Functional and Morphological Adaptations in the Heart of Children Aged 12–14 Years following Two Different Endurance Training Protocols
Abstract
:1. Introduction
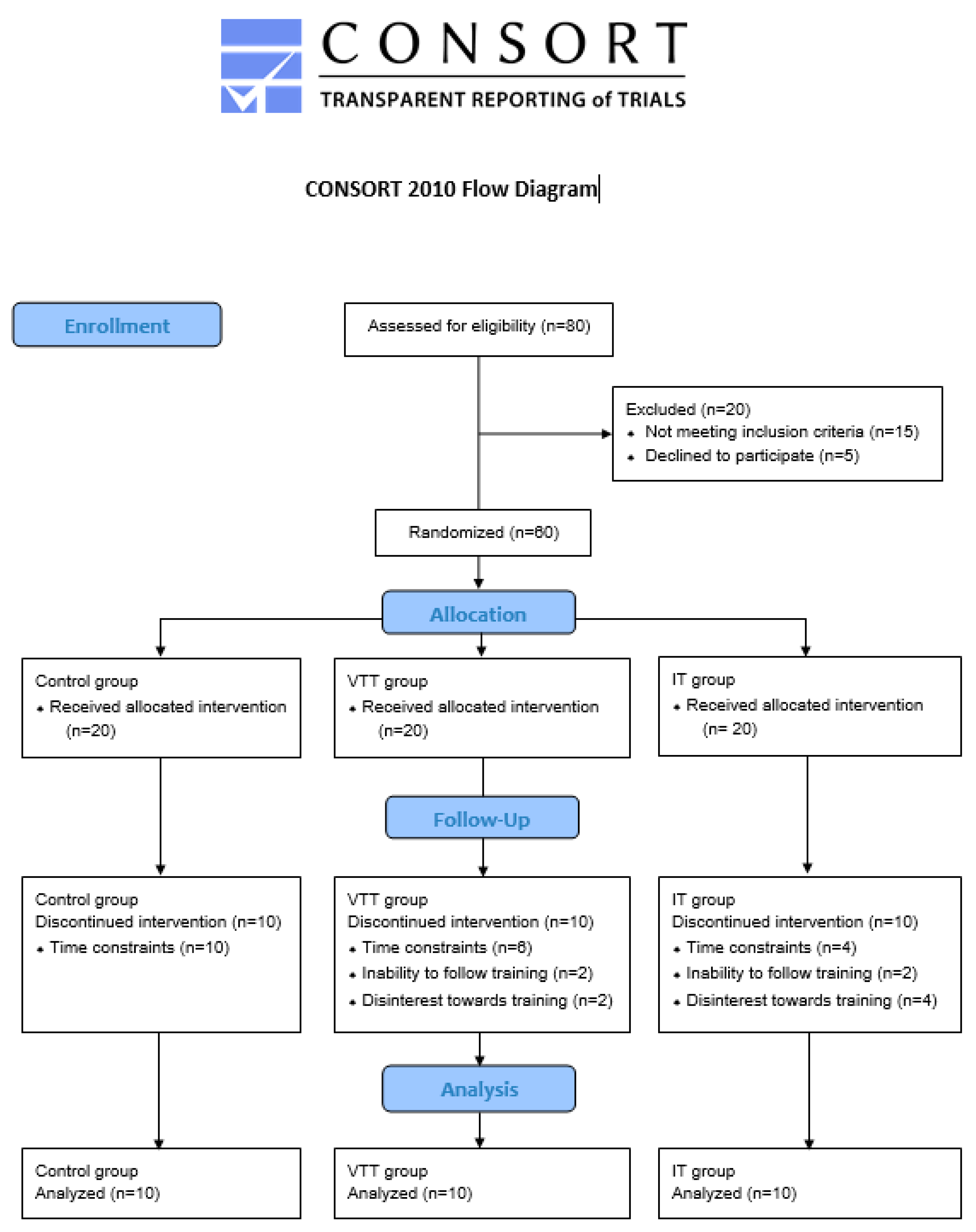
2. Material and Methods
2.1. Participants
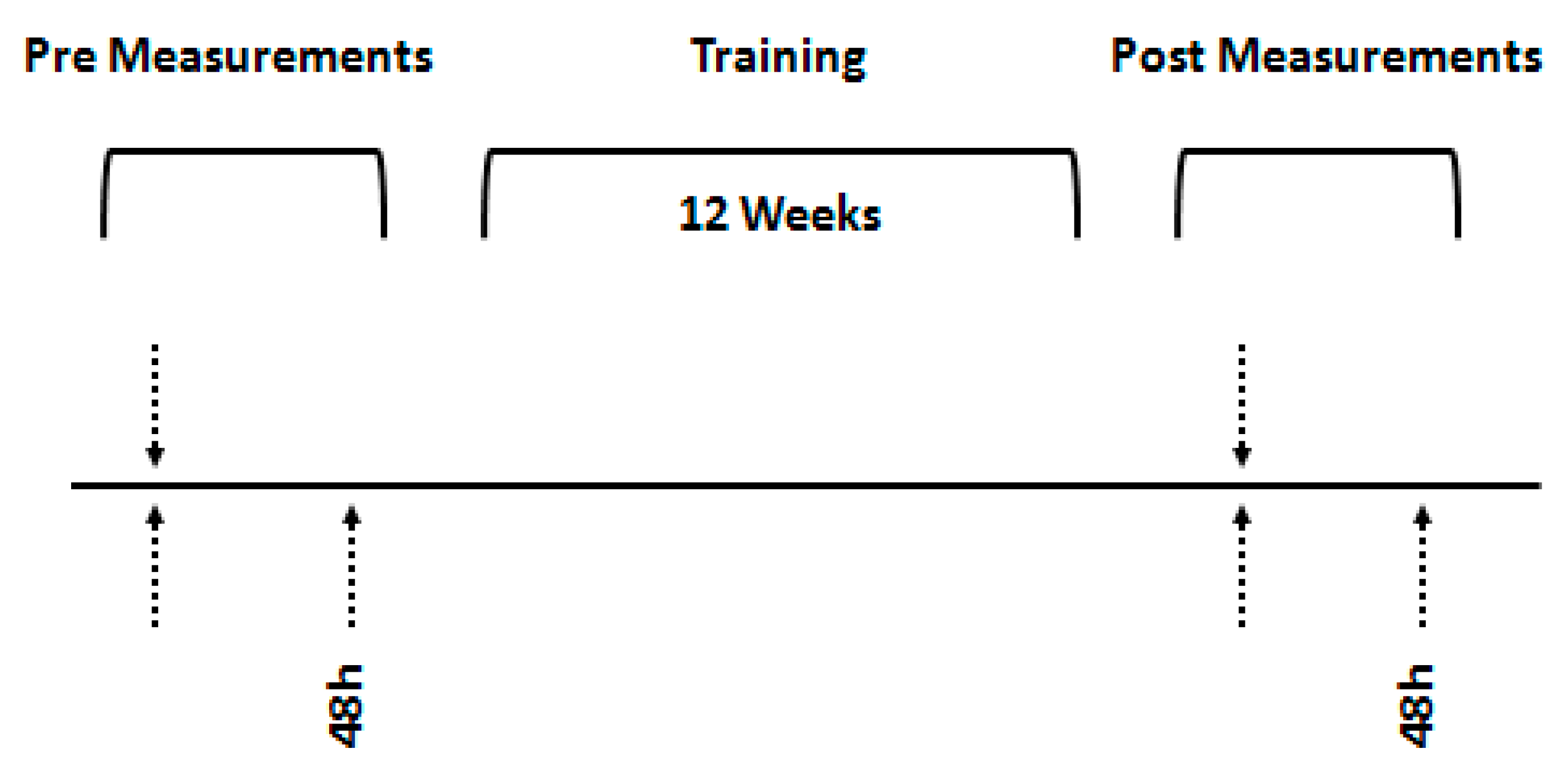
2.2. Experimental Design
2.3. Anthropometric Measures
2.4. Maximal Oxygen Uptake
2.5. Time to Exhaustion
2.6. Echocardiogram
2.7. Training Protocol
2.8. Statistical Analysis
3. Results
Correlation Analysis
4. Discussion
4.1. Summary
4.2. Performance
4.3. Morphological Adaptations
4.4. Functional Adaptations
4.5. Correlation of Cardiovascular and Ergospirometric Data
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basavarajaiah, S.; Wilson, M.; Whyte, G.; Shah, A.; McKenna, W.; Sharma, S. Prevalence of hypertrophic cardiomyopathy in highly trained athletes: Relevance to pre-participation screening. J. Am. Coll. Cardiol. 2008, 51, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, J.C.; Kelly, A.R.; Gonyea, W.J.; Mitchell, J.H. Echocardiographic left ventricular masses in distance runners and weight lifters. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Maron, B.J. Physiologic left ventricular cavity dilatation in elite athletes. Ann. Intern. Med. 1999, 130, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Pelliccia, A.; Proschan, M.A.; Granata, M.; Spataro, A.; Bellone, P.; Caselli, G.; Biffi, A.; Vecchio, C.; Maron, B.J. Morphology of the “athlete’s heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am. J. Cardiol. 1994, 74, 802–806. [Google Scholar] [CrossRef]
- Rost, R. Athlete’s heart: A 100-year long discussion. In Sports Cardiology: Exercise in Health and Cardiovascular Disease; Fagard, R.H., Bekaert, I.E., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 12–19. [Google Scholar] [CrossRef]
- Lewis, E.J.; McKillop, A.; Banks, L. The Morganroth hypothesis revisited: Endurance exercise elicits eccentric hypertrophy of the heart. J. Physiol. 2012, 590, 2833–2834. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Samuel, T.J.; Nelson, M.D.; La Gerche, A. Athlete’s Heart: Is the Morganroth Hypothesis Obsolete? Heart Lung Circ. 2018, 27, 1037–1041. [Google Scholar] [CrossRef]
- Kusy, K.; Błażejewski, J.; Gilewski, W.; Karasek, D.; Banach, J.; Bujak, R.; Zieliński, J.; Sinkiewicz, W.; Grześk, G. Aging Athlete’s Heart: An Echocardiographic Evaluation of Competitive Sprint- versus Endurance-Trained Master Athletes. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 34, 1160–1169. [Google Scholar] [CrossRef]
