The Participation of Trans Women in Competitive Fencing and Implications on Fairness: A Physiological Perspective Narrative Review
Abstract
1. Introduction
1.1. Transgender Inclusion Policies and Guidelines
1.2. Needs Analysis of Fencing
1.2.1. Physiological Demands of Fencing
1.2.2. Lunge
1.3. Methodological Considerations and Aims
2. Physiological Cisgender Male vs. Cisgender Female Differences
3. Cisgender Male vs. Cisgender Female Fencing Performance Differences
4. Testosterone and Females
5. Testosterone Suppression and Sporting Performance
6. Opinions to Be Considered
7. Open or Third Gender Category
8. Fairness in Elite Fencing: Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, C. An everlasting gender gap? Science 2004, 305, 639–640. [Google Scholar] [CrossRef] [PubMed]
- Thibault, V.; Guillaume, M.; Berthelot, G.; El Helou, N.; Schaal, K.; Quinquis, L.; Nassif, H.; Tafflet, M.; Escolano, S.; Hermine, O.; et al. Women and Men in Sport Performance: The Gender Gap has not Evolved since 1983. J. Sport. Sci. Med. 2010, 9, 214–223. [Google Scholar]
- Sandbakk, Ø.; Solli, G.S.; Holmberg, H.C. Sex differences in world-record performance: The influence of sport discipline and competition duration. Int. J. Sport. Physiol. Perform. 2018, 13, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.; Steensma, T.D.; Blanchard, R.; VanderLaan, D.P.; Wood, H.; Fuentes, A.; Spegg, C.; Wasserman, L.; Ames, M.; Fitzsimmons, C.L.; et al. Evidence for an altered sex ratio in clinic-referred adolescents with gender dysphoria. J. Sex. Med. 2015, 12, 756–763. [Google Scholar] [CrossRef]
- Arcelus, J.; Bouman, W.P.; Van Den Noortgate, W.; Claes, L.; Witcomb, G.; Fernandez-Aranda, F. Systematic Review and Meta-Analysis of Prevalence Studies in Transsexualism. Eur. Psychiatry 2015, 30, 807–815. [Google Scholar] [CrossRef]
- Scott, S. Cyclist Emily Bridges Had “Physical Threats” after PM’s Comments on Trans Participation in Sport. 2022. Available online: https://www.itv.com/news/2022-06-07/emily-bridges-had-physical-threats-after-pms-trans-participation-comments (accessed on 17 March 2023).
- Olympics.com. Laurel Hubbard: Who Is the Transgender Weightlifter Making History at Tokyo 2020? 2021. Available online: https://olympics.com/en/news/laurel-hubbard-new-zealand-weightlifter-history-tokyo-2020-2021-games (accessed on 17 March 2023).
- Knox, T.; Anderson, L.C.; Heather, A. Transwomen in elite sport: Scientific and ethical considerations. J. Med. Ethics 2019, 45, 395–403. [Google Scholar] [CrossRef]
- Jones, B.A.; Arcelus, J.; Bouman, W.P.; Haycraft, E. Sport and Transgender People: A Systematic Review of the Literature Relating to Sport Participation and Competitive Sport Policies. Sport. Med. 2017, 47, 701–716. [Google Scholar] [CrossRef]
- Bianchi, A. Transgender women in sport. J. Philos. Sport. 2017, 44, 229–242. [Google Scholar] [CrossRef]
- Pitsiladis, Y.; Harper, J.; Betancurt, J.O.; Martinez-Patino, M.-J.; Parisi, A.; Wang, G.; Pigozzi, F. Beyond Fairness: The Biology of Inclusion for Transgender and Intersex Athletes. Curr. Sport. Med. Rep. 2016, 15, 386–388. [Google Scholar] [CrossRef]
- UK Parliament POST. Performance, Inclusion and Elite Sports-Transgender Athletes. 2022. Available online: https://researchbriefings.files.parliament.uk/documents/POST-PN-0683/POST-PN-0683.pdf (accessed on 16 March 2023).
