Precision of an Inertial System to Evaluate the Finger Tapping Test in Women with Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Procedures
2.3. Data Treatment and Statistical Analysis
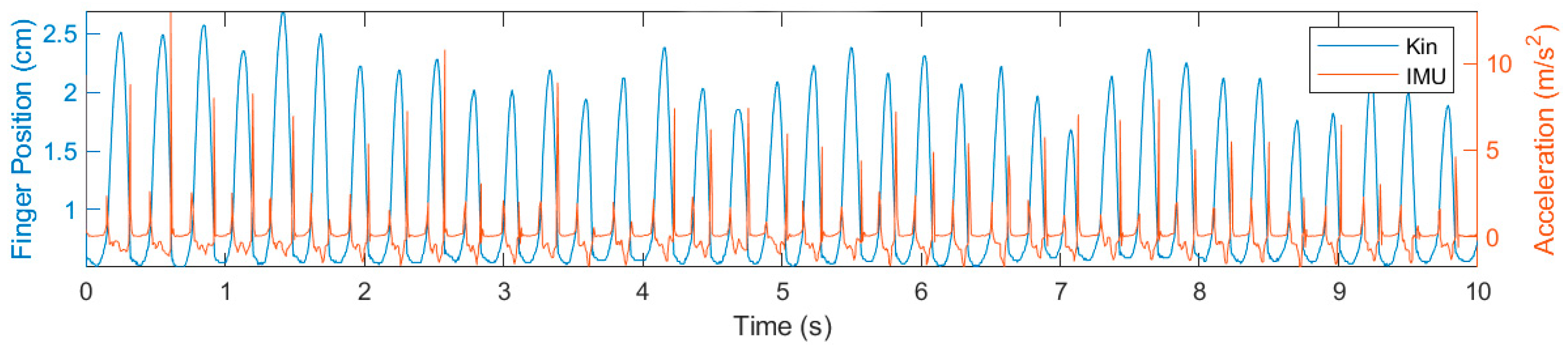
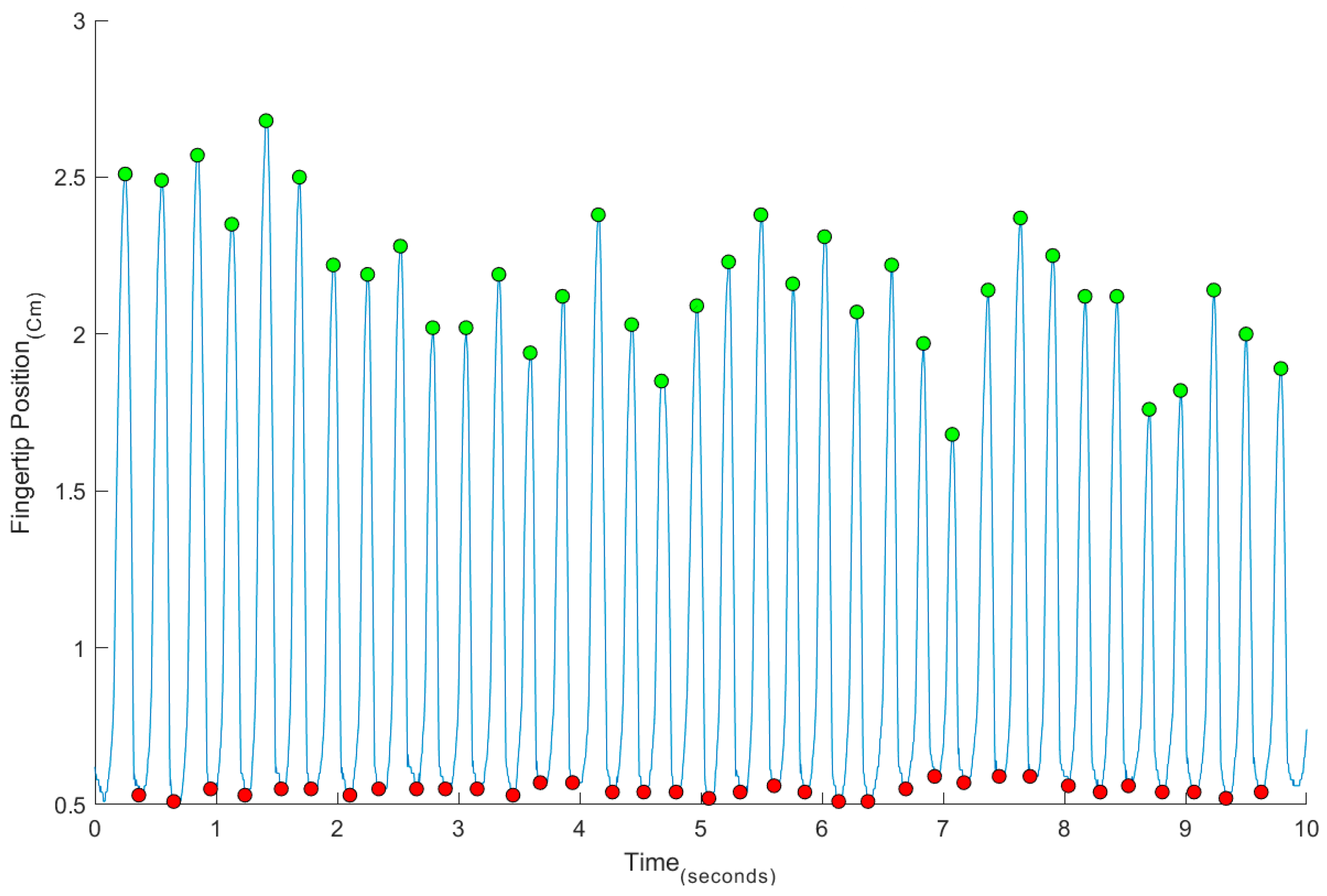
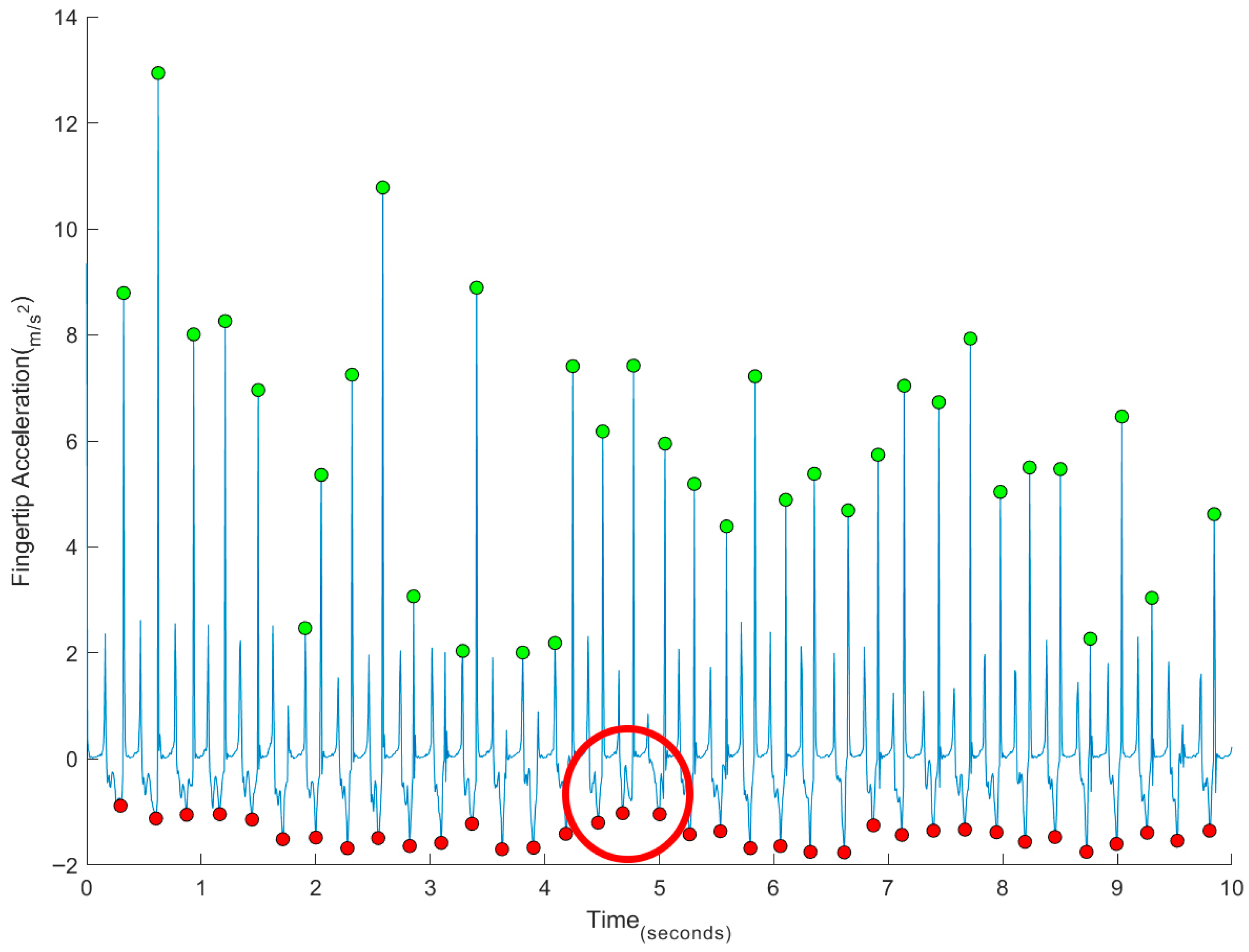
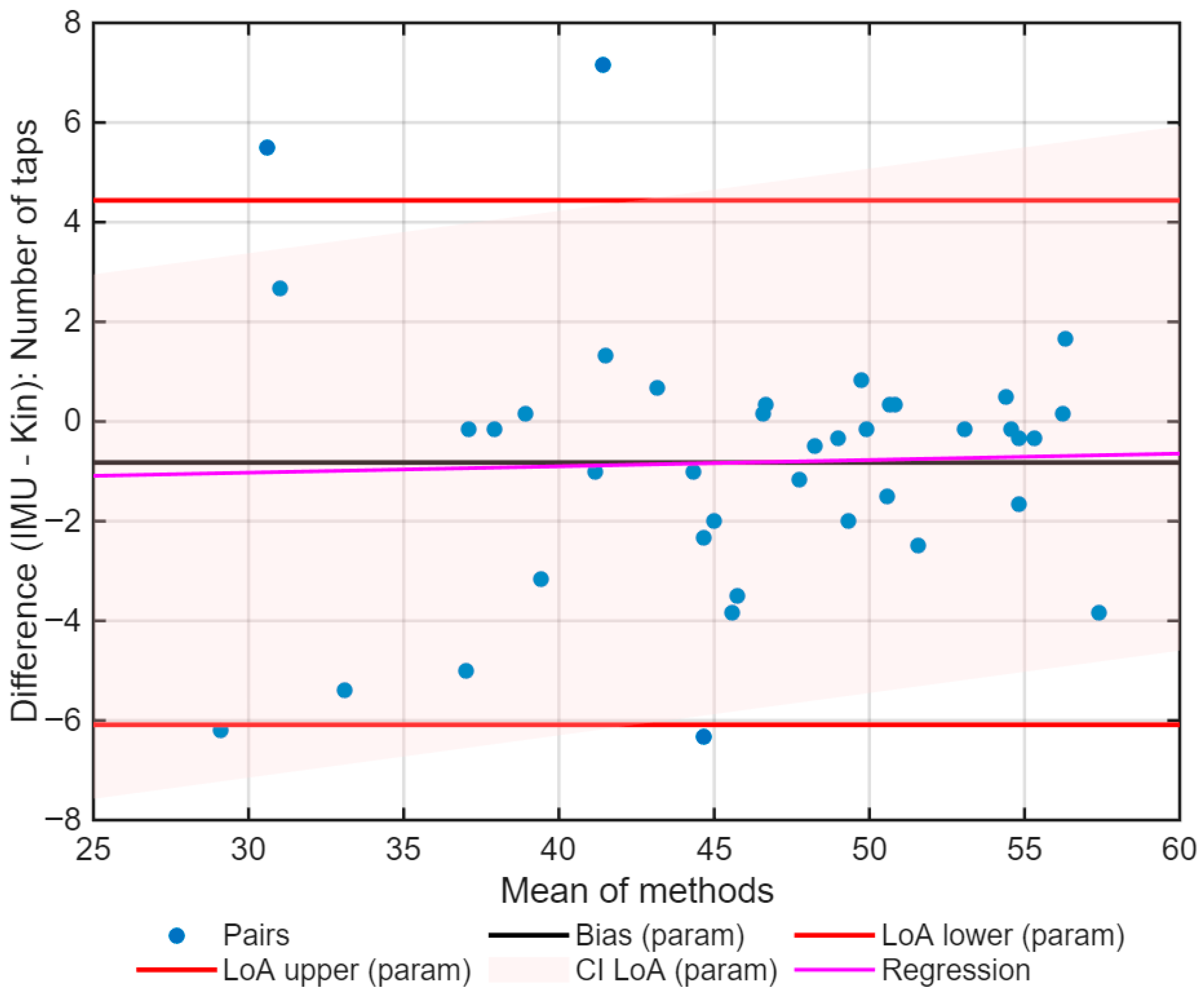
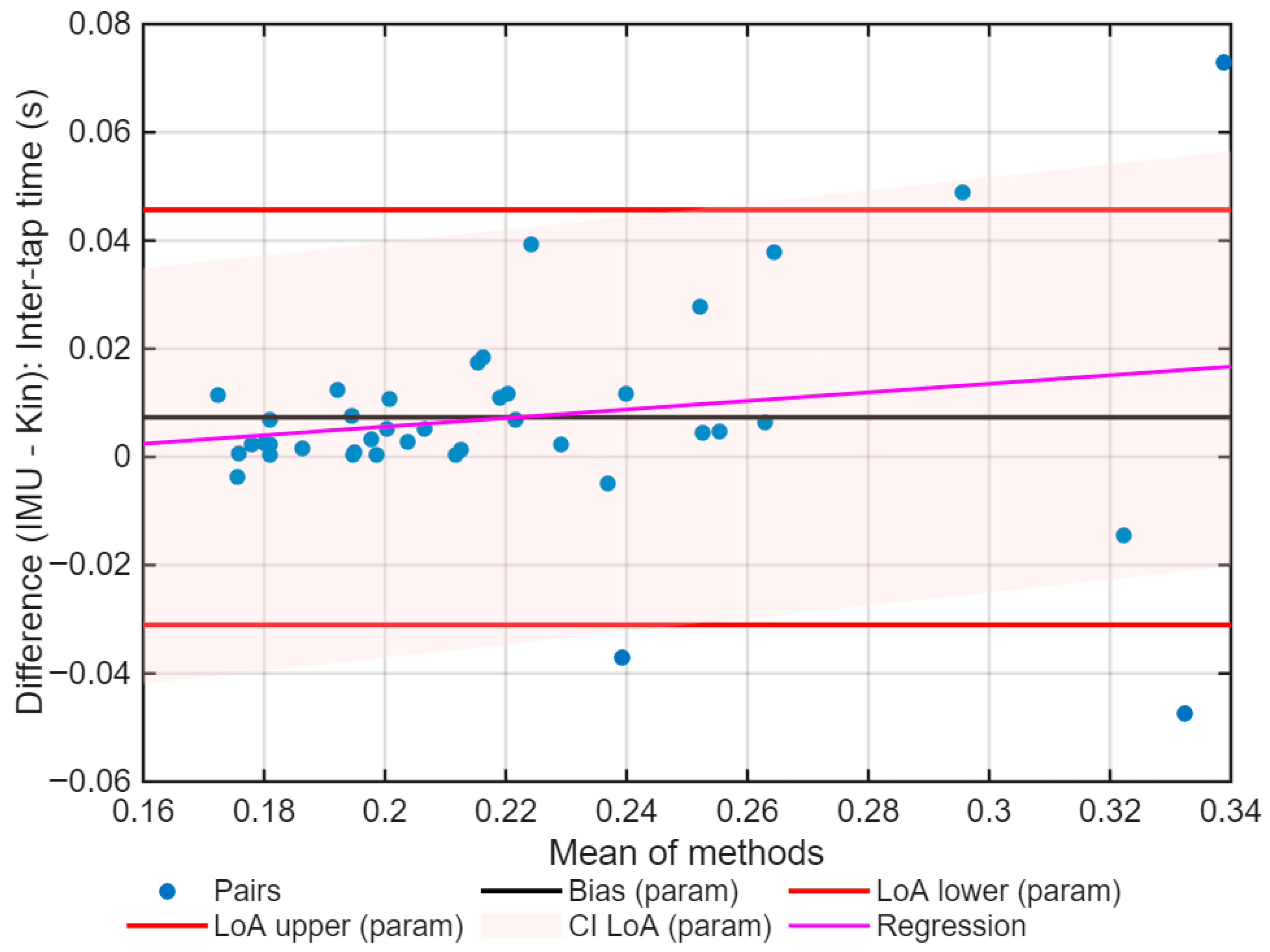
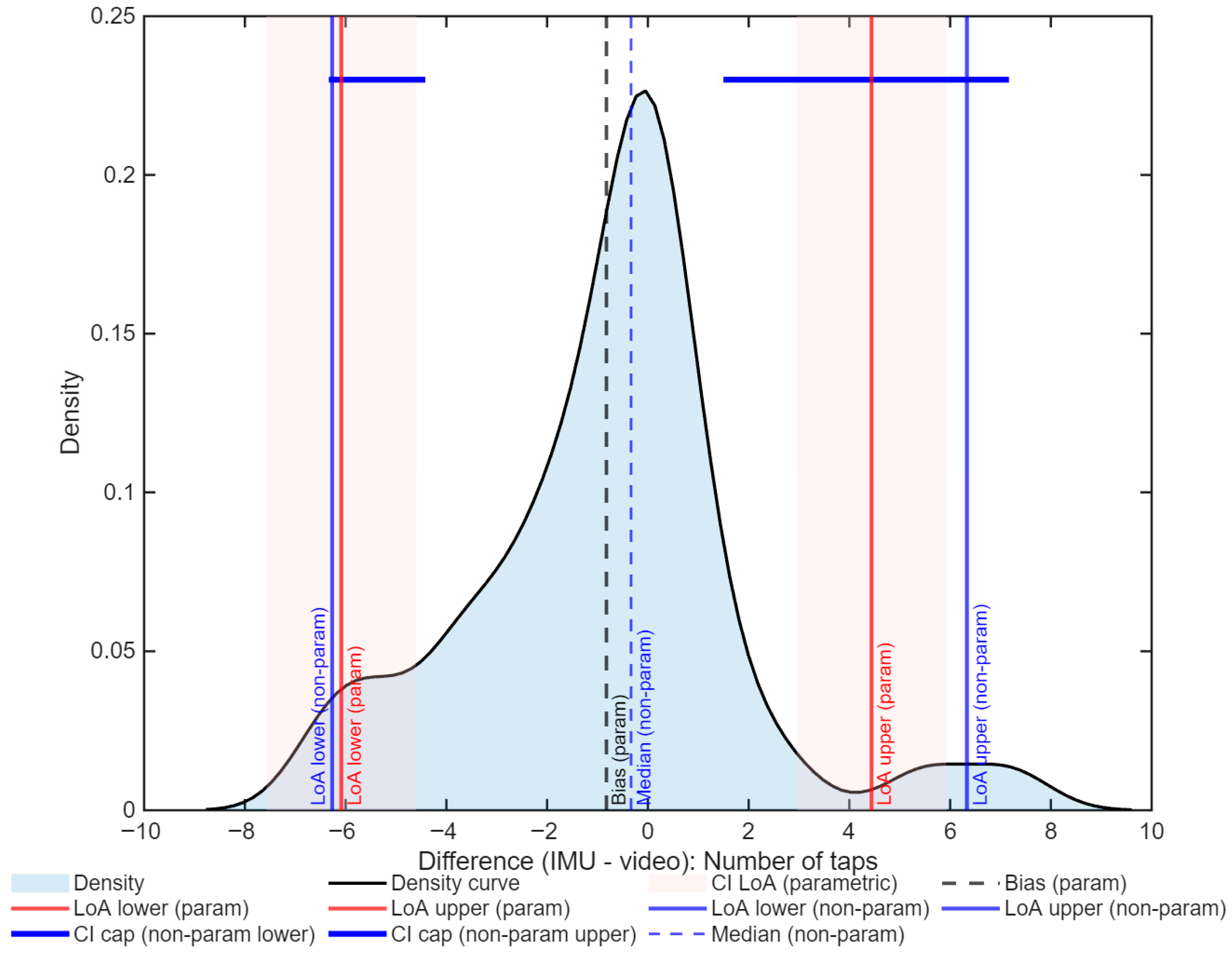
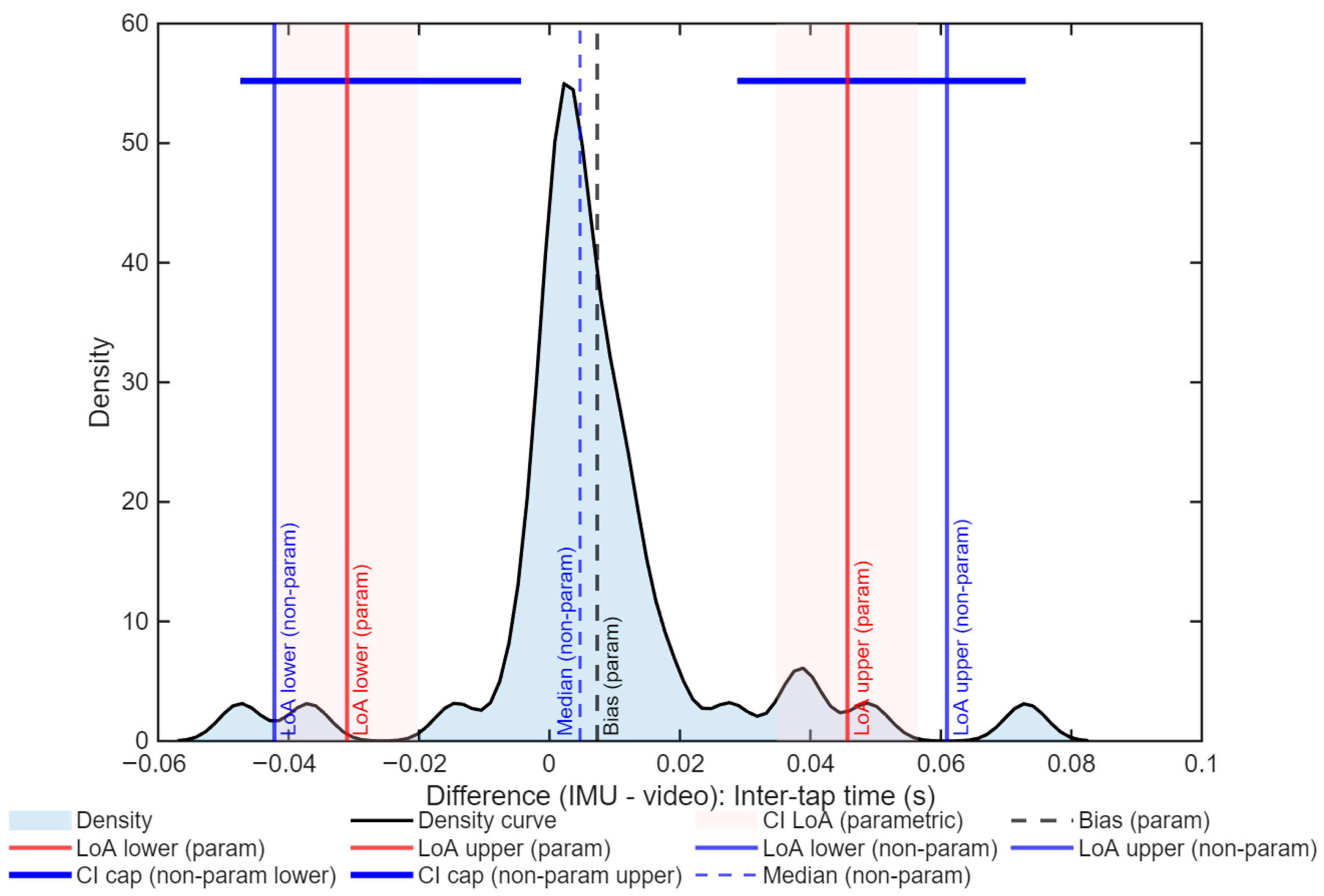
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalra, A. Decoding the Bland–Altman plot: Basic review. J. Pract. Cardiovasc. Sci. 2017, 3, 36–38. [Google Scholar] [CrossRef]
- Plant, A.L.; Becker, C.A.; Hanisch, R.J.; Boisvert, R.F.; Possolo, A.M.; Elliott, J.T. How measurement science can improve confidence in research results. PLoS Biol. 2018, 16, e2004299. [Google Scholar] [CrossRef]
- Nordberg, S.S.; McAleavey, A.A.; Moltu, C. Continuous quality improvement in measure development: Lessons from building a novel clinical feedback system. Qual. Life Res. 2021, 30, 3085–3096. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation; Neuropsychology Press: Tucson, AZ, USA, 1985. [Google Scholar]
- Goldstein, G.; Incagnoli, T. Contemporary Approaches to Neuropsychological Assessment; Plenum Press: New York, NY, USA, 1997. [Google Scholar]
- Halstead, W.C. Brain and Intelligence; a Quantitative Study of the Frontal Lobes; University of Chicago Press: Chicago, IL, USA, 1947; p. xiii, 206. [Google Scholar]