- Nagashima, J.; Musha, H.; Takada, H.; Murayama, M. New upper limit of physiologic cardiac hypertrophy in Japanese participants in the 100-km ultramarathon. J. Am. Coll. Cardiol. 2003, 42, 1617–1623. [Google Scholar] [CrossRef]
- Venckunas, T.; Lionikas, A.; Marcinkeviciene, J.E.; Raugaliene, R.; Alekrinskis, A.; Stasiulis, A. Echocardiographic parameters in athletes of different sports. J. Sports Sci. Med. 2008, 7, 151–156. [Google Scholar]
- Kim, Y.J.; Park, K.M. Effects of Super-ultramarathon Running on Cardiac Structure and Function in Middle-aged Men. J. Cardiovasc. Imaging 2020, 28, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Maingourd, Y.; Bourges-Petit, E.; Tanguy, C.; Quintard, J.M.; Medelli, J.; Freville, M. Peripubertal longitudinal study by echocardiography of left heart development in a group of ice hockey players. Arch. Mal. Coeur Vaiss. 1990, 83, 371–375. [Google Scholar] [PubMed]
- Owen, A.; Theakston, S.C.; O’Donovan, G.; Bird, S.R. Right and left ventricular diastolic function of male endurance athletes. Int. J. Cardiol. 2004, 95, 231–235. [Google Scholar] [CrossRef]
- Pavlik, G.; Major, Z.; Varga-Pintér, B.; Jeserich, M.; Kneffel, Z. The athlete’s heart Part I (Review). Acta Physiol. Hung. 2010, 97, 337–353. [Google Scholar] [CrossRef]
- Pavlik, G.; Major, Z.; Csajági, E.; Jeserich, M.; Kneffel, Z. The athlete’s heart. Part II: Influencing factors on the athlete’s heart: Types of sports and age (review). Acta Physiol. Hung. 2013, 100, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef]
- Csanády, M.; Forster, T.; Högye, M. Comparative echocardiographic study of junior and senior basketball players. Int. J. Sports Med. 1986, 7, 128–132. [Google Scholar] [CrossRef]
- Csanády, M.; Forster, T.; Högye, M.; Gruber, N.; Móczó, I. Three-year echocardiographic follow-up study on canoeist boys. Acta Cardiol. 1986, 41, 413–425. [Google Scholar]
- Makan, J.; Sharma, S.; Firoozi, S.; Whyte, G.; Jackson, P.G.; McKenna, W.J. Physiological upper limits of ventricular cavity size in highly trained adolescent athletes. Heart 2005, 91, 495–499. [Google Scholar] [CrossRef]
