Predictive Validity of Multifactorial Injury Risk Models and Associated Clinical Measures in the U.S. Population
Abstract
1. Introduction
2. Materials and Methods
Case–Control
3. Results
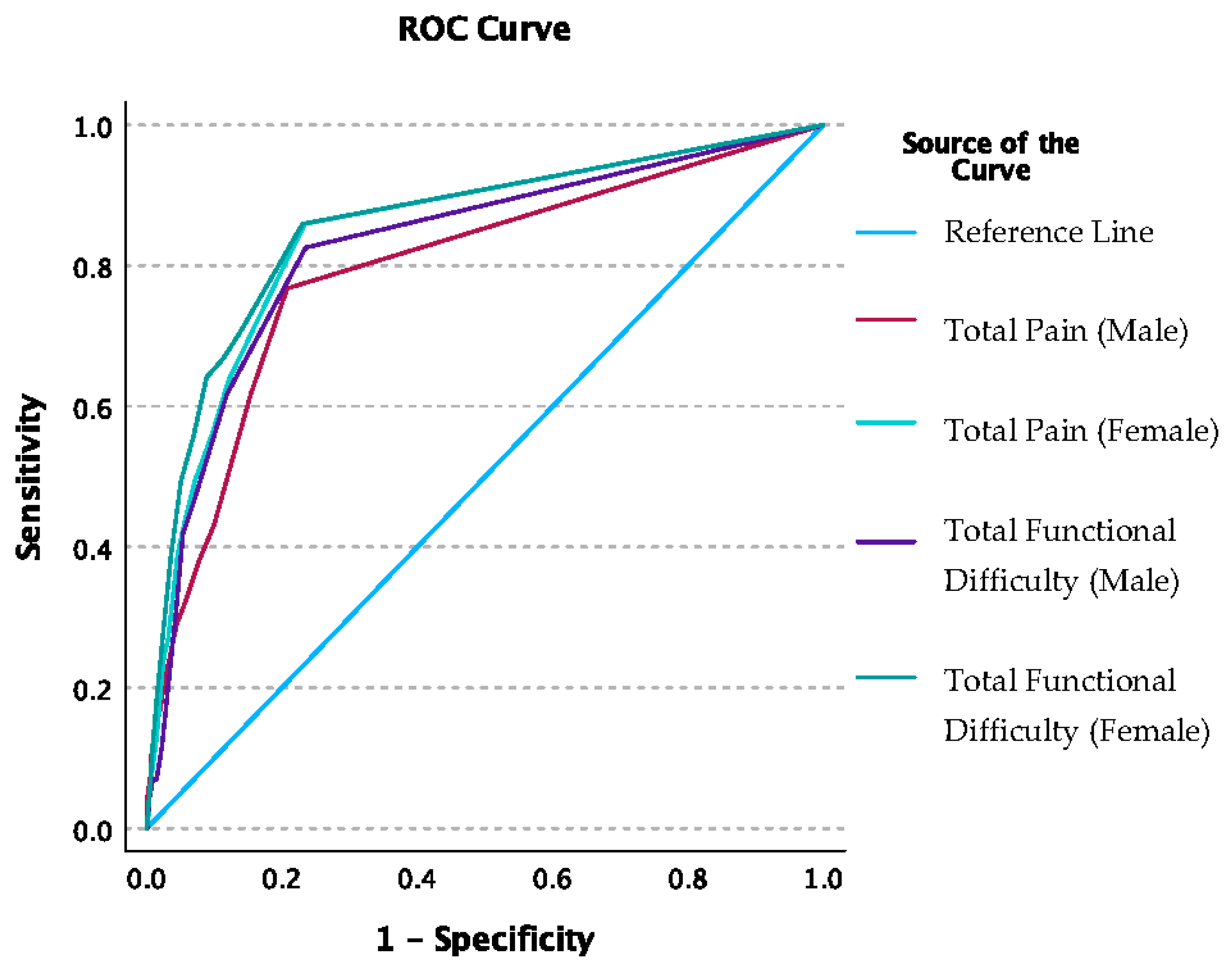
3.1. Injury Risk Models
3.2. Sensitivity Analysis
3.3. Case–Control Analysis
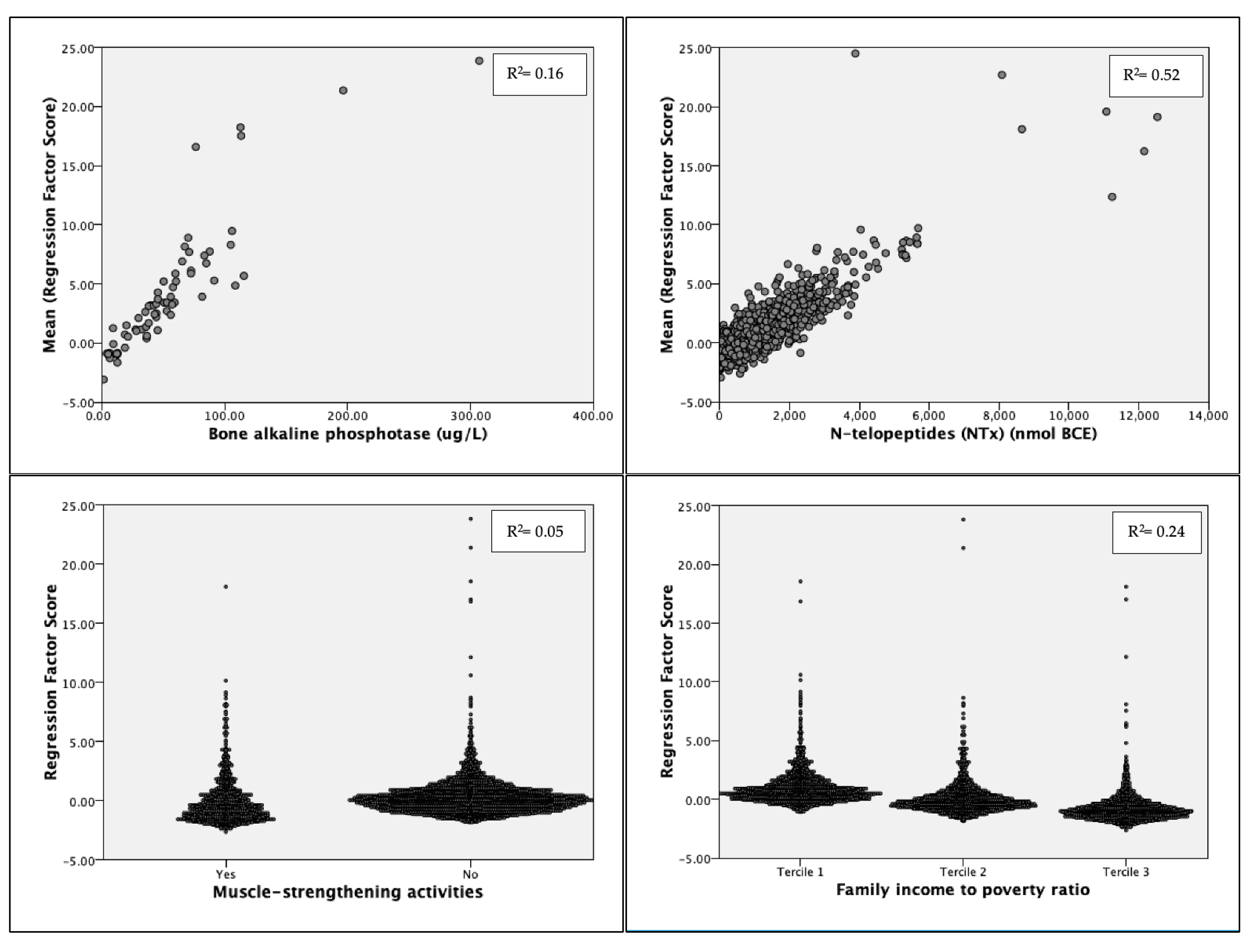
Principal Component Analysis
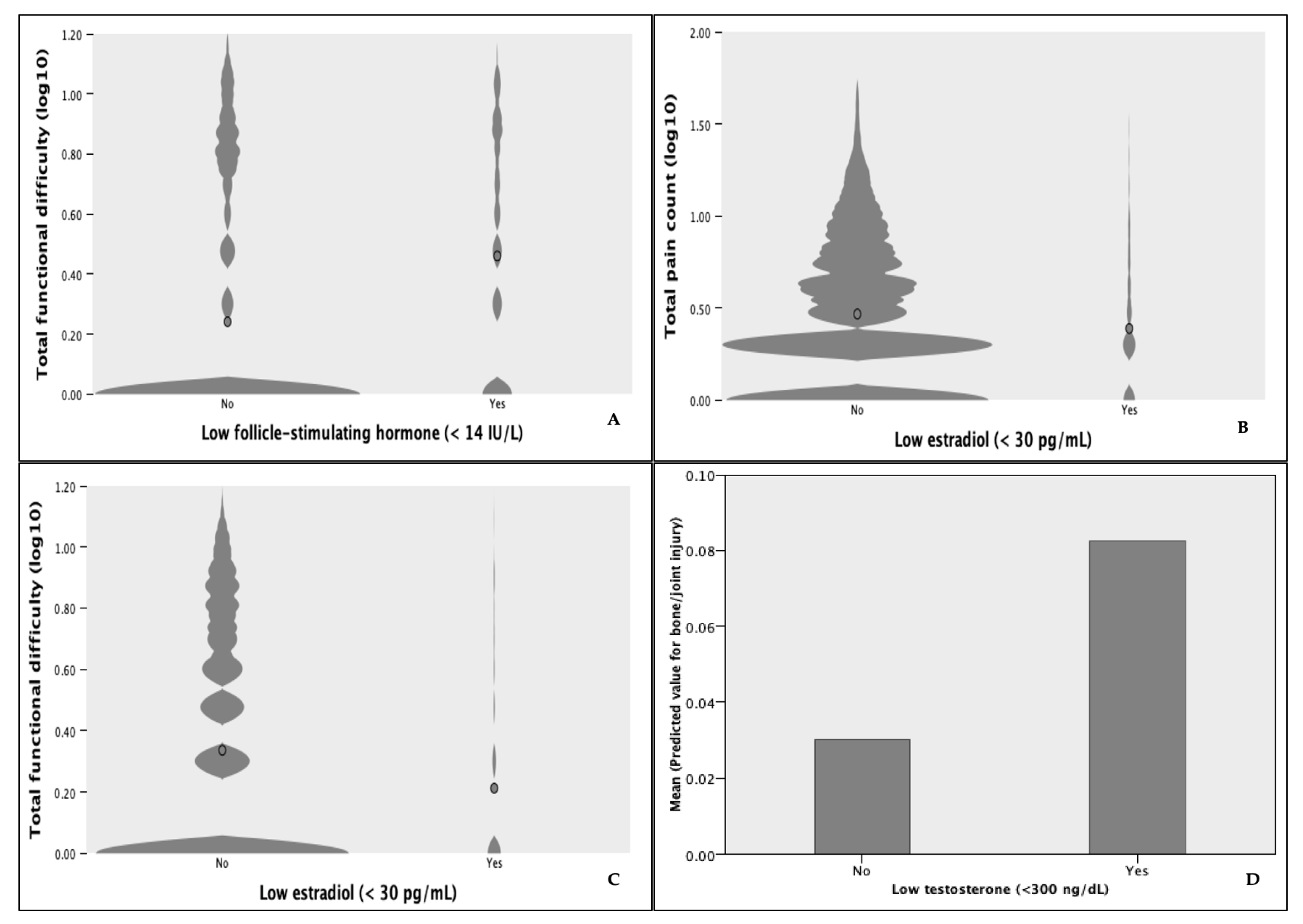
3.4. Post Hoc Analyses
4. Discussion
4.1. Limitations
4.2. Practical and Clinical Considerations
- Pain not due to recent injury is prevalent in the general population and among those with trouble during ADLs. Pain and inflammation are commonly indicative of current MSI but could be caused by previous injury, underlying disease processes, or a combination thereof. Our findings highlight the interdependent nature of chronic disease, pain, and MSIs and emphasize the need to differentiate the causes of pain, which should dictate the process of care. Furthermore, these relationships demonstrate the need for holistic approaches to MSI and chronic disease management.
- Identification of lifestyle risk factor clusters should be prioritized during routine clinical care given their strong associations with injuries, pain, and functional difficulties. Likewise, elevated risk due to demographic factors including veteran or socioeconomic status should be considered in injury prevention strategies. In practice, injury risk could be systematically assessed by capturing accessible information through intake questionnaires, which are commonly used within clinical and non-clinical settings.
