Integrative Physiological Strategies for Monitoring Demands in Functional Fitness
Abstract
1. Introduction
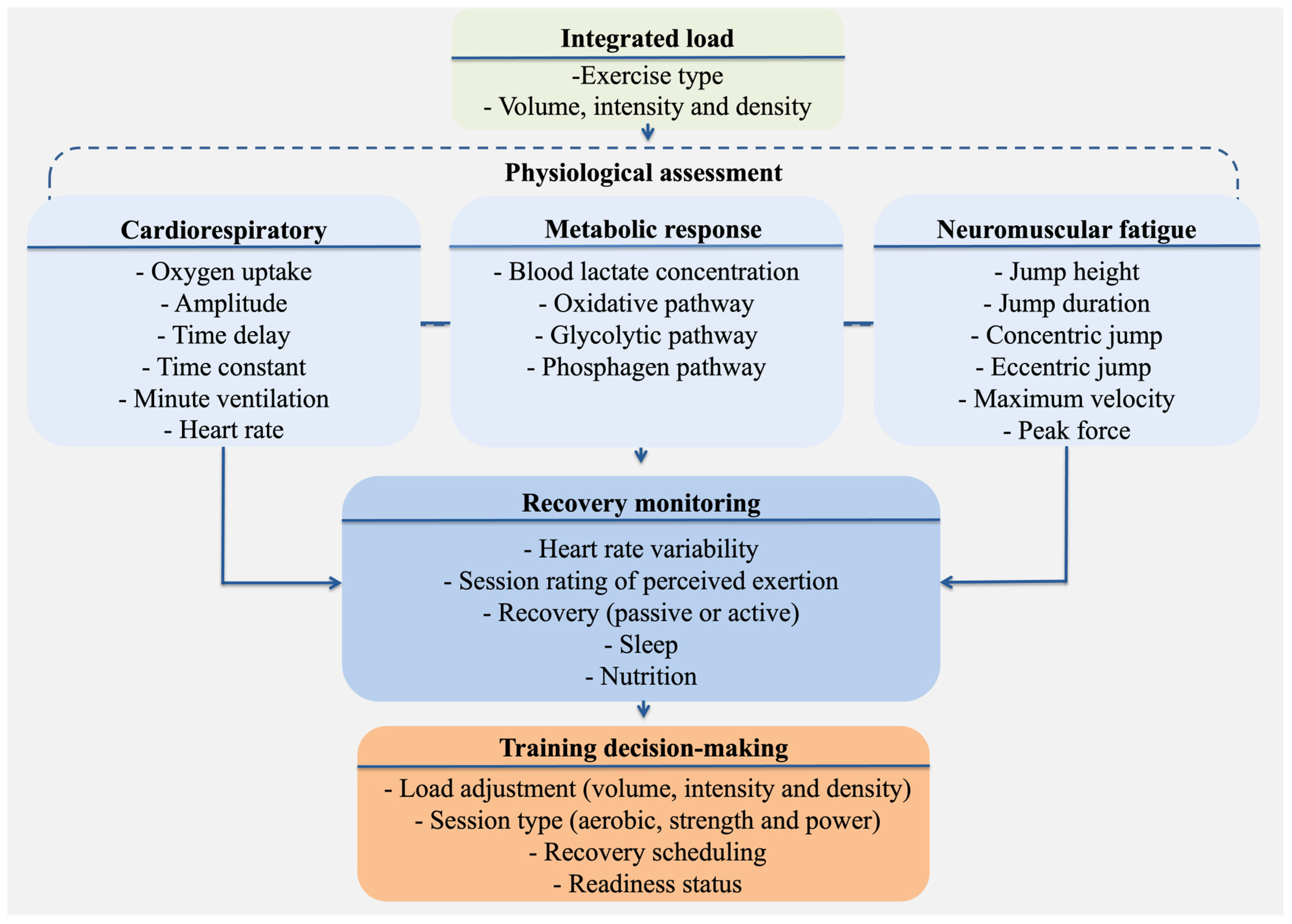
2. An Integrated Physiological Model
2.1. Cardiorespiratory Function
2.2. Bioenergetic Profiling
2.3. Neuromuscular Fatigue
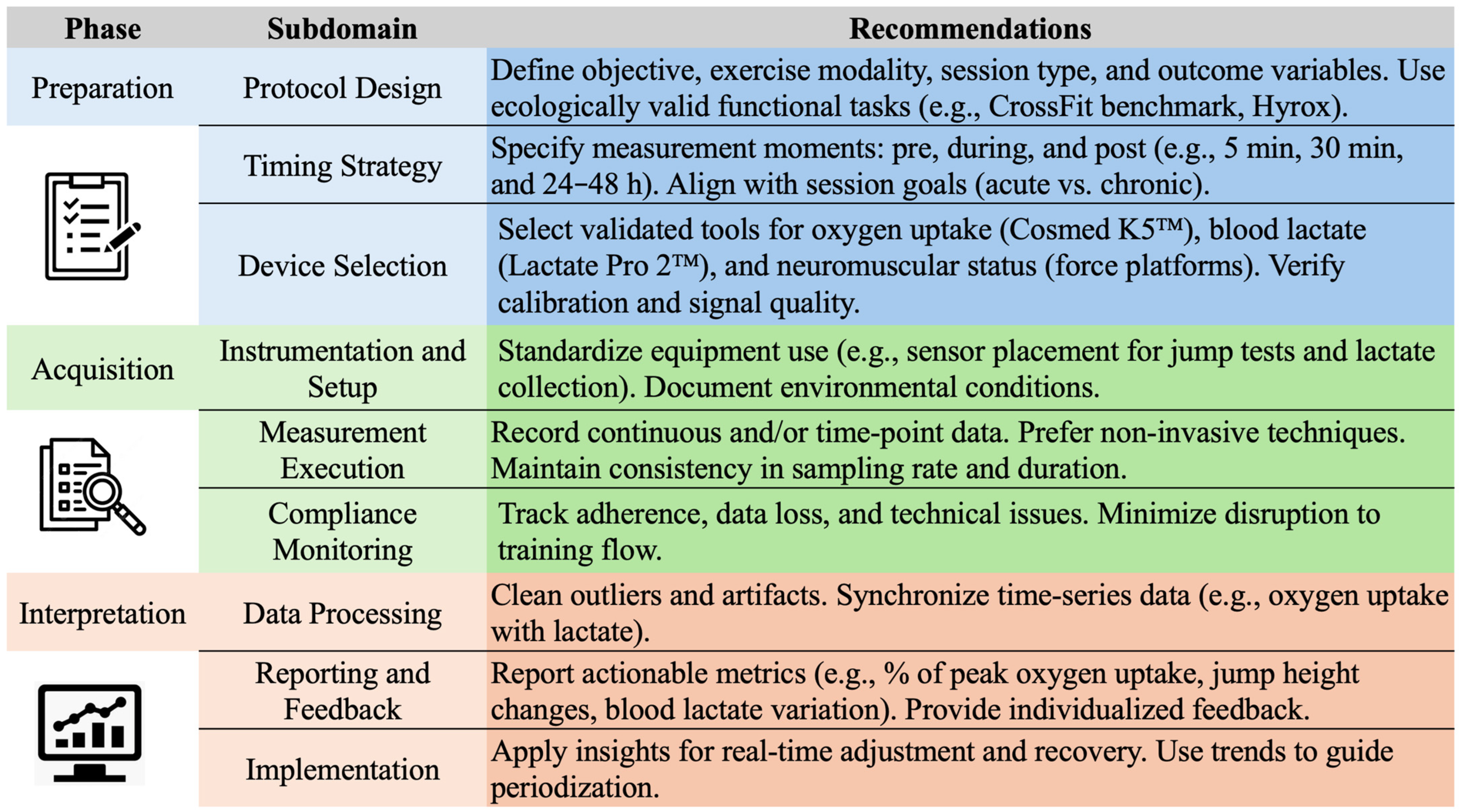
3. Integrating Technology and Physiology
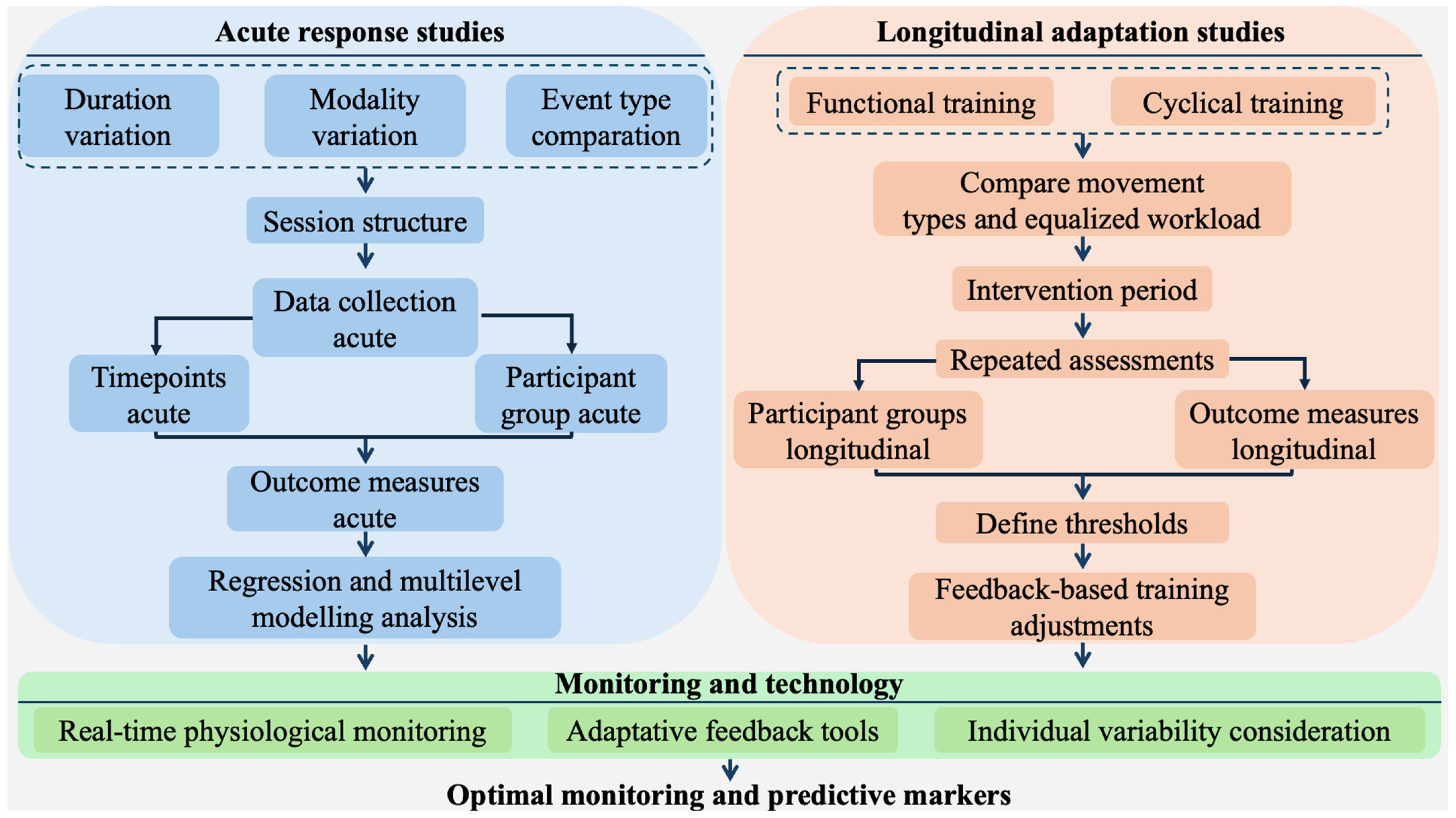
4. Future Directions
5. Practical Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wojna, J.; Zuwala-Jagiello, J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J. Physiol. Pharmacol. 2015, 66, 811–821. [Google Scholar]
- Heinrich, K.M.; Becker, C.; Carlisle, T.; Gilmore, K.; Hauser, J.; Frye, J.; Harms, C. High–intensity functional training improves functional movement and body composition among cancer survivors: A pilot study. Eur. J. Cancer Care 2015, 24, 812–817. [Google Scholar] [CrossRef]
- Dominski, F.H.; Tibana, R.A.; Andrade, A. “Functional Fitness Training”, CrossFit, HIMT, or HIFT: What Is the Preferable Terminology? Front. Sports Act. Living 2022, 4, 882195. [Google Scholar] [CrossRef] [PubMed]
- Martinho, D.V.; Rebelo, A.; Gouveia, É.R.; Field, A.; Costa, R.; Ribeiro, A.S.; Casonatto, J.; Amorim, C.; Sarmento, H. The physical demands and physiological responses to CrossFit®: A scoping review with evidence gap map and meta-correlation. BMC Sports Sci. Med. Rehabil. 2024, 16, 196. [Google Scholar] [CrossRef]