- McClean, G.; Riding, N.R.; Ardern, C.L.; Farooq, A.; Pieles, G.E.; Watt, V.; Adamuz, C.; George, K.P.; Oxborough, D.; Wilson, M.G. Electrical and structural adaptations of the paediatric athlete’s heart: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 230. [Google Scholar] [CrossRef]
- Bjerring, A.W.; Landgraff, H.E.; Leirstein, S.; Haugaa, K.H.; Edvardsen, T.; Sarvari, S.I.; Hallén, J. From talented child to elite athlete: The development of cardiac morphology and function in a cohort of endurance athletes from age 12 to 18. Eur. J. Prev. Cardiol. 2021, 28, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M. The bi-(or multi-) phasic response of cardiac remodelling to endurance exercise related to the article: ‘From talented child to elite athlete: The development of cardiac morphology and function in a cohort of endurance athletes from age 12 to 18’ by Bjerring and colleagues. Eur. J. Prev. Cardiol. 2020, 28, 1058–1060. [Google Scholar] [CrossRef]
- Obert, P.; Stecken, F.; Courteix, D.; Lecoq, A.M.; Guenon, P. Effect of long-term intensive endurance training on left ventricular structure and diastolic function in prepubertal children. Int. J. Sports Med. 1998, 19, 149–154. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Valentini, F.; Malandrino, A.; Natali, B.M.; Barbati, R.; Focardi, M.; Bonifazi, M.; Mondillo, S. Training-induced right ventricular remodelling in pre-adolescent endurance athletes: The athlete’s heart in children. Int. J. Cardiol. 2017, 236, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, A.W.; Landgraff, H.E.; Leirstein, S.; Aaeng, A.; Ansari, H.Z.; Saberniak, J.; Murbræch, K.; Bruun, H.; Stokke, T.M.; Haugaa, K.H.; et al. Morphological changes and myocardial function assessed by traditional and novel echocardiographic methods in preadolescent athlete’s heart. Eur. J. Prev. Cardiol. 2018, 25, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Billat, V.L.; Blondel, N.; Berthoin, S. Determination of the velocity associated with the longest time to exhaustion at maximal oxygen uptake. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 159–161. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Welsman, J.R.; Armstrong, N.; Withers, S. Responses of young girls to two modes of aerobic training. Br. J. Sports Med. 1997, 31, 139–142. [Google Scholar] [CrossRef]
- McNarry, M.; Jones, A. The influence of training status on the aerobic and anaerobic responses to exercise in children: A review. Eur. J. Sport Sci. 2014, 14 (Suppl. S1), S57–S68. [Google Scholar] [CrossRef]
