurance. According to a systematic review by Hall et al. [
6], patients who engaged in preoperative exercise had a 40% lower risk of postoperative complications. Furthermore, nutritional evaluations, which include diets high in protein and micronutrient supplements, are necessary to sustain energy reserves and boost immunological function, both of which are vital for the healing process following surgery. Reducing preoperative anxiety and improving mental resilience also require psychological support via mindfulness-based therapies or cognitive–behavioral therapy (CBT) [
6]. These interventions improve pain management and accelerate functional recovery, aligning with ERAS goals. Prehabilitation is generally initiated in the outpatient setting during the preadmission phase, often 2–6 weeks before surgery, depending on the time available between diagnosis and the scheduled intervention. Programs are commonly delivered in hospital-based rehabilitation facilities, community centers, or as home-based supervised exercise plans, sometimes using telemedicine support to enhance accessibility. The duration of interventions varies across studies, but most range from 2 to 8 weeks, aiming to optimize functional capacity prior to surgery.
1.3. Scope of the Review
Despite the growing body of research, significant gaps remain in the current literature. Prehabilitation programs involving exercise vary widely in terms of type (aerobic vs. resistance), frequency, duration, and intensity, with no consensus on standardized protocols applicable across surgical contexts [
4]. Furthermore, many studies suffer from methodological limitations, including small sample sizes, heterogeneous outcome measures, and inconsistent follow-up, which limit the generalizability of their findings. Some investigations report clear benefits in functional capacity and recovery, while others show less pronounced or inconclusive results, underscoring the presence of controversy within the field. These issues highlight the need for systematic synthesis of the evidence to inform future standardization of exercise-based prehabilitation strategies within ERAS pathways. In the context of the ERAS paradigm, this review will evaluate cohort studies, randomized controlled trials, and systematic reviews to give an overall view of how these interventions improve important outcomes across a range of surgical specialties, including lowering postoperative complications, reducing hospital length of stay, and improving functional recovery.
2. Materials and Methods
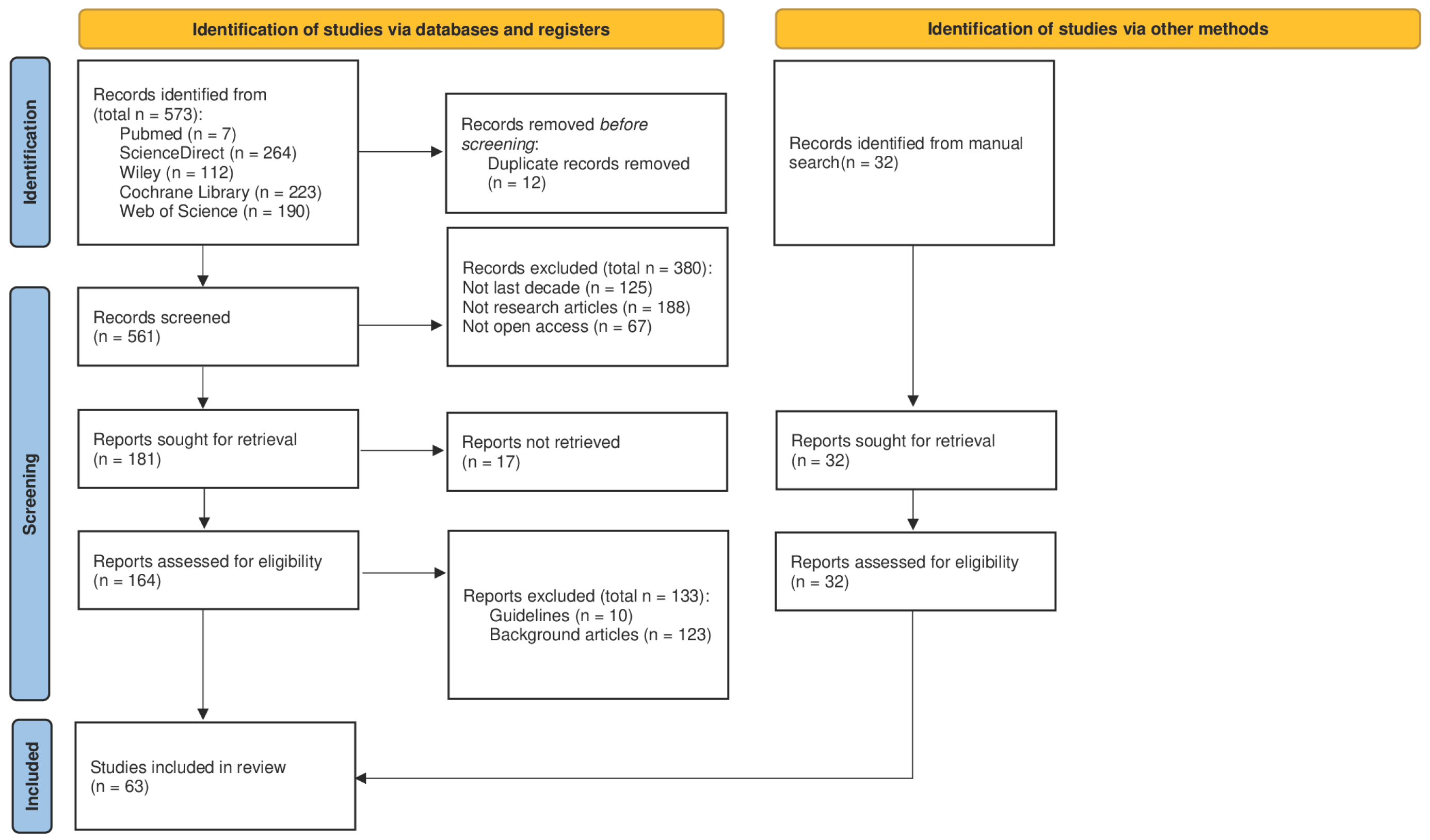
This review was conducted in accordance with the PRISMA 2020 statement (
Figure 1); however, no protocol was prospectively registered in PROSPERO or other registries. This represents a methodological limitation that may reduce transparency and reproducibility. The review process included a structured multi-phase screening (identification, screening, inclusion), a clearly defined search strategy, and independent reviewer assessment of studies. The systematic scoping review checklist is included as
Supplementary Materials.
2.1. Identification Phase
The identification phase was carried out through a systematic search of the PubMed, ScienceDirect, Cochrane Library, Web of Science, and Wiley databases, covering publications from the last decade up to June 2025. This cutoff was selected to ensure the inclusion of evidence generated after the widespread implementation and consolidation of ERAS protocols in clinical practice. Restricting the search to more recent years also allowed us to capture advancements in perioperative care, exercise prescription, and nutritional support, thereby reflecting the most current scientific developments in the field. The search strategy combined predefined keywords and MeSH terms relevant to the topic, including “prehabilitation”, “structured exercise”, “Enhanced Recovery After Surgery”, “postoperative recovery”, and “surgical outcomes”. The following search string and search