- Rios, M.; Pyne, D.B.; Fernandes, R.J. The Effects of CrossFit(®) Practice on Physical Fitness and Overall Quality of Life. Int. J. Environ. Res. Public Health 2024, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Mangine, G.T.; Tankersley, J.E.; McDougle, J.M.; Velazquez, N.; Roberts, M.D.; Esmat, T.A.; VanDusseldorp, T.A.; Feito, Y. Predictors of CrossFit open performance. Sports 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, R.; Valenzuela, P.L.; Alejo, L.B.; Gil-Cabrera, J.; Montalvo-Pérez, A.; Talavera, E.; Lucia, A.; Moral-González, S.; Barranco-Gil, D. Physiological predictors of competition performance in CrossFit athletes. Int. J. Environ. Res. Public Health 2020, 17, 3699. [Google Scholar] [CrossRef]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; Cañuelo-Márquez, A.M.; Guodemar-Pérez, J.; García-Fernández, P.; Lozano-Estevan, M.D.C.; Alonso-Melero, R.; Sánchez-Calabuig, M.A.; Ruíz-López, M.; et al. Cardiometabolic and Muscular Fatigue Responses to Different CrossFit® Workouts. J. Sports Sci. Med. 2018, 17, 668–679. [Google Scholar]
- Rios, M.; Becker, K.M.; Monteiro, A.S.; Fonseca, P.; Pyne, D.B.; Reis, V.M.; Moreira-Gonçalves, D.; Fernandes, R.J. Effect of the Fran CrossFit Workout on Oxygen Uptake Kinetics, Energetics, and Postexercise Muscle Function in Trained CrossFitters. Int. J. Sports Physiol. Perform. 2024, 19, 299–306. [Google Scholar] [CrossRef]
- Fernández, J.F.; Solana, R.S.; Moya, D.; Marin, J.M.S.; Ramón, M.M. Acute physiological responses during crossfit® workouts. Eur. J. Hum. Mov. 2015, 35, 114–124. [Google Scholar]
- Leitão, L.; Dias, M.; Campos, Y.; Vieira, J.G.; Sant’Ana, L.; Telles, L.G.; Tavares, C.; Mazini, M.; Novaes, J.; Vianna, J. Physical and physiological predictors of FRAN CrossFit® WOD athlete’s performance. Int. J. Environ. Res. Public Health 2021, 18, 4070. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; De Sousa, N.M.F.; Prestes, J.; Voltarelli, F.A. Lactate, Heart Rate and Rating of Perceived Exertion Responses to Shorter and Longer Duration CrossFit(®) Training Sessions. J. Funct. Morphol. Kinesiol. 2018, 3, 60. [Google Scholar] [CrossRef]