- Reitan, R.M. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed.; Neuropsychology Press: Tucson, AZ, USA, 1993. [Google Scholar]
- Nussbaum, N.L.; Bigler, E.D. Halstead-Reitan Neuropsychological Test Batteries for Children. In Handbook of Clinical Child Neuropsychology; Reynolds, C.R., Fletcher-Janzen, E., Eds.; Springer: Boston, MA, USA, 1997; pp. 219–236. [Google Scholar]
- Heilbronner, R.L.; Sweet, J.J.; Morgan, J.E.; Larrabee, G.J.; Millis, S.R.; Conference, P. American Academy of Clinical Neuropsychology Consensus Conference Statement on the Neuropsychological Assessment of Effort, Response Bias, and Malingering. Clin. Neuropsychol. 2009, 23, 1093–1129. [Google Scholar] [CrossRef]
- Roalf, D.R.; Rupert, P.; Mechanic-Hamilton, D.; Brennan, L.; Duda, J.E.; Weintraub, D.; Trojanowski, J.Q.; Wolk, D.; Moberg, P.J. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J. Neurol. 2018, 265, 1365–1375. [Google Scholar] [CrossRef]
- Suzumura, S.; Osawa, A.; Nagahama, T.; Kondo, I.; Sano, Y.; Kandori, A. Assessment of finger motor skills in individuals with mild cognitive impairment and patients with Alzheimer’s disease: Relationship between finger-to-thumb tapping and cognitive function. Jpn. J. Compr. Rehabil. Sci. 2016, 7, 19–28. [Google Scholar] [CrossRef]
- Eken, A.; Gökçay, D.; Yılmaz, C.; Baskak, B.; Baltacı, A.; Kara, M. Association of Fine Motor Loss and Allodynia in Fibromyalgia: An fNIRS Study. J. Mot. Behav. 2018, 50, 664–676. [Google Scholar] [CrossRef]
