The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
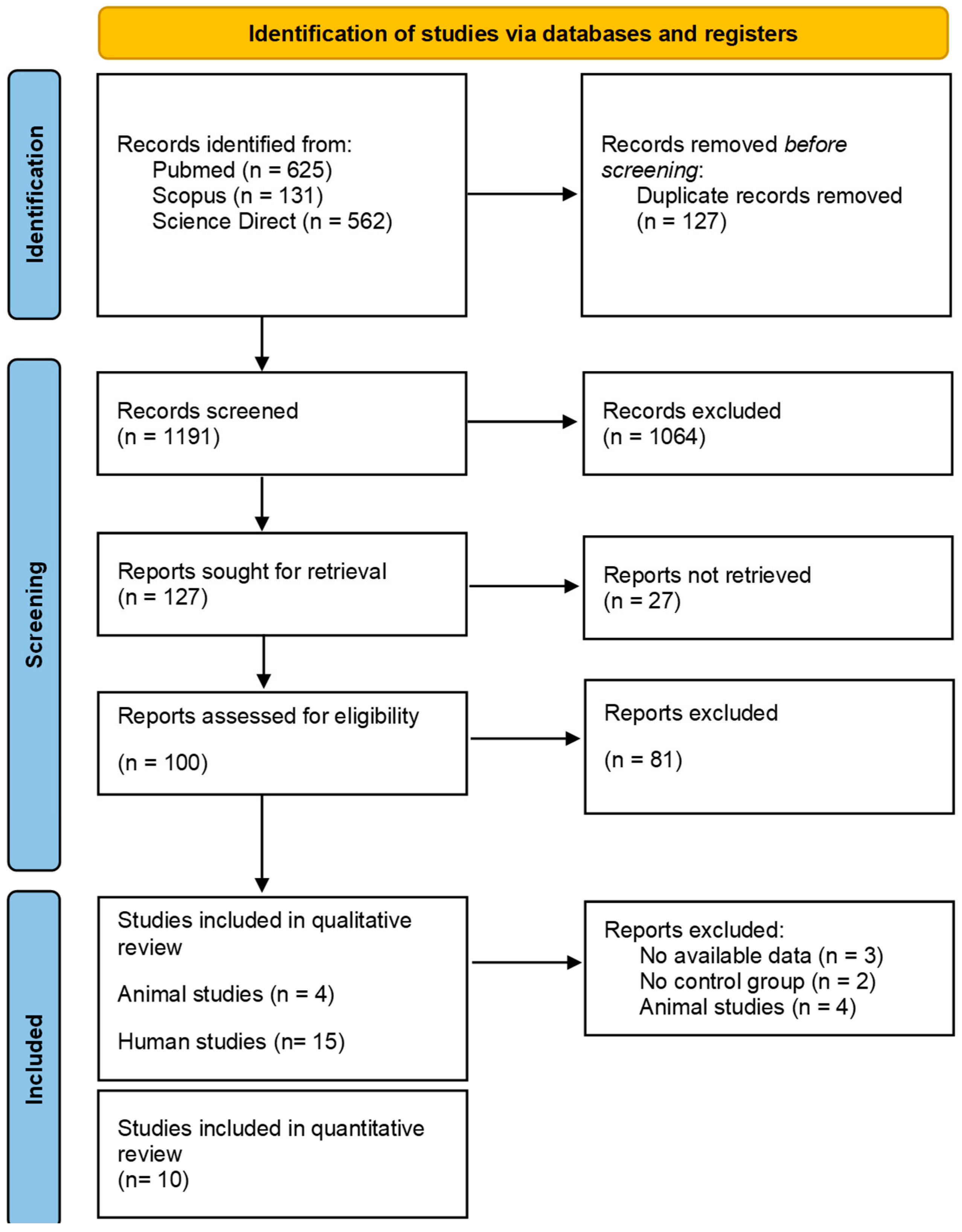
2.1. Selection Protocol and Search Strategy
2.2. Data Extraction Process and Quality Assessment
2.3. Data Synthesis
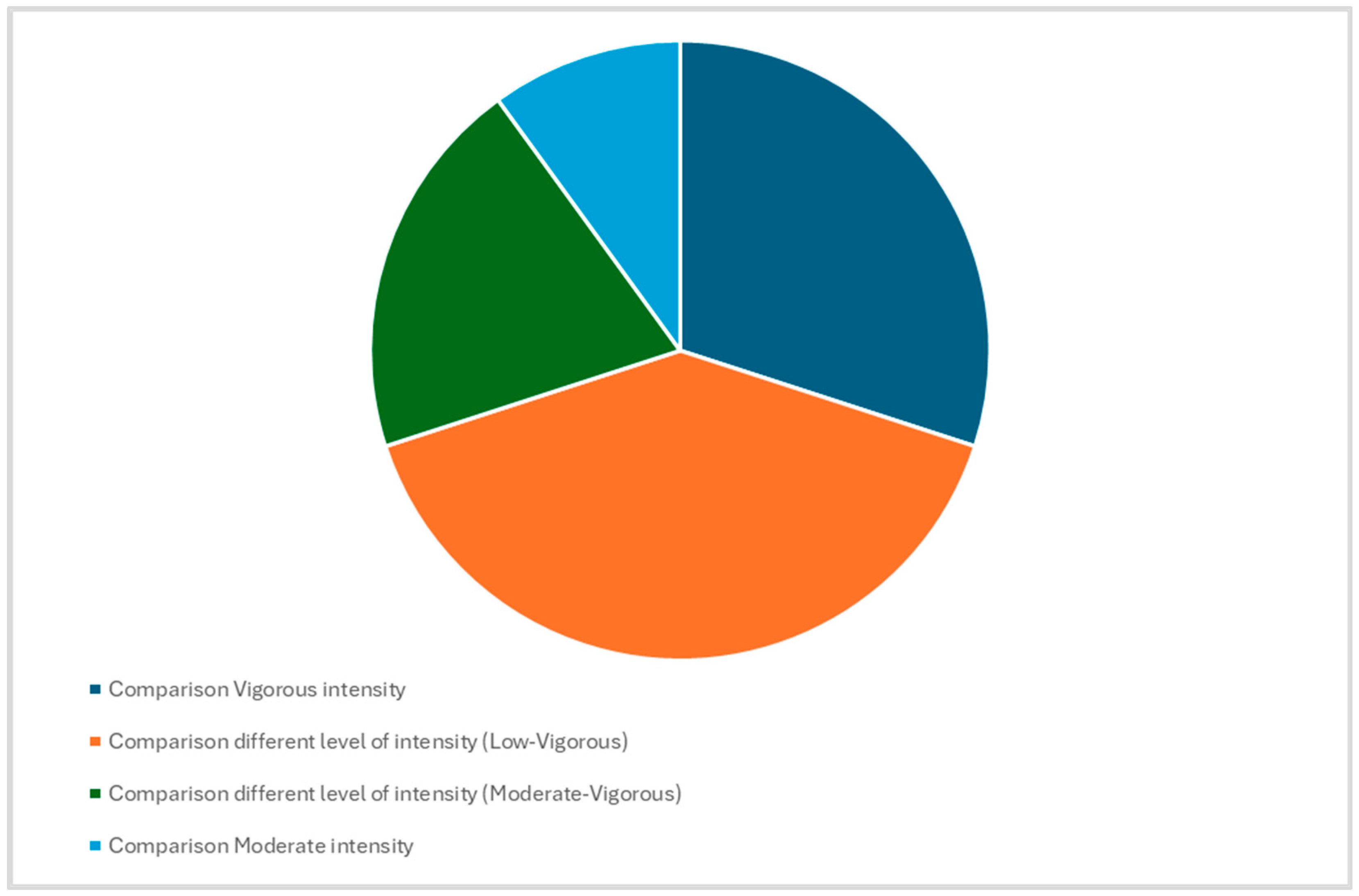
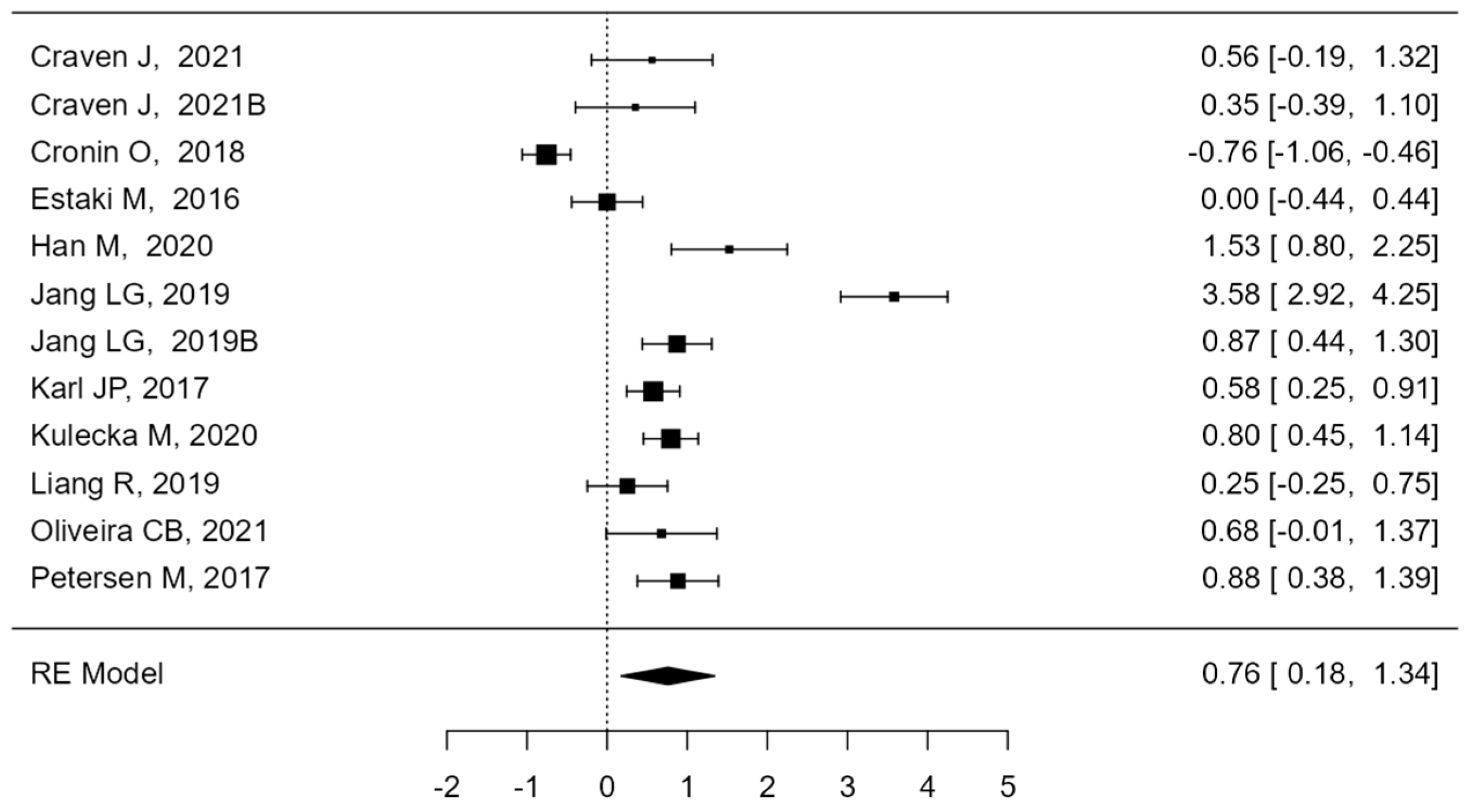
3. Results
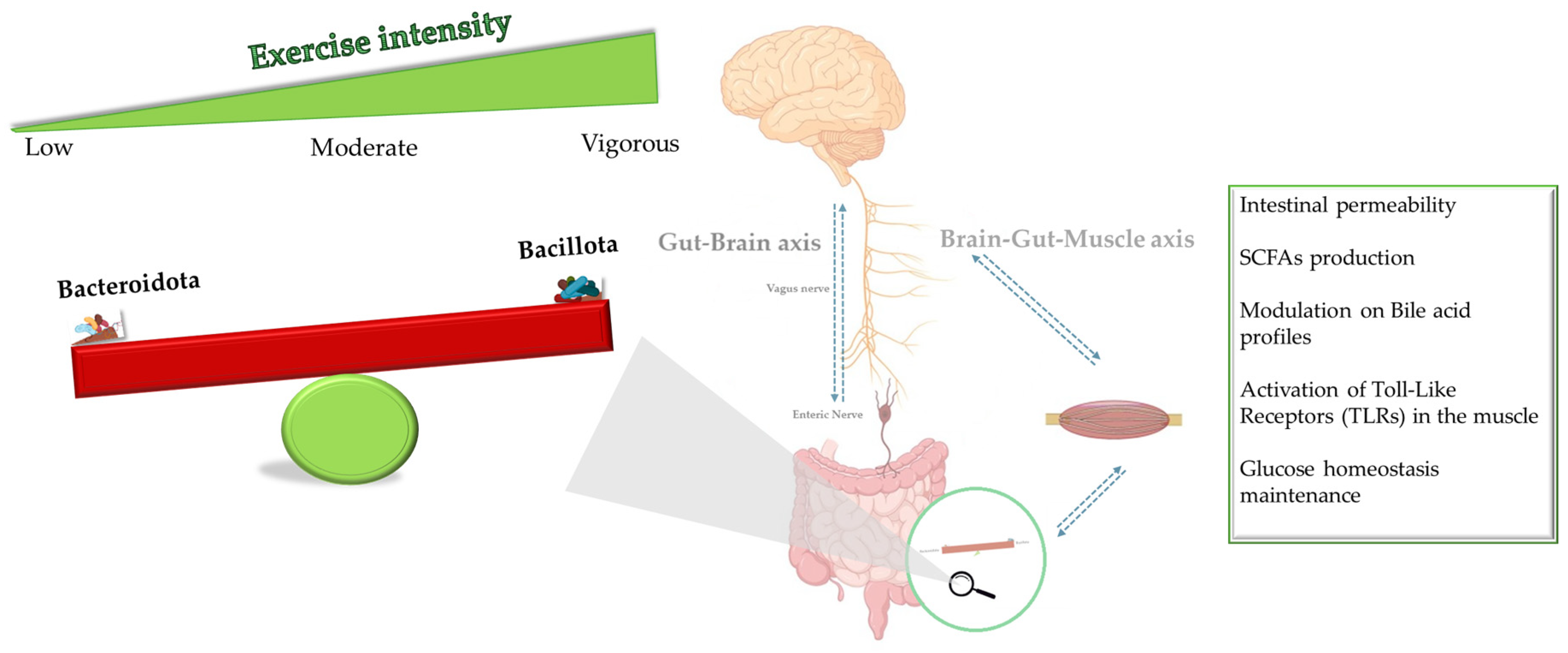
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.M.; Reimer, R.A. Unlocking a Novel Determinant of Athletic Performance: The Role of the Gut Microbiota, Short-Chain Fatty Acids, and “Biotics” in Exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Stulberg, E.; Fravel, D.; Proctor, L.M.; Murray, D.M.; LoTempio, J.; Chrisey, L.; Garland, J.; Goodwin, K.; Graber, J.; Harris, M.C.; et al. An Assessment of US Microbiome Research. Nat. Microbiol. 2016, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Romano Spica, V.; Valeriani, F.; Orsini, M.; Clementi, M.E.; Seguella, L.; Gianfranceschi, G.; Liddo, R.D.; Sante, G.D.; Ubaldi, F.; Ria, F.; et al. S100B Affects Gut Microbiota Biodiversity. Int. J. Mol. Sci. 2023, 24, 2248. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Christensen, L.H. Personal Diet-Microbiota Interactions and Weight Loss. Proc. Nutr. Soc. 2022, 81, 243–254. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Ubaldi, F.; Spica, V.R.; Liguori, G.; Calimeri, S.; Bono, R.; Privitera, G.; Fabiani, L.; et al. Exploring the Association between Physical Activity and Gut Microbiota Composition: A Review of Current Evidence. Ann. Ig. Med. Prev. E Comunita 2019, 31, 582–589. [Google Scholar]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between Physical Activity and Changes in Intestinal Microbiota Composition: A Systematic Review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. Health Benefits of Physical Activity: A Systematic Review of Current Systematic Reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk–Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical Activity Induced Alterations of Gut Microbiota in Humans: A Systematic Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Díaz, J.; Jorquera-Aguilera, C.; Rodríguez-Rodríguez, A.; Rodríguez-Rodríguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Res. Public Health 2022, 19, 9518. [Google Scholar] [CrossRef]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C.; et al. A Microbiome-Dependent Gut–Brain Pathway Regulates Motivation for Exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef]
- Furber, M.J.W.; Young, G.R.; Holt, G.S.; Pyle, S.; Davison, G.; Roberts, M.G.; Roberts, J.D.; Howatson, G.; Smith, D.L. Gut Microbial Stability Is Associated with Greater Endurance Performance in Athletes Undertaking Dietary Periodization. mSystems 2022, 7, e00129-22. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef]