- Baquet, G.; van Praagh, E.; Berthoin, S. Endurance training and aerobic fitness in young people. Sports Med. 2003, 33, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Medved, R.; Fabecić-Sabadi, V.; Medved, V. Echocardiographic findings in children participating in swimming training. Int. J. Sports Med. 1986, 7, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Obert, P.; Mandigout, S.; Vinet, A.; N’Guyen, L.D.; Stecken, F.; Courteix, D. Effect of aerobic training and detraining on left ventricular dimensions and diastolic function in prepubertal boys and girls. Int. J. Sports Med. 2001, 22, 90–96. [Google Scholar] [CrossRef]
- Delicce, A.V.; Makaryus, A.N. Physiology, Frank Starling Law. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chaui-Berlinck, J.G.; Monteiro, L.H.A. Frank-Starling mechanism and short-term adjustment of cardiac flow. J. Exp. Biol. 2017, 220, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Manolas, V.M.; Pavlik, G.; Bánhegyi, A.; Faludi, J.; Sidó, Z.; Olexó, Z. Echocardiographic changes in the development of the athlete’ s heart in 9 to 20-year-old male subjects. Acta Physiol. Hung. 2001, 88, 259–270. [Google Scholar] [CrossRef]
- Rowland, T.W.; Unnithan, V.B.; MacFarlane, N.G.; Gibson, N.G.; Paton, J.Y. Clinical manifestations of the ‘athlete’s heart’ in prepubertal male runners. Int. J. Sports Med. 1994, 15, 515–519. [Google Scholar] [CrossRef]
- Conti, V.; Migliorini, F.; Pilone, M.; Barriopedro, M.I.; Ramos-Álvarez, J.J.; Montero, F.J.C.; Maffulli, N. Right heart exercise-training-adaptation and remodelling in endurance athletes. Sci. Rep. 2021, 11, 22532. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F. Exercise improves vascular health: Role of mitochondria. Free Radic. Biol. Med. 2021, 177, 347–359. [Google Scholar] [CrossRef]
- Morganroth, J.; Maron, B.J.; Henry, W.L.; Epstein, S.E. Comparative left ventricular dimensions in trained athletes. Ann. Intern. Med. 1975, 82, 521–524. [Google Scholar] [CrossRef]
- Fernandes, T.; Soci, U.P.; Oliveira, E.M. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz. J. Med. Biol. Res. 2011, 44, 836–847. [Google Scholar] [CrossRef]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Popkin, C.A.; Bayomy, A.F.; Ahmad, C.S. Early Sport Specialization. J. Am. Acad. Orthop. Surg. 2019, 27, e995–e1000. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.T.; LaBotz, M. Sport specialization in young athletes. JAAPA Off. J. Am. Acad. Physician Assist. 2020, 33, 47–50. [Google Scholar] [CrossRef] [PubMed]