- de Sousa Neto, I.V.; de Sousa, N.M.F.; Neto, F.R.; Falk Neto, J.H.; Tibana, R.A. Time Course of Recovery Following CrossFit(®) Karen Benchmark Workout in Trained Men. Front. Physiol. 2022, 13, 899652. [Google Scholar] [CrossRef]
- Ponce-García, T.; García-Romero, J.; Carrasco-Fernández, L.; Castillo-Domínguez, A.; Benítez-Porres, J. Sex differences in anaerobic performance in CrossFit® athletes: A comparison of three different all-out tests. PeerJ 2025, 13, e18930. [Google Scholar] [CrossRef]
- Brandt, T.; Ebel, C.; Lebahn, C.; Schmidt, A. Acute physiological responses and performance determinants in Hyrox(©)—A new running-focused high intensity functional fitness trend. Front. Physiol. 2025, 16, 1519240. [Google Scholar] [CrossRef] [PubMed]
- Davids, C.J. A Performance Analysis of HYROX: A Review of the Physiologic, Mechanical, and Technical Demands. Strength Cond. J. 2022, 10, 1519. [Google Scholar] [CrossRef]
- Rios, M.; Zacca, R.; Azevedo, R.; Fonseca, P.; Pyne, D.B.; Reis, V.M.; Moreira-Gonçalves, D.; Fernandes, R.J. Bioenergetic Analysis and Fatigue Assessment During the Fran Workout in Experienced Crossfitters. Int. J. Sports Physiol. Perform. 2023, 18, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; de Sousa, N.M.F.; Cunha, G.V.; Prestes, J.; Fett, C.; Gabbett, T.J.; Voltarelli, F.A. Validity of Session Rating Perceived Exertion Method for Quantifying Internal Training Load during High-Intensity Functional Training. Sports 2018, 6, 68. [Google Scholar] [CrossRef]
