Exposures to Elevated Core Temperatures during Football Training: The Impact on Autonomic Nervous System Recovery and Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Procedures
2.3.1. Exercise Training Sessions
2.3.2. Measurements
2.3.3. Core Temperature
2.3.4. 24 h ANS Recovery and Function
2.3.5. Baseline HR
2.3.6. HR Recovery
2.3.7. HRV
2.4. Statistical Analysis
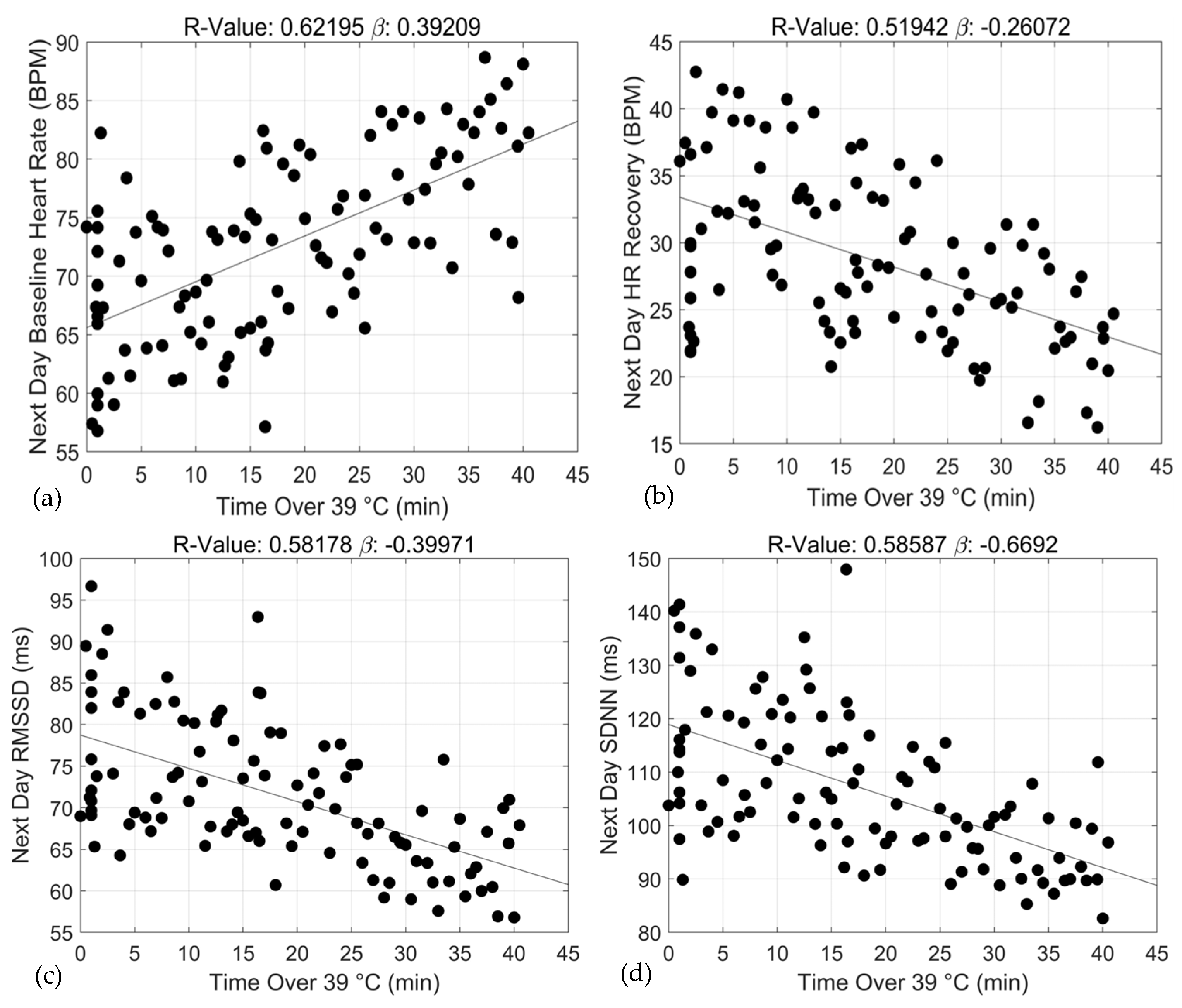
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J.; et al. Recovery and Performance in Sport: Consensus Statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Bompa, T.O. A Physiological Review of American Football. Sports Med. 1997, 23, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Fealey, R.D. Chapter 7—Interoception and Autonomic Nervous System Reflexes Thermoregulation. In Handbook of Clinical Neurology; Buijs, R.M., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 117, pp. 79–88. ISBN 0072-9752. [Google Scholar]
- Cramer, M.N.; Jay, O. Biophysical Aspects of Human Thermoregulation during Heat Stress. Auton. Neurosci. 2016, 196, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ftaiti, F.; Grélot, L.; Coudreuse, J.M.; Nicol, C.; Coudreuse, J.M. Combined Effect of Heat Stress, Dehydration and Exercise on Neuromuscular Function in Humans. Eur. J. Appl. Physiol. 2001, 84, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Johnson, E.C.; Casa, D.J.; Ganio, M.S.; McDermott, B.P.; Yamamoto, L.M.; Lopez, R.M.; Emmanuel, H. The American Football Uniform: Uncompensable Heat Stress and Hyperthermic Exhaustion. J. Athl. Train. 2010, 45, 117–127. [Google Scholar] [CrossRef]
- Krohn, A.R.; Sikka, R.; Olson, D.E. Heat Illness in Football: Current Concepts. Curr. Sports Med. Rep. 2015, 14, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yeargin, S.W.; Kerr, Z.Y.; Casa, D.J.; Djoko, A.; Hayden, R.; Parsons, J.T.; Dompier, T.P. Epidemiology of Exertional Heat Illnesses in Youth, High School, and College Football. Med. Sci. Sports Exerc. 2016, 48, 1523–1529. [Google Scholar] [CrossRef]
- Kay, D.; Marino, F.E. Fluid Ingestion and Exercise Hyperthermia: Implications for Performance, Thermoregulation, Metabolism and the Development of Fatigue. J. Sports Sci. 2000, 18, 71–82. [Google Scholar] [CrossRef]
- Nybo, L.; Nielsen, B. Hyperthermia and Central Fatigue during Prolonged Exercise in Humans. J. Appl. Physiol. 2001, 91, 1055–1060. [Google Scholar] [CrossRef]
- Beal, H.; Corbett, J.; Davis, D.; Barwood, M.J. Marathon Performance and Pacing in the Doha 2019 Women’s IAAF World Championships: Extreme Heat, Suboptimal Pacing, and High Failure Rates. Int. J. Sports Physiol. Perform. 2022, 17, 1119–1125. [Google Scholar] [CrossRef]
- Racinais, S.; Nichols, D.; Travers, G.; Moussay, S.; Belfekih, T.; Farooq, A.; Schumacher, Y.O.; Périard, J.D. Health Status, Heat Preparation Strategies and Medical Events among Elite Cyclists Who Competed in the Heat at the 2016 UCI Road World Cycling Championships in Qatar. Br. J. Sports Med. 2020, 54, 1003. [Google Scholar] [CrossRef] [PubMed]
