Feasibility and Preliminary Effects of Community-Based High-Intensity Functional Training for Adults with Mobility Disabilities and Overweight/Obesity: A Pilot Study
Abstract
1. Introduction
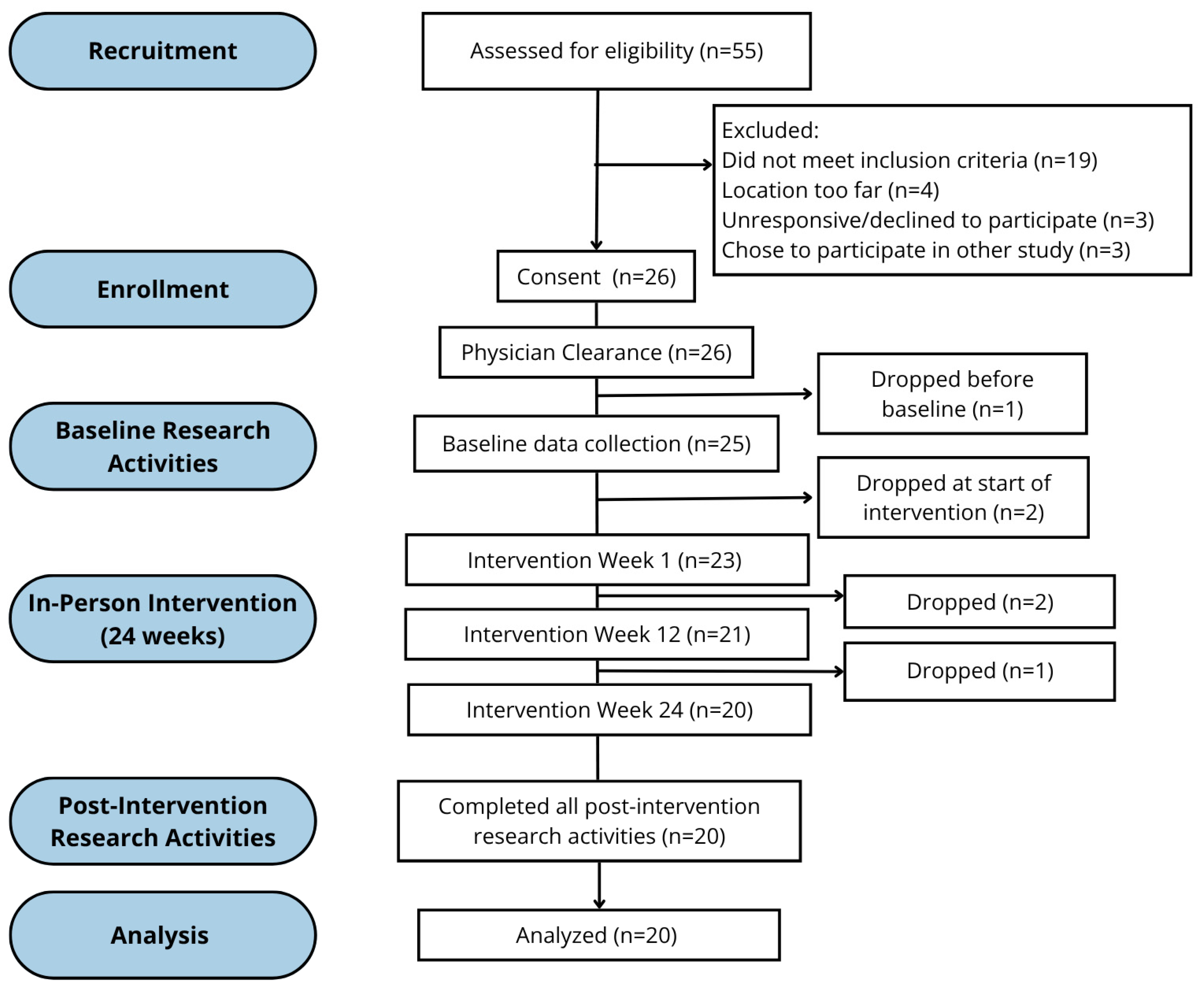
2. Method
3. Measures
4. Data Analysis
5. Results
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIT | Adaptive and Inclusive Trainer |
| AMRAP | As Many Rounds As Possible |
| ATA | Adaptive Training Academy |
| BBS | Berg Balance Scale |
| BHADP | Barriers to Health Activities for Daily Practice |
| BMC | Bone Mineral Content |
| BMI | Body Mass Index |
| COPM | Canadian Occupational Performance Measure |
| RPE | Rate of Perceived Exertion |
| DB | Dumbbell |
| DXA | Dual-Energy X-ray Absorptiometry |
| EMOM | Every Minute on the Minute |
| FIST | Function in Sitting Test |
| HIFT | High-Intensity Functional Training |
| QOL | Quality of Life |
| ICC | Intraclass Correlation Coefficient |
| MCID | Minimal Clinically Important Difference |
| MD | Mobility Disabilities |
| RFT | Rounds for Time |
| SCI | Spinal Cord Injury |
| WHOQOL-BREF | World Health Organization Quality of Life-BREF |
| WGSS | Washington Group Short Set on Functioning |
| WOD | Workout of the Day |
References
- Okoro, C.A. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 882–887. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Wang, E.; Yamaki, K.; Davis, B. Documenting disparities in obesity and disability. Focus: A Publ. Natl. Cent. Dissem. Disabil. Res. 2010, 24, 1–16. [Google Scholar]
- Froehlich-Grobe, K.; Lee, J.; Washburn, R.A. Disparities in obesity and related conditions among Americans with disabilities. Am. J. Prev. Med. 2013, 45, 83–90. [Google Scholar] [CrossRef]
- Krahn, G.L.; Walker, D.K.; Correa-De-Araujo, R. Persons with disabilities as an unrecognized health disparity population. Am. J. Public Health 2015, 105 (Suppl. S2), S198–S206. [Google Scholar] [CrossRef]
- Holmgren, M.; De Munter, J.; Rasmussen, F.; Sandberg, M.; Ahlström, G. Is Obesity More Than a Double Burden among People with Mobility Disability? The Effect of Obesity on HRQoL and Participation in Society. Healthcare 2017, 5, 79. [Google Scholar] [CrossRef]
- Holmgren, M.; Lindgren, A.; de Munter, J.; Rasmussen, F.; Ahlström, G. Impacts of mobility disability and high and increasing body mass index on health-related quality of life and participation in society: A population-based cohort study from Sweden. BMC Public Health 2014, 14, 381. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Schiller, W.; Chen, M.-D. Effects of disability-associated low energy expenditure deconditioning syndrome. Exerc. Sport Sci. Rev. 2012, 40, 22–29. [Google Scholar] [CrossRef]
