Abstract
Adolescence represents a critical period of neurodevelopment during which brain-derived neurotrophic factor (BDNF) plays a fundamental role in neuronal survival and synaptic plasticity. While exercise-BDNF relationships are well-documented in adults, evidence in adolescents remains limited and inconsistent. This systematic review examined the effects of exercise modalities on circulating BDNF concentrations in adolescent populations. A systematic search was conducted following PRISMA guidelines across multiple databases (FECYT, PubMed, SPORTDiscus, ProQuest Central, SCOPUS, Cochrane Library) through June 2025. Inclusion criteria comprised adolescents, exercise interventions, BDNF outcomes, and randomized controlled trial design. Methodological quality was assessed using the PEDro scale. From 130 initially identified articles, 8 randomized controlled trials were included, with 4 rated as excellent and the other 4 as good quality. Exercise modalities included aerobic, resistance, concurrent, high-intensity interval training, Taekwondo, and whole-body vibration, with durations ranging 6–24 weeks. Four studies demonstrated statistically significant BDNF increases following exercise interventions, four showed no significant changes, and one reported transient reduction. Positive outcomes occurred primarily with vigorous-intensity protocols implemented for a minimum of six weeks. Meta-analysis was not feasible due to high heterogeneity in populations, interventions, and control conditions. Moreover, variation in post-exercise sampling timing further limited comparability of BDNF results. Future research should standardize protocols and examine longer interventions to clarify exercise-BDNF relationships in adolescents.
1. Introduction
Adolescence represents a critical period of neurodevelopment characterized by extensive neuroplastic changes, particularly in prefrontal cortical regions that govern executive functions essential for cognitive performance and academic achievement [1]. During this developmental stage, the brain exhibits heightened sensitivity to environmental influences, making it an optimal window for interventions aimed at enhancing neural health and cognitive capacity. Physical exercise has emerged as a promising modulator of brain function and development, with mounting evidence suggesting its potential to promote neuroplasticity through various molecular mechanisms [2,3].
Brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin in the mammalian brain, with high concentrations found in the hippocampus, cerebral cortex, hypothalamus, and cerebellum [4,5]. This protein plays a fundamental role in neuronal survival, differentiation, and synaptic plasticity, serving as a critical mediator of learning, memory formation, and cognitive function [6,7]. BDNF exerts its effects through binding to the tropomyosin receptor kinase B (TrkB), initiating signaling cascades that promote neurogenesis, dendritic branching, and synaptic strengthening [8]. Importantly, BDNF can cross the blood-brain barrier bidirectionally, allowing peripheral concentrations in serum and plasma to serve as accessible biomarkers of central nervous system BDNF activity [9].
The relationship between physical exercise and BDNF has been extensively documented in adult populations, with systematic reviews and meta-analyses by Szuhany et al. [10] and Dinoff et al. [11] demonstrating that both acute and chronic exercise interventions can modulate peripheral BDNF concentrations, with moderate-to-vigorous intensity aerobic exercise producing the most robust BDNF elevations [12,13]. However, the evidence regarding exercise-induced BDNF modulation in adolescent populations (12–18 years) remains substantially more limited and inconsistent, with only a handful of studies specifically examining this relationship in pediatric populations.
While systematic reviews by Donnelly et al. [14] and Carson et al. [15] (examining early childhood populations) have established clear associations between physical activity and cognitive development in younger populations, and recent meta-analyses by Menezes-Junior et al. [16] and Acevedo et al. [17] have demonstrated exercise effects on neurocognitive outcomes in children and adolescents, the specific neurobiological mechanisms underlying these relationships, particularly the role of BDNF, require further elucidation and actualization. The few studies that have examined BDNF responses to exercise in adolescents have yielded mixed results, with some investigations reporting significant increases following aerobic training protocols [18,19], while others have found no meaningful changes across various exercise modalities [20,21].
Recent systematic reviews have highlighted the need for updated analyses focusing specifically on adolescent populations and higher-quality study designs [22,23]. Several factors unique to adolescent populations may contribute to heterogeneity in findings, including ongoing neurodevelopmental processes, hormonal fluctuations, varying baseline fitness levels, and the presence of comorbid conditions such as obesity or mental health disorders [24,25]. Additionally, methodological considerations such as exercise prescription parameters (intensity, frequency, duration), biological sample matrix (serum versus plasma), timing of sample collection, and population characteristics may contribute to the observed variability in BDNF responses [26,27].
Understanding the exercise-BDNF relationship in adolescents has significant clinical and public health implications, particularly given the rising prevalence of sedentary behavior, obesity, and mental health concerns in this population [28,29]. If exercise interventions can reliably modulate BDNF levels in adolescents, this knowledge could inform evidence-based strategies for optimizing cognitive development, academic performance, and overall brain health during this critical developmental period [30,31].