- Grundstein, A.J.; Hosokawa, Y.; Casa, D.J. Fatal Exertional Heat Stroke and American Football Players: The Need for Regional Heat-Safety Guidelines. J. Athl. Train. 2018, 53, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Eichner, E.R. The Heat Is On: Exertional Heatstroke in Football. Curr. Sports Med. Rep. 2021, 20, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Eichner, E.R. Fatal Exertional Heat Stroke in Football: The Coaches Are the Culprits. Curr. Sports Med. Rep. 2019, 18, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.K.; Baker, L.B.; Barnes, K.; Ungaro, C.; Stofan, J. Thermoregulation, Fluid Balance, and Sweat Losses in American Football Players. Sports Med. 2016, 46, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Launstein, E.D.; Miller, K.C.; O’Connor, P.; Adams, W.M.; Abrego, M.L. American Football Uniforms Elicit Thermoregulatory Failure during a Heat Tolerance Test. Temperature 2021, 8, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.; Wishon, M.J.; Wittels, H.; Lee, S.J.; Hendricks, S.; Davila, H.; Wittels, S.H. Single Limb Electrocardiogram Using Vector Mapping: Evaluation and Validation of a Novel Medical Device. J. Electrocardiol. 2021, 67, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wittels, S.H.; Renaghan, E.; Wishon, M.J.; Wittels, H.L.; Chong, S.; Wittels, E.D.; Hendricks, S.; Hecocks, D.; Bellamy, K.; Girardi, J.; et al. Recovery of the Autonomic Nervous System Following Football Training among Division I Collegiate Football Athletes: The Influence of Intensity and Time. Heliyon 2023, 9, e18125. [Google Scholar] [CrossRef]
- Renaghan, E.; Wittels, H.L.; Feigenbaum, L.A.; Wishon, M.J.; Chong, S.; Wittels, E.D.; Hendricks, S.; Hecocks, D.; Bellamy, K.; Girardi, J.; et al. Exercise Cardiac Load and Autonomic Nervous System Recovery during In-Season Training: The Impact on Speed Deterioration in American Football Athletes. J. Funct. Morphol. Kinesiol. 2023, 8, 134. [Google Scholar] [CrossRef]
- Wittels, S.H.; Renaghan, E.; Wishon, M.J.; Wittels, H.L.; Chong, S.; Wittels, E.D.; Hendricks, S.; Hecocks, D.; Bellamy, K.; Girardi, J.; et al. A Novel Metric “Exercise Cardiac Load” Proposed to Track and Predict the Deterioration of the Autonomic Nervous System in Division I Football Athletes. J. Funct. Morphol. Kinesiol. 2023, 8, 143. [Google Scholar] [CrossRef]
- Gómez-Romero, F.J.; Fernández-Prada, M.; Fernández-Suárez, F.E.; Gutiérrez-González, C.; Estrada-Martínez, M.; Cachero-Martínez, D.; Suárez-Fernández, S.; García-González, N.; Picatto-Hernández, M.D.; Martínez-Ortega, C.; et al. Intra-Operative Temperature Monitoring with Two Non-Invasive Devices (3M Spoton® and Dräger Tcore®) in Comparison with the Swan-Ganz Catheter. Cirugía Cardiovasc. 2019, 26, 191–196. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative Temperature Monitoring. Anesthesiology 2021, 134, 111–118. [Google Scholar] [CrossRef]
