Muscle Synergy During Cutting Movements in Athletes with a History of Groin Pain
Abstract
1. Introduction
1.1. Background of Groin Pain
1.2. Muscle Activity and Ground Reaction Force (GRF) Characteristics of Groin Pain
1.3. Muscle Synergy Analysis
1.4. Current Gap
1.5. Purpose and Hypotheses
2. Materials and Methods
2.1. Participants
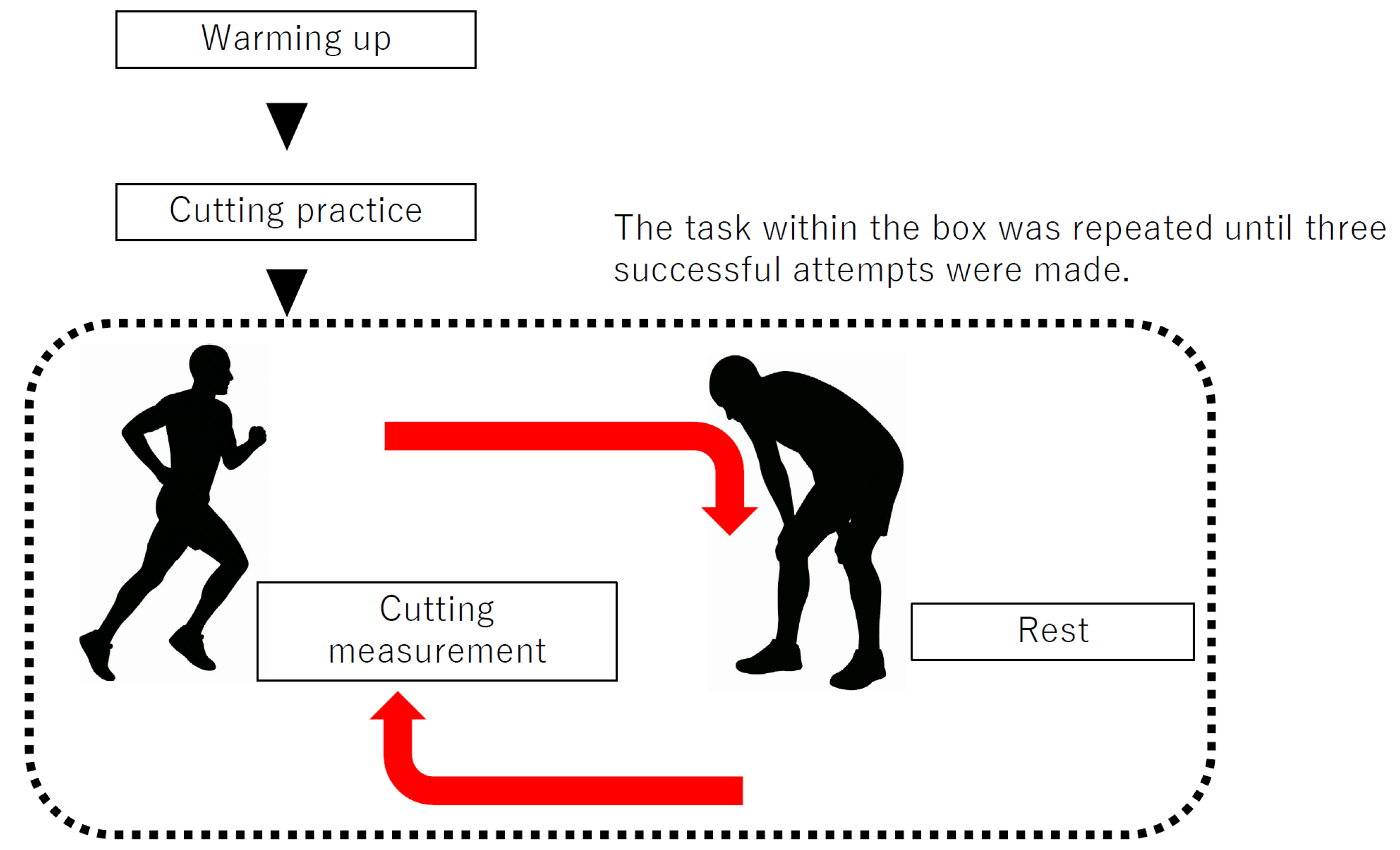
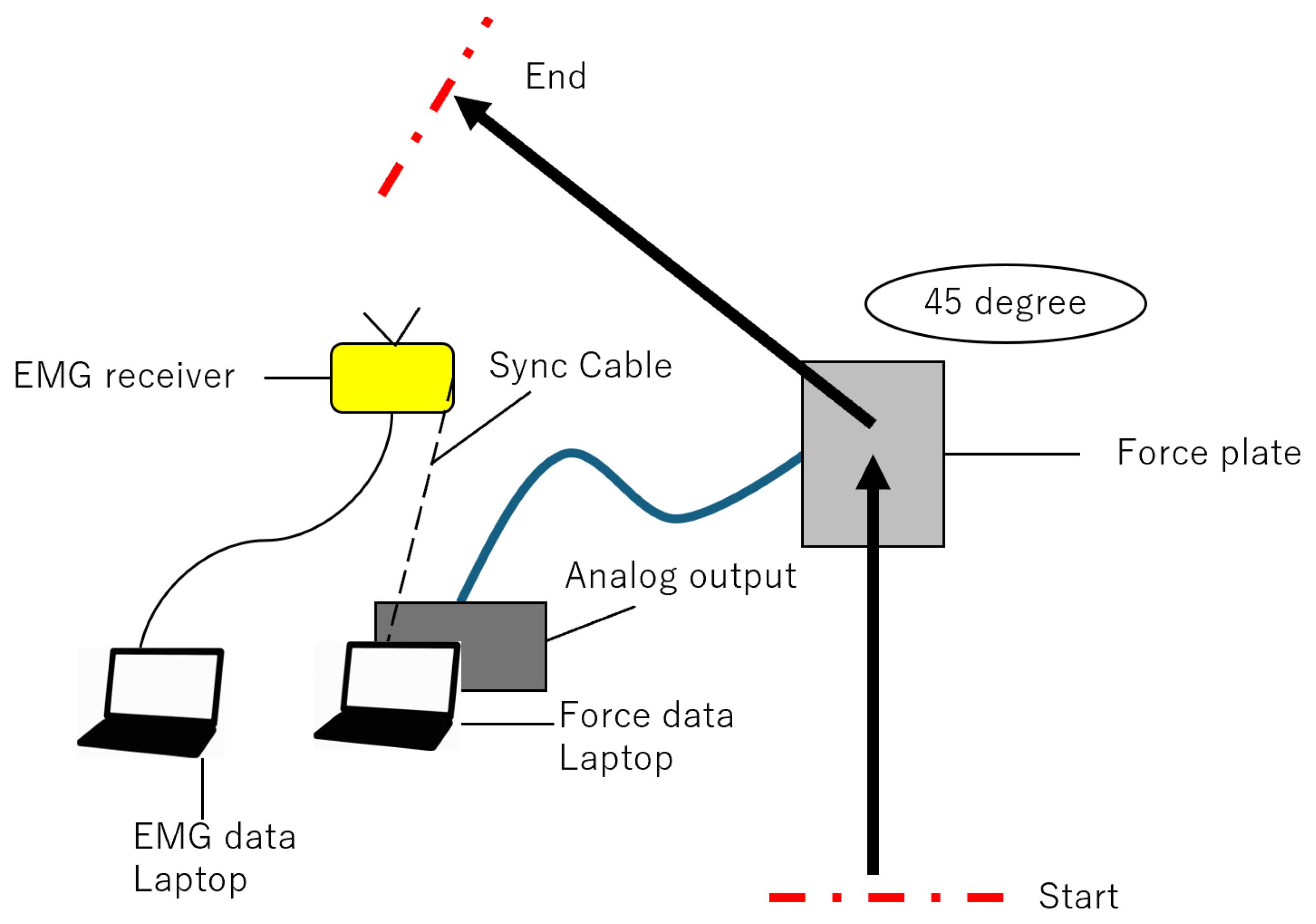
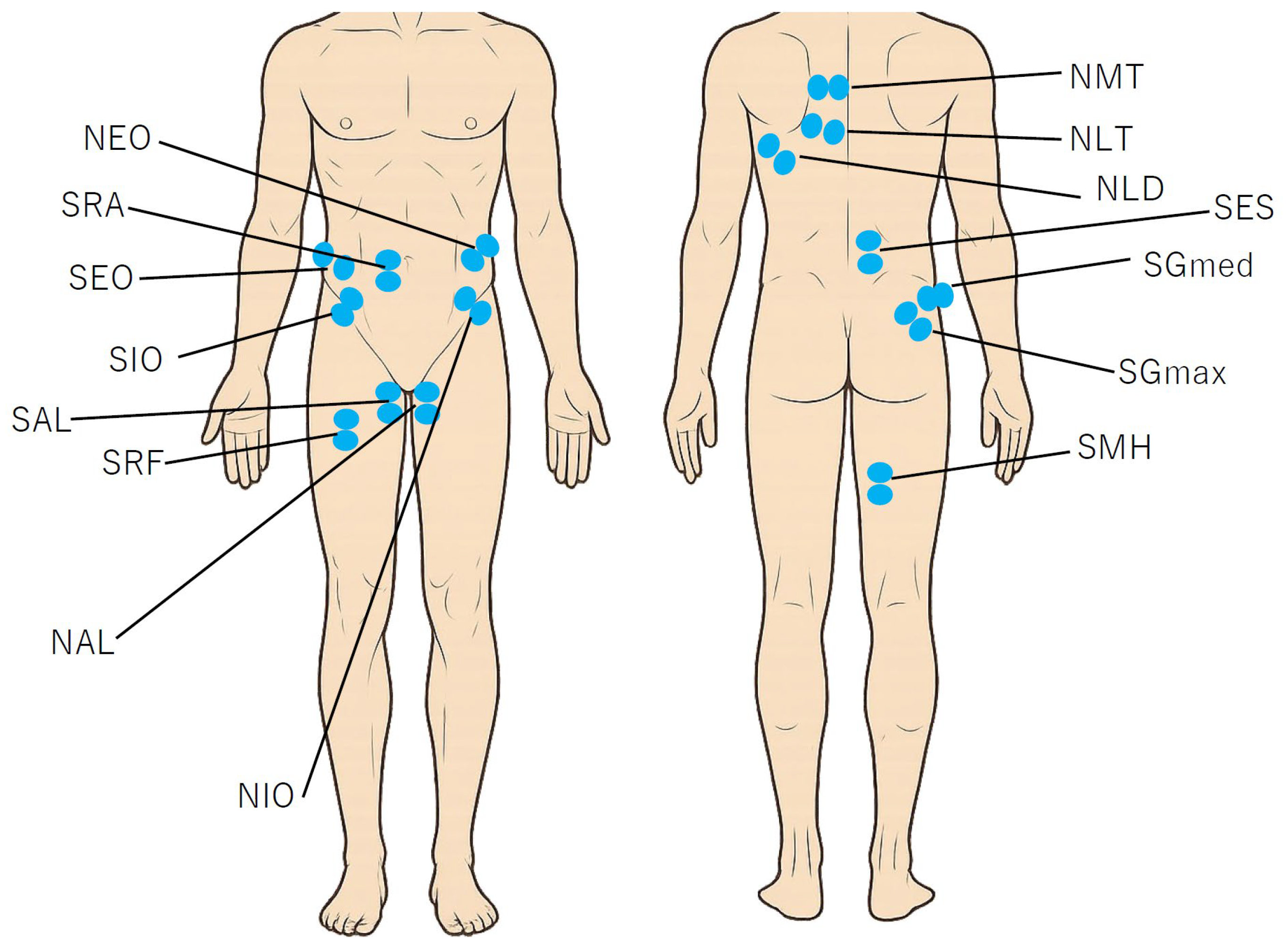
2.2. Data Collection
2.3. GRF and EMG Procedure
2.4. Extraction of Muscle Synergies
2.5. Statistical Analysis
3. Results
3.1. Participants
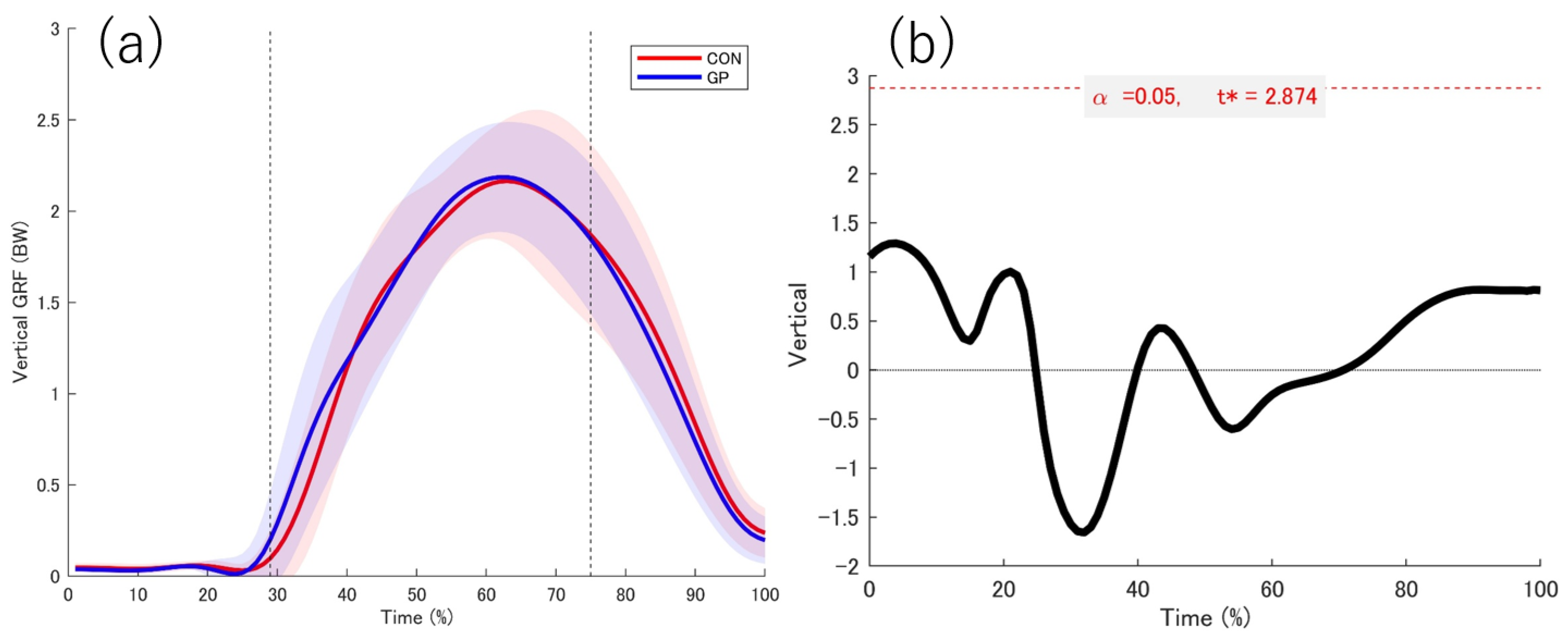
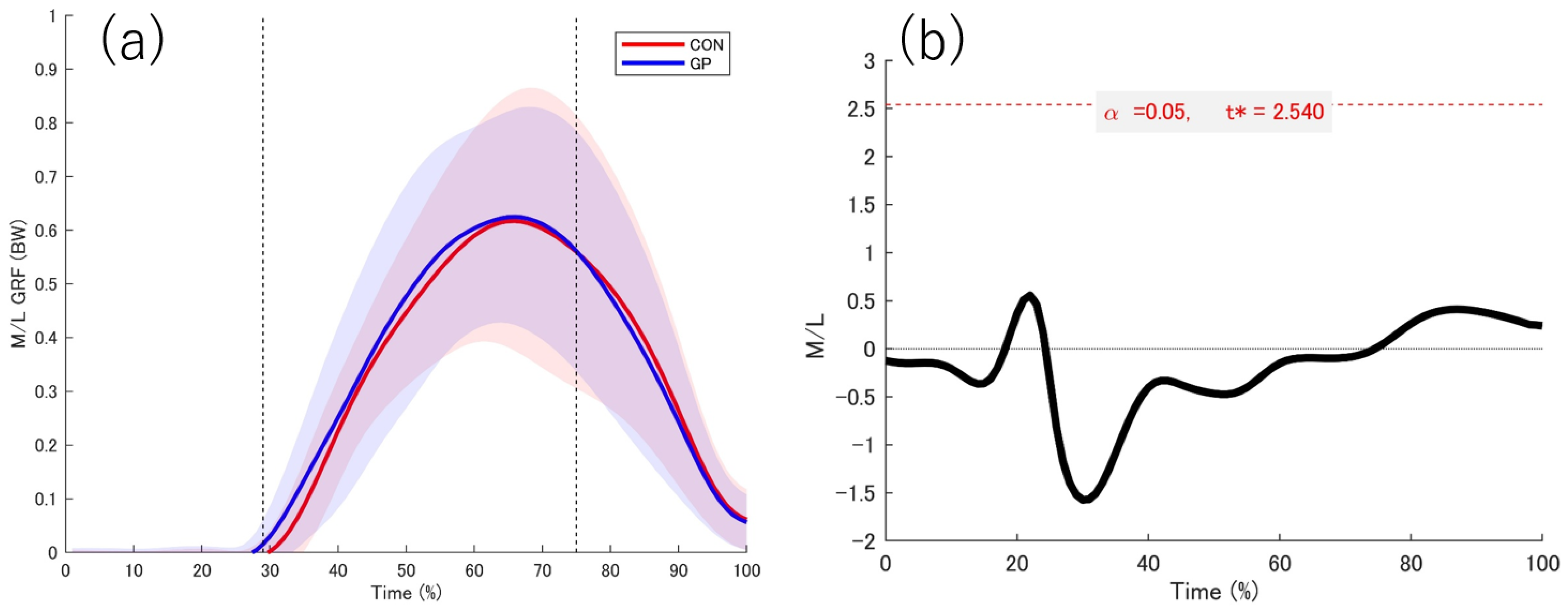
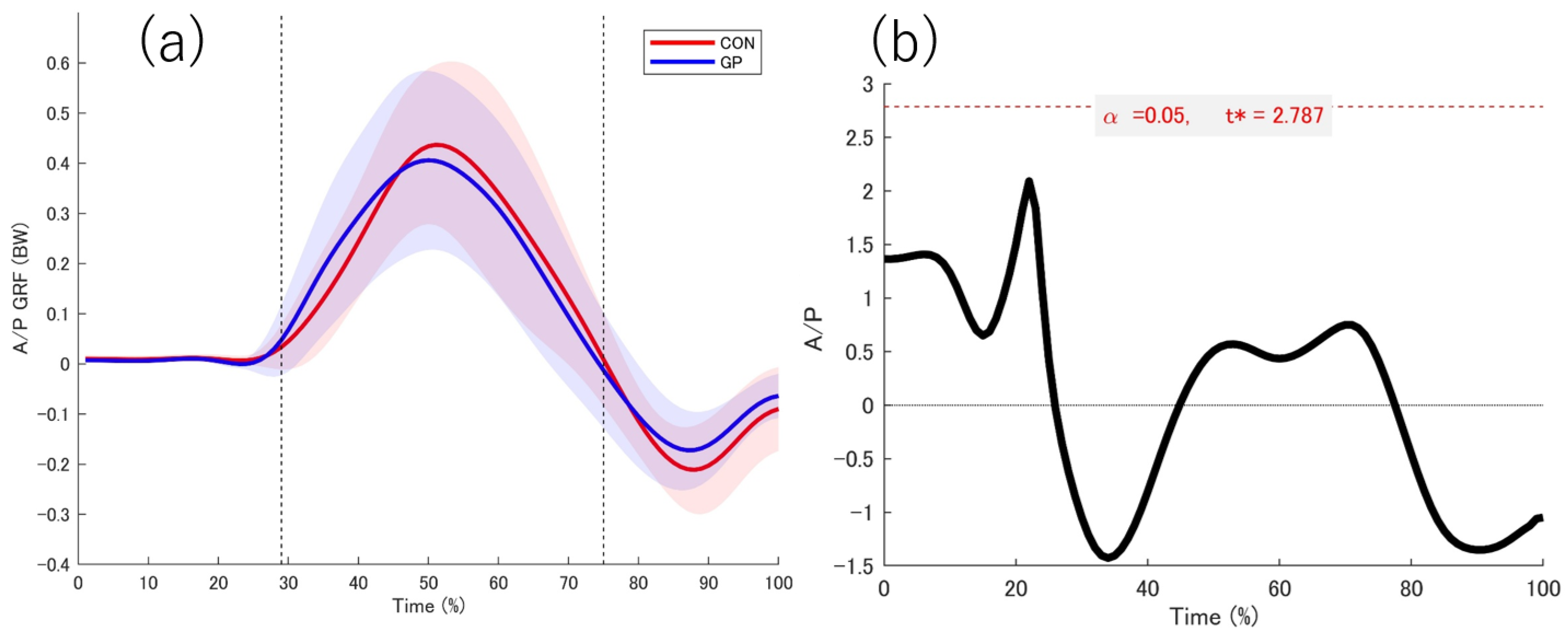
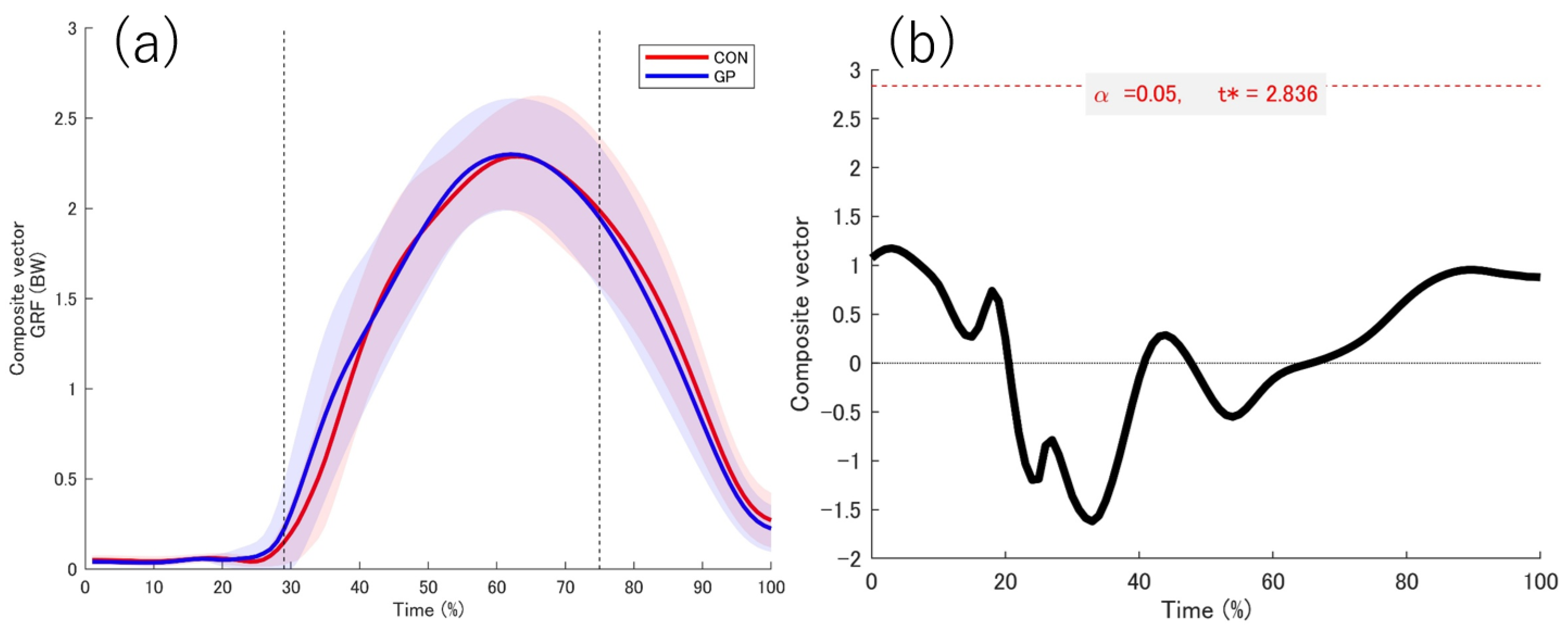
3.2. Kinetic Date
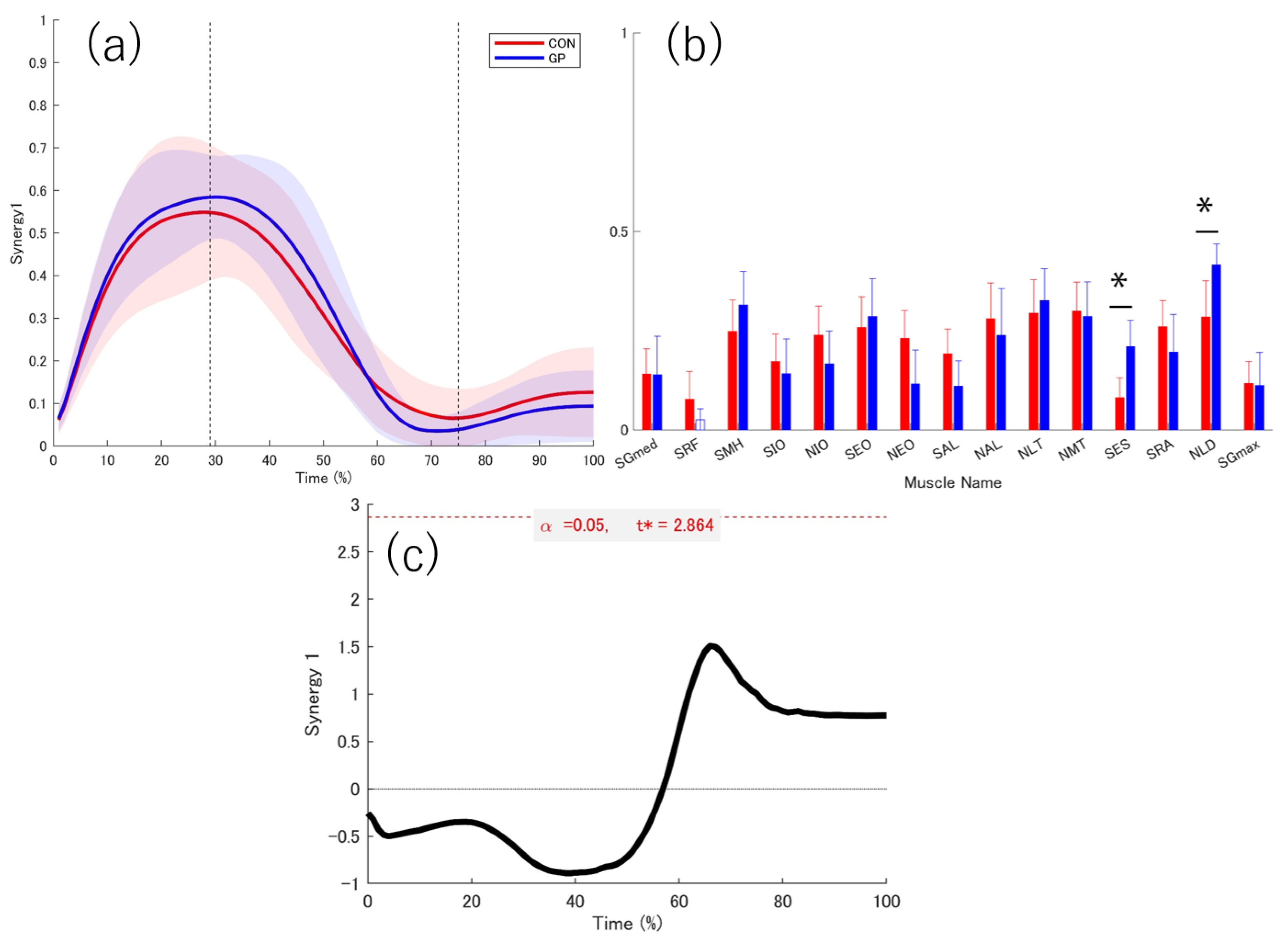
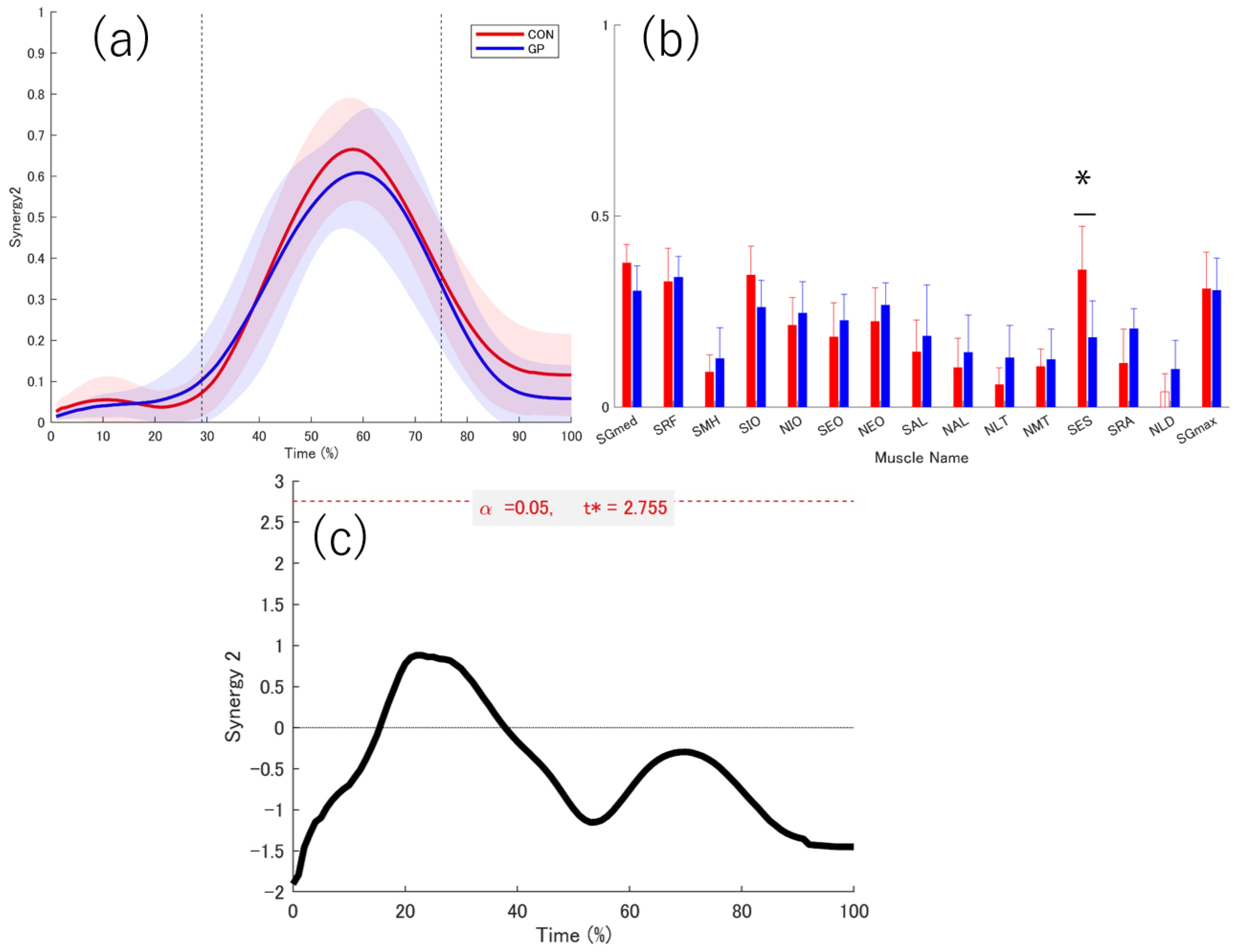
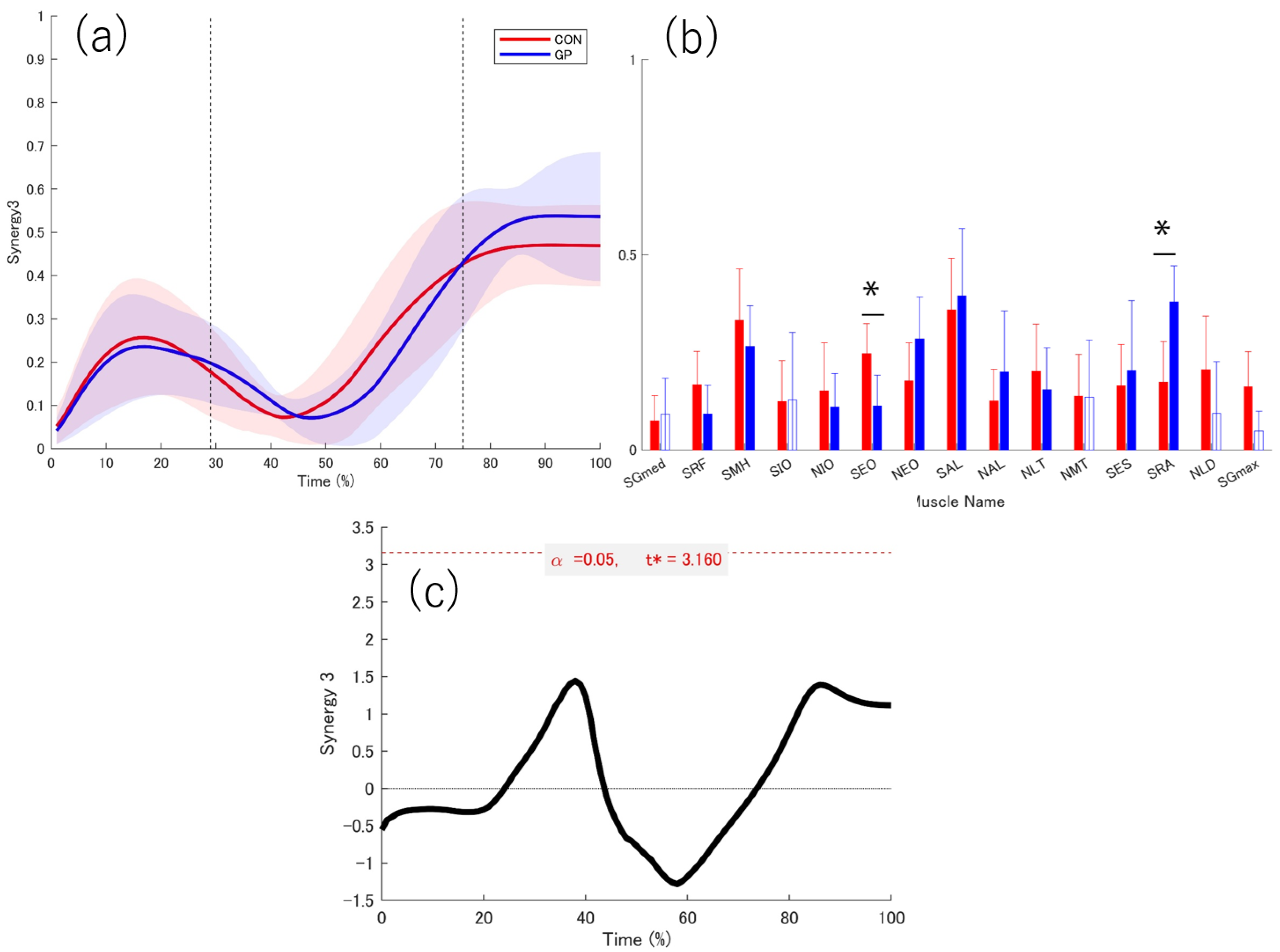
3.3. Extracted Muscle Synergy
3.4. Activation Coefficient
3.5. Weightings
4. Discussion
4.1. GRF
4.2. Function of Muscle Synergy
4.3. Contact of the Support Leg
4.4. Deceleration of the Support Leg
4.5. Acceleration Phase of the Support Leg
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GRF | Ground reaction force |
| GP | Group of with a history of groin pain |
| CON | Control |
| SGmed | Support leg gluteus medius |
| SRF | Support leg rectus femoris |
| SMH | Support leg medial hamstring |
| SIO | Support leg internal oblique |
| NIO | Non-support leg internal oblique |
| SEO | Support leg external oblique |
| NEO | Non-support leg external oblique |
| SAL | Support leg adductor longus |
| NAL | Non-support leg adductor longus |
| NLT | Non-support leg lower trapezius |
| NMT | Non-support leg middle trapezius |
| SES | Support leg erector spinae |
| SRA | Support leg rectus abdominis |
| NLD | Non-support leg latissimus dorsi |
| SGmax | Support leg gluteus maximus |
| EMG | Electromyography |
| HAGOS | Copenhagen Hip and Groin Outcome Score |
References
- Serner, A.; Mosler, A.B.; Tol, J.L.; Bahr, R.; Weir, A. Mechanisms of acute adductor longus injuries in male football players: A systematic visual video. Br. J. Sports Med. 2019, 53, 158. [Google Scholar] [CrossRef]
- Coughlan, G.F.; Delahunt, E.; Caulfield, B.M.; Forde, C.; Green, B.S. Normative Adductor Squeeze Test Values in Elite Junior Rugby Union Players. Clin. J. Sport Med. 2014, 24, 315–319. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Small, C.; Maffey, L.; Emery, C.A. Risk factors for groin injury in sport: An updated systematic review. Br. J. Sports Med. 2015, 49, 803–809. [Google Scholar] [CrossRef]