- Adriaansen, J.J.E.; Van Asbeck, F.W.; Lindeman, E.; Van Der Woude, L.H.; De Groot, S.; Post, M.W. Secondary health conditions in persons with a spinal cord injury for at least 10 years: Design of a comprehensive long-term cross-sectional study. Disabil. Rehabil. 2013, 35, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.N.; Kendall, M.D.; Amsters, D.I.; Pershouse, K.J.; Haines, T.P.; Kuipers, P. The relationship between quality of life and disability across the lifespan for people with spinal cord injury. Spinal Cord 2009, 47, 149–155. [Google Scholar] [CrossRef] [PubMed]
- CDC Increasing Physical Activity Among Adults with Disabilities. 2024. Available online: https://www.cdc.gov/ncbddd/disabilityandhealth/pa.html (accessed on 9 January 2024).
- Rimmer, J.; Lai, B. Framing new pathways in transformative exercise for individuals with existing and newly acquired disability. Disabil. Rehabil. 2017, 39, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Young, H.J.; Bickel, C.S.; Motl, R.W.; Rimmer, J.H. Current trends in exercise intervention research, technology, and behavioral change strategies for people with disabilities: A scoping review. Am. J. Phys. Med. Rehabil. 2017, 96, 748–761. [Google Scholar] [CrossRef]
- Robson, E.K.; Hodder, R.K.; Kamper, S.J.; O’Brien, K.M.; Williams, A.; Lee, H.; Wolfenden, L.; Yoong, S.; Wiggers, J.; Barnett, C.; et al. Effectiveness of weight-loss interventions for reducing pain and disability in people with common musculoskeletal disorders: A systematic review with meta-analysis. J. Orthop. Sports Phys. Ther. 2020, 50, 319–333. [Google Scholar] [CrossRef]
- Graham, K.; Yarar-Fisher, C.; Li, J.; McCully, K.M.; Rimmer, J.H.; Powell, D.; Bickel, C.S.; Fisher, G. Effects of high-intensity interval training versus moderate-intensity training on cardiometabolic health markers in individuals with spinal cord injury: A pilot study. Top. Spinal Cord Inj. Rehabil. 2019, 25, 248–259. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Ginis, K.A.M.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828.e3. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.; Martin Ginis, K.A.; Pelletier, C.A.; Ditor, D.S.; Foulon, B.; Wolfe, D.L. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: A systematic review. Spinal Cord 2011, 49, 1103–1127. [Google Scholar] [CrossRef] [PubMed]
- Ravesloot, C.; Myers, A.; Santasier, A.; Ward, B. Effects of an Exercise Intervention on Participation Reported by People with Disabilities: A Mixed Methods Randomized Trial. Disabil. Health J. 2022, 15, 101272. [Google Scholar] [CrossRef]
- Morgan, K.A.; Taylor, K.L.; Walker, C.W.; Tucker, S.; Dashner, J.L.; Hollingsworth, H. Mobility disability and exercise: Health outcomes of an accessible community-based center. Front. Rehabil. Sci. 2022, 3, 836655. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Malone, E.; Lekiachvili, A.; Briss, P. Health care industry insights: Why the use of preventive services is still low. Prev. Chronic Dis. 2019, 16, E30. [Google Scholar] [CrossRef]
- Tederko, P.; Krasuski, T.; Krasuski, M.; Długołęcka, A.; Tarnacka, B. Determinants of health knowledge and health perceptions from the perspective of health-related education of patients with spinal cord injury: A systematic review. Int. J. Rehabil. Res. 2017, 40, 97–106. [Google Scholar] [CrossRef]