- Speed, C.; Arneil, T.; Harle, R.; Wilson, A.; Karthikesalingam, A.; McConnell, M.; Phillips, J. Measure by Measure: Resting Heart Rate across the 24-Hour Cycle. PLoS Digit. Health 2023, 2, e0000236. [Google Scholar] [CrossRef] [PubMed]
- Nandi, P.S.; Spodick, D.H. Recovery from Exercise at Varying Work Loads. Time Course of Responses of Heart Rate and Systolic Intervals. Br. Heart J. 1977, 39, 958–966. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology; North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Reilly, T.; Ekblom, B. The Use of Recovery Methods Post-exercise. J. Sports Sci. 2005, 23, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Altarriba-Bartes, A.; Pena, J.; Vicens-Bordas, J.; Mila-Villaroel, R.; Calleja-Gonzalez, J. Post-Competition Recovery Strategies in Elite Male Soccer Players. Effects on Performance: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0240135. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Hanani, M. The Actions of Hyperthermia on the Autonomic Nervous System: Central and Peripheral Mechanisms and Clinical Implications. Auton. Neurosci. 2012, 168, 4–13. [Google Scholar] [CrossRef]
- Stamatis, A.; Magnusen, M. Nontraumatic Injuries in the NCAA: Collegiate Football Strength Coaches Should Exercise Caution This Off-Season. Int. J. Exerc. Sci. 2021, 14, 980–983. [Google Scholar]
- Mtibaa, K.; Thomson, A.; Nichols, D.; Hautier, C.; Racinais, S. Hyperthermia-Induced Neural Alterations Impair Proprioception and Balance. Med. Sci. Sports Exerc. 2018, 50, 46–53. [Google Scholar] [CrossRef]
- Nybo, L.; Rasmussen, P.; Sawka, M.N. Performance in the Heat-Physiological Factors of Importance for Hyperthermia-Induced Fatigue. Compr. Physiol. 2014, 4, 657–689. [Google Scholar] [PubMed]
- Periard, J.D.; Racinais, S. Performance and Pacing during Cycle Exercise in Hyperthermic and Hypoxic Conditions. Med. Sci. Sports Exerc. 2016, 48, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Nadel, E.R.; Cafarelli, E.; Roberts, M.F.; Wenger, C.B. Circulatory Regulation during Exercise in Different Ambient Temperatures. J. Appl. Physiol. 1979, 46, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Wimer, G.S.; Culver, M.N.; Flatt, A.A.; Grosicki, G.J. Fan Cooling Improves Submaximal Exercise Capacity in an Indoor Thermoneutral Environment. Res. Quart. Exerc. Sport. 2023, 94, 124–130. [Google Scholar] [CrossRef]
- Bradley, L.J.; Miller, K.C.; Wiese, B.W.; Novak, J.R. Precooling’s Effect on American Football Skills. J. Strength. Cond. Res. 2019, 33, 2616–2621. [Google Scholar] [CrossRef]




| Mean (SD) | Median (Min, Max) | |
|---|---|---|
| No. of Training Sessions | 128 | ----- |
| Duration of Sessions (min) | 161.1 (40.6) | 157.1 (90.1, 339.6) |
| Duration (min) Under Temperature Thresholds | ||
| ≥37 °C | 30.4 (35.9) | 18.0 (0.0, 121.5) |
| ≥38 °C | 19.5 (21.5) | 15.0 (0.0, 81.0) |
