Bioelectrical Impedance Analysis in Professional and Semi-Professional Football: A Scoping Review
Abstract
1. Introduction
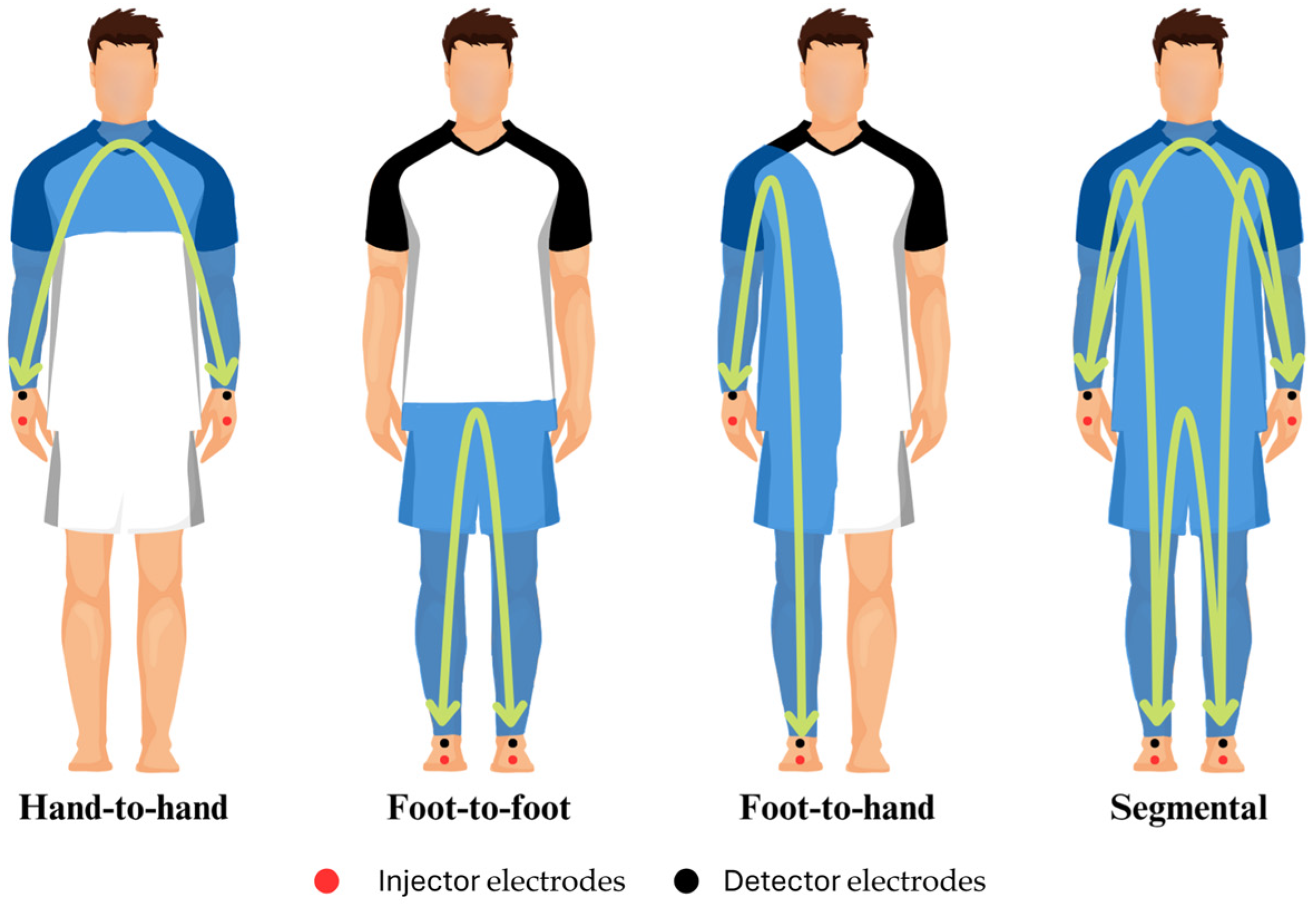
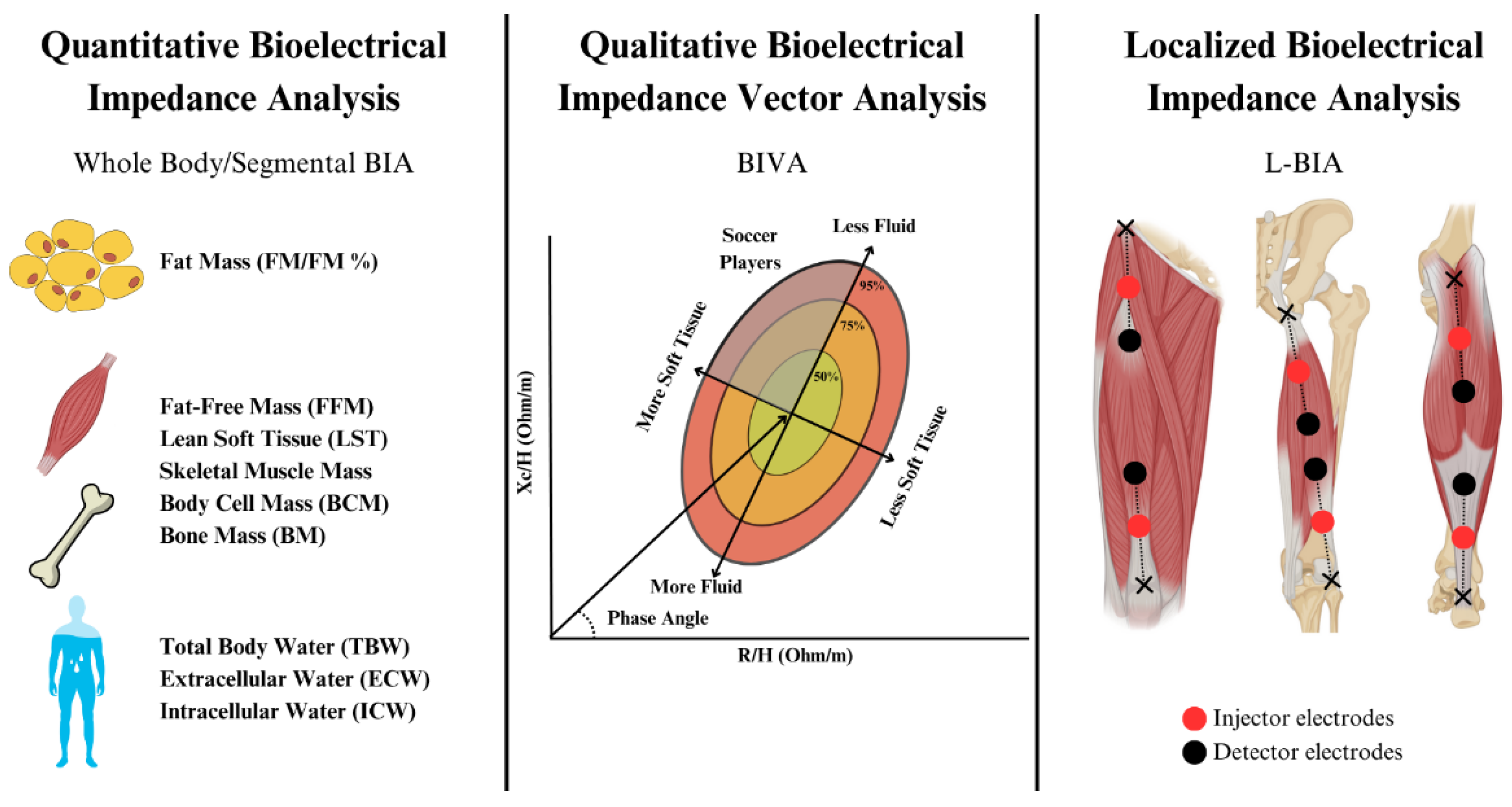
2. Materials and Methods
- Population: Healthy or injured (sport-related) professional or semi-professional football players ≥ 16 years old (mean), independent of playing position, sex, or ethnicity.