- Dorelli, B.; Gallè, F.; Vito, C.D.; Duranti, G.; Iachini, M.; Zaccarin, M.; Standoli, J.P.; Ceci, R.; Romano, F.; Liguori, G.; et al. Can Physical Activity Influence Human Gut Microbiota Composition Independently of Diet? A Systematic Review. Nutrients 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, F.; Gallè, F.; Cattaruzza, M.S.; Antinozzi, M.; Gianfranceschi, G.; Postiglione, N.; Spica, V.R.; Liguori, G. Are Nutrition and Physical Activity Associated with Gut Microbiota? A Pilot Study on a Sample of Healthy Young Adults. Ann. Ig. Med. Prev. E Comunita 2020, 32, 521–527. [Google Scholar]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Rankin, A.; O’Donavon, C.; Madigan, S.M.; O’Sullivan, O.; Cotter, P.D. ‘Microbes in Sport’-The Potential Role of the Gut Microbiota in Athlete Health and Performance. Br. J. Sports Med. 2017, 51, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Hsu, T.H.; Chiu, C.C.; Wang, Y.C.; Chen, T.H.; Chen, Y.H.; Lee, Y.P.; Hung, S.W.; Wu, C.P.; Chuang, H.L. Supplementation with Beef Extract Improves Exercise Performance and Reduces Post-Exercise Fatigue Independent of Gut Microbiota. Nutrients 2018, 10, 1740. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Covidence-Better Systematic Review Management. Available online: https://www.covidence.org/ (accessed on 1 August 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Quality Assessment—Systematic Reviews. 2021. Available online: https://guides.lib.unc.edu/systematic-reviews (accessed on 27 May 2024).