- Hägglund, M.; Waldén, M.; Ekstrand, J. Risk factors for lower extremity muscle injury in professional soccer: The UEFA injury study. Am. J. Sports Med. 2013, 41, 327–335. [Google Scholar] [CrossRef]
- Cowan, S.M.; Schache, A.G.; Brukner, P.; Bennell, K.L.; Hodges, P.W.; Coburn, P.; Crossley, K.M. Delayed onset of transversus abdominus in long-standing groin pain. Med. Sci. Sports Exerc. 2004, 36, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.; Graham, J.; Screen, H.; Sinha, A.; Small, C.; Twycross-Lewis, R.; Woledge, R. Coronal plane hip muscle activation in football code athletes with chronic adductor groin strain injury during standing hip flexion. Man. Ther. 2012, 17, 145–149. [Google Scholar] [CrossRef]
- Sugaya, T.; Sakamoto, M.; Nakazawa, R.; Wada, N. Relationship between spinal range of motion and trunk muscle activity during trunk rotation. J. Phys. Ther. Sci. 2016, 28, 589–595. [Google Scholar] [CrossRef]
- Yamauchi, T.; Hasegawa, S.; Matsumura, A.; Nakamura, M.; Ibuki, S.; Ichihashi, N. The effect of trunk rotation during shoulder exercises on the activity of the scapular muscle and scapular kinematics. J. Shoulder Elb. Surg. 2015, 24, 955–964. [Google Scholar] [CrossRef]
- Baida, S.; King, E.; Gore, S.; Richter, C.; Franklyn-Miller, A.; Moran, K. Movement Variability and Loading Characteristics in Athletes With Athletic Groin Pain: Changes After Successful Return to Play and Compared With Uninjured Athletes. Orthop. J. Sports Med. 2022, 10, 23259671221125159. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Brooke, H.C.; Cook, J.L. Distinct cut task strategy in Australian football players with a history of groin pain. Phys. Ther. Sport 2017, 23, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of Muscle Synergy Outcomes in Clinics, Robotics, and Sports: A Systematic Review. Appl. Bionics Biomech. 2018, 2018, 3934698. [Google Scholar] [CrossRef]
- Kim, H.; Palmieri-Smith, R.; Kipp, K. Muscle Synergies in People With Chronic Ankle Instability During Anticipated and Unanticipated Cutting Tasks. J. Athl. Train. 2021, 58, 143–152. [Google Scholar] [CrossRef]
- Matsuura, Y.; Matsunaga, N.; Iizuka, S.; Akuzawa, H.; Kaneoka, K. Muscle Synergy of the Underwater Undulatory Swimming in Elite Male Swimmers. Front. Sports Act. Living 2020, 2, 62. [Google Scholar] [CrossRef]
- Marras, W.S.; Davis, K.G.; Granata, K.P. Trunk muscle activities during asymmetric twisting motions. J. Electromyogr. Kinesiol. 1998, 8, 247–256. [Google Scholar] [CrossRef]