- Total FD was found to be an independent predictor of bone/joint injury. Presumably, FD ratings vary according to subjective norms for physical functioning performance and reflect relative decreases in movement competence. This questions the validity of movement screens scored on movement pattern ideals. Moreover, idealized movement criteria would be rendered invalid for movement contexts in which the supposed ideal performance departs from movement strategies deemed functional by the individual. Ratings of FD during ADLs may be useful in building programs that incorporate individualized prescriptions; however, more research is needed to determine the usefulness of FD ratings in identifying high-risk groups.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collaborators, U. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008; U.S. Department of Health and Human Services: Washington, DC, USA, 2008.
- Bonazza, N.A.; Smuin, D.; Onks, C.A.; Silvis, M.L.; Dhawan, A. Reliability, validity, and injury predictive value of the functional movement screen: A systematic review and meta-analysis. Am. J. Sports Med. 2017, 45, 725–732. [Google Scholar] [CrossRef]
- Teyhen, D.; Bergeron, M.F.; Deuster, P.; Baumgartner, N.; Beutler, A.I.; de la Motte, S.J.; Jones, B.H.; Lisman, P.; Padua, D.A.; Pendergrass, T.L.; et al. Consortium for health and military performance and American College of Sports Medicine Summit: Utility of functional movement assessment in identifying musculoskeletal injury risk. Curr. Sports Med. Rep. 2014, 13, 52–63. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Lai, W.; Vail, J.; Rugg, C.M.; Hame, S.L. Predictive value of the functional movement screen for sports-related injury in NCAA division I athletes. Orthop. J. Sports Med. 2017, 5, 2325967117S00132. [Google Scholar] [CrossRef]
- O’connor, F.G.; Deuster, P.A.; Davis, J.; Pappas, C.G.; Knapik, J.J. Functional movement screening: Predicting injuries in officer candidates. Med. Sci. Sports Exerc. 2011, 43, 2224–2230. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Shaffer, S.W.; Butler, R.J.; Goffar, S.L.; Kiesel, K.B.; Rhon, D.I.; Williamson, J.N.; Plisky, P.J. What risk factors are associated with musculoskeletal injury in US Army Rangers? A prospective prognostic study. Clin. Orthop. Relat. Res. 2015, 473, 2948–2958. [Google Scholar] [CrossRef]
- Moore, E.; Chalmers, S.; Milanese, S.; Fuller, J.T. Factors influencing the relationship between the functional movement screen and injury risk in sporting populations: A systematic review and meta-analysis. Sports Med. 2019, 49, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Chimera, N.J.; Smith, C.A.; Warren, M. Injury history, sex, and performance on the functional movement screen and Y balance test. J. Athl. Train. 2015, 50, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.; Wright, K.; Kelly, M.; Zebrosky, B.; Zanis, M.; Drvol, C.; Butler, R. Injury risk is altered by previous injury: A systematic review of the literature and presentation of causative neuromuscular factors. Int. J. Sports Phys. Ther. 2014, 9, 583. [Google Scholar] [PubMed]
- Toohey, L.A.; Drew, M.K.; Cook, J.L.; Finch, C.F.; Gaida, J.E. Is subsequent lower limb injury associated with previous injury? A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, E.; Garvey, K. Sex differences in common sports-related injuries. NASN Sch. Nurse 2019, 34, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Uehli, K.; Mehta, A.J.; Miedinger, D.; Hug, K.; Schindler, C.; Holsboer-Trachsler, E.; Leuppi, J.D.; Künzli, N. Sleep problems and work injuries: A systematic review and meta-analysis. Sleep Med. Rev. 2014, 18, 61–73. [Google Scholar] [CrossRef]