- The Newcastle-Ottawa Scale (Nos) for Assessing the Quality of Non-randomised Studies in Meta-Analyses. 2021. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 29 July 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Friede, T.; Koch, A.; Kuss, O.; Schlattmann, P.; Schwarzer, G.; Skipka, G. Methods for Evidence Synthesis in the Case of Very Few Studies. Res. Synth. Methods 2018, 9, 382–392. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; LWW: Philadelphia, PA, USA, 2021; Available online: https://www.acsm.org/education-resources/books (accessed on 1 August 2024).
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The Human Gut Microbiome of Athletes: Metagenomic and Metabolic Insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, S.; Lensu, S.; Nokia, M.; Vanhatalo, S.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Intrinsic Aerobic Capacity Governs the Associations between Gut Microbiota Composition and Fat Metabolism Age-Dependently in Rat Siblings. Physiol. Genom. 2017, 49, 733–746. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell. Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Mach, N.; Moroldo, M.; Rau, A.; Lecardonnel, J.; Moyec, L.L.; Robert, C.; Barrey, E. Understanding the Holobiont: Crosstalk Between Gut Microbiota and Mitochondria During Long Exercise in Horse. Front. Mol. Biosci. 2021, 8, 656204. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Hsu, Y.J.; Liao, C.C.; Ho, S.T.; Huang, C.C.; Huang, W.C. Physiological and Biochemical Effects of Intrinsically High and Low Exercise Capacities Through Multiomics Approaches. Front. Physiol. 2019, 10, 1201. [Google Scholar]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in Intestinal Microbiota Composition and Metabolism Coincide with Increased Intestinal Permeability in Young Adults under Prolonged Physiological Stress. Am. J. Physiol. Gastroin.-Test Liver Physiol. 2017, 312, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wiklund, P.; Tan, X.; Wu, N.; Zhang, X.; Tikkanen, O.; Zhang, C.; Munukka, E.; Cheng, S. The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients 2017, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Tabone, M.; Bressa, C.; García-Merino, J.A.; Moreno-Pérez, D.; Van, E.C.; Castelli, F.A.; Fenaille, F.; Larrosa, M. The Effect of Acute Moderate-Intensity Exercise on the Serum and Fecal Metabolomes and the Gut Microbiota of Cross-Country Endurance Athletes. Sci. Rep. 2021, 11, 3558. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Marques, C.; Abreu, R.; Figueiredo, P.; Calhau, C.; Brito, J.; Sousa, M. Gut Microbiota of Elite Female Football Players Is Not Altered during an Official International Tournament. Scand. J. Med. Sci. Sports 2022, 32, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.; Cox, A.J.; Bellinger, P.; Desbrow, B.; Irwin, C.; Buchan, J.; McCartney, D.; Sabapathy, S. The Influence of Exercise Training Volume Alterations on the Gut Microbiome in Highly Trained Middle-Distance Runners. Eur. J. Sport Sci. 2022, 22, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Moitinho-Silva, L.; Wegener, M.; May, S.; Schrinner, F.; Akhtar, A.; Boysen, T.J.; Schaeffer, E.; Hansen, C.; Schmidt, T.; Rühlemann, M.C.; et al. Short-Term Physical Exercise Impacts on the Human Holobiont Obtained by a Randomised Intervention Study. BMC Microbiol. 2021, 21, 162. [Google Scholar] [CrossRef]
- Han, M.; Yang, K.; Yang, P.; Zhong, C.; Chen, C.; Wang, S.; Lu, Q.; Ning, K. Stratification of Athletes’ Gut Microbiota: The Multifaceted Hubs Associated with Dietary Factors, Physical Characteristics and Performance. Gut Microbes 2020, 12, 1842991. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, S.; Peng, X.; Yang, W.; Xu, Y.; Wu, P.; Chen, J.; Cai, Y.; Zhou, J. Characteristics of the Gut Microbiota in Professional Martial Arts Athletes: A Comparison between Different Competition Levels. PLoS ONE 2019, 14, e0226240. [Google Scholar] [CrossRef]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef]
- Kulecka, M.; Fraczek, B.; Mikula, M.; Zeber-Lubecka, N.; Karczmarski, J.; Paziewska, A.; Ambrozkiewicz, F.; Jagusztyn-Krynicka, K.; Cieszczyk, P.; Ostrowski, J. The Composition and Richness of the Gut Microbiota Differentiate the Top Polish Endurance Athletes from Sedentary Controls. Gut Microbes 2020, 11, 1374–1384. [Google Scholar] [CrossRef]
- Rettedal, E.A.; Cree, J.M.E.; Adams, S.E.; MacRae, C.; Skidmore, P.M.L.; Cameron-Smith, D.; Gant, N.; Blenkiron, C.; Merry, T.L. Short-Term High-Intensity Interval Training Exercise Does Not Affect Gut Bacterial Community Diversity or Composition of Lean and Overweight Men. Exp. Physiol. 2020, 105, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Jang, L.G.; Choi, G.; Kim, S.W.; Kim, B.Y.; Lee, S.; Park, H. The Combination of Sport and Sport-Specific Diet Is Associated with Characteristics of Gut Microbiota: An Observational Study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Cronin, O.; Garcia-Perez, I.; Whiston, R.; Holmes, E.; Woods, T.; Molloy, C.B.; Molloy, M.G.; Shanahan, F.; Cotter, P.D.; et al. The Effects of Sustained Fitness Improvement on the Gut Microbiome: A Longitudinal, Repeated Measures Case-Study Approach. Transl. Sports Med. 2021, 4, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Margolis, L.M.; Murphy, N.E.; McClung, H.L.; Martini, S.; Gundersen, Y.; Castellani, J.W.; Karl, J.P.; Teien, H.K.; Madslien, E.H.; et al. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol. Rep. 2016, 4, e12820. [Google Scholar] [CrossRef]
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut Microbiota Composition Is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef]
- Min, L.; Ablitip, A.; Wang, R.; Luciana, T.; Wei, M.; Ma, X. Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1070. [Google Scholar] [CrossRef]
- Álvarez-Herms, J.; Odriozola, A. Microbiome and physical activity. Adv. Genet. 2024, 111, 409–450. [Google Scholar]
