The Criterion Validity of a Newly Developed Ballroom Aerobic Test (BAT) Protocol Against Objective Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Study Participants
2.3. The BAT Protocol
2.4. The MetaMax® 3B Outcomes
2.5. The KF1 and Bruce Testing Protocols
2.6. Data Analysis
3. Results
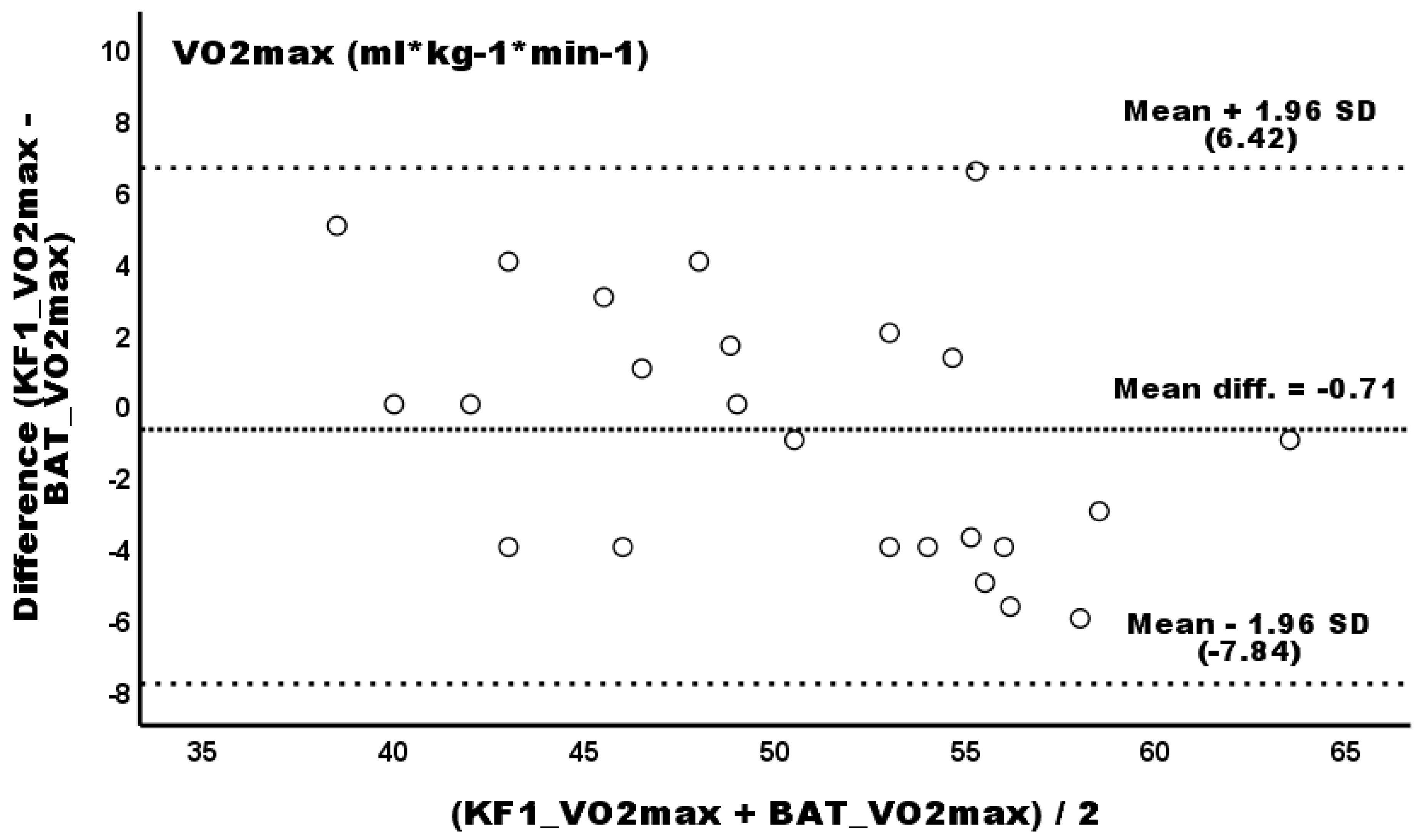
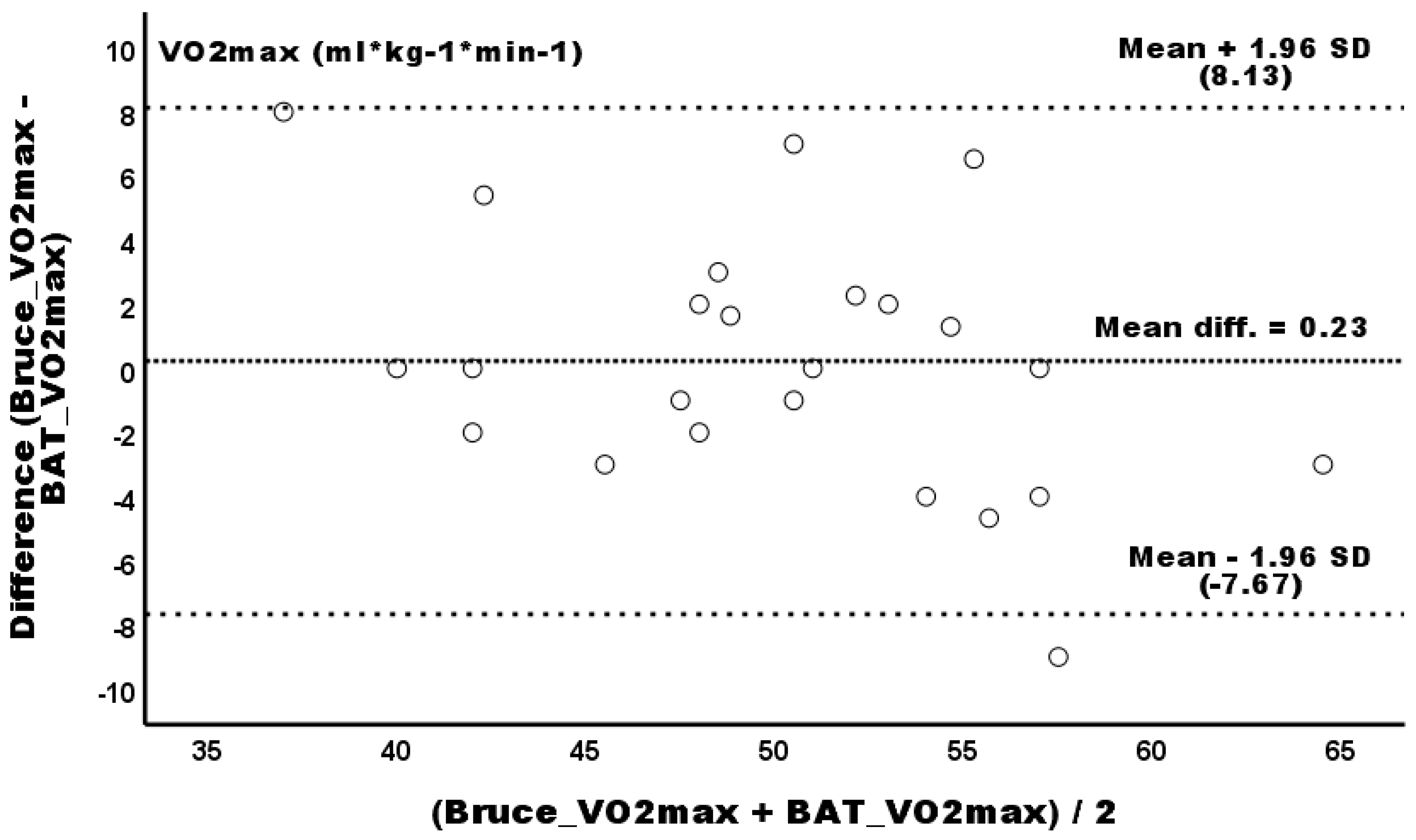
3.1. Primary Outcomes
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koutedakis, Y.; Jamurtas, A. The dancer as a performing athlete: Physiological considerations. Sports Med. 2004, 34, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcalá, M.; Carcelén-Fraile, M.D.C.; Vico-Rodríguez, P.; Cano-Orihuela, M.; Carcelén-Fraile, M.D.M. Effects of dance-based aerobic training on functional capacity and risk of falls in older adults with mild cognitive impairment. J. Clin. Med. 2025, 14, 5900. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, N.; Yang, X. A meta-analysis of the effects of strength training on physical fitness in dancers. Front. Physiol. 2025, 16, 1511833. [Google Scholar] [CrossRef] [PubMed]
- Tiemens, A.; van Rijn, R.M.; Koes, B.W.; Stubbe, J.H. A systematic review of cardiorespiratory fitness tests used in dance. J. Danc. Med. Sci. Off. Publ. Int. Assoc. Danc. Med. Sci. 2023, 27, 27–40. [Google Scholar] [CrossRef]
- Wyon, M.A.; Abt, G.; Redding, E.; Head, A.; Sharp, N.C. Oxygen uptake during modern dance class, rehearsal, and performance. J. Strength Cond. Res. 2004, 18, 646–649. [Google Scholar]
- Zaferiou, A.M. Dance-themed National Biomechanics Day community engagement to inspire our future STEAM leaders. J. Biomech. 2023, 150, 111511. [Google Scholar] [CrossRef]
- Russell, J.A. Preventing dance injuries: Current perspectives. Open Access J. Sports Med. 2013, 4, 199–210. [Google Scholar] [CrossRef]
- Giustino, V.; Patti, A. Biomechanics and sports performances. Sports 2025, 13, 73. [Google Scholar] [CrossRef]
- Wallman, K.; Goodman, C.; Morton, A.; Grove, R.; Dawson, B. Test-retest reliability of the aerobic power index component of the tri-level fitness profile in a sedentary population. J. Sci. Med. Sport 2003, 6, 443–454. [Google Scholar] [CrossRef]