- Concept: Application of bioelectrical impedance analysis (BIA) to estimate body composition outcomes or report/analyze raw bioelectrical parameters.
- Context: Routine training and match-play context in professional and semi-professional football, independent of the competitive or non-competitive period (i.e., off-season, pre-season, in-season).
3. Results
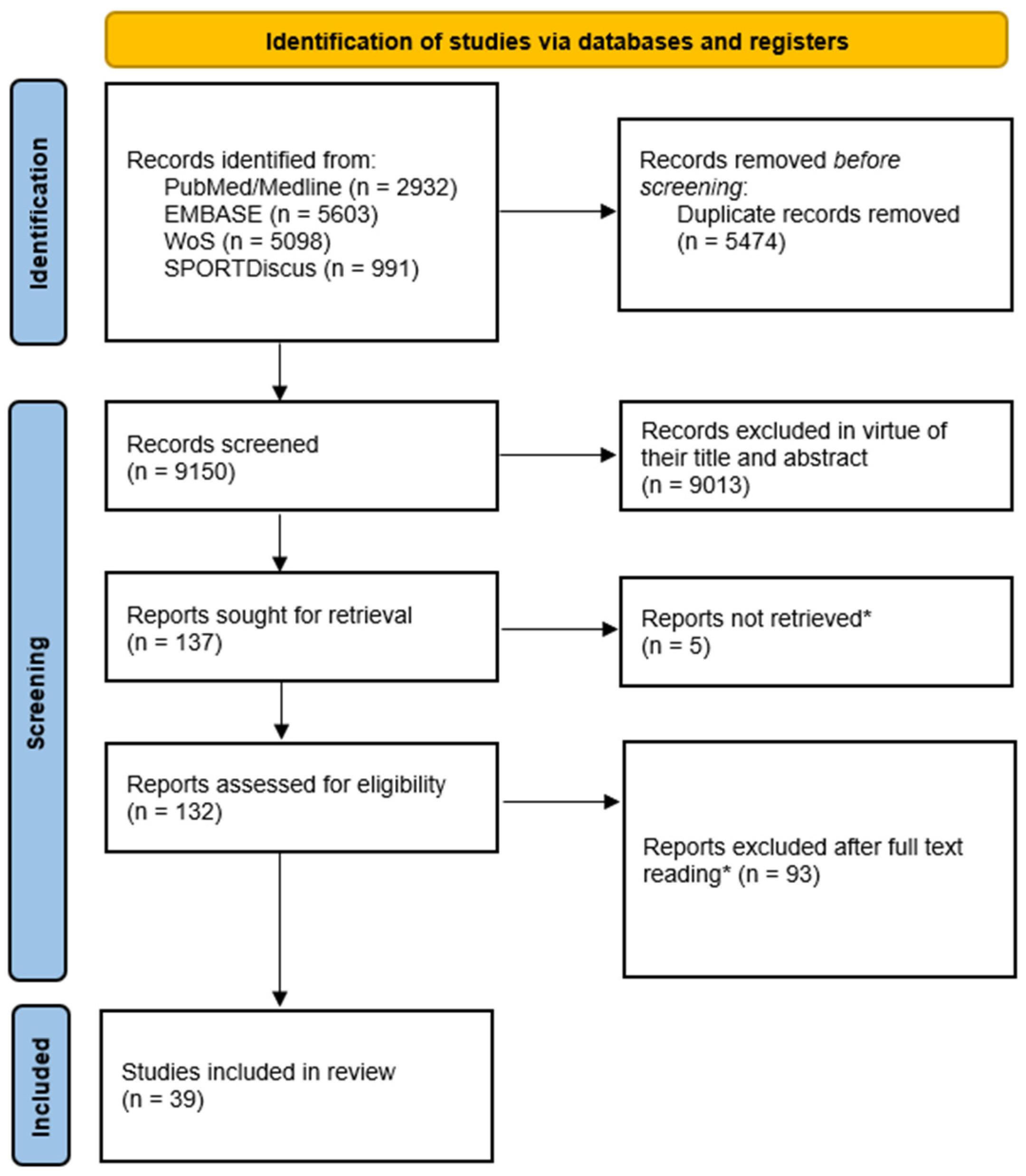
3.1. Identification, Screening, and Record Selection Process
3.2. Characteristics of Included Studies
3.3. Data Synthesis
4. Discussion
4.1. Quantitative Body Composition Assessment
4.1.1. Cross-Method Validation of BIA Quantitative Assessments
4.1.2. Development of New Predictive Equations/Models
4.1.3. Hydration Assessment
4.2. Qualitative and Semi-Quantitative Body Composition Analysis
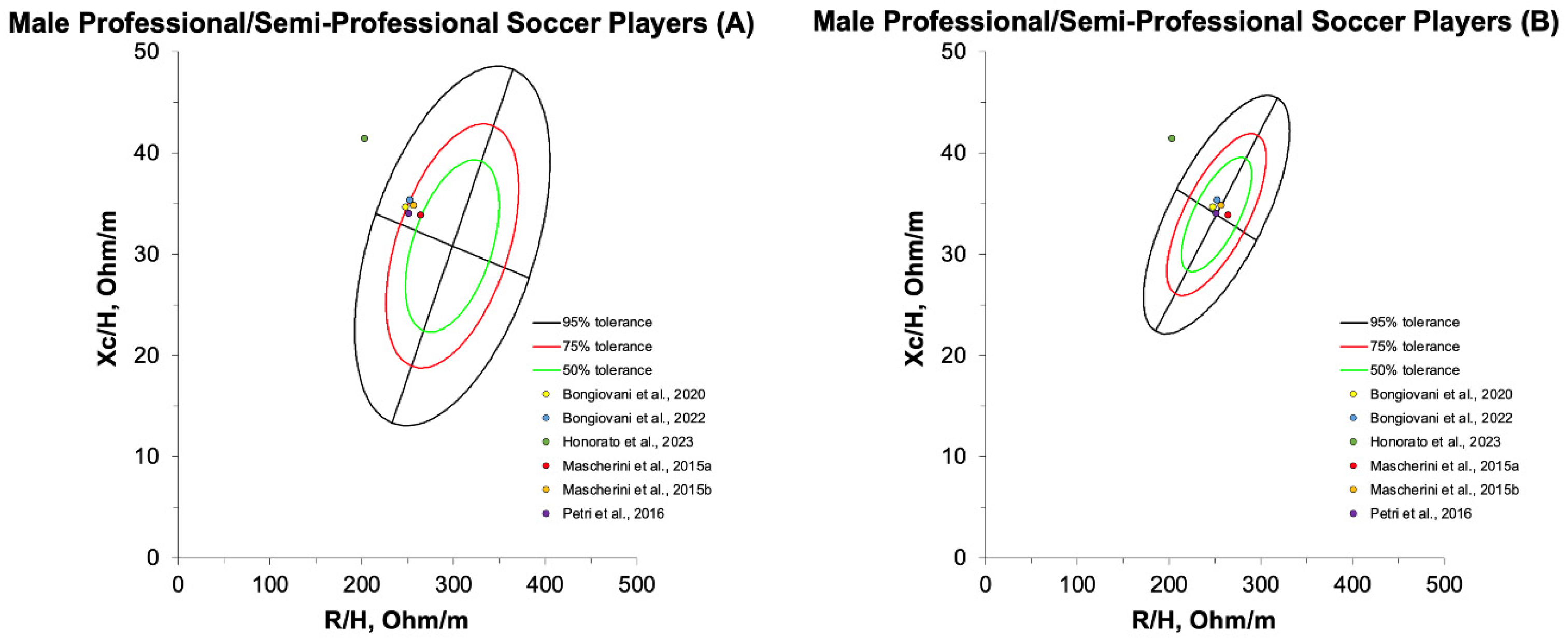
4.2.1. Assessment of Professional/Semi-Professional Football-Specific Phenotypes
4.2.2. Longitudinal Changes in Bioelectrical Impedance Vector Analysis
4.3. Assessment of Muscle Health and Performance
4.3.1. Phase Angle as an Indicator of Player Load and Performance
4.3.2. Localized Bioelectrical Impedance Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, L.C. Human body composition: Yesterday, today, and tomorrow. Eur. J. Clin. Nutr. 2018, 72, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz Marcos, S.; Redondo del Río, M.P.; de Mateo Silleras, B. Applications of Bioelectrical Impedance Vector Analysis (BIVA) in the Study of Body Composition in Athletes. Appl. Sci. 2021, 11, 9781. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef]
- Ackland, T.R.; Lohman, T.G.; Sundgot-Borgen, J.; Maughan, R.J.; Meyer, N.L.; Stewart, A.D.; Müller, W. Current status of body composition assessment in sport: Review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the IOC Medical Commission. Sports Med. 2012, 42, 227–249. [Google Scholar] [CrossRef]
- Campa, F.; Gobbo, L.A.; Stagi, S.; Cyrino, L.T.; Toselli, S.; Marini, E.; Coratella, G. Bioelectrical impedance analysis versus reference methods in the assessment of body composition in athletes. Eur. J. Appl. Physiol. 2022, 122, 561–589. [Google Scholar] [CrossRef]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of Body Composition in Athletes: A Narrative Review of Available Methods with Special Reference to Quantitative and Qualitative Bioimpedance Analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef]
- Lukaski, H.; Raymond-Pope, C.J. New Frontiers of Body Composition in Sport. Int. J. Sports Med. 2021, 42, 588–601. [Google Scholar] [CrossRef]
- Requena, B.; García, I.; Suárez-Arrones, L.; Sáez de Villarreal, E.; Naranjo Orellana, J.; Santalla, A. Off-Season Effects on Functional Performance, Body Composition, and Blood Parameters in Top-Level Professional Soccer Players. J. Strength Cond. Res. 2017, 31, 939–946. [Google Scholar] [CrossRef]
- Seow, D.; Massey, A. Correlation between preseason body composition and sports injury in an English Premier League professional football team. BMJ Open Sport Exerc. Med. 2022, 8, e001193. [Google Scholar] [CrossRef]
- Figueiredo, D.H.; Dourado, A.C.; Stanganelli, L.C.R.; Gonçalves, H.R. Evaluation of body composition and its relationship with physical fitness in professional soccer players at the beginning of pre-season. Retos Nuevas Tend. En Educ. Física Deporte Y Recreación 2021, 40, 117–125. [Google Scholar]
- Bradley, P.S.; Archer, D.T.; Hogg, B.; Schuth, G.; Bush, M.; Carling, C.; Barnes, C. Tier-specific evolution of match performance characteristics in the English Premier League: It’s getting tougher at the top. J. Sports Sci. 2016, 34, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Nédélec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part I—Post-match fatigue and time course of recovery. Sports Med. 2012, 42, 997–1015. [Google Scholar]
- Mills, C.D.; De Ste Croix, M.B.; Cooper, S.-M. The importance of measuring body composition in professional football players: A commentary. Sport Exerc. Med. Open J. 2017, 3, 24–29. [Google Scholar] [CrossRef]