- Pergolotti, M.; Lavery, J.; Reeve, B.B.; Dusetzina, S.B. Therapy caps and variation in cost of outpatient occupational therapy by provider, insurance status, and geographic region. Am. J. Occup. Ther. 2018, 72, 7202205050p1–7202205050p9. [Google Scholar] [CrossRef]
- Persson, G.; Brorsson, A.; Ekvall Hansson, E.; Troein, M.; Strandberg, E.L. Physical activity on prescription (PAP) from the general practitioner’s perspective–a qualitative study. BMC Fam. Pract. 2013, 14, 128. [Google Scholar] [CrossRef]
- Letts, L.; Ginis, K.A.M.; Faulkner, G.; Colquhoun, H.; Levac, D.; Gorczynski, P. Preferred methods and messengers for delivering physical activity information to people with spinal cord injury: A focus group study. Rehabil. Psychol. 2011, 56, 128. [Google Scholar] [CrossRef]
- Levins, S.M.; Redenbach, D.M.; Dyck, I. Individual and societal influences on participation in physical activity following spinal cord injury: A qualitative study. Phys. Ther. 2004, 84, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Kehn, M.; Kroll, T. Staying physically active after spinal cord injury: A qualitative exploration of barriers and facilitators to exercise participation. BMC Public Health 2009, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Barfield, J.; Brasher, J.D. Perceived benefits and barriers to exercise among persons with physical disabilities or chronic health conditions within action or maintenance stages of exercise. Disabil. Health J. 2012, 5, 254–260. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Riley, B.; Wang, E.; Rauworth, A.; Jurkowski, J. Physical activity participation among persons with disabilities: Barriers and facilitators. Am. J. Prev. Med. 2004, 26, 419–425. [Google Scholar] [CrossRef]
- Sharon-David, H.; Siekanska, M.; Tenenbaum, G. Are gyms fit for all? A scoping review of the barriers and facilitators to gym-based exercise participation experienced by people with physical disabilities. Perform. Enhanc. Health 2021, 9, 100170. [Google Scholar] [CrossRef]
- Williams, T.L.; Smith, B.; Papathomas, A. The barriers, benefits and facilitators of leisure time physical activity among people with spinal cord injury: A meta-synthesis of qualitative findings. Health Psychol. Rev. 2014, 8, 404–425. [Google Scholar] [CrossRef]
- Glassman, G. Official CrossFit Affiliate Map; Official CrossFit: Santa Cruz, CA, USA, 2017. [Google Scholar]
- Kercher, V.M.M.; Kercher, K.; Levy, P.; Bennion, T.; Alexander, C.; Amaral, P.C.; Batrakoulis, A.; Chávez, L.F.J.G.; Cortés-Almanzar, P.; Haro, J.L.; et al. Fitness Trends from around the Globe. ACSM’s Health Fit. J. 2023, 27, 19–30. [Google Scholar] [CrossRef]
- YMCA. YMCA Key Facts & Figures. 2025. Available online: https://www.ymca.org/who-we-are/our-reach/key-facts (accessed on 10 April 2025).