- Buzuvis, E.E. Caster Semenya and the Myth of a Level Playing Field. Mod. Am. 2010, 6, 36–42. [Google Scholar]
- Harper, J.; Martinez-Patino, M.J.; Pigozzi, F.; Pitsiladis, Y. Implications of a Third Gender for Elite Sports. Curr. Sport. Med. Rep. 2018, 17, 42–44. [Google Scholar] [CrossRef]
- Martínez-Patiño, M.J.; Vilain, E.; Bueno-Guerra, N. The unfinished race: 30 years of gender verification in sport. Lancet 2016, 388, 541–543. [Google Scholar] [CrossRef]
- International Olympic Committee. IOC Framework Fairness Inclusion and Non-Discrimination on the Basis of Gender Identity and Sex Variations. 2021. Available online: https://stillmed.olympics.com/media/Documents/News/2021/11/IOC-Framework-Fairness-Inclusion-Non-discrimination-2021.pdf?_ga=2.152369019.39347725.1657800938-773674365.1652626215 (accessed on 16 January 2023).
- Erdener, U.; Ljungqvist, A.; Bermon, S.; Beloff, M.; Conway, G.; Genel, M.; Harper, J.; Hirschberg, A.; Martinez-Patino, M.; Ritzen, M.; et al. IOC Consensus Meeting on Sex Reassignment and Hyperandrogenism. 2015. Available online: https://stillmed.olympic.org/Documents/Commissions_PDFfiles/Medical_commission/2015-11_ioc_consensus_meeting_on_sex_reassignment_and_hyperandrogenism-en.pdf (accessed on 12 June 2022).
- Hilton, E.N.; Lundberg, T.R. Transgender Women in the Female Category of Sport: Perspectives on Testosterone Suppression and Performance Advantage. Sport. Med. 2021, 51, 199–214. [Google Scholar] [CrossRef]
- Martowicz, M.; Budgett, R.; Pape, M.; Mascagni, K.; Engebretsen, L.; Dienstbach-Wech, L.; Pitsiladis, Y.P.; Pigozzi, F.; Erdener, U. Position statement: IOC framework on fairness, inclusion and non-discrimination on the basis of gender identity and sex variations. Br. J. Sport. Med. 2023, 57, 26–32. [Google Scholar] [CrossRef]
- Union Cycliste Internationale. UCI Management Committee Approves the Federation’s Agenda 2030 and Awards the First UCI Gravel World Championships. 2022. Available online: https://www.uci.org/pressrelease/uci-management-committee-approves-the-federations-agenda-2030-and-awards-the/2YzsHNKvfDZTytpsYw5e6p (accessed on 6 August 2022).
- World Triathlon. World Triathlon Executive Board approves Transgender Policy. 2022. Available online: https://www.triathlon.org/news/article/world_triathlon_executive_board_approves_transgender_policy (accessed on 27 November 2022).
- World Rugby. Transgender Women Guidelines. 2021. Available online: https://www.world.rugby/the-game/player-welfare/guidelines/transgender (accessed on 6 August 2022).
- FINA. Policy on Eligibility for the Men’s and Women’s Competition Categories. 2022. Available online: https://resources.fina.org/fina/document/2022/06/19/525de003-51f4-47d3-8d5a-716dac5f77c7/FINA-INCLUSION-POLICY-AND-APPENDICES-FINAL-.pdf (accessed on 5 September 2022).
- World Athletics. Eligibility Regulations for Transgender Athletes. 2023. Available online: https://worldathletics.org/news/press-releases/council-meeting-march-2023-russia-belarus-female-eligibility (accessed on 5 April 2023).