- Taniguchi, M.; Umehara, J.; Yamagata, M.; Yagi, M.; Motomura, Y.; Okada, S.; Okada, S.; Nakazato, K.; Fukumoto, Y.; Kobayashi, M.; et al. Understanding muscle coordination during gait based on muscle synergy and its association with symptoms in patients with knee osteoarthritis. Clin. Rheumatol. 2024, 43, 743–752. [Google Scholar] [CrossRef]
- Kubota, K.; Hanawa, H.; Yokoyama, M.; Kita, S.; Hirata, K.; Fujino, T.; Kokubun, T.; Ishibashi, T.; Kanemura, N.; Sonoo, M. Usefulness of Muscle Synergy Analysis in Individuals with Knee Osteoarthritis during Gait. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 239–248. [Google Scholar] [CrossRef]
- Serrancolí, G.; Monllau, J.C.; Font-Llagunes, J.M. Analysis of muscle synergies and activation–deactivation patterns in subjects with anterior cruciate ligament deficiency during walking. Clin. Biomech. 2016, 31, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, V.; Lopez-Valenciano, A.; Barbado, D.; Moreside, J.; Elvira, J.L.L.; Vera-Garcia, F.J. Comparisons of hip strength and countermovement jump height in elite tennis players with and without acute history of groin injuries. Musculoskelet. Sci. Pract. 2017, 29, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Severin, A.C.; Mellifont, D.B.; Sayers, M.G.L. Influence of previous groin pain on hip and pelvic instep kick kinematics. Sci. Med. Footb. 2017, 1, 80–85. [Google Scholar] [CrossRef]
- Beddows, T.; Weir, A.; Agricola, R.; Tak, I.; Piscaer, T.; Verhaar, J.; van Klij, P. Hip and groin pain in male field hockey players: Prevalence, incidence and associations with patient reported outcome scores and hip muscle strength. Phys. Ther. Sport 2023, 61, 66–72. [Google Scholar] [CrossRef]
- Harøy, J.; Clarsen, B.; Thorborg, K.; Hölmich, P.; Bahr, R.; Andersen, T.E. Groin Problems in Male Soccer Players Are More Common Than Previously Reported. Am. J. Sports Med. 2017, 45, 1304–1308. [Google Scholar] [CrossRef]
- Zacharias, A.J.; Spiker, A.M. Evaluation and treatment of hip pain in the lacrosse athlete. J. Cartil. Jt. Preserv. 2022, 2, 100087. [Google Scholar] [CrossRef]
- Thorborg, K.; Branci, S.; Stensbirk, F.; Jensen, J.; Hölmich, P. Copenhagen hip and groin outcome score (HAGOS) in male soccer: Reference values for hip and groin injury-free players. Br. J. Sports Med. 2014, 48, 557–559. [Google Scholar] [CrossRef] [PubMed]
- De Sèze, M.; Gille, O. Functional anatomy of the erector spinae: Review. In Spinal Anatomy: Modern Concepts; Springer: Cham, Switzerland, 2019; pp. 321–327. [Google Scholar]
- Nelson-Wong, E.; Gregory, D.E.; Winter, D.A.; Callaghan, J.P. Gluteus medius muscle activation patterns as a predictor of low back pain during standing. Clin. Biomech. 2008, 23, 545–553. [Google Scholar] [CrossRef]