Given the limitations of previous reviews that included mixed study designs or focused on broader age ranges, and the critical need to establish causal relationships between exercise interventions and BDNF responses for clinical translation, the present systematic review specifically focused on randomized controlled trials—the gold standard for maximizing causal inference and minimizing bias when evaluating intervention efficacy. Therefore, this systematic review aimed to: (1) examine the effects of various exercise modalities on circulating BDNF concentrations in adolescent populations through exclusive analysis of randomized controlled trials, (2) evaluate the methodological quality of available studies, and (3) identify optimal intervention parameters to guide future research and evidence-based clinical practice.
2. Materials and Methods
2.1. Experimental Approach to the Problem
The present systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Materials S1 and S2) [32] and adhered to established guidelines for conducting systematic reviews within the domain of sport sciences [33]. The review protocol was developed with the objective of ensuring comprehensive coverage of the relevant literature while maintaining methodological rigor. This systematic review was registered in PROSPERO (CRD420251071792).
2.2. Information Sources
The following bibliographic databases were consulted: FECYT (Web of Sciences, CCC, CIDW, KJD, MEDLINE, RSCI, and SCIELO), PubMed, SPORTDiscus, ProQuest Central, SCOPUS, and Cochrane Library. The search encompassed all published literature prior to 10 June 2025. The combination of databases was selected to ensure broad coverage of both medical and sports science literature.
2.3. Search Strategy
The PICO (Patient, Problem, or Population—Intervention or Exposure—Comparison, Control, or Comparator—Outcome[s]) framework was implemented to structure the search strategy and ensure systematic coverage of relevant literature. In the interest of maintaining transparency, the authors were not blinded to journal names or manuscript authors. The final search string was as follows:
(adolescent*) AND (exercise OR movement OR activity OR sport OR fitness OR aerobic OR training OR performance) AND (BDNF OR “brain-derived neurotrophic factor”) AND (“randomized controlled trial”)
2.4. Eligibility Criteria
The authors initiated the search string on databases and downloaded the title, authors’ names, journal, and date of all the articles that appeared in the search. Following the organization of the Excel spreadsheet, the process of removing all duplicates was initiated, and the remaining articles were subjected to a rigorous evaluation to ascertain their eligibility (Table 1).

Table 1.
Inclusion and exclusion criteria.
2.5. Data Extraction
A standardized data extraction process was implemented using an Excel spreadsheet developed in accordance with the Cochrane Consumers and Communication Review Group’s data extraction template. The spreadsheet enabled a systematic evaluation of the inclusion and exclusion requirements for all the selected studies. The extraction process was conducted independently by two authors, with any disagreements being resolved through discussion until consensus was reached. Inter-rater agreement for study inclusion was assessed using Cohen’s kappa coefficient (≥0.80).
Extracted data included: study characteristics, participant demographics, intervention details (exercise modality, intensity, frequency, duration), BDNF measurement protocols (sample matrix, timing, assay method), and outcome measures. For incomplete data, corresponding authors were contacted via email with up to two follow-up attempts over four weeks. Non-responses were documented and considered in quality assessment. A full record was kept of all articles that were not included, including the particular reasons for exclusion.
2.6. Assessment of Study Methodology
The Physiotherapy Evidence Database (PEDro) scale was utilized to evaluate the methodological quality of pre-test post-test studies with experimental (EXP) and control (CON) groups that were randomly selected. The scale employs a range of 0 (low methodological quality) to 10 (high methodological quality) to score the internal study validity. The score that each section is awarded can range from 0 (“no”) to 1 (“yes”), depending on the quality obtained by each point. The quality of the studies were categorized according to the following cut-off points: excellent (9–10), good (6–8), fair (4–5), and poor (<3) [34]. The scale in question comprises ten items. Quality assessment was performed independently by two authors (A.M.-V. and M.R.-G.), with inter-rater reliability calculated using intraclass correlation coefficient (ICC ≥ 0.85). Disagreements were resolved through discussion, with third author (C.D.G.-C.) consultation if needed.
3. Results
After analyzing all databases (FECYT: 5; PubMed: 5; SPORTDiscus: 1; ProQuest Central: 1; SCOPUS: 68; Cochrane Library: 46; external sources: 2), the contents of 130 articles were checked, detecting, at initial stage, 46 duplicate articles. Then, the authors analysed if each of the remaining 84 articles meet all inclusion criterion, resulting in the elimination of 74 articles by exclusion criteria number one (n = 9), exclusion criteria number two (n = 56) and exclusion criteria number four (n = 2). The remaining eight articles were included in the qualitative synthesis of the systematic review (Figure 1).