- Tallis, J.; Morris, R.O.; Duncan, M.J.; Eyre, E.L.J.; Guimaraes-Ferreira, L. Agreement between Force Platform and Smartphone Application-Derived Measures of Vertical Jump Height in Youth Grassroots Soccer Players. Sports 2023, 11, 117. [Google Scholar] [CrossRef]
- Jones, A.M.; Poole, D.C. Oxygen uptake dynamics: From muscle to mouth--an introduction to the symposium. Med. Sci. Sports Exerc. 2005, 37, 1542–1550. [Google Scholar] [CrossRef]
- Sousa, A.; Figueiredo, P.; Zamparo, P.; Pyne, D.B.; Vilas-Boas, J.P.; Fernandes, R.J. Exercise Modality Effect on Bioenergetical Performance at V˙O2max Intensity. Med. Sci. Sports Exerc. 2015, 47, 1705–1713. [Google Scholar] [CrossRef]
- Rios, M.; Becker, K.M.; Cardoso, F.; Pyne, D.B.; Reis, V.M.; Moreira-Gonçalves, D.; Fernandes, R.J. Assessment of Cardiorespiratory and Metabolic Contributions in an Extreme Intensity CrossFit(®) Benchmark Workout. Sensors 2024, 24, 513. [Google Scholar] [CrossRef]
- Ferguson, H.A.; Harnish, C.; Chase, J.G. Using Field Based Data to Model Sprint Track Cycling Performance. Sports Med.–Open 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; Sousa, N.M.F.; Prestes, J.; Feito, Y.; Ferreira, C.E.; Voltarelli, F.A. Monitoring Training Load, Well-Being, Heart Rate Variability, and Competitive Performance of a Functional-Fitness Female Athlete: A Case Study. Sports 2019, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes: Opening the door to effective monitoring. Sports Med. 2013, 43, 773–781. [Google Scholar] [CrossRef]