- Bottoms, L.; Sinclair, J.; Rome, P.; Gregory, K.; Price, M. Development of a lab based epee fencing protocol. Int. J. Perform. Anal. Sport. 2013, 13, 11–22. [Google Scholar] [CrossRef]
- Roi, G.S.; Bianchedi, D. The Science of Fencing Implications for Performance and Injury Prevention. Sport. Med. 2008, 38, 465–481. [Google Scholar] [CrossRef]
- Aquili, A.; Tancredi, V.; Triossi, T.; De Sanctis, D.; Padua, E.; D’Arcangelo, G.; Melchiorri, G. Performance analysis in saber. J. Strength Cond. Res. 2013, 27, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Oates, L.W.; Campbell, I.G.; Iglesias, X.; Price, M.J.; Muniz-Pumares, D.; Bottoms, L.M. The physiological demands of elite epée fencers during competition. Int. J. Perform. Anal. Sport. 2019, 19, 76–89. [Google Scholar] [CrossRef]
- Bottoms, L.M.; Sinclair, J.; Gabrysz, T.; Szmatlan-Gabrysz, U.; Price, M.J. Physiological responses and energy expenditure to simulated epee fencing in elite female fencers. Physiol. Fenc. Serb. J. Sport. Sci. 2011, 5, 17–20. [Google Scholar]
- Bottoms, L.; Cawdron, R.; Kemp, S.; Oates, L. Fencing. In Sport and Exercise Physiology Testing Guidelines: Volume I—Sport Testing; Routledge: Oxfordshire, UK, 2022; pp. 325–329. [Google Scholar] [CrossRef]
- Turner, A.; James, N.; Dimitriou, L.; Greenhalgh, A.; Moody, J.; Fulcher, D.; Mias, E.; Kilduff, L. Determinants of Olympic fencing performance and implications for strength and conditioning training. J. Strength Cond. Res. 2014, 28, 3001–3011. [Google Scholar] [CrossRef]
- Turner, A.; Miller, S.; Stewart, P.; Cree, J.; Ingram, R.; Dimitriou, L.; Moody, J.; Kilduff, L. Strength and Conditioning for Fencing. Strength Cond. J. 2013, 35, 1–9. [Google Scholar] [CrossRef]
- Gholipour, M.; Tabrizi, A. Kinematics analysis of lunge fencing using stereophotogrametry. World J. Sport. Sci. 2008, 1, 32–37. [Google Scholar]
- Turner, A.; Baker, E.; Miller, S. Increasing the impact force of the rear hand punch. Strength Cond. J. 2011, 33, 2–9. [Google Scholar] [CrossRef]
- Guilhem, G.; Giroux, C.; Couturier, A.; Chollet, D.; Rabita, G. Mechanical and muscular coordination patterns during a high-level fencing assault. Med. Sci. Sport. Exerc. 2014, 46, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef]
- Ntai, A.; Zahou, F.; Paradisis, G.; Smirniotou, A.; Tsolakis, C. Anthropometric parameters and leg power performance in fencing. Age, sex and discipline related differences. Sci. Sport. 2017, 32, 135–143. [Google Scholar] [CrossRef]
- Asçi, A.; Açikada, C. Power production among different sports with similar maximum strength. J. Strength Cond. Res. 2007, 21, 10–16. Available online: http://journals.lww.com/nsca-jscr (accessed on 24 April 2022).
- Williams, L.R.T.; Walmsley, A. Response timing and muscular coordination fencing: A comparison of elite and novice fencers. J. Sci. Med. Sport. 2000, 3, 460–475. [Google Scholar] [CrossRef]
- Hassan, S.E.A.; Klauck, J. Kinematics of Lower and Upper Extremities Motions during the Fencing Lunge: Results and Training Implications. 1998. Available online: https://ojs.ub.uni-konstanz.de/cpa/article/view/965 (accessed on 15 April 2023).
- Klauck, J.; Hassan, S.E.A. Lower and Upper Extremity Coordination Parameters during the Fencing Lunge. 1998. Available online: https://ojs.ub.uni-konstanz.de/cpa/article/view/1620 (accessed on 15 April 2023).
- Zhang, B.; Chu, D.; Hong, Y. Biomechanical Analysis of the Lunge Technique in the Elite Female Fencers. 1999. Available online: https://ojs.ub.uni-konstanz.de/cpa/article/view/4053 (accessed on 15 April 2023).