- Gentile, E.; Brunetti, A.; Ricci, K.; Delussi, M.; Bevilacqua, V.; De Tommaso, M. Mutual interaction between motor cortex activation and pain in fibromyalgia: EEG-fNIRS study. PLoS ONE 2020, 15, e0228158. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Ricci, K.; Vecchio, E.; Libro, G.; Delussi, M.; Casas-Barragàn, A.; de Tommaso, M. A Simple Pattern of Movement is not Able to Inhibit Experimental Pain in FM Patients and Controls: An sLORETA Study. Brain Sci. 2020, 10, 190. [Google Scholar] [CrossRef]
- Gentile, E.; Ricci, K.; Delussi, M.; de Tommaso, M. Motor cortex function in fibromyalgia: A pilot study involving near-infrared spectroscopy and co-recording of laser-evoked potentials. Funct. Neurol. 2019, 34, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Brígida, N.; Catela, D.; Mercê, C.; Branco, M. Predictability and Complexity of Fine and Gross Motor Skills in Fibromyalgia Patients: A Pilot Study. Sports 2024, 12, 90. [Google Scholar] [CrossRef]
- Brígida, N.; Catela, D.; Mercê, C.; Branco, M. Variability of gross and fine motor control in different tasks in fibromyalgia patients. Retos 2024, 54, 151–158. [Google Scholar] [CrossRef]
- Schmitt, L. Finger-Tapping Test. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer International Publishing: Cham, Switzerland, 2021; p. 2030. [Google Scholar]