- Barreto, A.C.; Medeiros, A.P.; da Silva Araujo, G.; Vale, R.; Vianna, J.M.; Alkimin, R.; Serra, R.; Leitão, L.; Reis, V.M.; da Silva Novaes, J. Variabilidad de la frecuencia cardíaca y de la presión arterial durante y después de tres sesiones de CrossFit®(Heart rate variability and blood pressure during and after three CrossFit® sessions). Retos 2023, 47, 311. [Google Scholar] [CrossRef]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Rios, M.; Reis, V.M.; Soares, S.; Moreira-Gonçalves, D.; Fernandes, R.J. Pros and cons of two methods of anaerobic alactic energy assessment in a high-intensity CrossFit® workout. Oxygen 2022, 2, 621–627. [Google Scholar] [CrossRef]
- Claudino, J.G.; Cronin, J.; Mezêncio, B.; McMaster, D.T.; McGuigan, M.; Tricoli, V.; Amadio, A.C.; Serrão, J.C. The countermovement jump to monitor neuromuscular status: A meta-analysis. J. Sci. Med. Sport 2017, 20, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44 (Suppl. 2), S139–S147. [Google Scholar] [CrossRef] [PubMed]
- de Beukelaar, T.T.; Mantini, D. Monitoring Resistance Training in Real Time with Wearable Technology: Current Applications and Future Directions. Bioengineering 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Varley, M.C. Wearable Training-Monitoring Technology: Applications, Challenges, and Opportunities. Int. J. Sports Physiol. Perform. 2017, 12, S255–S262. [Google Scholar] [CrossRef]
- Zacca, R.; Azevedo, R.; Figueiredo, P.; Vilas-Boas, J.P.; Castro, F.A.S.; Pyne, D.B.; Fernandes, R.J. VO2FITTING: A Free and Open-Source Software for Modelling Oxygen Uptake Kinetics in Swimming and other Exercise Modalities. Sports 2019, 7, 31. [Google Scholar] [CrossRef]