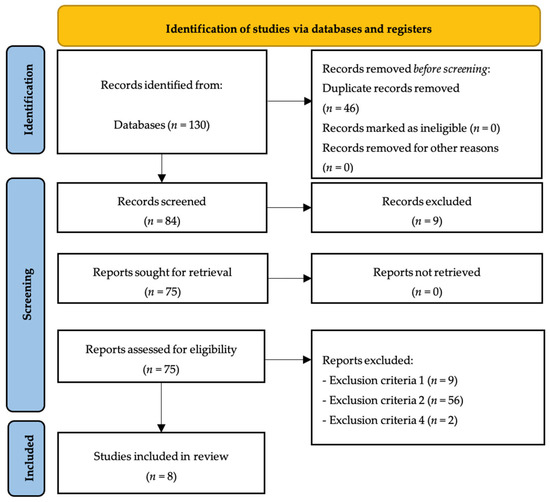
Figure 1.
PRISMA flow diagram.
3.1. Methodological Quality
The quality assessment for this systematic review can be found in Table 2. Four of the eight studies included in this review were rated as good [18,20,21,35], whereas the remaining four were of fair quality [19,36,37,38]. Common methodological weaknesses included complete absence of subject and therapist blinding across all studies, which is understandable given the nature of exercise interventions. Assessor blinding was achieved in only four studies (50%) [20,21,35,38], and allocation concealment was poorly implemented, with only Wunram et al. [35] meeting this criterion. Follow-up rates were problematic, adequate retention above 85% was only achieved by two studies [18,19]. Notably, Seok-Min and Chol-Hyoung [37] demonstrated the poorest baseline group similarity reporting. All studies appropriately reported between-group statistical comparisons and provided point measures with variability measures.

Table 2.
Methodological assessment of the included studies.
3.2. Study Characteristics
The characteristics of included studies are presented in Table 3. Eight randomized clinical trials examining the effect of physical exercise on BDNF levels in adolescents were included, with substantial heterogeneity in methodological and population characteristics [18,19,20,21,35,36,37,38].

Table 3.
Main characteristics and findings about the effects of exercise on adolescents’ brain-derived neurotrophic factor.
Sample sizes ranged from 18 to 304 participants, with ages spanning 12.55 to 18 years. Four studies included exclusively male participants [18,19,37,38], while others included mixed populations. Study populations were diverse, including healthy adolescents [18,19,38], adolescents with obesity [20,21,36,37], and those with major depressive disorder [35]. Most studies excluded participants with regular physical activity backgrounds [18,19,20,21,36].
Exercise modalities varied considerably, including aerobic training [18,19,38], resistance training [20,21], combined protocols [20,21,37], high-intensity interval training [19,38], and specialized disciplines such as Taekwondo [36] and whole-body vibration [35]. Intervention durations ranged from 6 weeks [35] to 24 weeks [20,21], with training frequencies between 3 and 5 sessions per week. Exercise intensities varied from 40% to 100% of maximum or reserve parameters.
All studies assessed BDNF levels using commercial ELISA kits, with seven studies analyzing serum samples [18,19,20,21,35,36,37] and one using plasma [38]. Sample collection protocols were consistent across studies, requiring 8–12 h fasting conditions and standardized timing to avoid acute exercise effects. Several studies incorporated complementary measures including growth factors [18,19,35], metabolic parameters [20,21], cognitive assessments [19,35,38], and oxidative stress markers [36].
3.3. Main Outcomes
Regarding the effects of exercise on BDNF levels, four studies showed statistically significant increases after intervention [18,19,35,36], while four studies found no significant changes [20,21,37,38]. Jeon and Ha [18] demonstrated a significant increase in serum BDNF levels following 8 weeks of moderate-intensity aerobic exercise in healthy male adolescents. In their subsequent study [19], the same authors reported intensity-dependent effects after 12 weeks of aerobic training, with significant increases observed in moderate-intensity and high-intensity groups, while low-intensity and control groups showed no significant changes.
Wunram et al. [35] found that 6 weeks of ergometer cycling training significantly increased BDNF levels compared to controls in adolescents with major depressive disorder. Whole-body vibration training showed a trend toward significance. Roh et al. [36] reported a significant time × group interaction after 16 weeks of Taekwondo training in overweight/obese adolescents, with the exercise group showing increased BDNF levels compared to controls.
Four studies failed to demonstrate significant BDNF changes. Walsh et al. [20] and Goldfield et al. [21] examined exercise interventions in adolescents with obesity and found no significant group × time interactions for BDNF after 24 weeks of aerobic, resistance, or combined training compared to diet-only controls. Gejl et al. [38] found no significant pre-post differences in plasma BDNF levels after 9 weeks of either high-intensity or moderate-intensity training in healthy adolescents. Similarly, Seok-Min and Chol-Hyoung [37] reported non-significant increases in BDNF after 12 weeks of combined aerobic and strength training in adolescents with obesity.