terms were used for the research: preoperative optimization OR enhanced recovery after surgery OR prehabilitation OR preoperative recovery AND physical exercise OR structured exercise AND nutritional support AND surgical outcomes. Filters were applied to retrieve only open access publications written in English. Studies were selected if they reported on structured exercise interventions within ERAS protocols applied to major surgeries. The initial list of references was exported and screened using the RAYYAN web-based platform, with duplicate records removed prior to analysis. No additional software was employed.
2.2. Screening Phase
The screening process involved a two-step evaluation of titles and abstracts conducted independently by two reviewers. Disagreements during this phase were discussed and resolved by consensus; if unresolved, a third reviewer was consulted. Eligible study designs included randomized controlled trials, cohort studies, and meta-analyses that addressed key outcomes such as postoperative complications, hospital length of stay, and global patient recovery. Articles were excluded if they were narrative reviews, clinical guidelines, conference abstracts, or book chapters. Additionally, studies published before 2015 or those not available in open access were excluded. While this criterion facilitated transparency and reproducibility, it may have limited the number of eligible studies, potentially excluding relevant evidence published behind paywalls. No restrictions were placed on surgical discipline, provided the intervention was clearly part of an ERAS pathway.
2.3. Inclusion Phase
Following the screening phase, full-text versions of the selected articles were retrieved and assessed for eligibility based on the predefined inclusion and exclusion criteria. Data extraction was independently performed by two reviewers using a standardized extraction form, recording variables such as authors, year of publication, country, study design, sample size, and surgical procedure. Specific attention was paid to intervention characteristics, particularly the exercise protocols, which were detailed in terms of type (e.g., aerobic, resistance, multimodal), frequency, duration, and intensity. Additional variables included nutritional support, psychological interventions, and outcomes such as length of hospital stay, postoperative complications, functional recovery, and quality of life. These variables were selected to capture the multifactorial nature of ERAS prehabilitation and to allow for meaningful comparisons across studies. Risk of bias was assessed using appropriate tools: the Cochrane RoB 2 tool for randomized controlled trials and the Newcastle–Ottawa Scale (NOS) for cohort studies. Each study was rated as having low, moderate, or high risk of bias. Effect measures were reported as risk ratios (RRs) or odds ratios (ORs) for complications, mean differences (MD) for length of stay, and MD or standardized mean differences (SMDs) for continuous outcomes related to recovery and quality of life. Due to the methodological heterogeneity of the included studies, a meta-analysis was not feasible. Instead, a narrative and thematic synthesis was conducted to summarize the role of structured exercise within ERAS protocols. Key themes and outcomes were identified by systematically comparing study designs, participant characteristics, intervention types, frequency, duration, and outcome measures. Heterogeneity was assessed by examining variations in study methodology, population characteristics, intervention components, and reported outcomes. Findings were integrated by highlighting both convergent and divergent results across studies, allowing us to achieve a comprehensive understanding of the multifactorial effects of structured exercise on postoperative recovery. This qualitative synthesis was conducted following the recommendations outlined in the PRISMA 2020 guidelines, ensuring a systematic, transparent, and reproducible approach.