- Campa, F.; Bongiovanni, T.; Rossi, A.; Cerullo, G.; Casolo, A.; Martera, G.; Trecroci, A.; Moro, T.; Paoli, A. Athletic bioimpedance-based equations underestimate fat free mass components in male elite soccer players: Development and validation of new soccer-specific predictive models. J. Transl. Med. 2023, 21, 912. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, L.B.; Correia, I.R.; Magalhães, J.P.; Júdice, P.B.; Silva, A.M.; Hetherington-Rauth, M. Development and validation of BIA prediction equations of upper and lower limb lean soft tissue in athletes. Eur. J. Clin. Nutr. 2020, 74, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Comfort, P.; Stewart, A.; Bloom, L.; Clarkson, B. Relationships Between Strength, Sprint, and Jump Performance in Well-Trained Youth Soccer Players. J. Strength Cond. Res. 2014, 28, 173–177. [Google Scholar] [CrossRef]
- Marini, E.; Campa, F.; Buffa, R.; Stagi, S.; Matias, C.N.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin. Nutr. 2020, 39, 447–454. [Google Scholar] [CrossRef]
- Castizo-Olier, J.; Irurtia, A.; Jemni, M.; Carrasco-Marginet, M.; Fernández-García, R.; Rodríguez, F.A. Bioelectrical impedance vector analysis (BIVA) in sport and exercise: Systematic review and future perspectives. PLoS ONE 2018, 13, e0197957. [Google Scholar] [CrossRef]
- Honorato, R.d.C.; Soares Marreiros Ferraz, A.; Kassiano, W.; Martins, P.C.; Silva, D.A.S.; Ceccatto, V.M. Regional phase angle, not whole-body, is augmented in response to pre-season in professional soccer players. Res. Sports Med. 2023, 31, 831–845. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Localized bioimpedance to assess muscle injury. Physiol. Meas. 2013, 34, 237. [Google Scholar] [CrossRef]
- Moon, J.R. Body composition in athletes and sports nutrition: An examination of the bioimpedance analysis technique. Eur. J. Clin. Nutr. 2013, 67, S54–S59. [Google Scholar] [CrossRef]
- Levi Micheli, M.; Pagani, L.; Marella, M.; Gulisano, M.; Piccoli, A.; Angelini, F.; Burtscher, M.; Gatterer, H. Bioimpedance and Impedance Vector Patterns as Predictors of League Level in Male Soccer Players. Int. J. Sports Physiol. Perform. 2014, 9, 532–539. [Google Scholar] [CrossRef]
- Foster, K.R.; Lukaski, H.C. Whole-body impedance--what does it measure? Am. J. Clin. Nutr. 1996, 64, 388s–396s. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, E.C.; Meador, C.K.; Simpson, D.C. Correlation of whole-body impedance with total body water volume. J. Appl. Physiol. 1969, 27, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sagayama, H.; Yamada, Y.; Ichikawa, M.; Kondo, E.; Yasukata, J.; Tanabe, Y.; Higaki, Y.; Takahashi, H. Evaluation of fat-free mass hydration in athletes and non-athletes. Eur. J. Appl. Physiol. 2020, 120, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.C.; Bartok, C.; Schoeller, D.A. The validity of bioelectrical impedance models in clinical populations. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2004, 19, 433–446. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Vega Diaz, N.; Talluri, A.; Nescolarde, L. Classification of hydration in clinical conditions: Indirect and direct approaches using bioimpedance. Nutrients 2019, 11, 809. [Google Scholar] [CrossRef]
- Lukaski, H.C. Biological indexes considered in the derivation of the bioelectrical impedance analysis. Am. J. Clin. Nutr. 1996, 64, 397S–404S. [Google Scholar] [CrossRef]
- Buendia, R.; Seoane, F.; Lindecrantz, K.; Bosaeus, I.; Gil-Pita, R.; Johannsson, G.; Ellegård, L.; Ward, L. Estimation of body fluids with bioimpedance spectroscopy: State of the art methods and proposal of novel methods. Physiol. Meas. 2015, 36, 2171. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Piccoli, A.; Pastori, G. BIVA Software, 2002; Department of Medical and Surgical Sciences, University of Padova: Padova, Italy, 2002.