| ≥39 °C | 10.5 (12.9) | 2.8 (0.0, 40.5) |
| 24 h ANS Recovery and Function | ||
| Baseline HR (bpm) | 61.4 (8.6) | 60.1 (44.8, 118.2) |
| 30 s HR Recovery (bpm) | 30.6 (6.0) | 31.0 (11.2, 49.6 |
| rMSSD (ms) | 72.0 (7.0) | 72.5 (55.4, 93.2) |
| SDNN (ms) | 106.7 (12.2) | 107.6 (81.1, 141.8) |
| Slope (β) | SE | Adjusted R2 | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Baseline HR (bpm) | |||||
| Duration Under Temperature Thresholds | |||||
| ≥37 °C | 0.01 | 0.01 | 0.03 | (−0.02, 0.02) | 0.698 |
| ≥38 °C | 0.10 | 0.02 | 0.26 | (0.06, 0.14) | p < 0.0000 |
| ≥39 °C | 0.39 | 0.05 | 0.62 | (0.30, 4.49) | p < 0.0000 |
| HR Recovery (bpm) | |||||
| Duration Under Temperature Thresholds | |||||
| ≥37 °C | 0.003 | 0.01 | 0.02 | (−0.01, 0.02) | 0.609 |
| ≥38 °C | −0.06 | 0.02 | 0.21 | (−0.10, −0.03) | 0.0002 |
| ≥39 °C | −0.26 | 0.04 | 0.52 | (−0.34, −0.18) | p < 0.0000 |
| Slope (β) | SE | Adjusted R2 | 95% CI | p-Value | |
|---|---|---|---|---|---|
| rMSSD | |||||
| Duration Under Temperature Thresholds | |||||
| ≥37 °C | −0.01 | 0.01 | 0.04 | −0.03, 0.02 | 0.635 |
| ≥38 °C | −0.11 | 0.02 | 0.24 | −0.15, −0.06 | p < 0.0000 |
| ≥39 °C | −0.37 | 0.05 | 0.58 | −0.47, −0.26 | p < 0.0000 |
| SDNN | |||||
| Duration Under Temperature Thresholds | |||||
| ≥37 °C | −0.01 | 0.02 | 0.027 | −0.05, 0.03 | 0.529 |
| ≥38 °C | −0.16 | 0.04 | 0.22 | −0.24, −0.08 | p < 0.0000 |
| ≥39 °C | −0.67 | 0.09 | 0.58 | −0.85, −0.49 | p < 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renaghan, E.; Wittels, H.L.; Feigenbaum, L.A.; Wishon, M.J.; Chong, S.; Wittels, E.D.; Hendricks, S.; Hecocks, D.; Bellamy, K.; Girardi, J.; et al. Exposures to Elevated Core Temperatures during Football Training: The Impact on Autonomic Nervous System Recovery and Function. Sports 2024, 12, 8. https://doi.org/10.3390/sports12010008
Renaghan E, Wittels HL, Feigenbaum LA, Wishon MJ, Chong S, Wittels ED, Hendricks S, Hecocks D, Bellamy K, Girardi J, et al. Exposures to Elevated Core Temperatures during Football Training: The Impact on Autonomic Nervous System Recovery and Function. Sports. 2024; 12(1):8. https://doi.org/10.3390/sports12010008
Chicago/Turabian StyleRenaghan, Eric, Harrison L. Wittels, Luis A. Feigenbaum, Michael J. Wishon, Stephanie Chong, Eva D. Wittels, Stephanie Hendricks, Dustin Hecocks, Kyle Bellamy, Joe Girardi, and et al. 2024. "Exposures to Elevated Core Temperatures during Football Training: The Impact on Autonomic Nervous System Recovery and Function" Sports 12, no. 1: 8. https://doi.org/10.3390/sports12010008
APA StyleRenaghan, E., Wittels, H. L., Feigenbaum, L. A., Wishon, M. J., Chong, S., Wittels, E. D., Hendricks, S., Hecocks, D., Bellamy, K., Girardi, J., Lee, S., Vo, T., McDonald, S. M., & Wittels, S. H. (2024). Exposures to Elevated Core Temperatures during Football Training: The Impact on Autonomic Nervous System Recovery and Function. Sports, 12(1), 8. https://doi.org/10.3390/sports12010008