Studies reporting positive effects were predominantly conducted in male-only samples [18,19] or included specific clinical populations such as adolescents with depression [35] or overweight/obesity [36]. In contrast, studies with null findings included larger proportions of females [20,21] or examined healthy populations [38]. Exercise modalities varied from traditional aerobic training [18,19,38] to specialized activities such as Taekwondo [36], with intervention durations ranging from 6 weeks [35] to 24 weeks [20,21]. Additional variables examined across studies included oxidative stress markers, growth factors (IGF-1), metabolic parameters, and cognitive assessments. Notable secondary findings included associations between BDNF changes and improved glucose metabolism and beta cell function in adolescents with obesity [20].
3.4. Narrative Synthesis of BDNF Outcomes
A narrative synthesis of BDNF outcomes across the eight included studies revealed considerable heterogeneity in exercise-induced responses. Table 4 presents the pre- and post-intervention BDNF values, standard deviations, percentage changes, and p-values for each intervention group. (Table 4). The percentage changes in resting BDNF concentrations ranged from −4.51% to 27.7%, with four intervention groups achieving statistical significance. The synthesis identified three distinct response patterns: studies demonstrating significant BDNF increases, studies showing non-significant but notable changes, and studies reporting minimal alterations.

Table 4.
Summary of pre-post BDNF values, percentage changes, and statistical significance across intervention groups.
Studies showing significant BDNF increases were primarily those investigating exercise intensity effects and general fitness interventions. Jeon and Ha [19] demonstrated an intensity-dependent response pattern, with high-intensity exercise producing the largest increase (19.22%), moderate-intensity showing a modest but significant increase (6.99%), and low-intensity exhibiting minimal change (1.05%). Their subsequent investigation [18] confirmed exercise efficacy with an 18.19% increase compared to 3.53% in controls. Interestingly, Roh et al. [36] reported a paradoxical finding where the control group achieved significance (4.14% increase) while the exercise group, despite a larger magnitude increase (16.17%), did not reach statistical significance.
The remaining studies contributed to a pattern of non-significant responses across diverse populations and intervention types. Walsh et al. [20] examined participants with diabetes risk factors, finding minimal changes in both groups. Wunram et al. [35] investigated depression and cognitive function through three modalities: ergometer cycling (0.19% increase), whole-body vibration (0.33% decrease), and control conditions (1.42% increase). Gejl et al. [38] evaluated cardiorespiratory fitness interventions, with moderate-intensity training showing the largest single change across all studies (27.7%), though this remained non-significant. Goldfield et al. [21] compared exercise modalities in adolescents, revealing variable responses: aerobic training (0.42%), resistance training (2.05%), combined training (−4.51%), and controls (10.63%). Seok-Min and Chol-Hyoung [37] reported a 6.08% increase in exercise participants compared to minimal change (0.09%) in controls.
4. Discussion
This systematic review sought to analyze whether physical exercise can modulate circulating BDNF concentrations in adolescents. The search identified eight RCTs, each of good-to-excellent methodological quality, encompassing aerobic, resistance, concurrent, and high-intensity protocols, in addition to discipline-specific interventions such as Taekwondo and whole-body vibration. The resulting evidence may prove especially valuable to exercise professionals, healthcare practitioners, and school-based program designers interested in neurodevelopment and overall well-being in adolescents.
The main findings of this review yield mixed results. Although four trials demonstrated statistically significant post-intervention elevations in circulating BDNF [18,19,35,36], four detected no significant changes [20,21,37,38]. Collectively, these observations indicate that physical exercise is a plausible, though not definitive, modulator of BDNF in adolescents. The magnitude and direction of this effect likely hinge on exercise modality, dose (i.e., intensity, duration, and frequency), participant characteristics, and additional contextual factors.
Of the two studies that implemented resistance training [20,21], neither reported a significant post-intervention increase in BDNF. In this regard, other reviews on the effects of resistance training on BDNF levels in adults have also reported mixed findings. Babiarz et al. [39] found inconclusive evidence after analyzing ten studies on the effects of resistance training, with only four reporting positive results. Nevertheless, they concluded that intensities ≥ 70% of 1RM combined with short recovery periods are required to induce appreciable increases in BDNF. Similarly, the review by Huang et al. [40] found that five out of seven resistance training trials did not show significant changes in peripheral BDNF concentrations. The few reported increases were observed in studies lacking a control group.
Of the five included studies that implemented an aerobic exercise intervention, three reported statistically significant positive outcomes [18,19,35]. The trial conducted by Wunram et al. [35] demonstrated significantly greater improvements in adolescents with major depressive disorder following a six-week stationary cycling program. In contrast, previous research by Jeon and Ha [18,19] in obese adolescents reported significant increases in serum levels of BDNF after treadmill-based aerobic exercise (three to four sessions per week), supporting the hypothesis that the neurobiological benefits of exercise may be modulated by individual participant characteristics.