- Mika, A.; Fleshner, M. Early-life exercise may promote lasting brain and metabolic health through gut bacterial metabolites. Immunol. Cell. Biol. 2016, 94, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, R.; Konstanti, P.; Smidt, H.; Hermus, A.; Thijssen, D.H.J.; Hopman, M.T.E. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country, Ref | Study Design | Subjects (Animals) | Sample Size | Age (Mean Value ± SD, Range/Age Group %) | Gender | Type of Sample | Type of Exercise | Duration, Frequency, Training Volume | Outcome | Quality Assessment (NOS Scale) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pekkala, 2017, Finland [42] | Cohort study | Mice | 20 | 7 weeks | Male and female | Fecal | Endurance exercise treadmill running | ND | Intrinsic aerobic capacity governs the microbiome, which may influence body weight, metabolism, and gene expression | 8 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Yang, 2021, China [44] | Cohort study | Mice | 30 | 5 weeks old | Male | Fecal | Moderate intensity treadmill exercise | 3 days a week, 10–20 min a day, slope 0, speed 10–13 mile per minute | Moderate-intensity treadmill exercise significantly increased the exercise capacity of mice and alters the core bacteria and bacterial metabolic activities | 6 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Mach, 2021, France [45] | Cohort study | Horse | 20 | 10 ± 1.69 years | Male and female | Fecal, Blood | Endurance exercises | 120 and 160 km race | Targeting the gut-mitochondria axis, therefore, appears to be a possible strategy to enhance athletic performance | 6 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Tung, 2019, Taiwan [46] | Cohort study | Mice | 100 | 7 weeks old | Male | Fecal | Exhaustive swimming | Exhaustive swimming test, with 5% body weight (BW) loading on the tail | The mice with various intrinsic exercise capacities have different gut microbiome as well as transcriptome and proteome of soleus muscle by applying multi-omics approaches. The main bacteria and controlling factors, including miRNA and functional proteins, may be too much correlated with the adoption of physiological functions and exercise capacity | 6 (Newcastle–Ottawa Quality Assessment Scale) Cohort Studies |
| Author, Year, Country | Study Design | Sample Size | Age (Mean Value ± SD, Range/Age Group %) | Gender | Type of Exercise | Duration, Frequency, Training Volume | Outcome | Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| Petersen, 2017, USA [43] | Cross-sectional study | 33 | 19–49 | Male and female | Cycling | All participants spent a minimum of 6 h exercising per week | No significant correlations were found between taxonomic cluster and being either a professional or amateur level cyclist, high abundance of the genus Prevotella (≥2.5%) was substantially correlated with time noted for exercising during an average week | 5 (Newcastle–Ottawa Quality Assessment Scale) Adapted for cross-sectional studies |
| Karl, 2017, USA [47] | RCT | 73 | >18 | Male and female | Fifty-one kilometer of cross-country ski-march | 4 days artic military training (51-km cross-country ski-march, during which volunteers skied in the 50:10-min work-to-rest ratios also carrying a ~45-kg pack) | Physiological stress is associated with the intestinal permeability, changes in gut microbiota by modifying diet and stress level can change intestinal permeability | 3 (Cochrane risk-of-bias tool for randomized trials) |
| Yang, 2017, China [48] | Cross-sectional study | 71 | 19–49 | Female | ND | 2-min warm-up at 50 W to access VO2 max | Cardiorespiratory fitness is associated with gut microbiota composition, independent of age and carbohydrate or fat intake | 6 (Newcastle–Ottawa Quality Assessment Scale) adapted for cross-sectional studies |
| Tabone,2021, France [49] | Cohort study | 40 | 18–50 | Male | Cross-country running | 10-min warm-up of continuous running on a treadmill at 60% of their maximum heart rate. After the warm-up, they ran with a slope of 1% at a speed of 10 km/h, with increase of 0.3 km per hour every 30 s until volitional exhaustion | The performance of a single exercise bout in cross-country non-professional athletes produces significant changes in the microbiota, in serum and fecal metabolome, which may have health implications | 8 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Oliveira, 2021, Portugal [50] | Cohort study | 17 | 18–25 | Female | Football | Perceived exertion method | The physical and physiological requirements of training and matches of an official international tournament did not vary the gut microbiota balance of elite female football players | 6 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Craven, 2021, Australia [51] | Cohort study | 14 | 18–25 | Male and female | Middle distance running | Three weeks of the normal training; 3 weeks of high-volume training (10, 20 and 30% increment in training volume during every successive week from NormTr); one-week taper (TaperTr; 55% exponential decrease in training volume from third HVolTr week) | The alpha-diversity and global formation of the gut microbiome were unaffected by differences in training volume. However, an increment in training volume led to various changes at the lower taxonomy levels that did not come back to pre-HVolTr levels following the taper period | 8 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Moitinho-Silva, 2021, Germany [52] | RCT | 42 | 20–45 | Male and female | Strength exercise, endurance exercise | Control group (general physical activity), Endurance group (at least 30 min of running three times per week), and Strength group (three days per week of whole-body hypertrophy strength training in the gym). The participants warmed up for five minutes on the treadmill, ergometer, or rowing machine before beginning their 30-min training session. One session included six distinct exercises, two for each leg, chest, and back, to develop the big and main muscle groups. The participants did one warm-up set (which was supposed to be 50% of the load set weight) and one load set for each activity. The weight for the load set was chosen so that eight repetitions were possible. If more than eight repetitions are doable on two consecutive training days. | Various types of exercise have different but balanced effects on the overall physiology of humans and very versatile microbial changes in the gut | 4 (Cochrane risk-of-bias tool for randomized trials) |