- Dawson, M.C. CrossFit: Fitness cult or reinventive institution? Int. Rev. Sociol. Sport 2015, 52, 361–379. [Google Scholar] [CrossRef]
- ATA. Adaptive and Inclusive Training: A Practical Guide for the Adaptive and Inclusive Trainer Certification Course; Adaptive Training Academy: San Diego, CA, USA, 2024. [Google Scholar]
- Glassman, G. The CrossFit Level 1 Training Guide; CrossFit, LLC.: Boulder, CO, USA, 2020; Available online: https://assets.crossfit.com/level-one-training-guide.pdf (accessed on 19 February 2020).
- Tafuri, S.; Notarnicola, A.; Monno, A.; Ferretti, F.; Moretti, B. CrossFit athletes exhibit high symmetry of fundamental movement patterns. A cross-sectional study. Muscles Ligaments Tendons J. 2016, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef]
- Willis, E.A.; Szabo-Reed, A.N.; Ptomey, L.T.; Honas, J.J.; Steger, F.L.; Washburn, R.A.; Donnelly, J.E. Energy expenditure and intensity of group-based high-intensity functional training: A brief report. J. Phys. Act. Health 2019, 16, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Patel, P.; Sal Redondo, A.; Heinrich, K.M. Effects of eight weeks of high intensity functional training on glucose control and body composition among overweight and obese adults. Sports 2019, 7, 51. [Google Scholar] [CrossRef]
- Koon, L.M.; Hall, J.P.; Arnold, K.A.; Donnelly, J.E.; Heinrich, K.M. High-Intensity Functional Training: Perceived Functional and Psychosocial Health-Related Outcomes from Current Participants with Mobility-Related Disabilities. Sports 2023, 11, 116. [Google Scholar] [CrossRef]
- Handlery, R.; Handlery, K.; Kahl, D.; Koon, L.; Regan, E.W. High intensity functional training for people with spinal cord injury & their care partners. Spinal Cord 2024, 62, 357–366. [Google Scholar]
- Handlery, R.; Handlery, K.; Kahl, D.; Koon, L.; Cabe, S.L.; Regan, E.W. High Intensity Functional Training for People with Parkinson’s & Their Care Partners: A Feasibility Study. Am. J. Health Promot. 2024, 38, 648–660. [Google Scholar]
- Fealy, C.E.; Nieuwoudt, S.; Foucher, J.A.; Scelsi, A.R.; Malin, S.K.; Pagadala, M.; Cruz, L.A.; Li, M.; Rocco, M.; Burguera, B.; et al. Functional high-intensity exercise training ameliorates insulin resistance and cardiometabolic risk factors in type 2 diabetes. Exp. Physiol. 2018, 103, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Ameur, R.; Maaloul, R.; Tagougui, S.; Neffati, F.; Hadj Kacem, F.; Najjar, M.F.; Ammar, A.; Hammouda, O. Unlocking the power of synergy: High-intensity functional training and early time-restricted eating for transformative changes in body composition and cardiometabolic health in inactive women with obesity. PLoS ONE 2024, 19, e0301369. [Google Scholar]
- Harris, A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics Books: Champaign, IL, USA, 1998. [Google Scholar]
- Tibana, R.A.; De Sousa, N.M.F.; Cunha, G.V.; Prestes, J.; Fett, C.; Gabbett, T.J.; Voltarelli, F.A. Validity of Session Rating Perceived Exertion Method for Quantifying Internal Training Load during High-Intensity Functional Training. Sports 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.A.; Drake, N.B.; Carper, M.J.; DeBlauw, J.; Heinrich, K.M. Validity, reliability, and application of the session-RPE method for quantifying training loads during high intensity functional training. Sports 2018, 6, 84. [Google Scholar] [CrossRef]
- Froehlich-Grobe, K.; Nary, D.E.; Van Sciver, A.; Lee, J.; Little, T.D. Measuring height without a stadiometer: Empirical investigation of four height estimates among wheelchair users. Am. J. Phys. Med. Rehabil. Assoc. Acad. Physiatr. 2011, 90, 658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lohman, T.J.; Roache, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar][Green Version]