- Svantesson, U.; Zander, M.; Klingberg, S.; Slinde, F. Body composition in male elite athletes, comparison of bioelectrical impedance spectroscopy with dual energy X-ray absorptiometry. J. Negat. Results Biomed. 2008, 7, 1. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Lacome, M.; Rodriguez, C.; Tinsley, G.M. Tracking Body Composition Over a Competitive Season in Elite Soccer Players Using Laboratory- and Field-Based Assessment Methods. J. Strength Cond. Res. 2024, 38, e104–e115. [Google Scholar] [CrossRef]
- Petri, C.; Pengue, L.; Bartolini, A.; Pistolesi, D.; Arrones, L.S. Body Composition Changes in Male and Female Elite Soccer Players: Effects of a Nutritional Program Led by a Sport Nutritionist. Nutrients 2024, 16, 334. [Google Scholar] [CrossRef]
- Ramírez-Munera, M.; Arcusa, R.; López-Román, F.J.; Victoria-Montesinos, D.; García-Muñoz, A.M.; Ávila-Gandía, V.; Pérez-Piñero, S.; Marhuenda, J. Anthropometric and Body Composition Changes during Pre-Season of Spanish Professional Female Soccer Players According to Playing Position. Nutrients 2024, 16, 2799. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Mascherini, G.; Genovesi, F.; Pasta, G.; Iaia, F.M.; Trecroci, A.; Ventimiglia, M.; Alberti, G.; Campa, F. Bioimpedance Vector References Need to Be Period-Specific for Assessing Body Composition and Cellular Health in Elite Soccer Players: A Brief Report. J. Funct. Morphol. Kinesiol. 2020, 5, 73. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Tinsley, G.; Martera, G.; Orlandi, C.; Genovesi, F.; Puleo, G.; Rossi, A.; Trecroci, A. Regional Lean Soft Tissue and Intracellular Water Are Associated with Changes in Lower-Body Neuromuscular Performance: A Pilot Study in Elite Soccer Players. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 882–892. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Trecroci, A.; Rossi, A.; Iaia, F.M.; Pasta, G.; Campa, F. Association between change in regional phase angle and jump performance: A pilot study in serie a soccer players. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 860–865. [Google Scholar] [CrossRef]
- Campa, F.; Bongiovanni, T.; Matias, C.N.; Genovesi, F.; Trecroci, A.; Rossi, A.; Iaia, F.M.; Alberti, G.; Pasta, G.; Toselli, S. A New Strategy to Integrate Heath-Carter Somatotype Assessment with Bioelectrical Impedance Analysis in Elite Soccer Player. Sports 2020, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Micheli, M.L.; Pompignoli, M.; Cannataro, R.; Gulisano, M.; Toselli, S.; Greco, G.; Coratella, G. The Influence of Menstrual Cycle on Bioimpedance Vector Patterns, Performance, and Flexibility in Elite Soccer Players. Int. J. Sports Physiol. Perform. 2022, 17, 58–66. [Google Scholar] [CrossRef]
- Campa, F.; Silva, A.M.; Talluri, J.; Matias, C.N.; Badicu, G.; Toselli, S. Somatotype and Bioimpedance Vector Analysis: A New Target Zone for Male Athletes. Sustainability 2020, 12, 4365. [Google Scholar] [CrossRef]
- Francavilla, V.C.; Bongiovanni, T.; Genovesi, F.; Minafra, P.; Francavilla, G. Localized bioelectrical impedance analysis: How useful is it in the follow-up of muscle injury? A case report. Med. Dello Sport 2015, 68, 323–334. [Google Scholar]
- Levi Micheli, M.; Cannataro, R.; Gulisano, M.; Mascherini, G. Proposal of a New Parameter for Evaluating Muscle Mass in Footballers through Bioimpedance Analysis. Biology 2022, 11, 1182. [Google Scholar] [CrossRef]
- Mascherini, G.; Castizo-Olier, J.; Irurtia, A.; Petri, C.; Galanti, G. Differences between the sexes in athletes’ body composition and lower limb bioimpedance values. Muscles Ligaments Tendons J. 2017, 7, 573–581. [Google Scholar] [CrossRef]
- Mascherini, G.; Gatterer, H.; Lukaski, H.; Burtscher, M.; Galanti, G. Changes in hydration, body-cell mass and endurance performance of professional soccer players through a competitive season. J. Sports Med. Phys. Fit. 2015, 55, 749–755. [Google Scholar]
- Mascherini, G.; Petri, C.; Galanti, G. Integrated total body composition and localized fat-free mass assessment. Sport Sci. Health 2015, 11, 217–225. [Google Scholar] [CrossRef]
- Mascherini, G.; Petri, C.; Galanti, G. Link between body cellular mass and left ventricular hypertrophy in female and male athletes. J. Sports Med. Phys. Fit. 2019, 59, 164–170. [Google Scholar] [CrossRef]
- Moya-Amaya, H.; Molina-López, A.; Berralaguilar, A.J.; Rojano-Ortega, D.; La Rosa, C.J.B.-D.; La Rosa, F.J.B.-D. Bioelectrical Phase Angle, Muscle Damage Markers and Inflammatory Response After a Competitive Match in Professional Soccer Players. Pol. J. Sport Tour. 2021, 28, 8–13. [Google Scholar] [CrossRef]
- Petri, C.; Mascherini, G.; Pengue, L.; Galanti, G. Dietary habits in elite soccer players. Sport Sci. Health 2016, 12, 113–119. [Google Scholar] [CrossRef]
- Suarez-Arrones, L.; Petri, C.; Maldonado, R.A.; Torreno, N.; Munguía-Izquierdo, D.; Di Salvo, V.; Méndez-Villanueva, A. Body fat assessment in elite soccer players: Cross-validation of different field methods. Sci. Med. Footb. 2018, 2, 203–208. [Google Scholar] [CrossRef]
- Martinez-Ferran, M.; Rafei, E.; Romero-Morales, C.; Pérez-Ruiz, M.; Lam-Meléndez, A.; Munguia-Izquierdo, D.; Pareja-Galeano, H. Optimizing Field Body Fat Percentage Assessment in Professional Soccer Players. Appl. Sci. 2022, 12, 727. [Google Scholar] [CrossRef]
- Munguia-Izquierdo, D.; Suarez-Arrones, L.; Di Salvo, V.; Paredes-Hernandez, V.; Alcazar, J.; Ara, I.; Kreider, R.; Mendez-Villanueva, A. Validation of field methods to assess body fat percentage in elite youth soccer players. Int. J. Sports Med. 2018, 39, 349–354. [Google Scholar] [CrossRef]
- Munguía-Izquierdo, D.; Suárez-Arrones, L.; Di Salvo, V.; Paredes-Hernández, V.; Ara, I.; Mendez-Villanueva, A. Estimating fat-free mass in elite youth male soccer players: Cross-validation of different field methods and development of prediction equation. J. Sports Sci. 2019, 37, 1197–1204. [Google Scholar] [CrossRef]
- Nescolarde, L.; Terricabras, J.; Mechó, S.; Rodas, G.; Yanguas, J. Differentiation Between Tendinous, Myotendinous and Myofascial Injuries by L-BIA in Professional Football Players. Front. Physiol. 2020, 11, 574124. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Effects of muscle injury severity on localized bioimpedance measurements. Physiol. Meas. 2014, 36, 27. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Terricabras, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Detection of muscle gap by L-BIA in muscle injuries: Clinical prognosis. Physiol. Meas. 2017, 38, L1–L9. [Google Scholar] [CrossRef]
- Núñez, F.J.; Munguía-Izquierdo, D.; Suárez-Arrones, L. Validity of Field Methods to Estimate Fat-Free Mass Changes Throughout the Season in Elite Youth Soccer Players. Front. Physiol. 2020, 11, 16. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Villegas-Mora, B.E.; Clemente-Suárez, V.J. Differences in Body Composition Analysis by DEXA, Skinfold and BIA Methods in Young Football Players. Children 2022, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Martins, A.D.; Clemente, F.M.; Brito, J.P.; Nobari, H.; Reis, V.; Oliveira, R. Variations of distance and accelerometry-based GPS measures and their influence on body composition in professional women soccer players. Proc. Inst. Mech. Eng.-Part P-J. Sports Eng. Technol. 2025, 239, 20–28. [Google Scholar]