- Chen, T.L.W.; Wong, D.W.C.; Wang, Y.; Ren, S.; Yan, F.; Zhang, M. Biomechanics of fencing sport: A scoping review. PLoS ONE 2017, 12, e0171578. [Google Scholar] [CrossRef]
- Sinclair, J.; Bottoms, L. Gender differences in the kinetics and lower extremity kinematics of the fencing lunge. Int. J. Perform. Anal. Sport. 2013, 13, 440–451. [Google Scholar] [CrossRef]
- Tsolakis, C.; Vagenas, G. Anthropometric, physiological and performance characteristics of elite and sub-elite fencers. J. Hum. Kinet. 2010, 23, 89–95. [Google Scholar] [CrossRef]
- Tsolakis, C.; Kostaki, E.; Vagenas, G. Anthropometric, flexibility, strength-power, and sport-specific correlates in elite fencing. Percept. Mot. Skills 2010, 110, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Agosti, V.; Autuori, M. Fencing functional training system (ffts): A new pedagogical-educational training project. Sport. Sci. 2020, 13, 118–122. [Google Scholar]
- McLarnon, M.; Thornton, J.; Knudson, G.; Jones, N.; Glover, D.; Murray, A.; Cummings, M.; Heron, N. A Scoping Review of Transgender Policies in the 15 Most Commonly Played UK Professional Sports. Int. J. Environ. Res. Public Health 2023, 20, 3568. [Google Scholar] [CrossRef]
- Harper, J.; O’Donnell, E.; Sorouri Khorashad, B.; McDermott, H.; Witcomb, G.L. How does hormone transition in transgender women change body composition, muscle strength and haemoglobin? Systematic review with a focus on the implications for sport participation. Br. J. Sport. Med. 2021, 55, 865–872. [Google Scholar] [CrossRef]
- Hamilton, B.R.; Lima, G.; Barrett, J.; Seal, L.; Kolliari-Turner, A.; Wang, G.; Karanikolou, A.; Bigard, X.; Löllgen, H.; Zupet, P.; et al. Integrating transwomen and female athletes with differences of sex development (DSD) into elite competition: The FIMS 2021 consensus statement. Sport. Med. 2021, 51, 1401–1415. [Google Scholar] [CrossRef]
- Stebbings, G.; Herbert, A.; Pielke, R., Jr.; Tucker, R.; Heffernan, S.; Williams, A. The BASES Expert Statement on Eligibility for Sex Categories in Sport: Trans Athletes. Br. Assoc. Sport. Exerc. Sci. Abstr. 2021, 39, 3–5. [Google Scholar] [CrossRef]
- Tatem, A.J.; Guerra, C.A.; Atkinson, P.M.; Hay, S.I. Momentous sprint at the 2156 Olympics? Nature 2004, 431, 525. [Google Scholar] [CrossRef]
- Whipp, B.; Ward, S. Will women soon outrun men? Nature 1992, 355, 25. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr. Rev. 2018, 39, 803–829. [Google Scholar] [CrossRef]
- Handelsman, D.J. Sex differences in athletic performance emerge coinciding with the onset of male puberty. Clin. Endocrinol. 2017, 87, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Sikaris, K.; Ly, L.P. Estimating age-specific trends in circulating testosterone and sex hormone-binding globulin in males and females across the lifespan. Ann. Clin. Biochem. 2016, 53, 377–384. [Google Scholar] [CrossRef]
- Pape, M.; Pielke, R. Science, sport, sex, and the case of Caster Semenya. Issues Sci. Technol. 2019, 36, 56–63. [Google Scholar] [CrossRef]
- Tønnessen, E.; Svendsen, I.S.; Olsen, I.C.; Guttormsen, A.; Haugen, T. Performance development in adolescent track and field athletes according to age, sex and sport discipline. PLoS ONE 2015, 10, e0129014. [Google Scholar] [CrossRef]
- Umapathysivam, M.; Grossmann, M.; Wittert, G.A. Effects of androgens on glucose metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101654. [Google Scholar] [CrossRef]
- Corona, G.; Giagulli, V.A.; Maseroli, E.; Vignozzi, L.; Aversa, A.; Zitzmann, M.; Saad, F.; Mannucci, E.; Maggi, M. Testosterone supplementation and body composition: Results from a meta-analysis of observational studies. J. Endocrinol. Investig. 2016, 39, 967–981. [Google Scholar] [CrossRef]
- Frank, A.P.; De Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Horwath, O.; Apró, W.; Moberg, M.; Godhe, M.; Helge, T.; Ekblom, M.; Hirschberg, A.L.; Ekblom, B. Fiber type-specific hypertrophy and increased capillarization in skeletal muscle following testosterone administration in young women. J. Appl. Physiol. 2020, 128, 1240–1250. [Google Scholar] [CrossRef]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Westfall, J.C.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2014, 69, 725–735. [Google Scholar] [CrossRef]