- Berg, K.O.; WoodDauphinee, S.L.; Williams, J.I. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar][Green Version]
- Major, M.J.; Fatone, S.; Roth, E.J. Validity and reliability of the Berg Balance Scale for community-dwelling persons with lower-limb amputation. Arch. Phys. Med. Rehabil. 2013, 94, 2194–2202. [Google Scholar] [CrossRef]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Abou, L.; Sung, J.; Sosnoff, J.J.; Rice, L.A. Reliability and validity of the function in sitting test among non-ambulatory individuals with spinal cord injury. J. Spinal Cord Med. 2020, 43, 846–853. [Google Scholar] [CrossRef]
- Gorman, S.L.; Harro, C.C.; Platko, C.; Greenwald, C. Examining the function in sitting test for validity, responsiveness, and minimal clinically important difference in inpatient rehabilitation. Arch. Phys. Med. Rehabil. 2014, 95, 2304–2311. [Google Scholar] [CrossRef]
- Gorman, S.L.; Radtka, S.; Melnick, M.E.; Abrams, G.M.; Byl, N.N. Development and validation of the function in sitting test in adults with acute stroke. J. Neurol. Phys. Ther. 2010, 34, 150–160. [Google Scholar] [CrossRef]
- Kim, H.-j.; Park, I.; joo Lee, H.; Lee, O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J. Exerc. Nutr. Biochem. 2016, 20, 46. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- May, L.A.; Butt, C.; Minor, L.; Kolbinson, K.; Tulloch, K. Measurement reliability of functional tasks for persons who self-propel a manual wheelchair. Arch. Phys. Med. Rehabil. 2003, 84, 578–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rikli, R.E.; Jones, C.J. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Cowan, R.E.; Callahan, M.K.; Nash, M.S. The 6-min push test is reliable and predicts low fitness in spinal cord injury. Med. Sci. Sports Exerc. 2012, 44, 1993–2000. [Google Scholar] [CrossRef]
- Law, M. COPM Canadian Occupational Performance Measure: Revised; Schulz-Kirchner Verlag GmbH: Idstein, Germany, 2022. [Google Scholar]
- Law, M.; Baptiste, S.; McColl, M.; Carswell, A.; Polatajko, H.; Pollock, N. Canadian Occupational Performance Measure (COPM) Manual; CAOT Publications ACE: Ottawa, ON, Canada, 1998. [Google Scholar]
- Raquel, C.-T.; Villafañe, J.H.; Medina-Porqueres, I.; Garcia-Orza, S.; Valdes, K. Convergent validity and responsiveness of the Canadian Occupational Performance Measure for the evaluation of therapeutic outcomes for patients with carpometacarpal osteoarthritis. J. Hand Ther. 2021, 34, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Lin, C.Y.; Lee, Y.C.; Chang, J.H. The Canadian occupational performance measure for patients with stroke: A systematic review. J. Phys. Ther. Sci. 2017, 29, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Washington Group on Disability Statistics. WG Short Set on Functioning (WG-SS). 2018. Available online: https://knowledge.unicef.org/feeding-and-disability-resource-bank/resource/washington-group-short-set-functioning-wg-ss (accessed on 15 August 2021).
- Washington Group on Disability Statistics. Washington Group Extended Set on Functioning (WG-ES). 2021. Available online: https://www.washingtongroup-disability.com/question-sets/wg-extended-set-on-functioning-wg-es/ (accessed on 15 August 2021).
- Crawford, D.A.; Drake, N.B.; Carper, M.J.; DeBlauw, J.; Heinrich, K.M. Are changes in physical work capacity induced by high-intensity functional training related to changes in associated physiologic measures? Sports 2018, 6, 26. [Google Scholar] [CrossRef]
- Glassman, G. Understanding CrossFit. CrossFit J. 2007, 56, 1–5. Available online: https://journal.crossfit.com/article/understanding-crossfit (accessed on 16 March 2020).
- Glassman, G. What Is Fitness? CrossFit J. 2002, 1, 1–11. Available online: https://journal.crossfit.com/article/what-is-fitness (accessed on 9 April 2020).