3. Results
ERAS protocols and prehabilitation programs have demonstrated substantial benefits across various surgical contexts, significantly improving recovery outcomes and reducing complications. Across the included studies, sample sizes ranged from 30 to over 300 participants, with most trials enrolling patients undergoing major abdominal surgery, followed by thoracic and urologic procedures. The majority of participants were aged between 18 and 85 years, with most cohorts clustered in the 55–75 range, and with a balanced representation of male and female patients where reported. Intervention durations varied from 2 to 8 weeks, and follow-up periods ranged from immediate postoperative outcomes to 12 months. Given the marked heterogeneity in study designs, populations, interventions, and outcome measures, a formal meta-analysis could not be conducted. Therefore, the findings were synthesized narratively, focusing on consistent patterns across studies. Considerable heterogeneity was observed regarding surgical specialties, intervention modalities, and outcome reporting. Additionally, several studies carried risks of bias related to small sample sizes, lack of blinding, or incomplete outcome data. These limitations should be taken into account when interpreting the results. The results are presented according to key outcome domains for clarity.
Table 1 provides an overview of the studies included in this review, grouped by thematic category.
3.1. Exercise/Physical Prehabilitation
Following video-assisted thoracoscopic lobectomy (VATS), physical activity levels declined postoperatively compared to pre-operative baselines (
p < 0.001;
p = 0.005;
p = 0.027). Pain scores increased both at rest (mean difference 1.2,
p < 0.001) and during walking (mean difference 1.4,
p < 0.001). Fatigue, as measured by the Christensen Fatigue Scale, also worsened postoperatively (mean difference 1.7,
p = 0.001), while sedentary activity and sleep duration remained unchanged. Functional recovery within seven days was not achieved, with fatigue (43%) and pain (33%) identified as the dominant barriers. APE interventions significantly enhanced recovery outcomes when integrated into ERAS protocols. These tailored programs improved cardiopulmonary capacity, muscle strength, and endurance. Aerobic exercise preoperatively increased VO2 max by 15% and reduced postoperative pulmonary complications by 20% in patients undergoing esophageal resection. High-intensity interval training (HIIT) reduced hospital stays by an average of two days for major abdominal surgery patients [
25]. Resistance training in colorectal cancer patients improved muscle strength by 25% and functional walking capacity, contributing to better postoperative recovery and fewer complications. Multimodal prehabilitation combining aerobic and resistance training reduced surgical complications by 25% and shortened hospital stays by three days [
4].
3.2. Nutritional Optimization
Targeted preoperative nutritional support, including protein supplementation, decreased postoperative complications by 20% and improved functional recovery. Specific interventions, such as low-AGE diets, reduced inflammatory markers and oxidative stress, supporting metabolic health during recovery. Protein-rich diets supplemented with leucine and vitamin D improved muscle strength and functional capacity, highlighting the importance of addressing nutritional deficiencies preoperatively [
30].
3.3. Pain Management
Pain management strategies within ERAS protocols shifted toward multimodal analgesia, reducing opioid reliance by 40% while enhancing postoperative recovery [
44]. Non-opioid medications such as acetaminophen, gabapentinoids, and NSAIDs effectively reduced pain, postoperative nausea and vomiting (PONV) by 30%, and restored bowel function 25% faster. The inclusion of dexmedetomidine further improved patient satisfaction and reduced pain levels [
43].
3.4. Psychological Interventions
Psychological preparation and interventions significantly impacted patient recovery. CBT and mindfulness-based stress reduction programs reduced preoperative anxiety by up to 40%, improving adherence to recovery protocols by 25% [
49]. Comprehensive preoperative education enhanced satisfaction and recovery outcomes. Mindfulness training, guided visualization, and relaxation techniques effectively managed anxiety and postoperative stress [
52].
3.5. LOS
ERAS protocols demonstrated measurable reductions in hospital length-of-stay across surgical settings. In colorectal surgery, LOS decreased from 5.4 to 4.3 days (
p < 0.001), and robotic liver resections reduced LOS to an average of 4.1 days compared to 5.7 days for laparoscopic approaches (
p = 0.002) [
54]. Integration of prehabilitation and ERAS strategies optimized recovery and highlighted the importance of a multidisciplinary approach.
In summary, the included studies suggest that prehabilitation and ERAS interventions are generally associated with improved postoperative outcomes, including functional recovery and reduced complications. However, the strength of evidence varies across specialties, and heterogeneity among studies warrants cautious interpretation. These findings are further contextualized in the Discussion.