- Murphy, W.G. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Patho-physiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Lang, T.F. The Bone-Muscle Relationship in Men and Women. J. Osteoporos. 2011, 2011, 702735. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Hegge, A.M.; Myhre, K.; Welde, B.; Holmberg, H.C.; Sandbakk, Ø. Are gender differences in upper-body power generated by elite cross-country skiers augmented by increasing the intensity of exercise? PLoS ONE 2015, 10, e0127509. [Google Scholar] [CrossRef]
- Jones, M.T.; Jagim, A.R.; Haff, G.G.; Carr, P.J.; Martin, J.; Oliver, J.M. Greater strength drives difference in power between sexes in the conventional deadlift exercise. Sports 2016, 4, 43. [Google Scholar] [CrossRef]
- Markovic, G.; Jaric, S. Is vertical jump height a body size-independent measure of muscle power? J. Sport. Sci. 2007, 25, 1355–1363. [Google Scholar] [CrossRef]
- Taylor, M.J.; Cohen, D.; Voss, C.; Sandercock, G.R. Vertical jumping and leg power normative data for English school children aged 10–15 years. J. Sport. Sci. 2010, 28, 867–872. [Google Scholar] [CrossRef]
- Butterfield, S.A.; Lehnhard, R.; Lee, J.; Coladarci, T. Growth rates in running speed and vertical jumping by boys and girls ages 11–13. Percept. Mot. Ski. 2004, 99, 225–234. [Google Scholar]
- Temfemo, A.; Hugues, J.; Chardon, K.; Mandengue, S.H.; Ahmaidi, S. Relationship between vertical jumping performance and anthropometric characteristics during growth in boys and girls. Eur. J. Pediatr. 2009, 168, 457–464. [Google Scholar] [CrossRef]
- Drakoulaki, V.; Kontochristopoulos, N.; Methenitis, S.; Simeonidis, T.; Cherouveim, E.; Koulouvaris, P.; Savvidou, O.; Tsolakis, C. Bilateral asymmetries in male and female young elite fencers in relation to fencing performance. Isokinet. Exerc. Sci. 2021, 29, 113–121. [Google Scholar] [CrossRef]
- Ntai, A.; Tsolakis, C.; Smirniotou, A. Anthropometric and Leg Power Factors Affect Offensive Kinetic Patterns in Fencing. Int. J. Exerc. Sci. 2021, 14, 919–931. [Google Scholar] [PubMed]
- Foddy, B.; Savulescu, J. Time to re-evaluate gender segregation in athletics? Br. J. Sport. Med. 2011, 45, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Sax, L. How common is lntersex? A response to Anne Fausto-Sterling. J. Sex. Res. 2002, 39, 174–178. [Google Scholar] [CrossRef]
- Ferguson-Smith, M.A.; Ferris, E.A. Gender verification in sport: The need for change? Br. J. Sport. Med. 1991, 25, 17–20. [Google Scholar] [CrossRef]
- Ferguson-Smith, M.A. Gender verification and the place of XY females in sport. In Oxford Textbook of Sports Medicine; Oxford University Press: Oxford, UK, 1998; pp. 355–365. [Google Scholar]
- Carmina, E.; Guastella, E.; Longo, R.A.; Rini, G.B.; Lobo, R.A. Correlates of increased lean muscle mass in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2009, 161, 583. [Google Scholar] [CrossRef]
- Douchi, T.; Oki, T.; Yamasaki, H.; Kuwahata, R.; Nakae, M.; Nagata, Y. Relationship of androgens to muscle size and bone mineral density in women with polycystic ovary syndrome. Obstet. Gynecol. 2001, 98, 445–449. [Google Scholar]
- Eklund, E.; Berglund, B.; Labrie, F.; Carlström, K.; Ekström, L.; Hirschberg, A.L. Serum androgen profile and physical performance in women Olympic athletes. Br. J. Sport. Med. 2017, 51, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Hagmar, M.; Berglund, B.; Brismar, K.; Linde, A. Hyperandrogenism may explain reproductive dysfunction in olympic athletes. Med. Sci. Sport. Exerc. 2009, 41, 1241–1248. [Google Scholar] [CrossRef]
- Rickenlund, A.; Carlström, K.; Ekblom, B.; Brismar, T.B.; Schoultz, B.; Hirschberg, A.L. Hyperandrogenicity is an alternative mechanism underlying oligomenorrhea or amenorrhea in female athletes and may improve physical performance. Fertil. Steril. 2003, 79, 947–955. [Google Scholar] [CrossRef]