- Heilman, K.M.; Valenstein, E. Clinical Neuropsychology; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- George, P.P.; Susan, R.B. Qualitative features of finger movement during the Halstead finger oscillation test following traumatic brain injury. J. Int. Neuropsychol. Soc. 2003, 9, 128–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimizu, Y.; Tanikawa, M.; Horiba, M.; Sahashi, K.; Kawashima, S.; Kandori, A.; Yamanaka, T.; Nishikawa, Y.; Matsukawa, N.; Ueki, Y.; et al. Clinical utility of paced finger tapping assessment in idiopathic normal pressure hydrocephalus. Front. Hum. Neurosci. 2023, 17, 1109670. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Barut, Ç.; Kızıltan, E.; Gelir, E.; Köktürk, F. Advanced Analysis of Finger-Tapping Performance: A Preliminary Study. Balk. Med. J. 2013, 30, 4. [Google Scholar] [CrossRef]
- Devrimsel, G.; Turkyilmaz, A.K.; Beyazal, M.S.; Karkucak, M. Assessment of hand function and disability in fibromyalgia. Z. Rheumatol. 2019, 78, 889–893. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Sackett, D.L. Bias in analytic research. J. Chronic. Dis. 1979, 32, 51–63. [Google Scholar] [CrossRef]
- Trajković, G. Measurement: Accuracy and Precision, Reliability and Validity; Springer: Dordrecht, The Netherlands, 2008; pp. 888–892. [Google Scholar]
- Glasser, B.J.; Goldhirsch, I. Scale dependence, correlations, and fluctuations of stresses in rapid granular flows. Phys. Fluids 2001, 13, 407–420. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Doğan, N. Bland-Altman analysis: A paradigm to understand correlation and agreement. Turk. J. Emerg. Med. 2018, 18, 139–141. [Google Scholar] [CrossRef]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends Supporting the In-Field Use of Wearable Inertial Sensors for Sport Performance Evaluation: A Systematic Review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef]
- Van Den Noort, J.C.; Kortier, H.G.; Beek, N.V.; Veeger, D.H.E.J.; Veltink, P.H. Measuring 3D Hand and Finger Kinematics—A Comparison between Inertial Sensing and an Opto-Electronic Marker System. PLoS ONE 2016, 11, e0164889. [Google Scholar] [CrossRef] [PubMed]
- Djurić-Jovičić, M.; Jovičić, N.S.; Roby-Brami, A.; Popović, M.B.; Kostić, V.S.; Djordjević, A.R. Quantification of Finger-Tapping Angle Based on Wearable Sensors. Sensors 2017, 17, 203. [Google Scholar] [CrossRef]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.-C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Bremm, R.P.; Pavelka, L.; Garcia, M.M.; Mombaerts, L.; Krüger, R.; Hertel, F. Sensor-Based Quantification of MDS-UPDRS III Subitems in Parkinson’s Disease Using Machine Learning. Sensors 2024, 24, 2195. [Google Scholar] [CrossRef]
- Schmidt, R.; Lee, T.; Winstein, C.; Wulf, G.; Zelaznik, H. Motor Control and Learning: A Behavioral Emphasis, 6th ed.; Library of Congress: Washington, DC, USA, 2019. [Google Scholar]
- Azami, H.; Rostaghi, M.; Abasolo, D.; Escudero, J. Refined Composite Multiscale Dispersion Entropy and its Application to Biomedical Signals. IEEE Trans. Biomed. Eng. 2017, 64, 2872–2879. [Google Scholar] [CrossRef]