4. Discussion
The integration of APE interventions into ERAS protocols appears to enhance surgical outcomes by supporting key physiological and psychological domains relevant to recovery. Across the included studies, exercise-based prehabilitation was generally associated with improved cardiopulmonary function, preservation of muscle mass, and accelerated postoperative recovery, while also reducing preoperative anxiety and supporting psychological resilience. When combined with nutritional and psychological interventions, these multimodal strategies underscore the holistic nature of ERAS and its potential to optimize perioperative care.
Nevertheless, the strength of evidence requires cautious interpretation. The included studies varied considerably in design, patient populations, surgical specialties, intervention modalities, and outcome measures. This heterogeneity, together with methodological limitations such as small sample sizes, lack of blinding, and incomplete reporting of follow-up, may limit the generalizability of results. Importantly, while several trials demonstrated significant improvements in outcomes such as cardiopulmonary capacity or reduced length of stay, effect sizes were often modest and context-dependent. A narrative synthesis was therefore adopted in this review, as the degree of variability precluded formal meta-analysis.
When considered by surgical specialty, APE and multimodal prehabilitation showed promising effects in major abdominal and colorectal surgery, with consistent reductions in complication rates and shorter hospital stays. In thoracic surgery, gains were primarily reflected in cardiopulmonary outcomes, whereas evidence in urological and gynecological procedures, although emerging, remains less robust. These differences highlight the importance of tailoring prehabilitation protocols to specific surgical populations and underscore the need for greater standardization in intervention design and outcome reporting.
From a practical standpoint, implementing APE in routine surgical care presents challenges, including resource availability, patient adherence, and integration within existing perioperative workflows. Variability in healthcare systems and patient populations may further influence the feasibility and scalability of such interventions. Addressing these barriers is essential to translate the benefits observed in controlled research settings into real-world practice.
This review has several limitations. First, the protocol was not prospectively registered in PROSPERO or other registries, which represents a methodological limitation that may reduce transparency and reproducibility, and potentially increase the risk of bias. Second, restricting the analysis to open access publications may have excluded some relevant studies. Third, substantial heterogeneity was observed across study designs, interventions, and outcomes, precluding a formal meta-analysis. Finally, variability in reporting of exercise intensity, adherence, and long-term follow-up limits the generalizability of the findings. These limitations should be considered when interpreting the results.
Future research should focus on large-scale, high-quality randomized controlled trials with standardized protocols, clearly defined outcomes, and longer follow-up periods to evaluate the sustainability of benefits. Studies directly comparing different exercise modalities, as well as investigating cost-effectiveness and patient-reported outcomes, would provide valuable insights. Moreover, pre-registration of review protocols should be prioritized to enhance transparency and reduce risk of bias in future systematic syntheses.
In summary, current evidence suggests that APE interventions, particularly when integrated with nutritional and psychological strategies, are generally associated with improved recovery within ERAS pathways. However, the variability and methodological limitations of the available studies necessitate cautious interpretation. Strengthening methodological rigor and addressing practical challenges will be essential for advancing the role of APE in perioperative care.
5. Conclusions
Prehabilitation, as defined within the ERAS framework, aims to optimize patients’ physical, nutritional, and psychological preparedness prior to surgery, and is generally associated with improved recovery outcomes. Evidence suggests that this integrated approach may contribute to fewer surgical complications, shorter recovery periods, and better overall well-being, largely through enhancing physiological fitness, supporting nutritional status, and improving psychological readiness.
Successful delivery of prehabilitation requires a coordinated, multidisciplinary effort involving surgeons, anesthesiologists, physiotherapists, dietitians, and psychologists. Standardization of exercise routines, nutritional support, and psychological interventions, alongside effective patient education, can help ensure feasibility and consistency across healthcare settings. Active patient engagement is particularly important to maximize adherence and the benefits of prehabilitation.
Future research should focus on long-term follow-up to evaluate sustained effects on recovery, recurrence, and quality of life, while also addressing variability across patient populations and surgical specialties. Investigating the biological mechanisms underpinning these benefits, as well as cost-effectiveness, will be essential to support wider implementation. Moreover, studies in implementation science are needed to identify strategies for scaling and integrating prehabilitation into routine surgical pathways.
In conclusion, current evidence supports prehabilitation as a promising strategy to enhance surgical care within ERAS protocols. However, variability among studies and methodological limitations warrant cautious interpretation, and further high-quality research is required to fully establish prehabilitation’s role in perioperative practice.