In this regard, available evidence in adults reveals mixed findings concerning BDNF responses to aerobic exercise. For instance, some short-term multi-session interventions (e.g., five weeks) have reported that only cognitive training, rather than structured aerobic exercise or mindfulness, induces sustained, modest increases in basal BDNF levels [41]. However, studies by Tsai et al. [42,43] have shown that while a single bout of moderate-intensity aerobic exercise can transiently elevate serum BDNF in young adults, these elevations do not significantly correlate with improvements in cognitive performance or neuroelectric indices (CNV and P3). Notably, only participants with higher cardiorespiratory fitness exhibit specific neurophysiological benefits, such as enhanced P3 amplitudes and reduced task-switching costs, suggesting that such effects may be driven more by fitness level than by BDNF per se. Thus, the mechanisms underlying exercise-induced cognitive enhancements may depend more on baseline physical fitness than on acute neurotrophic changes.
Therefore, several moderating factors may underlie the heterogeneous results observed across the included trials. Biological sex has been identified as a potential determinant of BDNF responsiveness to physical exercise [10,12], as estrogen is known to modulate both gene expression and peripheral release of this neurotrophin. However, most of the reviewed studies did not conduct sex-stratified analyses or adjust their statistical models accordingly, thereby limiting the interpretability of their findings. In addition, mental health status and body composition represent critical sources of variability. For instance, among adolescents with obesity, studies by Walsh et al. [20] and Goldfield et al. [21] found no significant changes in BDNF levels after aerobic, resistance, or combined training protocols. In contrast, Roh et al. [36] documented a significant increase in BDNF following a 16-week Taekwondo program in a similar population, suggesting that factors such as exercise modality, training intensity, and adherence may play a pivotal role in modulating neurotrophic responses. These discrepancies may reflect differences in exercise dose, baseline health status, initial fitness levels, or the influence of concomitant pharmacological treatments. Previous studies have suggested that session duration may influence acute BDNF levels [12]. On the other hand, during adolescence, the pubertal activation of the hormonal axis introduces additional complexity in the regulation of BDNF. Iughetti et al. [44] observed that boys in puberty exhibit significant lower plasma BDNF levels compared to their prepubertal peers and girls of similar age, suggesting a potential androgen-mediated modulation. Therefore, future RCTs should rigorously control for confounding variables such as sex, pubertal stage, menstrual cycle phase, and body composition, among others.
Despite the widespread use of commercial ELISA kits, seven studies analyzed serum samples [18,19,20,21,35,36,37] and only one utilized plasma [38]. This distinction may be relevant, as previous research by Tarassova et al. [26] highlighted that moderate-intensity exercise can increase plasma BDNF levels in older adults by up to 222%, whereas serum BDNF levels show only an 18% rise, with the effect dissipating within 35 min of rest. During the coagulation process, platelet activation induces the release of BDNF into serum [45], suggesting that clotting duration constitutes a critical methodological factor when quantifying serum BDNF concentrations [27]. Moreover, recent meta-analyses demonstrate an acute rise in BDNF levels after sessions of structured physical exercise, whereas long-term training programs do not increase circulating BDNF concentrations [46,47]. Additionally, extending the blood coagulation time to 30–60 min can substantially elevate BDNF levels [48]. In this regard, among the studies included in this review, blood sample collection occurred between 5 min and 72 h after the final exercise session. Given that BDNF typically returns to baseline within a few hours [49,50], such heterogeneous washout periods may have masked potential chronic adaptations. Therefore, future investigations should adopt standardized protocols for blood sample timing and handling to ensure the comparability and interpretability of findings.
Furthermore, although the present study has focused on BDNF levels as the primary variable, it is important to contextualize our findings in relation to the clinical effects observed in other secondary outcomes. For example, Jeon and Ha [19] reported a significant improvement in working memory. Numerous RCTs have consistently demonstrated improvements in cognitive function [51], academic performance [52], and mental health [53] in adolescents following physical exercise interventions.
Other studies, such as that by Walsh et al. [20], have shown that physical exercise interventions can improve glucose levels and HOMA-B indices, in line with previous findings [54]. Similarly, Seok-Min and Chol-Hyoung [37], Roh et al. [36], and Gejl et al. [38] observed significant improvements in body composition and physical fitness variables in adolescents. These results are consistent with previous systematic reviews suggesting that structured exercise programs are effective strategies for improving these outcomes in this population [55,56]. Therefore, physical exercise not only influences BDNF levels but may also provide adolescents with additional benefits in outcomes closely linked to their quality of life and overall functional capacity.
Although this original review includes high-quality and excellent RCTs, several limitations must be considered. First, the number of included studies was small, with limited sample sizes and a general lack of sex-stratified analyses. Second, a meta-analysis was not performed due to substantial heterogeneity in both study populations and interventions. Participants ranged from healthy individuals to those with obesity, depression, or anxiety, and the exercise protocols and control conditions varied widely, limiting the validity of pooled comparisons. Third, the timing of post-exercise sampling varied greatly across studies, which is a critical factor influencing BDNF levels and complicates cross-study comparisons. Finally, the adolescents assessed presented divergent health profiles (healthy, obese, and depressed), a variability that weakens external validity and further precludes meta-analysis.