| Week | Intensity | Duration | Volume | Frequency | ||
|---|---|---|---|---|---|---|
| Same for the Training Groups | Training | (min) | (km) | Same for the Training Groups | Training | |
| 1 | 55–60% vVo2max | @Ventilatory threshold | 180 | 21 | 2 | 2 |
| 2 | 180 | 22 | 2 | 2 | ||
| 3 | 180 | 22 | 2 | 2 | ||
| 4 | 180 | 23.5 | 2 | 2 | ||
| 5 | Increase 5% vVo2max | Increase 5% vVo2max | 180 | 24 | 2 | 2 |
| 6 | 180 | 24.5 | 2 | 2 | ||
| 7 | 180 | 24.5 | 2 | 2 | ||
| 8 | 180 | 25.5 | 2 | 2 | ||
| 9 | Increase 5% vVo2max | Increase 5% vVo2max | 180 | 26 | 2 | 2 |
| 10 | 180 | 26 | 2 | 2 | ||
| 11 | 180 | 26.5 | 2 | 2 | ||
| 12 | 180 | 27 | 2 | 2 | ||
| Week | Intensity | Duration | Volume | Frequency | ||
|---|---|---|---|---|---|---|
| Same for the Training Groups | Training | (min) | (km) | Same for the Training Groups | Training | |
| 1 | 55–60% vVo2max | 200–150–100 mRest 2 min120 vVO2max | 180 | 21 | 2 | 2 |
| 2 | 180 | 22 | 2 | 2 | ||
| 3 | 180 | 22 | 2 | 2 | ||
| 4 | 180 | 23.5 | 2 | 2 | ||
| 5 | Increase 5% vVo2max | Increase 5% vVO2max | 180 | 24 | 2 | 2 |
| 6 | 180 | 24.5 | 2 | 2 | ||
| 7 | 180 | 24.5 | 2 | 2 | ||
| 8 | 180 | 25.5 | 2 | 2 | ||
| 9 | Increase 5% vVo2max | Increase 5% vVO2max | 180 | 26 | 2 | 2 |
| 10 | 180 | 26 | 2 | 2 | ||
| 11 | 180 | 26.5 | 2 | 2 | ||
| 12 | 180 | 27 | 2 | 2 | ||
| Variable | CONTROL | VTΤ | IT | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Age (years) | 12.4 ± 0.7 | 12.8 ± 0.6 | 13.1 ± 0.3 | |||
| Body Mass (kg) | 39.9 ± 5.8 | 40.8 ± 6.3 ** | 40.5 ± 4.6 | 40.4 ± 4.5 | 47.7 ± 5.5 | 47.6 ± 5.5 |
| Height (cm) | 157.1 ± 4.3 | 157.4 ± 4.4 | 156.2 ± 4.6 | 156.8 ± 4.5 | 159.2 ± 4.9 | 159.4 ± 4.5 |
| Fat (%) | 13.9 ± 3.9 | 14.3 ± 4.1 | 16.2 ± 3.0 | 15.0 ± 3.9 *** | 15.0 ± 4.6 | 14.2 ± 3.9 * |
| Variable | CONTROL | VTT | IT | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| LVEDd (mm) | 43.7 ± 4.6 | 43.5 ± 4.6 | 44.1 ± 2.5 | 46.5 ± 2.0 *** | 43.9 ± 2.6 | 45.5 ± 2.7 *** |
| LVEDV (mL) | 88.1 ± 21.4 | 89.6 ± 22.4 | 81.3 ± 10.2 | 100.3 ± 12.5 *** | 83.8 ± 13.2 | 95.0 ± 12.3 *** |
| PWTLV (mm) | 8.15 ± 1.05 | 8.25 ± 1.03 | 8.00 ± 1.02 | 8.40 ± 1.02 | 8.15 ± 0.97 | 8.70 ± 1.25 |
| SV (mL) | 59.6 ± 16.0 | 60.4 ± 15.6 | 60.7 ± 9.2 | 75.9 ± 9.6 *** | 57.5 ± 9.4 | 67.3 ± 10.3 *** |