Author Contributions
Conceptualization, G.C. and A.A.V.; methodology, P.V. and A.D.S.; validation, G.C. and T.C.; formal analysis, A.A.V. and G.D.; resources, G.C. and A.A.V.; data curation, R.M. and G.D.; writing—original draft preparation, A.A.V. and P.V.; writing—review and editing, A.A.V., P.V., G.D. and R.M.; visualization, G.C.; supervision, A.A.V. and A.D.S.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union–NextGenerationEU, within the Italian National Recovery and Resilience Plan (PNRR), Ministry of University and Research (MUR)–M4C2–Investment 1.3, Public Call “Partenariati Estesi”-D.D. n. 341/2022. The APC was funded by the same project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The Authors acknowledge for support “HEAL ITALIA—Health Extended Alliance for Innovative Therapies, Advanced Lab-research and Integrated Approaches of Precision Medicine Cod. PE_00000019”.
Conflicts of Interest
The authors declare no conflicts of interest. The authors have no financial affiliation (including research funding) or involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript, except as disclosed and cited in the manuscript. Any other conflicts of interest (i.e., personal associations or involvement as a director, officer, or expert witness) are also disclosed and cited in the manuscript. The review was not registered. The protocol was not prepared.
References
- Gillis, C.; Loiselle, S.E.; Fiore, J.F., Jr.; Awasthi, R.; Wykes, L.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: A pilot double-blinded randomized placebo-controlled trial. J. Acad. Nutr. Diet. 2016, 116, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Pessoa, R.; Urkmez, A.; Kukreja, N.; Baack Kukreja, J. Enhanced recovery after surgery review and urology applications in 2020. BJUI Compass 2020, 1, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Ljungqvist, O.; Carli, F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br. J. Anaesth. 2022, 128, 434–448. [Google Scholar] [CrossRef]
- Tew, G.A.; Bedford, R.; Carr, E.; Harling, L. Prehabilitation for patients undergoing major abdominal surgery: A systematic review of randomised controlled trials. Am. J. Surg. 2018, 216, 607–612. [Google Scholar]
- McKechnie, T.; Parpia, S.; Bhandari, M.; Dionne, J.C.; Eskicioglu, C. Enhanced Recovery After Surgery (ERAS) protocols following emergency intra-abdominal surgery: A systematic review and meta-analysis protocol. PLoS ONE 2023, 18, e0291140. [Google Scholar] [CrossRef]
- Hall, D.E.; Youk, A.; Allsup, K.; Kennedy, K.; Byard, T.D.; Dhupar, R.; Chu, D.; Rahman, A.M.; Wilson, M.; Cahalin, L.P.; et al. Preoperative rehabilitation is feasible in the weeks prior to surgery and significantly improves functional performance. J. Frailty Aging 2023, 12, 267–276. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, E.; de Almeida, J.; Landoni, G.; Galas, F.R.B.G.; Fukushima, J.T.; Fominskiy, E.; De Brito, C.M.; Cavichio, L.B.; de Almeida, L.A.; Ribeiro, U., Jr.; et al. Early mobilization programme improves functional capacity after major abdominal cancer surgery: A randomized controlled trial. Br. J. Anaesth. 2017, 119, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Fenton, C.; Tan, A.R.; Abaraogu, U.O.; McCaslin, J.E. Prehabilitation exercise therapy before elective abdominal aortic aneurysm repair. Cochrane Database Syst. Rev. 2021, 7, CD013662. [Google Scholar] [CrossRef]
- Ferreira, V.; Minnella, E.M.; Awasthi, R.; Gamsa, A.; Ferri, L.; Mulder, D.; Sirois, C.; Spicer, J.; Schmid, S.; Carli, F. Multimodal Prehabilitation for Lung Cancer Surgery: A Randomized Controlled Trial. Ann. Thorac. Surg. 2021, 112, 1600–1608. [Google Scholar] [CrossRef]
- Fulop, A.; Lakatos, L.; Susztak, N.; Szijarto, A.; Banky, B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: A randomised clinical trial. Anaesthesia 2021, 76, 82–90. [Google Scholar] [CrossRef]
- Gillis, C.; Hasil, L.; Fenton, T. Pragmatic Prehabilitation for Colorectal Surgery: A Randomized Controlled Trial. JPEN J. Parenter. Enter. Nutr. 2023, 47, S49–S50. [Google Scholar] [CrossRef]
- Huang, G.H.; Ismail, H.; Murnane, A.; Kim, P.; Riedel, B. Structured exercise program prior to major cancer surgery improves cardiopulmonary fitness: A retrospective cohort study. Support. Care Cancer 2016, 24, 2277–2285. [Google Scholar] [CrossRef]