5. Conclusions and Practical Applications
The available evidence from RCT indicates that exercise interventions may modulate peripheral BDNF levels in adolescents, though the effects are inconsistent across studies. Of the eight high-quality trials examined, only four demonstrated statistically significant increases in circulating BDNF concentrations following exercise interventions.
For exercise professionals and healthcare practitioners working with adolescents, these findings suggest several practical considerations for program design:
- Exercise interventions involving moderate-to-vigorous intensity activities.
- These interventions should be performed at least 2–3 times per week over a minimum period of 6 weeks.
- Multi-modal training approaches that integrate metabolic stress with cognitive and coordinative demands may be particularly effective.
- Effective examples may include martial arts, circuit training, and sport-specific activities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sports13080253/s1, File S1: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. File S2: The PRISMA for Abstracts Checklist.
Author Contributions
Conceptualization, M.R.-G. and D.G.-D.; methodology, M.R.-G. and C.D.G.-C.; validation, D.G.-D. and A.M.-V.; formal analysis, M.R.-G. and D.G.-D.; investigation, M.R.-G., D.G.-D., C.D.G.-C. and A.M.-V.; resources, C.D.G.-C. and A.M.-V.; data curation, M.R.-G. and D.G.-D.; writing—original draft preparation, M.R.-G., D.G.-D., C.D.G.-C. and A.M.-V.; writing—review and editing, M.R.-G., D.G.-D., C.D.G.-C. and A.M.-V.; visualization, M.R.-G. and C.D.G.-C.; supervision, C.D.G.-C. and A.M.-V.; project administration, M.R.-G. and C.D.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the article is a systematic review and it does not include human or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, N.A.; Hillman, C.H. The Relation of Childhood Physical Activity and Aerobic Fitness to Brain Function and Cognition: A Review. Pediatr. Exerc. Sci. 2014, 26, 138–146. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Berthoud, H.-R.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of Exercise. Obesity 2006, 14, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. In Neurotrophic Factors; Lewin, G.R., Carter, B.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 223–250. ISBN 978-3-642-45106-5. [Google Scholar]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-Derived Neurotrophic Factor in the Control Human Brain, and in Alzheimer’s Disease and Parkinson’s Disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef] [PubMed]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF Mediates the Efficacy of Exercise on Synaptic Plasticity and Cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef]
- Sandhya, V.K.; Raju, R.; Verma, R.; Advani, J.; Sharma, R.; Radhakrishnan, A.; Nanjappa, V.; Narayana, J.; Somani, B.L.; Mukherjee, K.K.; et al. A Network Map of BDNF/TRKB and BDNF/p75NTR Signaling System. J. Cell Commun. Signal. 2013, 7, 301–307. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of Brain-Derived Neurotrophic Factor across the Blood–Brain Barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A Meta-Analytic Review of the Effects of Exercise on Brain-Derived Neurotrophic Factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The Effect of Acute Exercise on Blood Concentrations of Brain-Derived Neurotrophic Factor in Healthy Adults: A Meta-Analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The Effects of Cardiovascular Exercise on Human Memory: A Review with Meta-Analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef]
- Carson, V.; Hunter, S.; Kuzik, N.; Wiebe, S.A.; Spence, J.C.; Friedman, A.; Tremblay, M.S.; Slater, L.; Hinkley, T. Systematic Review of Physical Activity and Cognitive Development in Early Childhood. J. Sci. Med. Sport. 2016, 19, 573–578. [Google Scholar] [CrossRef]
- de Menezes-Junior, F.J.; Jesus, Í.C.; Brand, C.; Mota, J.; Leite, N. Physical Exercise and Brain-Derived Neurotrophic Factor Concentration in Children and Adolescents: A Systematic Review With Meta-Analysis. Pediatr. Exerc. Sci. 2022, 34, 44–53. [Google Scholar] [CrossRef]