- Eliakim, A.; Marom, N.; Galitskaya, L.; Nemet, D. Hyperandrogenism among elite adolescent female athletes. J. Pediatr. Endocrinol. Metab. 2010, 23, 755–758. [Google Scholar] [CrossRef]
- Cardinale, M.; Stone, M.H. Is testosterone influencing explosive performance? J. Strength Cond. Res. 2006, 20, 103–107. [Google Scholar] [CrossRef]
- Bermon, S.; Garnier, P. Serum androgen levels and their relation to performance in track and field: Mass spectrometry results from 2127 observations in male and female elite athletes. Br. J. Sport. Med. 2017, 51, 1309–1314. [Google Scholar] [CrossRef]
- Juppi, H.; Sipilä, S.; Cronin, N.J. Role of menopausal transition and physical activity in loss of lean and muscle mass: A follow-up study in middle-aged Finnish women. J. Clin. Med. 2020, 9, 1588. [Google Scholar] [CrossRef]
- Dobs, A.S.; Nguyen, T.; Pace, C.; Roberts, C.P. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 1509–1516. [Google Scholar] [CrossRef]
- Huang, G.; Basaria, S.; Travison, T.G. Testosterone dose-response relationships in hysterectomized women with and without oophorectomy: Effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause 2014, 21, 612–623. [Google Scholar] [CrossRef]
- Caenegem, V.; Wierckx, K.; Taes, Y. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case–controlled study (ENIGI). Eur. J. Endocrinol. 2015, 172, 163–171. [Google Scholar] [CrossRef]
- Elbers, J.M.H.; Asscheman, H.; Seidell, J.C.; Gooren, L.J.G. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am. J. Physiol. Metab. 1999, 276, E317–E325. [Google Scholar] [CrossRef]
- Andrade, S.R.d.L.; Mucida, Y.M.; Xavier, J.d.C.; Fernandes, L.N.; Silva, R.D.O.; Bandeira, F. Bone mineral density, trabecular bone score and muscle strength in transgender men receiving testosterone therapy versus cisgender men. Steroids 2021, 178, 108951. [Google Scholar] [CrossRef]
- Unger, C.A. Hormone therapy for transgender patients. Transl. Androl. Urol. 2016, 5, 877–884. [Google Scholar] [CrossRef]
- Schneider, F.; Kliesch, S.; Schlatt, S.; Neuhaus, N. Andrology of male-to-female transsexuals: Influence of cross-sex hormone therapy on testicular function. Andrology 2017, 5, 873–880. [Google Scholar] [CrossRef]
- Gooren, L.J.; Kreukels, B.; Lapauw, B.; Giltay, E.J. Patho physiology of cross-sex hormone administration to transsexual people: The potential impact of male–female genetic differences. Andrologia 2015, 47, 5–19. [Google Scholar] [CrossRef]
- Alvares, L.A.M.; Santos, M.R.; Souza, F.R.; Santos, L.M.; de Mendonça, B.B.; Costa, E.M.F.; Alves, M.J.N.N.; Domenice, S. Cardiopulmonary capacity and muscle strength in transgender women on long-term gender-affirming hormone therapy: A cross-sectional study. Br. J. Sport. Med. 2022, 56, 1292–1299. [Google Scholar] [CrossRef]
- Singh-ospina, N.; Maraka, S.; Rodriguez-gutierrez, R. Effect of sex steroids on the bone health of transgender individuals: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3904–3913. [Google Scholar] [CrossRef]
- Tiidus, P.M.; Lowe, D.A.; Brown, M. Estrogen replacement and skeletal muscle: Mechanisms and population health. J. Appl. Physiol. 2010, 115, 569–578. [Google Scholar] [CrossRef]
- Velders, M.; Schleipen, B.; Fritzemeier, K.H.; Zierau, O.; Diel, P. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012, 26, 1909–1920. [Google Scholar] [CrossRef]
- Parr, M.K.; Zhao, P.; Haupt, O. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol. Nutr. Food Res. 2014, 58, 1861–1872. [Google Scholar] [CrossRef]
- Bhasin, S.; Woodhouse, L.; Casaburi, R. Testosterone dose-response relationships in healthy young men. Am. J. Physiol.—Endocrinol. Metab. 2001, 281, 1172–1181. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Lee, H.; Burnett-Bowie, S.A.M. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013, 369, 1011–1022. [Google Scholar] [CrossRef]
- Campos, C.; Sotomayor, P.; Jerez, D. Exercise and prostate cancer: From basic science to clinical applications. Prostate 2018, 78, 639–645. [Google Scholar] [CrossRef]