- Huang, L.; Kehlet, H.; Petersen, R.H. Functional recovery after discharge in enhanced recovery video-assisted thoracoscopic lobectomy: A pilot prospective cohort study. Anaesthesia 2022, 77, 555–561. [Google Scholar] [CrossRef]
- Jack, S.; West, M.A.; Grocott, M.P.; Danjoux, G. Perioperative exercise training in elderly subjects undergoing major abdominal cancer surgery: A randomized controlled trial. Surgery 2019, 165, 839–846. [Google Scholar]
- Kaye, D.R.; Schafer, C.; Thelen-Perry, S.; Parker, C.; Iglay-Reger, H.; Daignault-Newton, S.; Qin, Y.; Morgan, T.M.; Weizer, A.Z.; Kaffenberger, S.D.; et al. The feasibility and impact of a presurgical exercise intervention program (prehabilitation) for patients undergoing cystectomy for bladder cancer. Urology 2020, 145, 106–112. [Google Scholar] [CrossRef]
- Koh, F.H.; Loh, C.H.; Tan, W.J.; Ho, L.M.L.; Yen, D.; Chua, J.M.W.; Kok, S.S.; Sivarajah, S.S.; Chew, M.H.; Foo, F.J. Structured presurgery prehabilitation for aged patients undergoing elective surgery significantly improves surgical outcomes and reduces cost: A nonrandomized sequential comparative prospective cohort study. Nutr. Clin. Pract. 2022, 37, 645–653. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.E.; Ramanakuma, A.; Ferri, L.; Carli, F. Prehabilitation improves functional capacity in esophago-gastric cancer surgery: A randomized control trial. Can. J. Anesth. 2018, 65, S91–S92. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Bousquet-Dion, G.; Ferreira, V.; Austin, B.; Audi, C.; Tanguay, S.; Aprikian, A.; Carli, F.; Kassouf, W. Multimodal Prehabilitation to Enhance Functional Capacity Following Radical Cystectomy: A Randomized Controlled Trial. Eur. Urol. Focus 2021, 7, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mithany, R.H.; Daniel, N.; Shahid, M.H.; Aslam, S.; Abdelmaseeh, M.; Gerges, F.; Gill, M.U.; Abdallah, S.B.; Hannan, A.; Saeed, M.T.; et al. Revolutionizing surgical care: The power of Enhanced Recovery After Surgery (ERAS). Cureus 2023, 15, e48795. [Google Scholar] [CrossRef]
- Molenaar, C.J.L.; van Rooijen, S.J.; Fokkenrood, H.J.P.; Roumen, R.M.H.; Janssen, L.; Slooter, G.D. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst. Rev. 2023, 5, CD013259. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Onerup, A.; Angenete, E.; Bonfre, P.; Börjesson, M.; Haglind, E.; Wessman, C.; Nilsson, H. Self-assessed preoperative level of habitual physical activity predicted postoperative complications after colorectal cancer surgery: A prospective observational cohort study. Eur. J. Surg. Oncol. 2019, 45, 2045–2051. [Google Scholar] [CrossRef]
- Shakya, P.; Poudel, S. Prehabilitation in patients before major surgery: A review article. JNMA J. Nepal Med Assoc. 2022, 60, 909–915. [Google Scholar] [CrossRef]
- Wang, B.; Shelat, V.G.; Chow, J.J.; Huey, T.C.; Low, J.K.; Woon, W.W.; Junnarkar, S.P. Prehabilitation program improves outcomes of patients undergoing elective liver resection. J. Surg. Res. 2020, 251, 119–125. [Google Scholar] [CrossRef]
- West, M.A.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.; Jack, S. Cardiopulmonary exercise testing in high-risk surgical patients. Br. J. Anaesth. 2015, 114, 472–482. [Google Scholar]
- Wu, F.; Laza-Cagigas, R.; Pagarkar, A.; Olaoke, A.; El Gammal, M.; Rampal, T. The feasibility of prehabilitation as part of the breast cancer treatment pathway. PM&R 2021, 13, 1237–1246. [Google Scholar]
- Wynter-Blyth, V.; Moorthy, K. Prehabilitation: Preparing patients for surgery. Br. Med. J. 2017, 358, j3702. [Google Scholar] [CrossRef]
- Yang, F.; Yuan, Y.; Liu, W.; Tang, C.; He, F.; Chen, D.; Xiong, J.; Huang, G.; Qian, K. Effect of prehabilitation exercises on postoperative frailty in patients undergoing laparoscopic colorectal cancer surgery. Front. Oncol. 2024, 14, 1411353. [Google Scholar] [CrossRef]
- Yu, P.; Luo, Z.; Wang, Y.; Lin, S.; Qin, D.; Jones, A.Y.; He, J. Preoperative inspiratory muscle training improves lung function prior to elective heart valve surgery and reduces postoperative lung function impairment and pulmonary complications: A randomised trial. J. Physiother. 2024, 71, 27–34. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.; Billson, H.A.; Lal, S.; Owen, K.A.; Muneer, A. Perioperative nutrition for the treatment of bladder cancer by radical cystectomy. Cochrane Database Syst. Rev. 2019, 5, CD010127. [Google Scholar] [CrossRef]