- de Azevedo, K.P.M.; de Oliveira, V.H.; de Medeiros, G.C.B.S.; Mata, Á.N.d.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. Expression of Brain-Derived Neurotrophic Factor, IGF-1 and Cortisol Elicited by Regular Aerobic Exercise in Adolescents. J. Phys. Ther. Sci. 2015, 27, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. The Effect of Exercise Intensity on Brain Derived Neurotrophic Factor and Memory in Adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; D’Angiulli, A.; Cameron, J.D.; Sigal, R.J.; Kenny, G.P.; Holcik, M.; Doucette, S.; Alberga, A.S.; Prud’homme, D.; Hadjiyannakis, S.; et al. Changes in the Brain-Derived Neurotrophic Factor Are Associated with Improvements in Diabetes Risk Factors after Exercise Training in Adolescents with Obesity: The HEARTY Randomized Controlled Trial. Neural Plast. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Goldfield, G.S.; Kenny, G.P.; Prud’homme, D.; Holcik, M.; Alberga, A.S.; Fahnestock, M.; Cameron, J.D.; Doucette, S.; Hadjiyannakis, S.; Tulloch, H.; et al. Effects of Aerobic Training, Resistance Training, or Both on Brain-Derived Neurotrophic Factor in Adolescents with Obesity: The Hearty Randomized Controlled Trial. Physiol. Behav. 2018, 191, 138–145. [Google Scholar] [CrossRef]
- Segundo, V.H.d.O.; de Azevedo, K.P.M.; de Medeiros, G.C.B.S.; Mata, Á.N.d.S.; Piuvezam, G. Association between Sedentary Behavior and Brain-Derived Neurotrophic Factor (BDNF) in Children and Adolescents: A Protocol for Systematic Review and Meta-Analysis. PLoS ONE 2024, 19, e0299024. [Google Scholar] [CrossRef]
- de Azevedo, K.P.M.; de Oliveira Segundo, V.H.; de Medeiros, G.C.B.S.; de Sousa Mata, Á.N.; García, D.Á.; de Carvalho Leitão, J.C.G.; Knackfuss, M.I.; Piuvezam, G. Effects of Exercise on the Levels of BDNF and Executive Function in Adolescents: A Protocol for Systematic Review and Meta-Analysis. Medicine 2019, 98, e16445. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF Mediates Adaptive Brain and Body Responses to Energetic Challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Gabbay, V.; Klein, R.G.; Alonso, C.M.; Babb, J.S.; Nishawala, M.; De Jesus, G.; Hirsch, G.S.; Hottinger-Blanc, P.M.Z.; Gonzalez, C.J. Immune System Dysregulation in Adolescent Major Depressive Disorder. J. Affect. Disord. 2009, 115, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tarassova, O.; Ekblom, M.M.; Moberg, M.; Lövdén, M.; Nilsson, J. Peripheral BDNF Response to Physical and Cognitive Exercise and Its Association With Cardiorespiratory Fitness in Healthy Older Adults. Front. Physiol. 2020, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Zanardini, R.; Gennarelli, M.; Bocchio-Chiavetto, L. [Letter to the Editor] Influence of Clotting Duration on Brain-Derived Neurotrophic Factor (BDNF) Dosage in Serum. BioTechniques 2014, 57, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R.; Arnett, D.K.; Eckel, R.H.; Gidding, S.S.; Hayman, L.L.; Kumanyika, S.; Robinson, T.N.; Scott, B.J.; Jeor, S.S.; Williams, C.L. Overweight in Children and Adolescents. Circulation 2005, 111, 1999–2012. [Google Scholar] [CrossRef]
- Carter, T.; Morres, I.D.; Meade, O.; Callaghan, P. The Effect of Exercise on Depressive Symptoms in Adolescents: A Systematic Review and Meta-Analysis. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 580–590. [Google Scholar] [CrossRef]
- Bustamante, E.E.; Williams, C.F.; Davis, C.L. Physical Activity Interventions for Neurocognitive and Academic Performance in Overweight and Obese Youth: A Systematic Review. Pediatr. Clin. 2016, 63, 459–480. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Biopsychosoc. Sci. Med. 2010, 72, 239. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rico-González, M.; Pino-Ortega, J.; Clemente, F.M.; Los Arcos, A. Guidelines for Performing Systematic Reviews in Sports Science. Biol. Sport 2022, 39, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Wunram, H.L.; Oberste, M.; Ziemendorff, A.; Hamacher, S.; Kapanci, T.; Heller, R.; Blick, S.; Bloch, W.; Clajus, T.C.; Schönau, E.; et al. Differential Effects of Ergometer-Cycling and Whole-Body-Vibration Training on Serological BDNF and IGF-1 in the Treatment of Adolescent Depression—Is There an Impact of BDNFp.Val66Met Variants? Physiol. Behav. 2021, 241, 113596. [Google Scholar] [CrossRef]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Effects of Regular Taekwondo Intervention on Oxidative Stress Biomarkers and Myokines in Overweight and Obese Adolescents. Int. J. Environ. Res. Public Heal. 2020, 17, 2505. [Google Scholar] [CrossRef]
- Shin, S.-M.; Kim, C.-H. Effects of Combined Exercise on Body Composition, Blood Lipids, and BDNF in Obese Adolescents. J. Life Sci. 2012, 22, 1231–1236. [Google Scholar] [CrossRef][Green Version]
- Gejl, A.K.; Bugge, A.; Ernst, M.T.; Mortensen, E.L.; Gejl, K.D.; Andersen, L.B. Effects of 9 Weeks of High- or Moderate-Intensity Training on Cardiorespiratory Fitness, Inhibitory Control, and Plasma Brain-Derived Neurotrophic Factor in Danish Adolescents—A Randomized Controlled Trial. Scand. Med. Sci. Sports 2024, 34, e14703. [Google Scholar] [CrossRef]