- Becker, H.; Stuifbergen, A.K.; Sands, D. Development of a scale to measure barriers to health promotion activities among persons with disabilities. Am. J. Health Promot. 1991, 5, 449–454. [Google Scholar] [CrossRef]
- Group, W. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Hammond, B.P.; Stotz, P.J.; Brennan, A.M.; Lamarche, B.; Day, A.G.; Ross, R. Individual variability in waist circumference and body weight in response to exercise. Med. Sci. Sports Exerc. 2019, 51, 315–322. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63 Pt. B, 2985–3023. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Giannopapas, V.; Stefanou, M.I.; Smyrni, V.; Kitsos, D.K.; Kosmidou, M.; Stasi, S.; Chasiotis, A.K.; Stavrogianni, K.; Papagiannopoulou, G.; Tzartos, J.S.; et al. Waist circumference and body mass index as predictors of disability progression in multiple sclerosis: A systematic review and meta-analysis. J. Clin. Med. 2024, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Hewlings, S.; Kalman, D. Body composition changes in weight loss: Strategies and supplementation for maintaining lean body mass, a brief review. Nutrients 2018, 10, 1876. [Google Scholar] [CrossRef]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, M.G.; Shin, S.J. What is the minimum clinically important difference in grip strength? Clin. Orthop. Relat. Res. 2014, 472, 2536–2541. [Google Scholar] [CrossRef]
- Lam, T.; Noonan, V.K.; Eng, J.J. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord 2008, 46, 246–254. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Learmonth, Y.C.; Dlugonski, D.D.; Pilutti, L.A.; Sandroff, B.M.; Motl, R.W. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult. Scler. J. 2013, 19, 1784–1791. [Google Scholar] [CrossRef]
- Larsson, U.E.; Reynisdottir, S. The six-minute walk test in outpatients with obesity: Reproducibility and known group validity. Physiother. Res. Int. 2008, 13, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, Y.; Kamimoto, T.; Sakai, K.; Yamada, M.; Kawakami, M. Estimation of minimal detectable change in the 10-meter walking test for patients with stroke: A study stratified by gait speed. Front. Neurol. 2023, 14, 1219505. [Google Scholar] [CrossRef] [PubMed]
- Alzyoud, J.; Medley, A.; Thompson, M.; Csiza, L. Responsiveness, minimal detectable change, and minimal clinically important difference of the sitting balance scale and function in sitting test in people with stroke. Physiother. Theory Pract. 2022, 38, 327–336. [Google Scholar] [CrossRef]
- Tamura, S.; Miyata, K.; Kobayashi, S.; Takeda, R.; Iwamoto, H. The minimal clinically important difference in Berg Balance Scale scores among patients with early subacute stroke: A multicenter, retrospective, observational study. Top. Stroke Rehabil. 2022, 29, 423–429. [Google Scholar] [CrossRef]
- Hiengkaew, V.; Jitaree, K.; Chaiyawat, P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch. Phys. Med. Rehabil. 2012, 93, 1201–1208. [Google Scholar] [PubMed]
- Cavaggioni, L.; Trecroci, A.; Formenti, D.; Hogarth, L.; Tosin, M.; Alberti, G. Seasonal changes in breathing pattern, trunk stabilization, and muscular power in Paralympic swimmers. Adapt. Phys. Act. Q. 2021, 38, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Romberg, A.; Virtanen, A.; Aunola, S.; Karppi, S.L.; Karanko, H.; Ruutiainen, J. Exercise capacity, disability and leisure physical activity of subjects with multiple sclerosis. Mult. Scler. J. 2004, 10, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.L.; Ma, J.K.; Ginis, K.A.M. Participant experiences and perceptions of physical activity-enhancing interventions for people with physical impairments and mobility limitations: A meta-synthesis of qualitative research evidence. Health Psychol. Rev. 2017, 11, 179–196. [Google Scholar] [CrossRef]