- Gazouli, A.; Georgiou, K.; Frountzas, M.; Tsourouflis, G.; Boyanov, N.; Nikiteas, N.; Gazouli, M.; Theodoropoulos, G.E. Perioperative nutritional assessment and management of patients undergoing gastrointestinal surgery. Ann. Gastroenterol. 2024, 37, 142–154. [Google Scholar] [CrossRef]
- Gündoğu, R.H. Current approach to perioperative nutrition in the ERAS age. Clin. Sci. Nutr. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Ho, C.; Daud, Z.A.M.; Mohd Yusof, B.N.; Abdul Majid, H. Perioperative immunonutrition intervention on postoperative outcomes among gynecological cancer patients under enhanced recovery after surgery setting: A study protocol of explanatory mixed method study. PLoS ONE 2024, 19, e0315568. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hu, Y.; Chen, J. Effectiveness of an ERAS-based exercise-nutrition management model in enhancing postoperative recovery for thoracoscopic radical resection of lung cancer: A randomized controlled trial. Medicine 2024, 103, e37667. [Google Scholar] [CrossRef]
- Khalooeifard, R.; Oraee-Yazdani, S.; Keikhaee, M.; Shariatpanahi, Z.V. Protein supplement and enhanced recovery after posterior spine fusion surgery: A randomized, double-blind, placebo-controlled trial. Clin. Spine Surg. 2022, 35, E356–E362. [Google Scholar] [CrossRef]
- Laza-Cagigas, R.; Chan, S.; Sumner, D.; Rampal, T. Effects and feasibility of a prehabilitation programme incorporating a low-carbohydrate, high-fat dietary approach in patients with type 2 diabetes: A retrospective study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The ketogenic diet and sport: A possible marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef]
- Sowerbutts, A.M.; Burden, S.; Sremanakova, J.; French, C.; Knight, S.R.; Harrison, E.M. Preoperative nutrition therapy in people undergoing gastrointestinal surgery. Cochrane Database Syst. Rev. 2024, 4, CD008879. [Google Scholar] [CrossRef]
- Williams, J.D.; Wischmeyer, P.E. Assessment of perioperative nutrition practices and attitudes—A national survey of colorectal and GI surgical oncology programs. Am. J. Surg. 2017, 213, 1010–1018. [Google Scholar] [CrossRef]
- Xu, B.; Chen, H.; Zhang, Q.; Chen, P. Supplemental parenteral nutrition improves patient outcomes after esophageal cancer surgery: A single-center randomized controlled study. Medicine 2022, 101, e31893. [Google Scholar] [CrossRef]
- Ivan, S.J.; Holck, H.W.; Robinson, M.M.; Shea, R.E.; Wallander, M.L.; Parker, B.; Matulay, J.T.; Gaston, K.E.; Clark, P.E.; Seymour, R.; et al. Persistent opioid and benzodiazepine use after radical cystectomy in enhanced recovery after surgery (ERAS) patients. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 432.e1. [Google Scholar] [CrossRef]
- Kaye, A.D.; Chernobylsky, D.J.; Thakur, P.; Siddaiah, H.; Kaye, R.J.; Eng, L.K.; Harbell, M.W.; Lajaunie, J.; Cornett, E.M. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) protocols for postoperative pain. Curr. Pain Headache Rep. 2020, 24, 21. [Google Scholar] [CrossRef]
- Rawal, N. Intrathecal opioids for the management of post-operative pain. Best Pract. Res. Clin. Anaesthesiol. 2023, 37, 123–132. [Google Scholar] [CrossRef]
- Simpson, J.C.; Bao, X.; Agarwala, A. Pain management in enhanced recovery after surgery (ERAS) protocols. Clin. Colon Rectal Surg. 2019, 32, 121–128. [Google Scholar] [CrossRef]
- Zwolinski, N.M.; Patel, K.S.; Vadivelu, N.; Kodumudi, G.; Kaye, A.D. ERAS protocol options for perioperative pain management of substance use disorder in the ambulatory surgical setting. Curr. Pain Headache Rep. 2023, 27, 65–79. [Google Scholar] [CrossRef]
- Garland, E.L.; Hanley, A.W.; Baker, A.K.; Howard, M.O. Mindfulness-oriented recovery enhancement reduces pain and opioid misuse in chronic pain patients: A randomized controlled trial. J. Consult. Clin. Psychol. 2017, 85, 539–551. [Google Scholar]