- Babiarz, M.; Laskowski, R.; Grzywacz, T. Effects of Strength Training on BDNF in Healthy Young Adults. Int. J. Environ. Res. Public Health 2022, 19, 13795. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The Effects of Physical Activity and Exercise on Brain-Derived Neurotrophic Factor in Healthy Humans: A Review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Ledreux, A.; Håkansson, K.; Carlsson, R.; Kidane, M.; Columbo, L.; Terjestam, Y.; Ryan, E.; Tusch, E.; Winblad, B.; Daffner, K.; et al. Differential Effects of Physical Exercise, Cognitive Training, and Mindfulness Practice on Serum BDNF Levels in Healthy Older Adults: A Randomized Controlled Intervention Study. J. Alzheimer’s Dis. 2019, 71, 1245–1261. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chen, F.-C.; Pan, C.-Y.; Wang, C.-H.; Huang, T.-H.; Chen, T.-C. Impact of Acute Aerobic Exercise and Cardiorespiratory Fitness on Visuospatial Attention Performance and Serum BDNF Levels. Psychoneuroendocrinology 2014, 41, 121–131. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Pan, C.-Y.; Chen, F.-C.; Wang, C.-H.; Chou, F.-Y. Effects of Acute Aerobic Exercise on a Task-Switching Protocol and Brain-Derived Neurotrophic Factor Concentrations in Young Adults with Different Levels of Cardiorespiratory Fitness. Exp. Physiol. 2016, 101, 836–850. [Google Scholar] [CrossRef]
- Iughetti, L.; Casarosa, E.; Predieri, B.; Patianna, V.; Luisi, S. Plasma Brain-Derived Neurotrophic Factor Concentrations in Children and Adolescents. Neuropeptides 2011, 45, 205–211. [Google Scholar] [CrossRef]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.; Sun, B.; Tandon, N.N. Brain-Derived Neurotrophic Factor Is Stored in Human Platelets and Released by Agonist Stimulation. Thromb. Haemost. 2017, 87, 728–734. [Google Scholar] [CrossRef]
- Ceylan, H.İ.; Silva, A.F.; Ramirez-Campillo, R.; Murawska-Ciałowicz, E. Exploring the Effect of Acute and Regular Physical Exercise on Circulating Brain-Derived Neurotrophic Factor Levels in Individuals with Obesity: A Comprehensive Systematic Review and Meta-Analysis. Biology 2024, 13, 323. [Google Scholar] [CrossRef]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Moncada-Jiménez, J.; Abellán-Aynés, O.; Rubio-Arias, J.Á. Immediate and Short-Term Effect of Physical Exercise on BDNF in Multiple Sclerosis Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2025, 15, 19696. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between Serum and Plasma Brain-Derived Neurotrophic Factor and Influence of Storage Time and Centrifugation Strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic Growth Factor Responses to Lower Body Resistance Training in Older Adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training Augments Resistance Exercise Induced Elevation of Circulating Brain Derived Neurotrophic Factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Tejero-Gonzalez, C.M.; Sallis, J.F.; Veiga, O.L. Physical Activity and Cognition in Adolescents: A Systematic Review. J. Sci. Med. Sport. 2015, 18, 534–539. [Google Scholar] [CrossRef]
- Haverkamp, B.F.; Wiersma, R.; Vertessen, K.; van Ewijk, H.; Oosterlaan, J.; Hartman, E. Effects of Physical Activity Interventions on Cognitive Outcomes and Academic Performance in Adolescents and Young Adults: A Meta-Analysis. J. Sports Sci. 2020, 38, 2637–2660. [Google Scholar] [CrossRef]
- Ruiz-Ranz, E.; Asín-Izquierdo, I. Physical Activity, Exercise, and Mental Health of Healthy Adolescents: A Review of the Last 5 Years. Sports Med. Health Sci. 2025, 7, 161–172. [Google Scholar] [CrossRef]
- García-Hermoso, A.; López-Gil, J.F.; Izquierdo, M.; Ramírez-Vélez, R.; Ezzatvar, Y. Exercise and Insulin Resistance Markers in Children and Adolescents With Excess Weight: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1276–1284. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Liu, Y.; Zhou, Y. Effects of Different Exercise Modalities and Intensities on Body Composition in Overweight and Obese Children and Adolescents: A Systematic Review and Network Meta-Analysis. Front. Physiol. 2023, 14, 1193223. [Google Scholar] [CrossRef]
- González Devesa, D.; Sánchez Lastra, M.A.; Meis García, D.; Ayán Pérez, C. Efectos del ejercicio acuático en variables relacionadas con la composición corporal en niños y adolescentes: Revisión sistemática. Arch. Med. Deporte 2024, 41, 120–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).