- Jones, P.A.; Herrington, L.C.; Graham-Smith, P. Technique determinants of knee abduction moments during pivoting in female soccer players. Clin. Biomech. 2016, 31, 107–112. [Google Scholar] [CrossRef]
- Franklyn-Miller, A.; Richter, C.; King, E.; Gore, S.; Moran, K.; Strike, S.; Falvey, E.C. Athletic groin pain (part 2): A prospective cohort study on the biomechanical evaluation of change of direction identifies three clusters of movement patterns. Br. J. Sports Med. 2017, 51, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Harris-Hayes, M.; Hillen, T.J.; Commean, P.K.; Harris, M.D.; Mueller, M.J.; Clohisy, J.C.; Salsich, G.B. Hip Kinematics During Single-Leg Tasks in People With and Without Hip-Related Groin Pain and the Association Among Kinematics, Hip Muscle Strength, and Bony Morphology. J. Orthop. Sports Phys. Ther. 2020, 50, 243–251. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, L.J.; Dare, M.; Louw, Q.; Crous, L.; Cockroft, J.; Williams, L.; Olivier, B. Pelvic and hip kinematics during single-leg drop-landing are altered in sports participants with long-standing groin pain: A cross-sectional study. Phys. Ther. Sport 2017, 26, 20–26. [Google Scholar] [CrossRef]
- Van Goeverden, W.; Langhout, R.F.H.; Barendrecht, M.; Tak, I.J.R. Active pelvic tilt is reduced in athletes with groin injury; a case-controlled study. Phys. Ther. Sport 2019, 36, 14–21. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Ting, L.H. Muscle synergies characterizing human postural responses. J. Neurophysiol. 2007, 98, 2144–2156. [Google Scholar] [CrossRef]
- Yang, S.; Ye, M. Global minima analysis of Lee and Seung’s NMF algorithms. Neural Process. Lett. 2013, 38, 29–51. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Macpherson, J.M.; Ting, L.H. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 2006, 96, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Karami, H.; Baniasad, M.; Shojaeefard, M.; Farahmand, F. The association between motor modules and movement primitives of gait: A muscle and kinematic synergy study. J. Biomech. 2022, 134, 110997. [Google Scholar] [CrossRef] [PubMed]
- Kibushi, B. Muscle coordination patterns in regulation of medial gastrocnemius activation during walking. Hum. Mov. Sci. 2023, 90, 103116. [Google Scholar] [CrossRef]
- Matsunaga, N.; Okubo, Y.; Isagawa, S.; Niitsuma, J.; Otsudo, T.; Sawada, Y.; Akasaka, K. Muscle fatigue in the gluteus maximus changes muscle synergies during single-leg landing. J. Bodyw. Mov. Ther. 2021, 27, 493–499. [Google Scholar] [CrossRef]