- Galvao, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Turner, D.; Newton, R.U. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: A comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009, 12, 198–203. [Google Scholar] [CrossRef]
- Keilani, M.; Hasenoehrl, T.; Baumann, L.; Ristl, R.; Schwarz, M.; Marhold, M.; Komandj, T.S.; Crevenna, R. Effects of resistance exercise in prostate cancer patients: A meta-analysis. Support. Care Cancer 2017, 25, 2953–2968. [Google Scholar] [CrossRef]
- Winters-stone, K.M.; Dobek, J.C.; Bennett, J.A. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: Evidence from a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 7–14. [Google Scholar] [CrossRef]
- Cormie, P.; Galvão, D.A.; Spry, N. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015, 115, 256–266. [Google Scholar] [CrossRef]
- Mina, D.S.; Alibhai, S.M.; Matthew, A.G.; Guglietti, C.L.; Pirbaglou, M.; Trachtenberg, J.; Ritvo, P. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J. Aging Phys. Act. 2013, 21, 455–478. [Google Scholar] [CrossRef]
- Segal, R.J.; Reid, R.D.; Courneya, K.S. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J. Clin. Oncol. 2009, 27, 344–351. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Lu, C.; Zeng, H.; Schumann, M.; Cheng, S. Supervised physical training enhances muscle strength but not muscle mass in prostate cancer patients undergoing androgen deprivation therapy: A systematic review and meta-analysis. Front. Physiol. 2019, 10, 843. [Google Scholar] [CrossRef]
- Staron, R.S.; Leonardi, M.J.; Karapondo, D.L.; Malicky, E.S.; Falkel, J.E.; Hagerman, F.C.; Hikida, R.S.; Stuart, C.A.; Lee, M.L.; South, M.A.; et al. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J. Appl. Physiol. 1991, 70, 631–640. [Google Scholar] [CrossRef]
- Bruusgaard, J.C.; Johansen, I.B.; Egner, I.M.; Rana, Z.A.; Gundersen, K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc. Natl. Acad. Sci. USA 2010, 107, 15111–15116. [Google Scholar] [CrossRef]
- Kadi, F.; Eriksson, A.; Holmner, S.; Thornell, L.E. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med. Sci. Sport. Exerc. 1999, 31, 1528–1534. [Google Scholar] [CrossRef]
- Cleland, J.; Cashmore, E.; Dixon, K. Why do sports fans support or oppose the inclusion of trans women in women’s sports? An empirical study of fairness and gender identity. Sport. Soc. 2022, 25, 2381–2396. [Google Scholar] [CrossRef]
- Burke, M. Trans women participation in sport: A feminist alternative to Pike’s position. J. Philos. Sport. 2022, 49, 212–229. [Google Scholar] [CrossRef]
- Devine, C. Female Olympians’ voices: Female sports categories and International Olympic Committee Transgender guidelines. Int. Rev. Sociol. Sport. 2022, 57, 335–361. [Google Scholar] [CrossRef]
- Burke, M. Trans women participation in sport: A commentary on the conservatism of gender critical feminism. Int. J. Sport. Policy Politics 2022, 14, 689–696. [Google Scholar] [CrossRef]
- Pigozzi, F.; Bigard, X.; Steinacker, J.; Wolfarth, B.; Badtieva, V.; Schneider, C.; Swart, J.; Bilzon, J.L.J.; Constantinou, D.; Dohi, M.; et al. Joint position statement of the International Federation of Sports Medicine (FIMS) and European Federation of Sports Medicine Associations (EFSMA) on the IOC framework on fairness, inclusion and non-discrimination based on gender identity and sex variations. BMJ Open Sport Exerc. Med. 2022, 8, 1273. [Google Scholar] [CrossRef]
- Menzel, T.; Braumuller, B.; Hartmann-Tews, I. The Relevance of Sexual Orientation and Gender Identity in Sport in Europe: Findings from the Outsport Survey; German Sport University Cologne, Institute of Sociology and Gender Studies: Cologne, Germany, 2019. [Google Scholar]
- Mermaids. IOC Guidelines: Mermaids Statement on the New Framework for Trans Athletes. 2021. Available online: https://mermaidsuk.org.uk/news/ioc-guidelines-mermaids-statement-on-the-new-framework/ (accessed on 17 March 2023).