- Twitchett, E.; Nevill, A.; Angioi, M.; Koutedakis, Y.; Wyon, M. Development, validity, and reliability of a ballet-specific aerobic fitness test. J. Danc. Med. Sci. Off. Publ. Int. Assoc. Danc. Med. Sci. 2011, 15, 123–127. [Google Scholar] [CrossRef]
- Wyon, M.; Redding, E.; Abt, G.; Head, A.; Sharp, N.C. Development, reliability, and validity of a multistage Dance Specific Aerobic Fitness Test (DAFT). J. Danc. Med. Sci. 2003, 7, 80–84. [Google Scholar] [CrossRef]
- Redding, E.; Weller, P.; Ehrenberg, S.; Irvine, S.; Quin, E.; Rafferty, S.; Wyon, M.; Cox, C. The development of a high intensity dance performance fitness test. J. Danc. Med. Sci. Off. Publ. Int. Assoc. Danc. Med. Sci. 2009, 13, 3–9. [Google Scholar] [CrossRef]
- Seifert, A.M.; Mullin, E.M.; Zehnder, S.; Paolone, V.J. Development and Validation of a Functional Capacity Test for Collegiate Dancers. J. Danc. Med. Sci. Off. Publ. Int. Assoc. Danc. Med. Sci. 2021, 25, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.K.; Lu, J.; Cloak, R.; Wong, D.P.; Devonport, T.; Wyon, M.A. Strength and conditioning in dance: A systematic review and meta-analysis. Eur. J. Sport Sci. 2024, 24, 637–652. [Google Scholar] [CrossRef]
- Liiv, H.; Jürimäe, T.; Mäestu, J.; Purge, P.; Hannus, A.; Jürimäe, J. Physiological characteristics of elite dancers of different dance styles. Eur. J. Sport Sci. 2014, 14, 429–436. [Google Scholar] [CrossRef]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Van Hooren, B.; Souren, T.; Bongers, B.C. Accuracy of respiratory gas variables, substrate, and energy use from 15 CPET systems during simulated and human exercise. Scand. J. Med. Sci. Sports 2024, 34, e14490. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.; Sue, D.; Casaburi, R.; Whipp, B. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; Appendix C; pp. 531–540. [Google Scholar]
- Kjertakov, M.; Dalip, M.; Hristovski, R.; Epstein, Y. Prediction of lactate threshold using the modified Conconi test in distance runners. Physiol. Int. 2016, 103, 262–270. [Google Scholar] [CrossRef]
- Bruce, R.A. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann. Clin. Res. 1971, 3, 323–332. [Google Scholar]