- Fischer, D.; Lombardi, D.A.; Folkard, S.; Willetts, J.; Christiani, D.C. Updating the “Risk Index”: A systematic review and meta-analysis of occupational injuries and work schedule characteristics. Chronobiol. Int. 2017, 34, 1423–1438. [Google Scholar] [CrossRef]
- Dzakpasu, F.Q.S.; Carver, A.; Brakenridge, C.J.; Cicuttini, F.; Urquhart, D.M.; Owen, N.; Dunstan, D.W. Musculoskeletal pain and sedentary behaviour in occupational and non-occupational settings: A systematic review with meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 159. [Google Scholar] [CrossRef]
- Macedo, L.G.; Battié, M.C. The association between occupational loading and spine degeneration on imaging—A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 489. [Google Scholar] [CrossRef]
- Rhon, D.I.; Molloy, J.M.; Monnier, A.; Hando, B.R.; Newman, P.M. Much work remains to reach consensus on musculoskeletal injury risk in military service members: A systematic review with meta-analysis. Eur. J. Sport Sci. 2022, 22, 16–34. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Shaffer, S.W.; Goffar, S.L.; Kiesel, K.; Butler, R.J.; Rhon, D.I.; Plisky, P.J. Identification of risk factors prospectively associated with musculoskeletal injury in a warrior athlete population. Sports Health 2020, 12, 564–572. [Google Scholar] [CrossRef]
- Afifi, N.; Medhat, B.M.; Ghani, A.M.A.; Hassan, H.G.E.M.A.; E Behiry, M. Value of albumin-fibrinogen ratio and CRP-albumin ratio as predictor marker of disease activity in Egyptian RA patients, correlated with musculoskeletal sonography. Open Access Rheumatol. Res. Rev. 2020, 12, 241–248. [Google Scholar] [CrossRef]
- Hughes, J.M.; Smith, M.A.; Henning, P.C.; Scofield, D.E.; Spiering, B.A.; Staab, J.S.; Hydren, J.R.; Nindl, B.C.; Matheny, R.W. Bone formation is suppressed with multi-stressor military training. Eur. J. Appl. Physiol. 2014, 114, 2251–2259. [Google Scholar] [CrossRef]
- Roberts, H.M.; Law, R.J.; Thom, J.M. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. Eur. J. Appl. Physiol. 2019, 119, 2401–2420. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhang, Q.; Ge, B.; Zhang, J.; Yu, L.; Cai, T.; Zhang, Y.; Xiong, H. Relationship between Helicobacter pylori infection and osteoporosis: A systematic review and meta-analysis. BMJ Open. 2019, 9, e027356. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Conte, C.; Eastell, R.; Ewing, S.K.; Bauer, D.C.; Strotmeyer, E.S.; Black, D.M.; Samelson, E.J.; Vittinghoff, E.; Schwartz, A.V. Bone turnover markers do not predict fracture risk in type 2 diabetes. J. Bone Miner. Res. 2020, 35, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Zetterman, T.; Markkula, R.; Kalso, E. Elevated highly sensitive C-reactive protein in fibromyalgia associates with symptom severity. Rheumatol. Adv. Pract. 2022, 6, rkac053. [Google Scholar] [CrossRef] [PubMed]
- Alkassabi, O.; Voogt, L.; Andrews, P.; Alhowimel, A.; Nijs, J.; Alsobayel, H. Risk factors to persistent pain following musculoskeletal injuries: A systematic literature review. Int. J. Environ. Res. Public Health 2022, 19, 9318. [Google Scholar] [CrossRef]
- Hinojosa, R.; Hinojosa, M.S. Activity-limiting musculoskeletal conditions in US veterans compared to non-veterans: Results from the 2013 National Health Interview Survey. PLoS ONE 2016, 11, e0167143. [Google Scholar] [CrossRef]