- Cheung, V.C.-K.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Region-of-interest analyses of one-dimensional biomechanical trajectories: Bridging 0D and 1D theory, augmenting statistical power. PeerJ 2016, 4, e2652. [Google Scholar] [CrossRef]
- Kabir, R.; Syed, H.Z.; Hayhoe, R.; Parsa, A.D.; Sivasubramanian, M.; Mohammadnezhad, M.; Sathian, B.; Rahana, K.; Jain, M.; Gandhi, A.P. Meta-analysis using SPSS: A simple guide for clinicians, public health, and allied health specialists. Evid. 2024, 2, 1. [Google Scholar]
- Scholes, M.J.; Crossley, K.M.; King, M.G.; Schache, A.G.; Kemp, J.L.; Semciw, A.I.; Sritharan, P.; Heerey, J.J.; Mentiplay, B.F. Running biomechanics in football players with and without hip and groin pain. A cross-sectional analysis of 116 sub-elite players. Phys. Ther. Sport 2021, 52, 312–321. [Google Scholar] [CrossRef]
- Ting, L.H.; Macpherson, J.M. A limited set of muscle synergies for force control during a postural task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.K.; D’Avella, A.; Tresch, M.C.; Bizzi, E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef] [PubMed]
- Maniar, N.; Schache, A.G.; Pizzolato, C.; Opar, D.A. Muscle function during single leg landing. Sci. Rep. 2022, 12, 11486. [Google Scholar] [CrossRef]
- Loukas, M.; Shoja, M.M.; Thurston, T.; Jones, V.L.; Linganna, S.; Tubbs, R.S. Anatomy and biomechanics of the vertebral aponeurosis part of the posterior layer of the thoracolumbar fascia. Surg. Radiol. Anat. 2008, 30, 125–129. [Google Scholar]
- Carvalhais, V.O.C.; Ocarino, J.M.; Araújo, V.L.; Souza, T.R.; Silva, P.L.P.; Fonseca, S.T. Myofascial force transmission between the latissimus dorsi and gluteus maximus muscles: An in vivo experiment. J. Biomech. 2013, 46, 1003–1007. [Google Scholar] [CrossRef]
- Pereira, G.R.; Leporace, G.; Chagas, D.D.V.; Furtado, L.F.L.; Praxedes, J.; Batista, L.A. Influence of hip external rotation on hip adductor and rectus femoris myoelectric activity during a dynamic parallel squat. J. Strength Cond. Res. 2010, 24, 2749–2754. [Google Scholar] [CrossRef]
- Chaudhari, A.M.W.; Jamison, S.T.; McNally, M.P.; Pan, X.; Schmitt, L.C. Hip adductor activations during run-to-cut manoeuvres in compression shorts: Implications for return to sport after groin injury. J. Sports Sci. 2014, 32, 1333–1340. [Google Scholar] [CrossRef]