- Altini, M.; Amft, O. HRV4Training: Large-scale longitudinal training load analysis in unconstrained free-living settings using a smartphone application. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 2610–2613. [Google Scholar] [CrossRef] [PubMed]
- Vondrasek, J.D.; Riemann, B.L.; Grosicki, G.J.; Flatt, A.A. Validity and Efficacy of the Elite HRV Smartphone Application during Slow-Paced Breathing. Sensors 2023, 23, 9496. [Google Scholar] [CrossRef]
- Heikura, I. Individual Adaptation to Endurance Training Guided by Heart Rate Variability. Master’s Thesis, Department of Biology of Physical Activity, University of Jyväskylä, Jyväskylä, Finland, 2015. Available online: https://jyx.jyu.fi/jyx/Record/jyx_123456789_45036 (accessed on 20 September 2025).
- Flatt, A.A.; Wilkerson, G.B.; Allen, J.R.; Keith, C.M.; Esco, M.R. Daily heart rate variability before and after concussion in an American college football player. Sports 2019, 7, 97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, M.; Pyne, D.B. Integrative Physiological Strategies for Monitoring Demands in Functional Fitness. Sports 2025, 13, 381. https://doi.org/10.3390/sports13110381
Rios M, Pyne DB. Integrative Physiological Strategies for Monitoring Demands in Functional Fitness. Sports. 2025; 13(11):381. https://doi.org/10.3390/sports13110381
Chicago/Turabian StyleRios, Manoel, and David B. Pyne. 2025. "Integrative Physiological Strategies for Monitoring Demands in Functional Fitness" Sports 13, no. 11: 381. https://doi.org/10.3390/sports13110381
APA StyleRios, M., & Pyne, D. B. (2025). Integrative Physiological Strategies for Monitoring Demands in Functional Fitness. Sports, 13(11), 381. https://doi.org/10.3390/sports13110381