- Hopkins, W.G.; Schabort, E.J.; Hawley, J.A. Reliability of power in physical performance tests. Sports Med. 2001, 31, 211–234. [Google Scholar] [CrossRef]
- Hopkins, W.G. How to interpret changes in an athletic performance test. Sportscience 2004, 8, 1–7. [Google Scholar]
- Mansournia, M.A.; Waters, R.; Nazemipour, M.; Bland, M.; Altman, D.G. Bland-Altman methods for comparing methods of measurement and response to criticisms. Glob. Epidemiol. 2020, 3, 100045. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Passing, H.; Bablok, W. Comparison of several regression procedures for method comparison studies and determination of sample sizes. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part II. J. Clin. Chem. Clin. Biochem. Z. Klin. Chem. Klin. Biochem. 1984, 22, 431–445. [Google Scholar] [CrossRef]
- Fotaki, A.; Triantafyllou, A.; Papagiannis, G.; Stasi, S.; Georgios, P.; Olga, S.; Tsolakis, C.K.; Koulouvaris, P. The science of biomechanics can promote dancers’ injury prevention strategies. Phys. Ther. Rev. 2020, 26, 94–101. [Google Scholar] [CrossRef]
- Pilch, W.; Tota, Ł.; Pokora, I.; Głowa, M.; Piotrowska, A.; Chlipalska, O.; Zuziak, R.; Czerwińska, O. Energy expenditure and lactate concentration in sports dancers in a simulated final round of the standard style competition. Hum. Mov. 2017, 18, 62–67. [Google Scholar] [CrossRef]
| Dance Styles | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 6 |
|---|---|---|---|---|---|---|
| English waltz | ||||||
| Beats | 75 | 82 | 89 | 96 | 103 | 110 |
| Time | 30 s | 60 s | 1.5 min | 2 min | 2.5 min | 3 min |
| Slow fox | ||||||
| Beats | 117 | 124 | 131 | 138 | 145 | |
| Time | 3.5 min | 4.0 min | 4.5 min | 5.0 min | 5.5 min | |
| Tango | ||||||
| Beats | 152 | 159 | 166 | 173 | 180 | |
| Time | 6.0 min | 6.5 min | 7.0 min | 7.5 min | 8.0 min | |
| Viennese waltz | ||||||
| Beats | 187 | 194 | 201 | |||
| Time | 8.5 min | 9.0 min | 9.5 min | |||
| Quick step | ||||||
| Beats | 208 | 215 | 222 | 229 | 236 | 243 |
| Time | 10.0 min | 10.5 min | 11.0 min | 11.5 min | 12.0 min | 12.5 min |
| Study Variables | KF1 | Bruce | BAT | Absolute Diff. (95% CI) | Relative Diff. (%) | ES | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | KF1–BAT | Bruce–BAT | KF1–BAT | Bruce–BAT | KF1–BAT | Bruce–BAT | |