- Briggs, M.S.; Givens, D.L.; Schmitt, L.C.; Taylor, C.A. Relations of C-reactive protein and obesity to the prevalence and the odds of reporting low back pain. Arch. Phys. Med. Rehabil. 2013, 94, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Feng, Z.; Liu, X.; Jia, G.; Geng, B.; Xia, Y. The saturation effect of body mass index on bone mineral density for people over 50 years old: A cross-sectional study of the US population. Front. Nutr. 2021, 8, 763677. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Huang, X.; Yu, X.; Li, Y.; Yu, F.; Zhou, W. Variation of Bone Turnover Markers in Childhood and Adolescence. Int. J. Clin. Pract. 2023, 2023, 5537182. [Google Scholar] [CrossRef]
- Nawai, A.; Foust, J.B.; Shi, L.; You, T.; Leveille, S.G. Is pain catastrophizing associated with poor mobility performance and falls in older adults? Arch. Gerontol. Geriatr. 2020, 91, 104219. [Google Scholar] [CrossRef]
- Kazman, J.B.; Galecki, J.M.; Lisman, P.; Deuster, P.A.; O’Connor, F.G. Factor structure of the functional movement screen in marine officer candidates. J. Strength Cond. Res. 2014, 28, 672–678. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Cirillo, D.J.; Eaton, C.B.; Stefanick, M.L.; Pettinger, M.; Carbone, L.D.; Johnson, K.C.; Simon, M.S.; Woods, N.F.; Wactawski-Wende, J. Estrogen alone and joint symptoms in the Women’s Health Initiative randomized trial. Menopause 2013, 20, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Testosterone and bone health in men: A narrative review. J. Clin. Med. 2021, 10, 530. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.D. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J. Bone Miner. Res. 2000, 15, 1526–1536. [Google Scholar] [CrossRef]
| Factors | Categories | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| BMI categories | Underweight | 0.075 * | 0.018 | 0.316 |
| Normal | ||||
| Overweight | 1.846 * | 1.252 | 2.722 | |
| Obese | 2.874 * | 2.088 | 3.955 | |
| Usually work 35 or more hours per week | 0.700 | 0.435 | 1.127 | |
| Avg level of physical activity each day | {you sit/he/she sits} during the day and {do/does} not walk about very much. | 3.053 * | 1.229 | 7.584 |
| {you stand or walk/he/she stands or walks} about a lot during the day, but {do/does} not have to carry or lift things very often. | 2.330 | 0.970 | 5.596 | |
| {you/he/she} lift(s) light load or {have/has} to climb stairs or hills often. | 1.539 | 0.715 | 3.314 | |
| {you/he/she} {do/does} heavy work or {carry/carries} heavy loads † | ||||
| Family PIR Tercile ‡ | 1.00 | 1.881 * | 1.226 | 2.885 |
| 2.00 | 1.134 | 0.793 | 1.622 | |
| 3.00 † | ||||
| Muscle-strengthening activities | None † | 0.563 * | 0.396 | 0.803 |
| Low back pain | None † | 2.552 * | 1.987 | 3.304 |
| Veteran/Military Status | No † | 1.520 * | 1.14 | 2.01 |
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Categories †/Units of Change | 95% Confidence Interval | 95% Confidence Interval | 95% Confidence Interval | ||||||
| Odds Ratio | Lower | Upper | Odds Ratio | Lower | Upper | Odds Ratio | Lower | Upper | ||
| Male sex | Female | 1.103 * | 1.020 | 1.193 | 0.965 | 0.827 | 1.125 | 0.801 * | 0.681 | 0.942 |
| Age group | 40–49 | 23.468 * | 17.547 | 31.387 | 12.342 * | 8.257 | 18.449 | 12.728 * | 8.163 | 19.848 |
| 50–59 | 31.080 * | 23.861 | 40.484 | 15.951 * | 11.235 | 22.646 | 20.663 * | 13.641 | 31.298 | |
| 60 and above | 25.645 * | 19.527 | 33.679 | 12.771 * | 8.408 | 19.398 | 16.484 * | 10.374 | 26.194 | |
| Veteran/Military Status | Yes | 2.135 * | 1.805 | 2.526 | 1.482 * | 1.215 | 1.808 | 1.582 * | 1.253 | 1.996 |
| Functional difficulties | 1.00 | 1.28 * | 1.245 | 1.31 | 1.30 * | 1.265 | 1.334 | 1.35 * | 1.31 | 1.390 |