- Janssen, T.L.; Steyerberg, E.W.; Faes, M.C.; Wijsman, J.H.; Gobardhan, P.D.; Ho, G.H.; van der Laan, L. Risk factors for postoperative delirium after elective major abdominal surgery in elderly patients: A cohort study. Int. J. Surg. 2019, 71, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Levett, D.Z.H.; Grocott, M.P. Psychological preparation for surgery: Impact on postoperative outcomes. J. Clin. Anesth. 2021, 72, 110325. [Google Scholar]
- McCartney, M.; Lewis, S. Enhancing recovery through psychological interventions: The impact on adherence to postoperative protocols. J. Surg. Res. 2022, 274, 229–238. [Google Scholar]
- Powell, R.; Davies, A.; Rowlinson-Groves, K.; French, D.P.; Moore, J.; Merchant, Z. Impact of a prehabilitation and recovery programme on emotional well-being in individuals undergoing cancer surgery: A multi-perspective qualitative study. BMC Cancer 2023, 23, 1232. [Google Scholar] [CrossRef]
- Villa, G.; Lanini, I.; Amass, T.; Bocciero, V.; Scirè Calabrisotto, C.; Chelazzi, C.; Romagnoli, S.; De Gaudio, A.R.; Lauro Grotto, R. Effects of psychological interventions on anxiety and pain in patients undergoing major elective abdominal surgery: A systematic review. Perioper. Med. 2020, 9, 38. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Wang, Y.; Akbari, M. Non-pharmacologic approaches in preoperative anxiety, a comprehensive review. Front. Public Health 2022, 10, 854673. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs. Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.P.; Liu, X.; Lo, S.H.; Chien, W.T.; Hui, S.K.; Choi, K.C.; Zhao, J. Perioperative enhanced recovery programmes for women with gynaecological cancers. Cochrane Database Syst. Rev. 2022, 3, CD008239. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Feijoo, B.; Agusti, N.; Sebio, R.; Sisó, M.; Carreras-Dieguez, N.; Domingo, S.; Díaz-Cambronero, O.; Torne, A.; Martinez-Palli, G.; Arguís, M.J. A multimodal prehabilitation program for the reduction of post-operative complications after surgery in advanced ovarian cancer under an ERAS pathway: A randomized multicenter trial (SOPHIE). Int. J. Gynecol. Cancer 2022, 32, 1463–1468. [Google Scholar] [CrossRef]
- Elfrink, A.K.E.; Alberga, A.J.; van Berge Henegouwen, M.I.; Scheurs, W.H.; van der Geest, L.G.M.; Verhagen, H.J.M.; Dekker, J.W.; Grünhagen, D.J.; Wouters, M.W.; Klaase, J.M.; et al. Outcomes after major surgical procedures in octogenarians: A nationwide cohort study. World J. Surg. 2022, 46, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Gemma, M.; Pennoni, F.; Braga, M. Studying Enhanced Recovery After Surgery (ERAS®) core items in colorectal surgery: A causal model with latent variables. World J. Surg. 2021, 45, 928–939. [Google Scholar] [CrossRef]
- Gonella, F.; Massucco, P.; Perotti, S.; Gianolio, S.; Vassallo, D.; Monasterolo, S.; Laezza, A.; Ponte, B.D.; Ricotti, A.; Ferrero, A. Prehab, ERAS, Rehab: A patient care continuum around colo-rectal surgery: Prehabilitation combined with ERAS and rehabilitation to reduce morbidity and hospital stay. Eur. J. Surg. Oncol. 2024, 50, 108688. [Google Scholar] [CrossRef]
- Hong, J.; de Roulet, A.; Foglia, C.; Saldinger, P.; Chao, S.Y. Outcomes of a colorectal enhanced recovery after surgery protocol modified for a diverse and urban community. J. Surg. Res. 2023, 286, 74–84. [Google Scholar] [CrossRef]
- Liu, G.; Cao, S.; Liu, X.; Tian, Y.; Li, Z.; Sun, Y.; Zhong, H.; Wang, K.; Zhou, Y. Short- and long-term outcomes following perioperative ERAS management in patients undergoing minimally invasive radical gastrectomy after neoadjuvant chemotherapy: A single-center retrospective propensity score matching study. Eur. J. Surg. Oncol. 2025, 51, 109459. [Google Scholar] [CrossRef] [PubMed]
- Merki-Künzli, C.; Kerstan-Huber, M.; Switalla, D.; Gisi, D.; Raptis, D.A.; Greco, N.; Greco, N.; Mungo, G.; Wirz, M.; Gloor, S.; et al. Assessing the value of prehabilitation in patients undergoing colorectal surgery according to the enhanced recovery after surgery (ERAS) pathway for the improvement of postoperative outcomes: Protocol for a randomized controlled trial. JMIR Res. Protoc. 2017, 6, e7972. [Google Scholar] [CrossRef] [PubMed]
- Miralpeix, E.; Mancebo, G.; Gayete, S.; Corcoy, M.; Solé-Sedeño, J.-M. Role and impact of multimodal prehabilitation for gynecologic oncology patients in an Enhanced Recovery After Surgery (ERAS) program. Int. J. Gynecol. Cancer 2019, 29, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).