- Kharitonov, V.N.; Namsaraev, Z.Z.; Brizitskii, R.V.; Samardak, A.S.; Ognev, A.V. Precise 6-DOF Motion Tracking of Fine Motor Skills of Fingers Based on Wearable Magnetic Induction Sensors. IEEE Sens. J. 2024, 24, 11295–11305. [Google Scholar] [CrossRef]
- Oliveira, A.; Dias, D.; Múrias Lopes, E.; Vilas-Boas, M.d.C.; Paulo Silva Cunha, J. SnapKi—An Inertial Easy-to-Adapt Wearable Textile Device for Movement Quantification of Neurological Patients. Sensors 2020, 20, 3875. [Google Scholar] [CrossRef] [PubMed]
- Bisi, M.C.; Stagni, R. Sensor-Based Quantitative Assessment of Children’s Fine Motor Competence: An Instrumented Version of the Placing Bricks Test. Sensors 2024, 24, 2192. [Google Scholar] [CrossRef]
- Liu, F.; Qiu, K.; Wang, H.; Dong, Y.; Yu, D. Decreased wrist rotation imitation abilities in children with autism spectrum disorder. Front. Psychiatry 2024, 15, 1349879. [Google Scholar] [CrossRef]
- Portaz, M.; Corbi, A.; Casas-Ortiz, A.; Santos, O.C. Exploring raw data transformations on inertial sensor data to model user expertise when learning psychomotor skills. User Model. User-Adapt. Interact. 2024, 34, 1283–1325. [Google Scholar] [CrossRef]
- Christianson, M.; Leathem, J. Development and standardisation of the Computerised Finger Tapping Test: Comparision with other finger tapping instruments. New Zealand J. Psychol. 2004, 33, 44–49. [Google Scholar]
- Charmant, J. & Contributors, “Kinovea”. 2021. Available online: https://www.kinovea.org (accessed on 22 October 2025).