- Takaki, S.; Kaneoka, K.; Okubo, Y.; Otsuka, S.; Tatsumura, M.; Shiina, I.; Miyakawa, S. Analysis of muscle activity during active pelvic tilting in sagittal plane. Phys. Ther. Res. 2016, 19, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Masuo, Y.; Nakamura, E.; Oda, S. Inter-sport variability of muscle volume distribution identified by segmental bioelectrical impedance analysis in four ball sports. Open Access J. Sports Med. 2013, 4, 97–108. [Google Scholar] [CrossRef]
- Shukla, A.; Dogra, D.K.; Pant, M.; Chakraborty, G. Comparative study on selected physical fitness variables among different team games players. Int. J. Phys. Educ. Sports Health 2020, 7, 31–34. [Google Scholar]
- McGill, S.M.; Sharratt, M.T. Relationship between intra-abdominal pressure and trunk EMG. Clin. Biomech. 1990, 5, 59–67. [Google Scholar] [CrossRef]
- Iida, Y.; Kanehisa, H.; Inaba, Y.; Nakazawa, K. Activity modulations of trunk and lower limb muscles during impact-absorbing landing. J. Electromyogr. Kinesiol. 2011, 21, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Macadam, P.; Feser, E.H. Examination of gluteus maximus electromyographic excitation associated with dynamic hip extension during body weight exercise: A systematic review. Int. J. Sports Phys. Ther. 2019, 14, 14–31. [Google Scholar] [CrossRef]
- Lewis, C.L.; Sahrmann, S.A.; Moran, D.W. Effect of position and alteration in synergist muscle force contribution on hip forces when performing hip strengthening exercises. Clin. Biomech. 2009, 24, 35–42. [Google Scholar] [CrossRef]
- Charnock, B.L.; Lewis, C.L.; Garrett, W.E.; Queen, R.M. Adductor longus mechanics during the maximal effort soccer kick. Sports Biomech. 2009, 8, 223–234. [Google Scholar] [CrossRef]
- Johnson, G.; Bogduk, N.; Nowitzke, A.; House, D. Anatomy and actions of the trapezius muscle. Clin. Biomech. 1994, 9, 44–50. [Google Scholar] [CrossRef]
- Siu, A.; Schinkel-Ivy, A.; Drake, J.D. Arm position influences the activation patterns of trunk muscles during trunk range-of-motion movements. Hum. Mov. Sci. 2016, 49, 267–276. [Google Scholar] [CrossRef]
- Price, D.; Ginn, K.A.; Halaki, M.; Reed, D. What is the contribution of latissimus dorsi to trunk movement and control? A systematic review and meta-analysis. Phys. Ther. Rev. 2024, 29, 47–63. [Google Scholar] [CrossRef]
- Tharnmanularp, S.; Muro, S.; Nimura, A.; Ibara, T.; Akita, K. Significant relationship between musculoaponeurotic attachment of the abdominal and thigh adductor muscles to the pubis: Implications for the diagnosis of groin pain. Anat. Sci. Int. 2024, 99, 190–201. [Google Scholar] [CrossRef]
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Comas, G.; Cardozo, E.; Abrams, S.; Herrera, J.E. Rectus abdominis and hip adductor tendons (‘athletic pubalgia/sports hernia’). In Tendinopathy: From Basic Science to Clinical Management; Springer: Cham, Switzerland, 2021; pp. 93–101. [Google Scholar]