- Leão, C.; Simões, M.; Silva, B.; Clemente, F.M.; Bezerra, P.; Camões, M. Body Composition Evaluation Issue among Young Elite Football Players: DXA Assessment. Sports 2017, 5, 17. [Google Scholar] [CrossRef]
- Oliveira, R.; Brito, J.P.; Fernandes, R.; Morgans, R.; Alves, S.; Santos, F.J.; Pinto, P.; Espada, M.C. The Effects of Pre-Season and Relationships with Physical, Physiological, Body Composition, and Load Markers: A Case Study Comparing Starters versus Non-Starters from an Elite Female Professional Soccer Team. Medicina 2023, 59, 2156. [Google Scholar] [CrossRef]
- Oliveira, R.; Francisco, R.; Fernandes, R.; Martins, A.; Nobari, H.; Clemente, F.M.; Brito, J.P. In-season body composition effects in professional women soccer players. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef]
- Nabuco, H.C.G.; Silva, A.M.; Sardinha, L.B.; Rodrigues, F.B.; Tomeleri, C.M.; Ravagnani, F.C.P.; Cyrino, E.S.; Ravagnani, C.F.C. Phase Angle is Moderately Associated with Short-term Maximal Intensity Efforts in Soccer Players. Int. J. Sports Med. 2019, 40, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Gatterer, H.; Schenk, K.; Ferrari, P.; Faulhaber, M.; Schopp, E.; Burtscher, M. Changes in hydration status of soccer players competing in the 2008 European Championship. J. Sports Med. Phys. Fit. 2011, 51, 89–94. [Google Scholar]
- Yargic, M.P.; Kurklu, G.B.; Celen, M.C.; Goktepe, E. Seasonal body composition alterations of an elite male soccer team evaluated with skinfold thickness equations and BIMP analysis. Comp. Exerc. Physiol. 2020, 16, 339–346. [Google Scholar] [CrossRef]
- Campa, F.; Thomas, D.M.; Watts, K.; Clark, N.; Baller, D.; Morin, T.; Toselli, S.; Koury, J.C.; Melchiorri, G.; Andreoli, A.; et al. Reference Percentiles for Bioelectrical Phase Angle in Athletes. Biology 2022, 11, 264. [Google Scholar] [CrossRef]
- Sebastiá-Rico, J.; Soriano, J.M.; González-Gálvez, N.; Martínez-Sanz, J.M. Body Composition of Male Professional Soccer Players Using Different Measurement Methods: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Padilla, S. Detraining: Loss of training-induced physiological and performance adaptations. Part I: Short term insufficient training stimulus. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef]
- Silva, J.R.; Brito, J.; Akenhead, R.; Nassis, G.P. The Transition Period in Soccer: A Window of Opportunity. Sports Med. 2016, 46, 305–313. [Google Scholar] [CrossRef]
- Suarez-Arrones, L.; Lara-Lopez, P.; Maldonado, R.; Torreno, N.; De Hoyo, M.; Nakamura, F.Y.; Di Salvo, V.; Mendez-Villanueva, A. The effects of detraining and retraining periods on fat-mass and fat-free mass in elite male soccer players. PeerJ 2019, 7, e7466. [Google Scholar] [CrossRef]
- Ostojic, S.M. Seasonal alterations in body composition and sprint performance of elite soccer players. J. Exerc. Physiol. 2003, 6, 11–14. [Google Scholar]
- Caldwell, B.P.; Peters, D.M. Seasonal Variation in Physiological Fitness of a Semiprofessional Soccer Team. J. Strength Cond. Res. 2009, 23, 1370–1377. [Google Scholar] [CrossRef]
- Clemente, F.M.; Ramirez-Campillo, R.; Sarmento, H. Detrimental Effects of the Off-Season in Soccer Players: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 795–814. [Google Scholar] [CrossRef]
- Martins, F.; França, C.; Henriques, R.; Ihle, A.; Przednowek, K.; Marques, A.; Lopes, H.; Sarmento, H.; Gouveia, É.R. Body composition variations between injured and non-injured professional soccer players. Sci. Rep. 2022, 12, 20779. [Google Scholar] [CrossRef]
- McEwan, G.P.; Drobnic, F.; Lizarraga, A.; Gómez Díaz, A.; Pons, E.; Dello Iacon, A.; Unnithan, V. Changes in markers of body composition of professional male soccer players during pre-season. Sports Med. Health Sci. 2020, 2, 166–171. [Google Scholar] [CrossRef]
- Milanese, C.; Cavedon, V.; Corradini, G.; De Vita, F.; Zancanaro, C. Seasonal DXA-measured body composition changes in professional male soccer players. J. Sports Sci. 2015, 33, 1219–1228. [Google Scholar] [CrossRef]
- Campa, F.; Coratella, G.; Cerullo, G.; Noriega, Z.; Francisco, R.; Charrier, D.; Irurtia, A.; Lukaski, H.; Silva, A.M.; Paoli, A. High-standard predictive equations for estimating body composition using bioelectrical impedance analysis: A systematic review. J. Transl. Med. 2024, 22, 515. [Google Scholar] [CrossRef]
- Matias, C.N.; Campa, F.; Santos, D.A.; Lukaski, H.; Sardinha, L.B.; Silva, A.M. Fat-free Mass Bioelectrical Impedance Analysis Predictive Equation for Athletes using a 4-Compartment Model. Int. J. Sports Med. 2021, 42, 27–32. [Google Scholar] [CrossRef]
- O’brien, C.; Young, A.; Sawka, M. Bioelectrical impedance to estimate changes in hydration status. Int. J. Sports Med. 2002, 23, 361–366. [Google Scholar] [CrossRef]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef]
- Collins, J.; Maughan, R.J.; Gleeson, M.; Bilsborough, J.; Jeukendrup, A.; Morton, J.P.; Phillips, S.M.; Armstrong, L.; Burke, L.M.; Close, G.L.; et al. UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. Br. J. Sports Med. 2021, 55, 416. [Google Scholar] [CrossRef]
- Bennett, J.P.; Cataldi, D.; Liu, Y.E.; Kelly, N.N.; Quon, B.K.; Gonzalez, M.C.; Heymsfield, S.B.; Shepherd, J.A. Variations in bioelectrical impedance devices impact raw measures comparisons and subsequent prediction of body composition using recommended estimation equations. Clin. Nutr. ESPEN 2024, 63, 540–550. [Google Scholar] [CrossRef]
- Matias, C.N.; Santos, D.A.; Júdice, P.B.; Magalhães, J.P.; Minderico, C.S.; Fields, D.A.; Lukaski, H.C.; Sardinha, L.B.; Silva, A.M. Estimation of total body water and extracellular water with bioimpedance in athletes: A need for athlete-specific prediction models. Clin. Nutr. 2016, 35, 468–474. [Google Scholar] [CrossRef]
- Matthews, E.L.; Hosick, P.A. Bioelectrical impedance analysis does not detect an increase in total body water following isotonic fluid consumption. Appl. Physiol. Nutr. Metab. 2019, 44, 1116–1120. [Google Scholar] [CrossRef]
- Francisco, R.; Jesus, F.; Gomes, T.; Nunes, C.L.; Rocha, P.; Minderico, C.S.; Heymsfield, S.B.; Lukaski, H.; Sardinha, L.B.; Silva, A.M. Validity of water compartments estimated using bioimpedance spectroscopy in athletes differing in hydration status. Scand. J. Med. Sci. Sports 2021, 31, 1612–1620. [Google Scholar] [CrossRef]
- Maughan, R.J.; Leiper, J.B. Fluid replacement requirements in soccer. J. Sports Sci. 1994, 12, S29–S34. [Google Scholar] [CrossRef]