- Klay, M. YAT, 2.4.1; SourceForge: San Diego, CA, USA, 2021. [Google Scholar]
- MATLAB, R2021a; The MatWorks, Inc.: Natick, MA, USA. 2021. Available online: https://www.mathworks.com/products/matlab.html (accessed on 22 October 2025).
- Reed, J.C.; Reed, H.B.C. The Halstead—Reitan Neuropsychological Battery. In Contemporary Approaches to Neuropsychological Assessment; Goldstein, G., Incagnoli, T.M., Eds.; Springer: Boston, MA, USA, 1997; pp. 93–129. [Google Scholar]
- Shimoyama, I.; Ninchoji, T.; Uemura, K. The finger-tapping test. A quantitative analysis. Arch. Neurol. 1990, 47, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Cui, J. Using the Bland-Altman method to measure agreement with repeated measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Lilliefors, H.W. On the Kolmogorov-Smirnov Test for Normality with Mean and Variance Unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- West, B.T.; Welch, K.B.; Galecki, A.T. Linear Mixed Models: A Practical Guide Using Statistical Software, 3rd ed.; Chapman and Hall, Hall/CRC: Boca Raton, FL, USA, 2022. [Google Scholar]
- Gueorguieva, R.; Krystal, J.H. Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch. Gen. Psychiatry 2004, 61, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.J.; Zhong, W.H.; Liu, Y.X.; Miao, H.Z.; Li, Y.C.; Ji, M.H. Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method. Int. J. Biostat. 2016, 12, 20150039. [Google Scholar] [CrossRef]
- Jones, M.; Dobson, A.; O’Brian, S. A graphical method for assessing agreement with the mean between multiple observers using continuous measures. Int. J. Epidemiol. 2011, 40, 1308–1313. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef]
- Turini, L.; Bonelli, F.; Lanatà, A.; Vitale, V.; Nocera, I.; Sgorbini, M.; Mele, M. Validation of a new smart textiles biotechnology for heart rate variability monitoring in sheep. Front. Vet. Sci. 2022, 9, 1018213. [Google Scholar] [CrossRef]
- Morrow, M.M.B.; Lowndes, B.; Fortune, E.; Kaufman, K.R.; Hallbeck, M.S. Validation of Inertial Measurement Units for Upper Body Kinematics. J. Appl. Biomech. 2017, 33, 227–232. [Google Scholar] [CrossRef]
- Zahn, A.; Koch, V.; Schreff, L.; Oschmann, P.; Winkler, J.; Gaßner, H.; Müller, R. Validity of an inertial sensor-based system for the assessment of spatio-temporal parameters in people with multiple sclerosis. Front. Neurol. 2023, 14, 1164001. [Google Scholar] [CrossRef]
- Adams, J.L.; Lizarraga, K.J.; Waddell, E.M.; Myers, T.L.; Jensen-Roberts, S.; Modica, J.S.; Schneider, R.B. Digital Technology in Movement Disorders: Updates, Applications, and Challenges. Curr. Neurol. Neurosci. Rep. 2021, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The use of wearable sensors to assess and treat the upper extremity after stroke: A scoping review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef] [PubMed]