| ΕF (%) | 69.10 ± 5.66 | 70.10 ± 5.97 | 72.40 ± 6.1 | 75.30 ± 0.56 | 72.20 ± 6.81 | 71.50 ± 5.38 |
| Variable | CONTROL | VTT | IT | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| MHR (bpm) | 206.2 ± 3.8 | 205.6 ± 3.6 | 205.0 ± 4.6 | 205.2 ± 4.9 | 206.2 ± 3.9 | 203.9 ± 4.9 |
| VO2max (mL/kg/min) | 46.8 ± 6.54 | 48.6 ± 7.37 | 46.0 ± 6.85 | 50.2 ± 8.46 *** | 48.7 ± 5.98 | 53.2 ± 6.88 *** |
| VT (mL/kg/min) | 36.13 ± 7.14 | 37.53 ± 7.96 | 35.05 ± 6.96 | 37.99 ± 8.48 *** | 37.57 ± 6.26 | 41.15 ± 6.73 *** |
| Tlim (min) | 3.35 ± 0.71 | 3.79 ± 0.77 | 3.59 ± 0.81 | 3.63 ± 1.15 | 4.63 ± 1.44 | 4.04 ± 1.29 |
| vVO2max (km/h) | 14.10 ± 0.93 | 14.70 ± 0.94 | 13.95 ± 0.98 | 14.75 ± 1.13 | 14.15 ± 1.52 | 14.31 ± 1.88 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. SV | - | |||
| 2. VO2max | 0.544 ** | - | ||
| 3. LVEDd | 0.830 ** | 0.505 ** | - | |
| 4. LVEDV | 0.897 ** | 0.656 ** | 0.868 ** | - |
| 5. PWTLV | 0.204 | 0.436 * | 0.213 | 0.167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafailakis, L.; Deli, C.K.; Fatouros, I.G.; Tsiokanos, A.; Draganidis, D.; Poulios, A.; Soulas, D.; Jamurtas, A.Z. Functional and Morphological Adaptations in the Heart of Children Aged 12–14 Years following Two Different Endurance Training Protocols. Sports 2023, 11, 157. https://doi.org/10.3390/sports11080157
Rafailakis L, Deli CK, Fatouros IG, Tsiokanos A, Draganidis D, Poulios A, Soulas D, Jamurtas AZ. Functional and Morphological Adaptations in the Heart of Children Aged 12–14 Years following Two Different Endurance Training Protocols. Sports. 2023; 11(8):157. https://doi.org/10.3390/sports11080157
Chicago/Turabian StyleRafailakis, Lefteris, Chariklia K. Deli, Ioannis G. Fatouros, Athanasios Tsiokanos, Dimitrios Draganidis, Athanasios Poulios, Dimitrios Soulas, and Athanasios Z. Jamurtas. 2023. "Functional and Morphological Adaptations in the Heart of Children Aged 12–14 Years following Two Different Endurance Training Protocols" Sports 11, no. 8: 157. https://doi.org/10.3390/sports11080157
APA StyleRafailakis, L., Deli, C. K., Fatouros, I. G., Tsiokanos, A., Draganidis, D., Poulios, A., Soulas, D., & Jamurtas, A. Z. (2023). Functional and Morphological Adaptations in the Heart of Children Aged 12–14 Years following Two Different Endurance Training Protocols. Sports, 11(8), 157. https://doi.org/10.3390/sports11080157