- Cutrufello, P.T.; Dixon, C.B.; Zavorsky, G.S. Hydration assessment among marathoners using urine specific gravity and bioelectrical impedance analysis. Res. Sports Med. 2016, 24, 219–227. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Ross, R.; Heymsfield, S.B. Does adipose tissue influence bioelectric impedance in obese men and women? J. Appl. Physiol. 1998, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Camina Martín, M.A.; de Mateo Silleras, B.; Nescolarde Selva, L.; Barrera Ortega, S.; Domínguez Rodríguez, L.; Redondo del Río, M.P. Bioimpedance vector analysis and conventional bioimpedance to assess body composition in older adults with dementia. Nutrition 2015, 31, 155–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marini, E.; Sergi, G.; Succa, V.; Saragat, B.; Sarti, S.; Coin, A.; Manzato, E.; Buffa, R. Efficacy of specific bioelectrical impedance vector analysis (BIVA) for assessing body composition in the elderly. J. Nutr. Health Aging 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Buffa, R.; Saragat, B.; Cabras, S.; Rinaldi, A.C.; Marini, E. Accuracy of specific BIVA for the assessment of body composition in the United States population. PLoS ONE 2013, 8, e58533. [Google Scholar] [CrossRef]
- Toselli, S.; Marini, E.; Maietta Latessa, P.; Benedetti, L.; Campa, F. Maturity Related Differences in Body Composition Assessed by Classic and Specific Bioimpedance Vector Analysis among Male Elite Youth Soccer Players. Int. J. Environ. Res. Public Health 2020, 17, 729. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.C.; Gobbo, L.A.; Silva, D.A.S. Bioelectrical impedance vector analysis (BIVA) in university athletes. J. Int. Soc. Sports Nutr. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.C.; Hansen, F.; Silva, A.M.; Silva, D.A.S. Fluid distribution and cell integrity indicators evaluated by bioelectrical impedance in university athletes: Comparison between team sports and individual sports. Physiol. Meas. 2019, 40, 015004. [Google Scholar] [CrossRef]
- Campa, F.; Toselli, S. Bioimpedance vector analysis of elite, subelite, and low-level male volleyball players. Int. J. Sports Physiol. Perform. 2018, 13, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.; Scott, M.; Wallace, J.; Reilly, T. Body composition of English Premier League soccer players: Influence of playing position, international status, and ethnicity. J. Sports Sci. 2009, 27, 1019–1026. [Google Scholar] [CrossRef]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- Campa, F.; Matias, C.; Gatterer, H.; Toselli, S.; Koury, J.C.; Andreoli, A.; Melchiorri, G.; Sardinha, L.B.; Silva, A.M. Classic bioelectrical impedance vector reference values for assessing body composition in male and female athletes. Int. J. Environ. Res. Public Health 2019, 16, 5066. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Rossi, A.; Trecroci, A.; Martera, G.; Iaia, F.M.; Alberti, G.; Pasta, G.; Lacome, M. Regional bioelectrical phase angle is more informative than whole-body phase angle for monitoring neuromuscular performance: A pilot study in elite young soccer players. Sports 2022, 10, 66. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Chapelle, L.; Tassignon, B.; Rommers, N.; Mertens, E.; Mullie, P.; Clarys, P. Pre-exercise hypohydration prevalence in soccer players: A quantitative systematic review. Eur. J. Sport Sci. 2020, 20, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Gatterer, H.; Schenk, K.; Laninschegg, L.; Schlemmer, P.; Lukaski, H.; Burtscher, M. Bioimpedance Identifies Body Fluid Loss after Exercise in the Heat: A Pilot Study with Body Cooling. PLoS ONE 2014, 9, e109729. [Google Scholar] [CrossRef]
- Silva, A.M.; Matias, C.N.; Nunes, C.L.; Santos, D.A.; Marini, E.; Lukaski, H.C.; Sardinha, L.B. Lack of agreement of in vivo raw bioimpedance measurements obtained from two single and multi-frequency bioelectrical impedance devices. Eur. J. Clin. Nutr. 2019, 73, 1077–1083. [Google Scholar] [CrossRef]
- Nescolarde, L.; Lukaski, H.; De Lorenzo, A.; de-Mateo-Silleras, B.; Redondo-del-Río, M.P.; Camina-Martín, M.A. Different displacement of bioimpedance vector due to Ag/AgCl electrode effect. Eur. J. Clin. Nutr. 2016, 70, 1401–1407. [Google Scholar] [CrossRef]
- Lukaski, H.C. Letter to the Editor: Normal Reference Plots of the Bioelectrical Impedance Vector for Healthy Korean Adults. J. Korean Med. Sci. 2019, 34, e274. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hetherington-Rauth, M.; Leu, C.G.; Júdice, P.B.; Correia, I.R.; Magalhães, J.P.; Sardinha, L.B. Whole body and regional phase angle as indicators of muscular performance in athletes. Eur. J. Sport Sci. 2021, 21, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, R.; Amaral, M.A.; Mundstock, E.; Ziegelmann, P.K. Reference values for the phase angle of the electrical bioimpedance: Systematic review and meta-analysis involving more than 250,000 subjects. Clin. Nutr. 2020, 39, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.C.; Teixeira, A.S.; Guglielmo, L.G.A.; Francisco, J.S.; Silva, D.A.S.; Nakamura, F.Y.; Lima, L.R.A. Phase Angle Is Related to 10 m and 30 m Sprint Time and Repeated-Sprint Ability in Young Male Soccer Players. Int. J. Environ. Res. Public Health 2021, 18, 4405. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Inami, T.; Ishida, H.; Nagata, N.; Murayama, M.; Morito, A.; Yamada, S.; Kohtake, N. Bioimpedance analysis for identifying new indicators of exercise-induced muscle damage. Sci. Rep. 2024, 14, 15299. [Google Scholar] [CrossRef]
- Pérez-Castillo, Í.M.; Rueda, R.; Bouzamondo, H.; López-Chicharro, J.; Mihic, N. Biomarkers of post-match recovery in semi-professional and professional football (soccer). Front. Physiol. 2023, 14, 1167449. [Google Scholar] [CrossRef] [PubMed]
- Nescolarde, L.; Talluri, A.; Yanguas, J.; Lukaski, H. Phase angle in localized bioimpedance measurements to assess and monitor muscle injury. Rev. Endocr. Metab. Disord. 2023, 24, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.T.; Smith, R.W.; Harty, P.S.; Rodriguez, C.; Johnson, B.A.; Dellinger, J.R.; Williams, A.D.; White, S.J.; Benavides, M.L.; Tinsley, G.M. Longitudinal agreement of four bioimpedance analyzers for detecting changes in raw bioimpedance during purposeful weight gain with resistance training. Eur. J. Clin. Nutr. 2021, 75, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Siedler, M.R.; Rodriguez, C.; Stratton, M.T.; Harty, P.S.; Keith, D.S.; Green, J.J.; Boykin, J.R.; White, S.J.; Williams, A.D.; DeHaven, B. Assessing the reliability and cross-sectional and longitudinal validity of fifteen bioelectrical impedance analysis devices. Br. J. Nutr. 2023, 130, 827–840. [Google Scholar] [CrossRef]
- Siedler, M.R.; Harty, P.S.; Stratton, M.T.; Rodriguez, C.; Keith, D.; Green, J.; Boykin, J.; Dellinger, J.; White, S.; Williams, A.D. Day-to-day precision error and least significant change for two commonly used bioelectrical impedance analysis devices. In Proceedings of the International Journal of Exercise Science: Conference Proceedings, In Virtual, 25–26 February 2021; p. 18. Available online: https://digitalcommons.wku.edu/ijesab/vol2/iss13/18/ (accessed on 20 September 2025).