| Family PIR Tercile | 1.00 | 0.599 * | 0.525 | 0.684 | 0.834 | 0.676 | 1.029 | 0.916 | 0.720 | 1.165 |
| 2.00 | 0.919 | 0.794 | 1.063 | 1.046 | 0.830 | 1.319 | 1.065 | 0.818 | 1.388 | |
| C-reactive protein (mg/dL) | 1.00 | 1.666 * | 1.454 | 1.909 | 1.390 * | 1.277 | 1.513 | 1.421 * | 1.289 | 1.566 |
| Fibrinogen (mg/dL) | 100.00 | 1.309 * | 1.180 | 1.451 | 1.61 * | 1.023 | 1.318 | 1.229 * | 1.070 | 1.411 |
| Bone alkaline phosphatase (ug/L) | 1.00 | 0.966 * | 0.963 | 0.969 | 0.973 * | 0.969 | 0.977 | 0.974 * | 0.969 | 0.978 |
| N-telopeptides (NTx) (nmol BCE) | 100.00 | 0.910 * | 0.897 | 0.924 | 0.922 * | 0.908 | 0.936 | 0.919 * | 0.902 | 0.938 |
| Helicobacter pylori (ISR) | 1.00 | 1.352 * | 1.281 | 1.427 | 1.252 * | 1.155 | 1.357 | 1.242 * | 1.108 | 1.393 |
| Age-Adjusted | BMI-Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Units of Change (Abs. Med. Diff.) | Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||
| Male | Female † | 0.869 | 0.639 | 1.183 | 0.983 | 0.658 | 1.467 | 0.916 | 0.638 | 1.314 |
| Age at screening | 24.00 | 3.545 * | 2.683 | 4.684 | 3.095 * | 2.26 | 4.24 | |||
| Body mass index (kg/m2) Change in BMI from 1 year ago | 4.50 4.50 | 1.528 * 0.943 | 1.291 0.775 | 1.81 1.147 | 1.36 * | 1.15 | 1.61 | |||
| Estimated VO2 max (ml/kg/min) | 2.13 | 0.961 | 0.896 | 1.030 | 0.983 | 0.90 | 1.076 | |||
| Total percent fat (DXA) | 5.70 | 1.348 * | 1.226 | 1.482 | 1.22 * | 1.11 | 1.348 | |||
| Total pain count | 4.00 | 2.403 * | 1.372 | 4.207 | 1.94 * | 1.51 | 3.72 | 2.17 * | 1.242 | 3.80 |
| Weeks of joint pain due to injury | 3.00 | 1.118 * | 1.052 | 1.188 | 1.17 * | 1.08 | 1.20 | 1.15 * | 1.06 | 1.26 |
| Total functional difficulties | 5.00 | 22.621 * | 11.517 | 44.431 | 13.16 * | 6.66 | 25.99 | 17.81 * | 9.05 | 35.04 |
| Bone mineral density (g/cm2) | 0.02 | 0.492 | 0.143 | 1.687 | 1.01 | 0.987 | 1.033 | 0.965 * | 0.944 | 0.986 |
| Bone alkaline phosphatase (ug/L) | 1.00 | 0.971 * | 0.956 | 0.986 | 0.986 | 0.969 | 1.003 | 0.973 * | 0.955 | 0.991 |
| C-reactive protein (mg/dL) | 0.12 | 1.024 | 0.968 | 1.084 | 1.003 | 0.967 | 1.041 | 1.003 | 0.978 | 1.028 |
| Fibrinogen (mg/dL) | 13.00 | 1.051 | 0.993 | 1.113 | 1.034 | 0.976 | 1.096 | 1.033 | 0.972 | 1.097 |
| Helicobacter pylori (ISR) ‡ | 0.15 | 1.043 | 1.00 | 1.088 | 1.02 | 0.975 | 1.07 | 1.033 | 0.990 | 1.078 |
| N-telopeptides (NTx) (nmol BCE) | 63.00 | 0.963 * | 0.941 | 0.985 | 0.986 | 0.964 | 1.01 | 0.963 * | 0.941 | 0.986 |
| Total factor count | 2.00 | 5.811 * | 4.286 | 7.877 | 3.907 * | 2.70 | 5.70 | 5.76 * | 4.02 | 8.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckart, A.C.; Ghimire, P.S.; Stavitz, J. Predictive Validity of Multifactorial Injury Risk Models and Associated Clinical Measures in the U.S. Population. Sports 2024, 12, 123. https://doi.org/10.3390/sports12050123
Eckart AC, Ghimire PS, Stavitz J. Predictive Validity of Multifactorial Injury Risk Models and Associated Clinical Measures in the U.S. Population. Sports. 2024; 12(5):123. https://doi.org/10.3390/sports12050123
Chicago/Turabian StyleEckart, Adam C., Pragya Sharma Ghimire, and James Stavitz. 2024. "Predictive Validity of Multifactorial Injury Risk Models and Associated Clinical Measures in the U.S. Population" Sports 12, no. 5: 123. https://doi.org/10.3390/sports12050123
APA StyleEckart, A. C., Ghimire, P. S., & Stavitz, J. (2024). Predictive Validity of Multifactorial Injury Risk Models and Associated Clinical Measures in the U.S. Population. Sports, 12(5), 123. https://doi.org/10.3390/sports12050123