| Han, 2020, China [53] | Cohort study | 19 | 12–26 | Female | Rowing | NA | Gut microbial communities depend upon the type of exercise associated with dietary factors and physical characteristics | 7 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Liang, 2019, China [54] | Cross-sectional study | 31 | 20–24 | Male and female | Professional martial arts | Higher-level group: exercise load (hours/week): 29.25 ± 9.48; lower-level group: 16.63 ± 6.82 | The higher-level athletes had increased diversity and higher metabolic proportions of the gut microbiome for it may positively impact athletic performance | 7 (Newcastle–Ottawa Quality Assessment Scale) adapted for cross-sectional studies |
| Cronin, 2018, Ireland [55] | RCT | 90 | 18–40 | Male and female | Aerobic and resistance exercise training | Eight-week combined aerobic and resistance exercise training program | The improved body composition with exercise is not depending on basic changes in the diversity of microbial populations in the gut. The various microbial characteristics already observed in long-term habitual athletes may be a late response to exercise and fitness enhancement | 5 (Cochrane risk-of-bias tool for randomized trials) |
| Kulecka, 2020, Poland [56] | Cross-sectional study | 71 | 14–72 | Male and female | Marathon running; cross-country skiing | Highest level of training endurance sports athletes trained on average 1.58 ± 0.58 times per day, taking 7.25 ± 2.17 training units in one week lasting 2.79 ± 1.74 h per day on an average | Excessive training is involved with the changes in composition and elevation of increased bacterial diversity | 6 (Newcastle–Ottawa Quality Assessment Scale) adapted for cross-sectional studies |
| Rettedal,2020, New Zealand [57] | Cross-sectional study | 29 | 20–45 | Male | HIIT training | Three weeks of high-intensity training (8–12 × 60 s cycle ergometer bouts at VO2 maximum power output interspersed by 75 s rest, three times per week) | The gut microbiome’s overall composition is not significantly altered by short-term HIIT, however some microbiome genera are linked to insulin sensitivity measures, and HIIT enhanced these markers in overweight subjects | 5 (Newcastle–Ottawa Quality Assessment Scale) adapted for cross-sectional studies |
| Estaki, 2016, Canada [58] | Cross-sectional study | 39 | 18–35 | Male and female | Not specific | NA | Cardiorespiratory fitness is correlated with increased microbial diversity in healthy humans and that the associated changes are anchored around a set of functional cores rather than specific taxa | 6 (Newcastle–Ottawa Quality Assessment Scale) adapted for cross-sectional studies |
| Jang, 2019, Korea [59] | cross-sectional study | 45 | 25 ± 3 (bodybuilders); 20 ± 1 (distance runners) Healthy men 26 (±2) years | Male | Bodybuilding; distance running | NA | Athlete type was significantly associated with the relative abundance of gut microbiota at the genus and species level: Faecalibacterium, Sutterella, Clostridium, Haemophilus, and Eisenbergiella were increased (p < 0.05) in bodybuilders, while Bifidobacterium and Parasutterella were decreased (p < 0.05). At the species level, intestinal beneficial bacteria widely used as probiotics (Bifidobacterium adolescentis group, Bifidobacterium longum group, Lactobacillus sakei group) and those were generating the short-chain fatty acids (Blautia wexlerae, Eubacterium hallii) were decreased in bodybuilders and the increased in controls | 6 (Newcastle–Ottawa Quality Assessment Scale) adapted for cross-sectional studies |
| Barton, 2021, Ireland [60] | Cohort study | 2 | 31.5 | Male | Marathon and triathlon | Regular aerobic exercise complimented with twice weekly resistance training | Sustained fitness improvements support alterations to gut microbiota and physiologically relevant metabolites | 6 (Newcastle–Ottawa Quality Assessment Scale—Cohort Studies) |
| Author, Country, Year [Ref] | Biodiversity Indicators, Alpha Diversity | Biodiversity Indicators, Beta Diversity | B/B Ratio | Bacillota | Bacteroidota | Diversity | Comparison between Groups | Bacillota/Bacteroidota | Type of Exercise and Control | Classification of Exercise and Sport Intensity Based VO2 Max [38] and Details | Classification of Exercise and Sport Intensity Based METs Values [39] and Details | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | CON | PA | CON | PA | |||||||||
| Craven, 2021, Australia [51] | ↔Alpha-diversity (Shannon index, Chao1) | Microbial communities are similar | (A) CON = 1.3; PA = 0.7; (B) CON = 1.2; PA = 0.8 | High-volume training: ↓ Lachnoclostridium (p = 0.02), S. parasagunis (p = 0.02) ↑ R. callidus (p = 0.03) Lachnoclostridium (bacillota) 21% H. parainfluenzae (pseudomonadota) 0.5% S. parasanguinis (bacillota) 3% R. callidus (bacillota) 15% | NA | Only three types of Bacillota identified including Lachnoclostridium, S. parasanguinis and R. callidus. No bacteroidota present. Overall in Bacillota only R.callidus percentage increased after high volume and taper training as compared to normal training while Lachnoclostridium and S. parasanguinis percentage decreased in high volume and taper training | Faecal samples were obtained before and instantly after each training (three weeks of the normal training, three weeks of the high-volume training and a one-week taper training) | Pasteurellaceae (pseudomonadota) 0.5% Haemophilus (pseudomonadota) 0.5% Lachnoclostridium (bacillota) 21% H. parainfluenzae (pseudomonadota) 0.5% S. parasanguinis (bacillota) 3% R. callidus (bacillota) 15% | Middle distance running CON = Normal training; PA = Higher level training | (A) Vigorous (60–84% VO2R) (B) Vigorous (60–84% VO2R) + Normal Training volume | (A) Vigorous (60–84% VO2R) (B) Vigorous (60–84% VO2R) + Training volume increasing during the activity | (A) Vigorous > 6 METs; (B) Vigorous > 6 METs | (A) Vigorous > 6 METs; (B) Vigorous > 6 METs |