- Caton, J.R.; Molé, P.A.; Adams, W.C.; Heustis, D.S. Body composition analysis by bioelectrical impedance: Effect of skin temperature. Med. Sci. Sports Exerc. 1988, 20, 489–491. [Google Scholar] [CrossRef]
| Reference | Characteristics of Participants and Measurement Time | BIA Device and Configuration | Fat Mass (%) | Fat-Free Mass (kg) | Agreement Between Calculated (DXA) and Estimated (BIA/BIS) Outcomes |
|---|---|---|---|---|---|
| Svantesson et al., 2008 [33] | Swedish first division male soccer players (n = 17; 24.1 ± 3.8 years old) (mean ± SD). (Timing not specified.) | Hydra 4200®, Xitron Technologies Inc., San Diego, CA, USA. Foot-to-hand. BIS. | DXA: 10.9 ± 3.5 BIS: 9.7 ± 3.6 | DXA: 72.4 ± 6.2 BIS: 72.8 ± 7.9 | BIS underestimates mean %FM by ~1.1% in professional players compared to DXA, albeit differences were not statistically significant. Agreement at individual level of BIS estimations was highly variable. |
| Leao et al., 2017 [61] | U19 National level male football players (n = 25; 17.28 ± 0.54 years old) (mean ± SD). In-season. | BC-418®, Tanita Corp., Tokyo, Japan. Foot-to-hand. SF-BIA. | DXA: 14.16 ± 1.91 SF-BIA: 11.97 ± 2.66 | -- | Only moderate correlations were found between DXA-calculated and SF-BIA-estimated %FM, with the latter technique underestimating %FM by 2.21% in U19 players (statistical significance not reported). |
| Suarez-Arrones et al., 2019 [71] | Italian first division male soccer players (n = 18; 27.6 ± 3.0 years old) (mean ± SD). End of the competitive season. | MC-180 MA III®, Tanita Corp., Tokyo, Japan. Foot-to-hand. MF-BIA. | DXA: 14.4 ± 1.3 MF-BIA: 9.5 ± 2.6 | -- | An unclear correlation was concluded between MF-BIA-estimated and DXA-calculated %FM values in professional players. MF-BIA substantially underestimated %FM compared to DXA. Differences were qualitatively classified as almost certainly lower than DXA. |
| Núñez et al., 2020 [58] | Spanish elite male football players (n = 40; 16.6 ± 0.5 years old) (mean ± SD). Pre-season (T0) and mid-season (T1). | Inbody 770®, Biospace, Seoul, South Korea. Foot-to-hand. MF-BIA. and BC-418®, Tanita Corp., Tokyo, Japan. Foot-to-hand. SF-BIA. | -- | DXA: 55.73 ± 4.04 (T0), 56.79 ± 4.15 (T1) MF-BIA (Inbody): 57.09 ± 4.61 (T0), 58.98 ± 4.77 (T1) SF-BIA (BC-418): 56.31 ± 4.24 (T0), 57.09 ± 4.38 (T1) | Tanita SF-BIA showed no statistically significant standardized difference compared to DXA-derived FFM values, whereas InBody MF-BIA demonstrated a significant standardized difference, indicating lower agreement with DXA. Both BIA techniques showed positive and very large correlations with DXA and identified significant changes in FFM from pre-season to mid-season in professional players. |
| Martinez-Ferran et al., 2022 [52] | Spanish first division male football players (n = 21; 26.3 ± 3.7 years old) (mean ± SD). First half of the competitive season. | BC-545N®, Tanita Corp, Tokyo, Japan. Foot-to-hand. SF-BIA. | DXA: 15.3 ± 2.0 MF-BIA: 13.0 ± 2.5 | -- | MF-BIA %FM only showed a moderate correlation with DXA, and standardized differences were qualitatively classified as almost certainly lower than DXA, indicating a consistent underestimation of %FM in professional players. |
| Tornero-Aguilera et al., 2022 [59] | National-level male (n = 70; 21.8 ± 5.0 years old) and female football players (n = 76; 22.2 ± 3.2 years old) (mean ± SD). Before pre-season. | Inbody 770®, Biospace, Seoul, South Korea. Foot-to-hand. MF-BIA. | DXA: 19.0 ± 3.7 (male), 29.2 ± 4.8 (female) MF-BIA: 9.3 ± 4.3 (male), 14.9 ± 5.6 (female) | -- | Statistically significant differences were observed between %FM values estimated via MF-BIA and assessed by DXA, with MF-BIA notably underestimating FM in both national-level male and female players. |
| Bongiovanni et al., 2024 [34] | Italian second division male football players (n = 21; 23.7 ± 4.8 years old) (Statistic type unspecified). Four time points throughout the competitive season (October (T0), December (T1), February (T2), April (T3)). | Inbody 770®, Biospace, Seoul, South Korea. Foot-to-hand. MF-BIA. | DXA: 12.2 ± 2.2 (T0), 12.4 ± 2.1 (T1), 12.3 ± 2.1 (T2), 12.6 ± 2.3 (T3) MF-BIA (Inbody): 8.4 ±2.8 (T0), 8.6 ± 2.7 (T1), 8.8 ± 2.9 (T2), 8.8 ± 2.7 (T3) | DXA: 70.8 ± 5.1 (T0), 71.6 ± 5.7 (T1), 72.5 ± 6.0 (T2), 71.7 ± 5.4 (T3) MF-BIA (Inbody): 73.9 ± 5.6 (T0), 74.2 ± 5.7 (T1), 74.9 ± 6.4 (T2), 74.3 ± 6.1 (T3) | Despite showing a strong correlation with DXA, MF-BIA demonstrated limited sensitivity in detecting seasonal changes in FFM. In contrast, MF-BIA-derived %FM values were significantly lower than those obtained via DXA, with statistically significant differences and variable agreement reflected in lower correlation coefficients. |
| Reference | Characteristics of Participants and Number of Injuries | Localized Bioelectric Impedance Analysis (L-BIA) | BIA Device and Configuration | |||
|---|---|---|---|---|---|---|