- Laughton, G.; Buchholz, A.C.; Martin Ginis, K.A.; Goy, R.E. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord 2009, 47, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Yarar-Fisher, C.; Chen, Y.; Jackson, A.B.; Hunter, G.R. Body mass index underestimates adiposity in women with spinal cord injury. Obesity 2013, 21, 1223–1225. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; Griffith, L.E.; Gilsing, A.; Beauchamp, M.K.; Kuspinar, A.; Raina, P. The association between self-reported and performance-based physical function with activities of daily living disability in the Canadian longitudinal study on aging. J. Gerontol. Ser. A 2020, 75, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kiadaliri, A.; Sirard, P.; Dahlberg, L.E.; Lohmander, L.S. Self-assessed performance-based function test versus patient-reported outcome measures for knee and hip osteoarthritis. BMC Sports Sci. Med. Rehabil. 2024, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.L.; Burch, K.; Mitchell, J.R.; Fox, A.B.; Baum, C.M.; Connor, L.T. Self-perception of physical function contributes to participation in cognitively-and physically-demanding activities after stroke. Front. Neurol. 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.P.; Kurth, N.K.; Goddard, K.S. Goddard. Assessing factors associated with social connectedness in adults with mobility disabilities. Disabil. Health J. 2022, 15, 101206. [Google Scholar] [CrossRef]
| Session Type | Standing | Seated |
|---|---|---|
| As many rounds as possible (AMRAP) | 10-min AMRAP: 10 Burpees; 5 squats; 10 push-ups; 5 dips | 10-min AMRAP: 10 slam balls; 5 dips; 5/5 shoulder presses; 3 dips |
| Rounds for Time (RFT) | 3 RFT: 20 squats; 20 sit-ups; 20 shoulder press; 20 kettlebell swings | 3 RFT: 12 dips; 40 weighted twists; 20 dumbbell shoulder press; 20 dumbbell front raise |
| Every minute on the minute (EMOM) | 12-min: Min 1: 18 alternating dumbbell row; Min 2: 200-m bike; Min 3: 9 deadlifts; Min 4: rest (repeat for rest of time) | 12-min: Min 1: 18 alternating dumbbell row; Min 2: 200-m bike; Min 3: 10 side-to-side deadlifts; Min 4: rest (repeat for rest of time) |
| Work Capacity | Description | Standardized Test Condition | Outcome Measured | Functional Relevance |
|---|---|---|---|---|
| 10 min AMRAP | Perform as many rounds + repetitions as possible: 9 Calorie Row + 15 Dumbbell Clean and Press (standing); 6 Calorie Row + 15 Ground-to-Overhead Plate (seated) | Rower damper setting Load used (DBs or plate) Rower serial # | Total rounds and repetitions completed in 10 min | Lifting/carrying under fatigue Multi-joint movement integration |
| 20 min AMRAP | Perform as many rounds + repetitions as possible: 5 Calorie Bike + 7 Push-ups + 9 Box Squats (standing); 2 Dips + 3 Calorie Arms-Only Bike + 8 Side-to-Side Deadlifts with Kettlebell (seated) | Push-up/dip bar height Box height/ Kettlebell weight Bike serial # Station setup for transition time | Total rounds and repetitions completed in 20 min | Transfers, toileting, lap-floor reach Total-body endurance in functional tasks |
| For Time | Complete: 250 m Row (standing); 180 m Ski (seated) | Damper setting Row/SkiErg serial # Consistent warm-up protocol | Time to completion | High-output, rapid work tasks (e.g., emergency transfers, sprint-carry, quick response in daily activities) |
| Group | N = 20 | ||
|---|---|---|---|
| Variable | Category | n | % |
| Sex | Female | 13 | 65.0% |
| Male | 7 | 35.0% | |
| Age | 18–35 | 3 | 15% |
| 36–54 | 7 | 35% | |
| 55–79 | 10 | 50% | |
| BMI * | 24.1–33.4 | 14 | 70% |
| 33.5–42.7 | 2 | 10% | |
| 42.8–54.1 | 4 | 20% | |
| Education | Graduate degree | 9 | 45% |
| College completed | 5 | 25% | |
| 1–3 years of college | 4 | 20% | |
| High School completed | 1 | 5% | |
| Other | 1 | 5% | |
| Race | White | 16 | 80% |
| Black/African American | 3 | 15% | |
| Do not wish to answer | 1 | 5% | |
| Disability Type | Neurological | ||
| (Ataxia, Multiple Sclerosis) | 6 | 30% | |
| Injury | |||
| (SCI/D) | 7 | 35% | |
| Health Impairment | |||
| (Stroke; GPA) | 6 | 30% | |
| Assistive Devices | Utilizes an assistive device | 15 | 75% |