| Cronin, 2018, Ireland [55] | α-diversity Shannon index changes were non-significant (no α-diversity value provided) | Microbial communities are statistically different. Bray-Curtis’s dissimilarity β-diversity changes were non-significant (no β-diversity value provided) | CON = 0.02; PA = 0.0015 | Borrelia hermsii (Spirochaetota) 2.3% Mycoplasma pneumoniae (Tenericutes) 6.6%; Streptococcus thermophilus 0.002% | NA | In training group the Bacillota are Streptococcus thermophillus and Lactobacillus lactis. and in one training group Bacillota are only Streptococcus thermophillus | Fecal samples collected before and after 8 weeks intervention (exercise only group, exercise and protein supplement group and control group) | Only exercise group: Borrelia hermsii (Spirochaetota) 2.3%; Mycoplasma pneumoniae (Tenericutes) 6.6%; only protein group: Streptococcus thermophilus 0.002% | Aerobic and resistance exercise training CON = Sedentary; PA = exercise | Low (20–39% VO2R) | High (60–84% VO2R) | Vigorous > 6 METs (METs consumption related to daily life) | Vigorous > 6 METs |
| Estaki, 2016, Canada [58] | ↑ * Shannon, Chao1, Simpson ↑ Alpha diversity (Chao, Shannon, Simpson) | Microbial communities are statistically different. Beta diversity (Bray–Curtis) | CON = 1.4; PA = 0.53 | ↑ * Lachnospiraceae ↑ * Christensenellaceae, ↑ * Ruminococcaceae ↑ * Clostridiales.(p = 0.026), Roseburia, Lachnospiraceae, Erysipelotrichaceae | NA | Fecal samples were collected in healthy adults before and after one week of VO2 testing | Erysipelotrichaceae, Coprococcus, Roseburia, Adlercreutzia, and unknown members of Clostridiales, Lachnospiraceae, and Erysipelotrichaceae. | CON = lower level exercise; PA = higher level exercise | Low (20–39% VO2R) | High (60–84% VO2R) | Vigorous > 6 METs (METs consumption related to daily life) | Vigorous > 6 METs | |
| Han, 2020, China [53] | ↑ * Shannon, Simpson ↑ Alpha diversity (Shannon, Simpson) | Microbial communities are statistically different. Beta diversity (Jac card, Unifrac), ↑ B/B | CON = 2.31; PA = 3.89 | ↑ * Ruminococcaceae, Clostridiales, Faecalibacterium and Lachnospiraceae. | ↑ * | Bacillota included are Ruminococcaceae, Clostridiales, Faecalibacterium and Lachnospiraceae. | Fecal samples were collected from adult elite athlete’s (AE), youth elite athlete’s (YE), and youth non-elite athlete’s (YN) during two months period | The average relative abundances of Bacillota and Proteobacteria of AE (76.27% and 8.73%) and YE (64.7% and 10.69%) were higher than those of YN (58.12% and 8.01%), the moderate relative richness of Bacteroidota in YN (26.19%) was significantly increased than that of AE (11.41%) and YE (16.63%). Ruminococcaceae_unclassified, Clostridiales_unclassified, Faecalibacterium and Lachnospiraceae_unclassified were highest in the AE cohort, and Bacteroides and Prevotella were prominent in the YE cohort and YN cohort | Rowing CON = non elite athletes; PA = elite athletes | Moderate (40–59% VO2R) | Vigorous (60–84% VO2R) | Vigorous > 6 METs | Vigorous > 6 METs |
| Jang, 2019, Korea [59] | ↓ * Shannon, OTUs, Chao1 Diversity between groups: → Alpha diversity (Chao1) ≠ Beta diversity (PC) Alpha and beta diversity remains same in both groups | Microbial communities are statistically different | (A) CON = 4.79; PA = 15.69; (B) CON = 4.79; PA = 3.96 | Faecalibacterium, Sutterella, Clostridium, Haemophilus, and Eisenbergiella were increased in (p < 0.05) in bodybuilders, while Bifidobacterium and Parasutterella were the decreased (p < 0.05) | NA | The Bacillota compromised of Blautia wexlerae, callidus, Faecalibacterium_uc Faecalibacterium prausnitzii, Clostridium innocuum Eubacterium hallii, Ruminococcus, Weissella confusa, Lactobacillus sakei. The Bacteroidota compromised of Bacteroides stercoris and Bacteroides caccae | Fecal samples were obtained from sedentary men as control and from bodybuilders and distance runners | Faecalibacterium, Sutterella, Clostridium, Haemophilus, and Eisenbergiella were the highest (p < 0.05) in bodybuilders, while Bifidobacterium and Parasutterella were the lowest (p < 0.05). At the species level, intestinal beneficial bacteria widely used as probiotics (Bifidobacterium adolescentis group, Bifidobacterium longum group, Lactobacillus sakei group) and those producing short-chain fatty acids (Blautia wexlerae, Eubacterium hallii) were the lowest in bodybuilders and the highest in controls | Body building; distance running (A) CON = not exercise; PA = bodybuilding; (B) CON = not exercise; PA = distance running | (A) Low (20–39% VO2R) (B) Vigorous (60–84% VO2R) | (A) Vigorous (60–84% VO2R) (B) Vigorous (60–84% VO2R) | (A) light < 3 METs (B) Vigorous > 6 METs | (A) Vigorous > 6 METs (B) Vigorous > 6 METs |