| Absolute VO2 (L * min−1) | 3.05 ± 0.74 | 3.05 ± 0.75 | 3.00 ± 0.65 | −0.05 (−0.19–0.10) | −0.05 (−0.20–0.11) | 1.64% | 1.64% | 0.07 (trivial) | 0.07 (trivial) |
| Relative VO2 (mL * kg−1 * min−1) | 50.92 ± 7.47 | 49.98 ± 7.55 | 50.21 ± 5.96 | −0.71 (−2.24–0.83) | 0.23 (−1.47–1.93) | 1.39% | 0.46% | 0.11 (trivial) | 0.03 (trivial) |
| RER | 1.09 ± 0.05 | 1.08 ± 0.04 | 1.07 ± 0.06 | −0.02 (−0.03–0.02) | −0.01 (−0.02–0.02) | 1.83% | 0.93% | 0.36 (small) | 0.20 (small) |
| VE (L * min−1) | 110.21 ± 28.43 | 110.43 ± 28.75 | 104.20 ± 26.37 | −6.01 (−12.22–0.20) | −6.23 (−13.83–1.37) | 5.45% | 5.64% | 0.22 (small) | 0.23 (small) |
| VT (L) | 1.93 ± 0.42 | 1.98 ± 0.54 | 1.77 ± 0.36 | −0.16 (−0.28–−0.04) | −0.22 (−0.54–−0.28) | 8.29% | 11.11% | 0.41 (small) | 0.46 (small) |
| VE/VO2 | 33.65 ± 3.82 | 33.85 ± 3.46 | 32.93 ± 2.93 | −0.71 (−1.67–0.24) | −0.91 (−1.78–0.04) | 2.11% | 2.69% | 0.21 (small) | 0.29 (small) |
| VE/VCO2 | 30.90 ± 3.83 | 30.16 ± 2.67 | 31.04 ± 3.51 | 0.14 (−0.89–1.17) | 0.88 (−0.03–1.73) | 0.45% | 2.92% | 0.04 (trivial) | 0.28 (small) |
| VD/VT | 0.15 ± 0.03 | 0.14 ± 0.03 | 0.15 ± 0.02 | 0.003 (−0.005–0.01) | 0.009 (−0.001–0.02) | 2.00% | 6.43% | 0.01 (trivial) | 0.39 (small) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Despot, T.; Plavec, D. The Criterion Validity of a Newly Developed Ballroom Aerobic Test (BAT) Protocol Against Objective Methods. Sports 2025, 13, 337. https://doi.org/10.3390/sports13100337
Despot T, Plavec D. The Criterion Validity of a Newly Developed Ballroom Aerobic Test (BAT) Protocol Against Objective Methods. Sports. 2025; 13(10):337. https://doi.org/10.3390/sports13100337
Chicago/Turabian StyleDespot, Tamara, and Davor Plavec. 2025. "The Criterion Validity of a Newly Developed Ballroom Aerobic Test (BAT) Protocol Against Objective Methods" Sports 13, no. 10: 337. https://doi.org/10.3390/sports13100337
APA StyleDespot, T., & Plavec, D. (2025). The Criterion Validity of a Newly Developed Ballroom Aerobic Test (BAT) Protocol Against Objective Methods. Sports, 13(10), 337. https://doi.org/10.3390/sports13100337