- King, E.; Franklyn-Miller, A.; Richter, C.; O’rEilly, E.; Doolan, M.; Moran, K.; Strike, S.; Falvey, É. Clinical and biomechanical outcomes of rehabilitation targeting intersegmental control in athletic groin pain: Prospective cohort of 205 patients. Br. J. Sports Med. 2018, 52, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Rivadulla, A.R.; Gore, S.; Preatoni, E.; Richter, C. Athletic groin pain patients and healthy athletes demonstrate consistency in their movement strategy selection when performing multiple repetitions of a change of direction test. J. Sci. Med. Sport 2020, 23, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Baida, S.R.; King, E.; Richter, C.; Gore, S.; Franklyn-Miller, A.; Moran, K. Hip Muscle Strength Explains Only 11% of the Improvement in HAGOS With an Intersegmental Approach to Successful Rehabilitation of Athletic Groin Pain. Am. J. Sports Med. 2021, 49, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
| No. | Sex | Age | Height (cm) | Weight (kg) | Level of Athletic Activity | Type of Sports | Competitive Level | Dominant Limb | Injured Limb | Types of Groin Pain |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 20 | 169.5 | 60.5 | Elite | Soccer | High | R | R | Adductor |
| 2 | M | 22 | 169.5 | 81.9 | Elite | Hockey | High | R | R | Iliopsoas |
| 3 | M | 21 | 173.2 | 61.2 | Elite | Soccer | High | L | L | Adductor |
| 4 | M | 20 | 164.0 | 50.0 | Elite | Hockey | High | R | R | N/A * |
| 5 | M | 20 | 163.0 | 66.0 | Elite | Lacrosse | High | R | R | Hip |
| 6 | M | 22 | 166.8 | 61.0 | Non-Elite | Hockey | High | R | L | Pubic |
| 7 | M | 21 | 162.0 | 55.7 | Elite | Soccer | Middle | R | R | Adductor |
| 8 | M | 22 | 169.4 | 76.0 | Elite | Lacrosse | High | R | R | Iliopsoas |
| 9 | M | 21 | 165.0 | 62.4 | Elite | Soccer | Middle | R | R | Adductor |
| 10 | M | 19 | 160.9 | 56.7 | Elite | Soccer | Middle | L | L | Adductor |
| 11 | F | 18 | 161.5 | 59.8 | Elite | Soccer | High | R | L | Pubic |
| 12 | F | 20 | 155.7 | 57.6 | Elite | Hockey | High | R | L | Hip |
| 13 | M | 21 | 169.4 | 63.2 | Elite | Hockey | High | R | R | Hip |
| 14 | M | 23 | 161.5 | 63.0 | Elite | Hockey | High | R | R | Adductor |
| 15 | F | 21 | 156.8 | 56.0 | Elite | Hockey | High | R | L | Hip |
| No. | Sex | Age | Height (cm) | Weight (kg) | Level of Athletic Activity | Type of Sports | Competitive Level | Dominant Limb |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 19 | 155.0 | 49.5 | Elite | Hockey | High | R |