| Karl, 2017, USA [47] | ↑ * Shannon, Chao1 ↑ Alpha diversity (Shannon) → Alpha-diversity (Chao1, OTU) ↑ B/B ratio | Bray-Curtis distances | CON = 1.28; PA = 1.86 | Pepto-streptococcus, Christensenella, Faecalibacterium, Staphylococcus, unassigned taxa within the Mogiobacteriaceae, Christensenellaceae, and Planococcaceae families. Verrucomicrobia *, TM7 *, Tenericutes *, Spirochaetes *, Proteobacteria, Lentisphaerae *, Fusobacteria *, Bacillota *, Euryarchaeota *, Cyanobacteria, Bacteroidota *, Actinobacteria | ↓ * | ↑ * | Fecal samples were collected from subjects the control group, the protein supplemented group and carbohydrate supplemented group two days before and one day after the stress exercises | Pepto-streptococcus, Christensenella, Faecalibacterium, Staphylococcus, unassigned taxa within the Mogiobacteriaceae, Christensenellaceae, and Planococcaceae families. Verrucomicrobia *, TM7 *, Tenericutes *, Spirochaetes *, Proteobacteria, Lentisphaerae *, Fusobacteria *, Bacillota *, Euryarchaeota *, Cyanobacteria, Bacteroidota *, Actinobacteria | Fifty-one kilometer of cross-country ski-march CON = pre-training; PA = post-training | Vigorous (60–84% VO2R) [61] | Vigorous (60–84% VO2R) [61] | Vigorous > 6 METs | Vigorous > 6 METs |
| Kulecka, 2020, Poland [56] | Chao1, Simpson (p = 0.025 & p = 0.00059) ↑ Alpha diversity (Shannon, Simpson, Chao1), ↑ B/B ratio | Microbial communities are statistically similar | CON = 1.83; PA = 10.04 | Bacteroides, Prevotella, Alistipes, Sutterella and Subdoligranulum, Ruminococcaceae family and Barnesiella genus, Bacillota; Clostridia; Clostridiales; Lachnospiraceae; Lachnoclostridium; Proteobacteria; Gammaproteobacteria; Pasteurellales; Pasteurellaceae; Haemophilus; Bacteroidia; Bacteroidales; Prevotellaceae; Prevotella_9 | Bacteroidota ↑ * Prevotella | Stools samples were obtained from the 14 marathon runners, 11 cross country skiers and the 46 inactive healthy controls | Bacteroides, Prevotella, Alistipes, Sutterella and Subdoligranulum, Ruminococcaceae family and Barnesiella genus, Bacillota; Clostridia; Clostridiales; Lachnospiraceae; Lachnoclostridium; Proteobacteria; Gammaproteobacteria; Pasteurellales; Pasteurellaceae; Haemophilus; Bacteroidia; Bacteroidales; Prevotellaceae; Prevotella_9 | Marathon running; cross-country skiing CON = lower level exercise; PA = higher level exercise | Low (20–39% VO2R) | Vigorous (60–84% VO2R) | (A) light < 3 METs | Vigorous > 6 METs | |
| Liang, 2019, China [54] | ↑ * Shannon (p = 0.019), Simpson(p = 0.001) | Microbial communities are statistically different | CON = 1.9; PA = 0.568 | ↑ * Parabacteroides (p < 0.001) Oscillibacter (p = 0.026 Bilophila (p = 0.036) Megasphaera (p = 0.04) Phascolarctobacterium (p = 0.028) | ↑ * Megasphaera (p = 0.04) | Veillonellaceae, Phascolarctobacterium, Oscillibacter and Megasphaera. The Bacteroidota are Porphyromonadaceae and Parabacteroides | Fecal samples were collected from 12 large scale and 16 small scale athletes | Higher level group: Porphyromonadaceae 4.4%; Veillonellaceae 0.9%; Parabacteroides 2.3%; Phascolarctobacterium 2.2%; Oscillibacter 0.7%; Megasphaera 0.008%; Bilophila 0.3%; Lower-level group: Porphyromonadaceae 2%; Veillonellaceae 4.5%; Parabacteroides 0.8%; Phascolarctobacterium 0.5%; Oscillibacter 0.3%; Megasphaera 0.069%; Bilophila 0.075% | Professional martial arts CON = Lower level group; PA = Higher level group | Moderate (40–59% VO2R) + Lower-level of Exercise load (hours/week) | Moderate (40–59% VO2R) + Higher level of Exercise load (hours/week) | Vigorous > 6 METs | Vigorous > 6 METs |
| Oliveira, 2021, Portugal [50] | ↑ * Shannon, Chao1, Simpson (p = 0.013) | Microbial communities are statistically different | CON = 1.78; PA = 1.677 | ↑ * Collinsella aerofaciens ↑ * Faecalibacterium prausnitzii | ↑ * Prevotella Copri (31%) | Bacillota included are Fecalibacterium and Bacteroidota included is Prevotella | Fecal samples were collected from subjects on 2nd, 3rd, 9th and 10th day of matches | At the beginning, 50% of bacteria were Bacillota (Frimicutes) followed by Bacteroidota (28%) and Actinobacteria (19%). Bacillota (Frimicutes) (52%) was the most prevalent type overall at the conclusion of the event followed by Bacteroidota (31%) and Actinobacteria (14%). At baseline, Faecalibacterium (29%) was the most prevalent bacterial genus followed by Collinsella (16%) and Prevotella (13%). At the conclusion of the competition Faecalibacterium was the most prevalent overall (29%) followed by Prevotella (17%) and Collinsella (12%). Faecalibacterium prausnitzii was the most prevalent by the end of the competition (30%) followed by Collinsella aerofaciens (13%) and Prevotella copri (12%) | Football CON = Baseline; PA = End of study | Moderate (40–59% VO2R) | Vigorous (60–84% VO2R) | Vigorous > 6 METs | Vigorous > 6 METs |
| Petersen, 2017, USA [43] | ↑ * Shannon (p = 0.0004), OTUs | Microbial communities are statistically different | CON = 2.05; PA = 2.33 | NA | ↑ * Prevotella | Fecal samples were obtained from 33 cyclists with extensive medical issues and no antibiotic use within the last year | NA | Cycling PA = professional level (the highest level) and CON = amateur level | Vigorous (60–84% VO2R) + lower level of Exercise load (h/week) | Vigorous (60–84% VO2R) + higher level of Exercise load (h/week) | Vigorous > 6 METs | Vigorous > 6 METs | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffar, T.; Ubaldi, F.; Volpini, V.; Valeriani, F.; Romano Spica, V. The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis. Sports 2024, 12, 221. https://doi.org/10.3390/sports12080221
Ghaffar T, Ubaldi F, Volpini V, Valeriani F, Romano Spica V. The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis. Sports. 2024; 12(8):221. https://doi.org/10.3390/sports12080221
Chicago/Turabian StyleGhaffar, Tehreema, Francesca Ubaldi, Veronica Volpini, Federica Valeriani, and Vincenzo Romano Spica. 2024. "The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis" Sports 12, no. 8: 221. https://doi.org/10.3390/sports12080221
APA StyleGhaffar, T., Ubaldi, F., Volpini, V., Valeriani, F., & Romano Spica, V. (2024). The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis. Sports, 12(8), 221. https://doi.org/10.3390/sports12080221