| (e.g., cane, walker, manual wheelchair, orthotic devices) | |||
| Does not utilize assistive devices | 5 | 25% | |
| Measure | N | Baseline Mean (SD) | Endpoint Mean (SD) | Mean Change (Pooled SD) | Effect Size (Cohen’s d) |
|---|---|---|---|---|---|
| Obesity-Related Outcomes | |||||
| Weight (kg) a | 20 | 97.64 (22.72) | 97.46 (23.24) | −0.18 (22.98) | −0.01 |
| Body Mass Index (weight (kg)/[height (m)]2) b | 20 | 34.39 (8.77) | 34.32 (8.82) | −0.08 (1.40) | −0.05 |
| Waist Circumference (cm) c | 20 | 106.29 (15.54) | 104.65 (16.86) | −1.64 (3.47) | −0.47 |
| Body Fat % d | 18 | 42.8% (9.8%) | 41.4% (9.3%) | −1.35% (0.03%) | −0.36 |
| Lean % d | 18 | 57.1% (9.8%) | 58.6% (9.3%) | +1.43% (3.76%) | 0.38 |
| Bone Mineral Content (g) d | 18 | 2814.89 (665.85) | 2813.11 (712.92) | −1.78 (110.90) | −0.02 |
| Performance-based Functional Health Outcomes | |||||
| Grip Strength (Dominant) e | 20 | 26.81 (11.98) | 29.56 (12.88) | 2.25 (2.72) | 1.01 * |
| Grip Strength (Non-Dominant) e | 19 | 23.43 (12.29) | 24.35 (12.35) | 0.91 (4.05) | 0.23 |
| Berg Balance Scale (BBS) f | 14 | 49.9 (4.4) | 51.2 (4.5) | 1.36 (4.22) | 0.32 |
| Function in Sitting Test (FIST) f | 6 | 38.2 (10.6) | 42.0 (6.4) | 3.8 (6.6) | 0.58 |
| 10-Meter Walk Time (m/s) g | 13 | 7.7 (2.8) | 6.9 (2.7) | −0.71 (0.74) | −0.95 * |
| 23-Meter Push Time (m/s) g | 4 | 9.8 (1.8) | 9.0 (1.4) | −0.83 (0.83) | −1.00 * |
| 6-Minute Walk Distance (m) h | 13 | 348.7 (132.3) | 422.3 (141.7) | 73.61 (57.63) | 1.28 * |
| 6-Minute Push Distance (m) h | 4 | 462.3 (37.9) | 539.6 (105.5) | 77.4 (24.1) | 0.68 |
| Self-Report Functional Health Outcomes | |||||
| COPM Performance i | 20 | 5.09 (1.41) | 6.58 (1.02) | 1.49 (1.19) | 1.25 * |
| COPM Satisfaction i | 20 | 4.36 (1.85) | 6.46 (1.38) | 2.12 (1.51) | 1.40 * |
| WGSS-6 + Upper Body Functioning Add-ons j | 20 | 12.45 (2.09) | 11.7 (1.66) | −0.85 (1.73) | −0.49 |
| Work Capacity | |||||
| Work Capacity (10 min AMRAP; rounds.repetitions) k | 18 | 3.69 (0.73) | 4.77 (1.07) | 1.08 (0.80) | 1.35 * |
| Work Capacity (20 min AMRAP; rounds.repetitions) k | 19 | 7.68 (1.90) | 11.20 (3.46) | 3.41 (2.45) | 1.39 * |
| Work Capacity (s) | 19 | 81.10 (27.61) | 64.25 (14.02) | −15.91 (21.44) | −0.74 |
| Psychological Outcomes | |||||
| Quality of Life (WHOQOL-BREF) l | 20 | 11.75 (1.92) | 12.15 (2.30) | 0.40 (1.60) | 0.25 |
| Barriers (BHADP) m | 19 | 26.53 (4.73) | 24.84 (5.29) | −3.00 (2.21) | −1.36 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koon, L.M.; Donnelly, J.E.; Sherman, J.R.; Rice, A.M.; Clina, J.G.; Thyfault, J.; Handlery, R.; Handlery, K.; Crawford, D.A. Feasibility and Preliminary Effects of Community-Based High-Intensity Functional Training for Adults with Mobility Disabilities and Overweight/Obesity: A Pilot Study. Sports 2025, 13, 361. https://doi.org/10.3390/sports13100361
Koon LM, Donnelly JE, Sherman JR, Rice AM, Clina JG, Thyfault J, Handlery R, Handlery K, Crawford DA. Feasibility and Preliminary Effects of Community-Based High-Intensity Functional Training for Adults with Mobility Disabilities and Overweight/Obesity: A Pilot Study. Sports. 2025; 13(10):361. https://doi.org/10.3390/sports13100361
Chicago/Turabian StyleKoon, Lyndsie M., Joseph E. Donnelly, Joseph R. Sherman, Anna M. Rice, Julianne G. Clina, John Thyfault, Reed Handlery, Kaci Handlery, and Derek A. Crawford. 2025. "Feasibility and Preliminary Effects of Community-Based High-Intensity Functional Training for Adults with Mobility Disabilities and Overweight/Obesity: A Pilot Study" Sports 13, no. 10: 361. https://doi.org/10.3390/sports13100361
APA StyleKoon, L. M., Donnelly, J. E., Sherman, J. R., Rice, A. M., Clina, J. G., Thyfault, J., Handlery, R., Handlery, K., & Crawford, D. A. (2025). Feasibility and Preliminary Effects of Community-Based High-Intensity Functional Training for Adults with Mobility Disabilities and Overweight/Obesity: A Pilot Study. Sports, 13(10), 361. https://doi.org/10.3390/sports13100361