- Vanmechelen, I.; Haberfehlner, H.; De Vleeschhauwer, J.; Van Wonterghem, E.; Feys, H.; Desloovere, K.; Aerts, J.-M.; Monbaliu, E. Assessment of movement disorders using wearable sensors during upper limb tasks: A scoping review. Front. Robot. AI 2023, 9, 1068413. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Caulfield, B.; Ward, T.; Johnston, W.; Doherty, C. Wearable Inertial Sensor Systems for Lower Limb Exercise Detection and Evaluation: A Systematic Review. Sports Med. 2018, 48, 1221–1246. [Google Scholar] [CrossRef]
- Wei, S.; Wu, Z. The Application of Wearable Sensors and Machine Learning Algorithms in Rehabilitation Training: A Systematic Review. Sensors 2023, 23, 7667. [Google Scholar] [CrossRef]
- Rast, F.M.; Labruyère, R. Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. Neuroeng. Rehabil. 2020, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; Von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef] [PubMed]
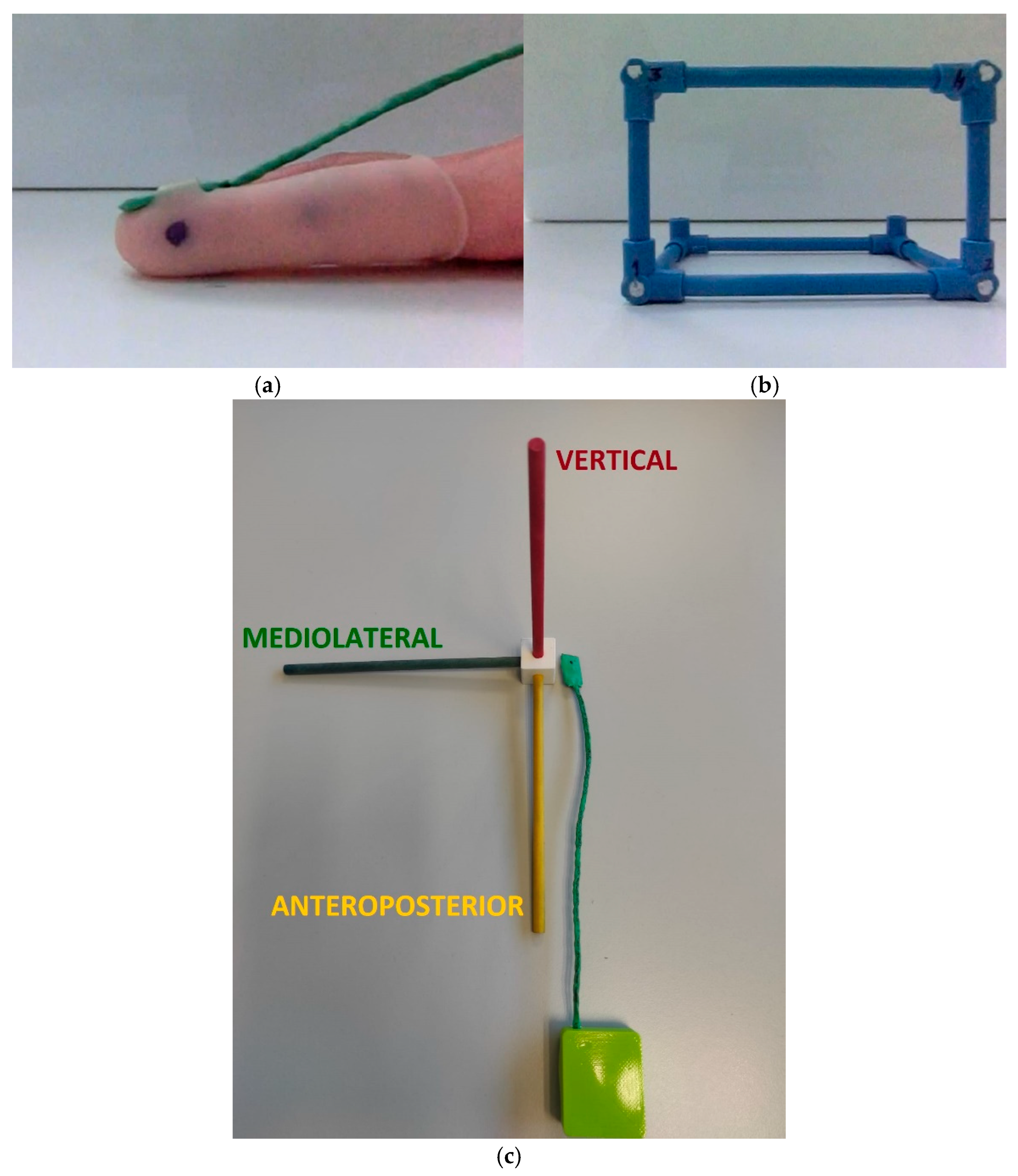
| Group | Age | Height | Weight | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Fibromyalgia | 46.400 | 12.714 | 162.900 | 5.243 | 63.000 | 10.536 |
| Control | 45.900 | 12.950 | 157.800 | 5.671 | 60.700 | 5.675 |
| Total | 46.150 | 12.835 | 160.350 | 6.027 | 61.850 | 8.540 |
| Variable | Number of Taps | Inter-Tap Time (s) |
|---|---|---|
| Normality (Lilliefors p) | 0.036 (rejected) | <0.001 (rejected) |
| Selected method | Non-parametric | Non-parametric |
| Bias | −0.33 | 0.005 |
| LoA (95%) | −6.27 to 6.33 | −0.042 to 0.061 |
| ICC(A,1) [95% CI] | 0.94 [0.89–0.96] | 0.89 [0.83–0.94] |
| Mixed-effects bias | −12.93 | −0.055 |
| Slope (p) | 0.263 (p < 0.001) | 0.283 (p < 0.001) |
| σ resid | 2.07 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brígida, N.; Catela, D.; Mercê, C.; Branco, M. Precision of an Inertial System to Evaluate the Finger Tapping Test in Women with Fibromyalgia. Sports 2025, 13, 373. https://doi.org/10.3390/sports13110373
Brígida N, Catela D, Mercê C, Branco M. Precision of an Inertial System to Evaluate the Finger Tapping Test in Women with Fibromyalgia. Sports. 2025; 13(11):373. https://doi.org/10.3390/sports13110373
Chicago/Turabian StyleBrígida, Nancy, David Catela, Cristiana Mercê, and Marco Branco. 2025. "Precision of an Inertial System to Evaluate the Finger Tapping Test in Women with Fibromyalgia" Sports 13, no. 11: 373. https://doi.org/10.3390/sports13110373
APA StyleBrígida, N., Catela, D., Mercê, C., & Branco, M. (2025). Precision of an Inertial System to Evaluate the Finger Tapping Test in Women with Fibromyalgia. Sports, 13(11), 373. https://doi.org/10.3390/sports13110373