| Nescolarde et al., 2013 [20] | Spanish first division male football players (n = 3 lower limb muscle injuries) | Grade I Muscle Injury (n = 1) | Baseline values | Injured (24 h post-injury) | Difference (%) | BIA-101®, Akern, Florence, Italy. Muscle Localized. SF 50 kHz. |
| R (Ohm) | 42 | 37 | −11.9 | |||
| Xc (Ohm) | 17 | 13 | −23.5 | |||
| PhA (°) | 22 | 19.3 | −12.3 | |||
| Grade II Muscle Injury (n = 1) | Baseline values | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 68 | 54 | −20.6 | |||
| Xc (Ohm) | 19 | 13 | −31.6 | |||
| PhA (°) | 15.6 | 13.5 | −13.5 | |||
| Grade III Muscle Injury (n = 1) | Baseline values | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 67 | 51.5 | −23.13 | |||
| Xc (Ohm) | 20 | 11 | −45 | |||
| PhA (°) | 16.6 | 12 | −27.7 | |||
| Nescolarde et al., 2014 [56] | Spanish first division male football players (n = 21 lower limb muscle injuries) | Grade I Muscle Injury (n = 11) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | BIA-101®, Akern, Florence, Italy. Muscle Localized. SF 50 kHz. |
| R (Ohm) | 40.4 ± 9.2 | 36.1 ± 7.6 | −10.4 | |||
| Xc (Ohm) | 15.3 ± 1.6 | 12.7 ± 1.6 | −17.5 | |||
| PhA (°) | 21.4 ± 3.9 | 19.9 ± 4 | −9.0 | |||
| Grade II Muscle Injury (n = 8) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 37.9 ± 5.9 | 30.9 ± 4.9 | −18.4 | |||
| Xc (Ohm) | 15.3 ± 2.5 | 10.2 ± 1.7 | −32.9 | |||
| PhA (°) | 22.1 ± 3.5 | 18.3 ± 2.3 | −16.6 | |||
| Grade III Muscle Injury (n = 2) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 44.8 ± 2.7 | 38.4 ± 1.8 | −14.1 ± 9.3 | |||
| Xc (Ohm) | 18.3 ± 1.7 | 8.6 ± 0.1 | −52.9 ± 3.6 | |||
| PhA (°) | 22.2 ± 0.7 | 12.7 ± 0.8 | −43.1 ± 1.8 | |||
| Francavilla et al., 2015 [43] | Italian first division male soccer player (n = 1 lower limb muscle injury) | Grade II Muscle Injury (n = 1) | Baseline values | Injured (24 h post-injury) | Difference (%) | BIA-101®, Akern, Florence, Italy. Muscle Localized. SF 50 kHz. |
| R (Ohm) | 23.3 | 21.2 | −9 | |||
| Xc (Ohm) | 1.7 | 1.2 | −29.4 | |||
| PhA (°) | 4.3 | 3.3 | −23.3 | |||
| Nescolarde et al., 2017 [57] | Spanish first division male football players (n = 22 lower limb muscle injuries) | Grade I Muscle Injury (n = 7) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | BIA-101®, Akern, Florence, Italy. Muscle Localized. SF 50 kHz |
| R (Ohm) | 37.3 ± 7.8 | 33.4 ± 6.6 | −10.2 | |||
| Xc (Ohm) | 14.9 ± 1.9 | 12.9 ± 1.7 | −13.4 | |||
| PhA (°) | 22.2 ± 3.8 | 21.5 ± 3.6 | −3.2 | |||
| Grade II (no gap) Muscle Injury (n = 8) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 42.1 ± 7.8 | 36.7 ± 7.0 | −12.8 | |||
| Xc (Ohm) | 15.1 ± 1.8 | 11.6 ± 1.4 | −23.5 | |||
| PhA (°) | 20.1 ± 2.9 | 17.8 ± 2.4 | −11.2 | |||
| Grade II (gap) Muscle Injury (n = 7) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 41.2 ± 13.3 | 32.8 ± 10.3 | −19.9 | |||
| Xc (Ohm) | 16.2 ± 2.8 | 10.1 ± 1.9 | −37.5 | |||
| PhA (°) | 22.4 ± 4.6 | 17.6 ± 3.1 | −20.5 | |||
| Nescolarde et al., 2020 [55] | Spanish first division male football players (n = 26 lower limb myotendinous junction injuries) | Grade I Muscle Injury (n = 11) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | BIA-101®, Akern, Florence, Italy. Muscle Localized. SF 50 kHz. |
| R (Ohm) | 40.4 ± 8.3 | 37.3 ± 8.4 | −7.9 | |||
| Xc (Ohm) | 15.1 ± 2.3 | 13.2 ± 2.3 | −12.3 | |||
| PhA (°) | 21.0 ± 4.5 | 20.2 ± 4.6 | −4.3 | |||
| Grade II Muscle Injury (n = 8) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 40.5 ± 9.0 | 36.7 ± 7.0 | −8.8 | |||
| Xc (Ohm) | 14.6 ± 1.7 | 11.7 ± 1.1 | −19.5 | |||
| PhA (°) | 20.3 ± 3.2 | 18.1 ± 2.9 | −10.8 | |||
| Grade III Muscle Injury (n = 7) | Non-injured limb | Injured (24 h post-injury) | Difference (%) | |||
| R (Ohm) | 39.2 ± 6.0 | 32.5 ± 6.0 | −17.1 | |||
| Xc (Ohm) | 15.3 ± 2.7 | 10.2 ± 1.7 | −32.7 | |||
| PhA (°) | 21.6 ± 4.0 | 17.7 ± 3.1 | −17.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Castillo, Í.M.; Valiño-Marques, A.; López-Chicharro, J.; Segura-Ortiz, F.; Rueda, R.; Bouzamondo, H. Bioelectrical Impedance Analysis in Professional and Semi-Professional Football: A Scoping Review. Sports 2025, 13, 348. https://doi.org/10.3390/sports13100348
Pérez-Castillo ÍM, Valiño-Marques A, López-Chicharro J, Segura-Ortiz F, Rueda R, Bouzamondo H. Bioelectrical Impedance Analysis in Professional and Semi-Professional Football: A Scoping Review. Sports. 2025; 13(10):348. https://doi.org/10.3390/sports13100348
Chicago/Turabian StylePérez-Castillo, Íñigo M., Alberto Valiño-Marques, José López-Chicharro, Felipe Segura-Ortiz, Ricardo Rueda, and Hakim Bouzamondo. 2025. "Bioelectrical Impedance Analysis in Professional and Semi-Professional Football: A Scoping Review" Sports 13, no. 10: 348. https://doi.org/10.3390/sports13100348
APA StylePérez-Castillo, Í. M., Valiño-Marques, A., López-Chicharro, J., Segura-Ortiz, F., Rueda, R., & Bouzamondo, H. (2025). Bioelectrical Impedance Analysis in Professional and Semi-Professional Football: A Scoping Review. Sports, 13(10), 348. https://doi.org/10.3390/sports13100348