| 2 | F | 22 | 160.9 | 55.9 | Elite | Lacrosse | High | L |
| 3 | F | 20 | 162.5 | 52.0 | Elite | Hockey | High | R |
| 4 | F | 19 | 160.6 | 51.6 | Elite | Soccer | High | R |
| 5 | M | 20 | 170.5 | 66.5 | Elite | Hockey | High | L |
| 6 | M | 20 | 181.8 | 76.0 | Elite | Soccer | Middle | R |
| 7 | F | 22 | 159.5 | 52.8 | Elite | Lacrosse | High | R |
| 8 | F | 22 | 164.0 | 61.4 | Elite | Soccer | High | R |
| 9 | F | 20 | 156.5 | 52.0 | Elite | Hockey | High | R |
| 10 | M | 22 | 182.4 | 76.2 | Elite | Soccer | High | R |
| 11 | F | 20 | 168.0 | 57.0 | Elite | Soccer | High | R |
| 12 | M | 23 | 163.7 | 57.1 | Elite | Hockey | High | R |
| 13 | F | 20 | 158.2 | 54.3 | Elite | Soccer | High | R |
| 14 | F | 19 | 155.0 | 51.6 | Elite | Hockey | High | R |
| HAGOS (%) | GP | CON | p Value | Effect Size d | ||
|---|---|---|---|---|---|---|
| HAGOS | 90.1 | 57.7–100.0 | 100.0 | 85.7–100.0 | 0.001 | 1.06 |
| Symptoms Score | 85.7 | 64.3–100.0 | 100.0 | 95.0–100.0 | <0.001 | 1.51 |
| Pain Score | 95.0 | 67.5–100.0 | 100.0 | 90.0–100.0 | 0.002 | 1.12 |
| Physical Function, Daily Living Score | 100.0 | 90.0–100.0 | 100.0 | 100.0–100.0 | 0.07 | 1.00 |
| Function, Sports, and Recreational Activities Score | 96.9 | 65.6–100 | 100.0 | 93.7–100.0 | 0.02 | 1.00 |
| Participation in Physical Activities Score | 100.0 | 12.5–100.0 | 100.0 | 100.0–100.0 | 0.07 | 0.70 |
| Quality of Life Score | 90.0 | 40.0–100.0 | 100.0 | 80.0–100.0 | 0.005 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, H.; Hakariya, N.; Laddawong, T.; Soga, T.; Moteki, T.; Kaneoka, K.; Matsunaga, N.; Hirose, N. Muscle Synergy During Cutting Movements in Athletes with a History of Groin Pain. Sports 2025, 13, 338. https://doi.org/10.3390/sports13100338
Saito H, Hakariya N, Laddawong T, Soga T, Moteki T, Kaneoka K, Matsunaga N, Hirose N. Muscle Synergy During Cutting Movements in Athletes with a History of Groin Pain. Sports. 2025; 13(10):338. https://doi.org/10.3390/sports13100338
Chicago/Turabian StyleSaito, Hiromi, Nadaka Hakariya, Teerapat Laddawong, Toshiaki Soga, Tatsuya Moteki, Koji Kaneoka, Naoto Matsunaga, and Norikazu Hirose. 2025. "Muscle Synergy During Cutting Movements in Athletes with a History of Groin Pain" Sports 13, no. 10: 338. https://doi.org/10.3390/sports13100338
APA StyleSaito, H., Hakariya, N., Laddawong, T., Soga, T., Moteki, T., Kaneoka, K., Matsunaga, N., & Hirose, N. (2025). Muscle Synergy During Cutting Movements in Athletes with a History of Groin Pain. Sports, 13(10), 338. https://doi.org/10.3390/sports13100338