- Sport Wales, Sport England, UK Sport, Sport Scotland, Sport Northern Ireland. The UK’s Sports Councils Guidance for Transgender Inclusion in Domestic Sport. 2021. Available online: https://equalityinsport.org/docs/300921/Guidance%20for%20Transgender%20Inclusion%20in%20Domestic%20Sport%202021.pdf (accessed on 12 March 2023).
- Pike, J. Safety, fairness, and inclusion: Transgender athletes and the essence of Rugby. J. Philos. Sport. 2021, 48, 155–168. [Google Scholar] [CrossRef]
- British Triathlon. FRG029-Transgender Policy; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2021; Volume 51. [Google Scholar]
- ILGA-Europe; Euro Central Asian Lesbian* Community (EL*C); TGEU; Organisation Intersex International Europe (OII Europe); European Gay; Lesbian Sport Federation (EGLSF). LBTI Women in Sport: Violence, Discrimination, and Lived Experiences. 2021. Available online: https://www.ilga-europe.org/report/lbti-women-in-sport-violence-discrimination-and-lived-experiences/ (accessed on 12 March 2023).
- Devine, C. Female Sports Participation, Gender Identity and the British 2010 Equality Act. Sport. Ethics Philos. 2022, 16, 503–525. [Google Scholar] [CrossRef]
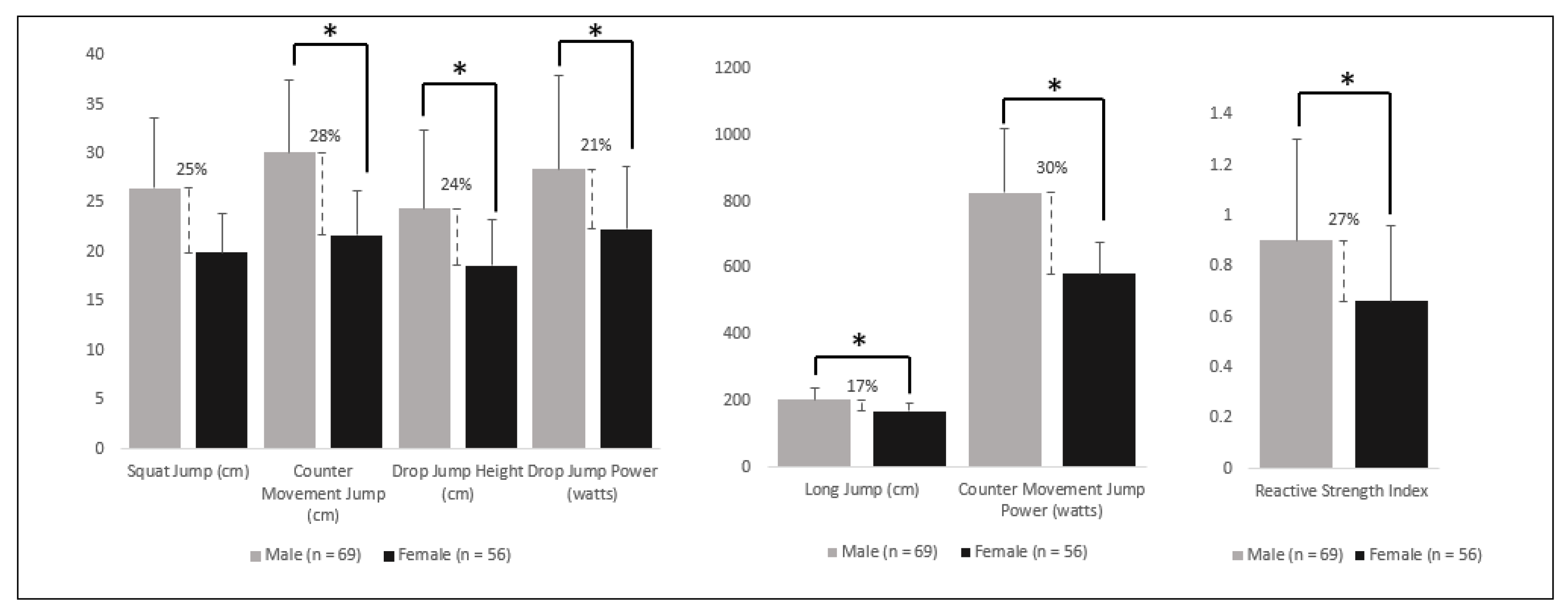
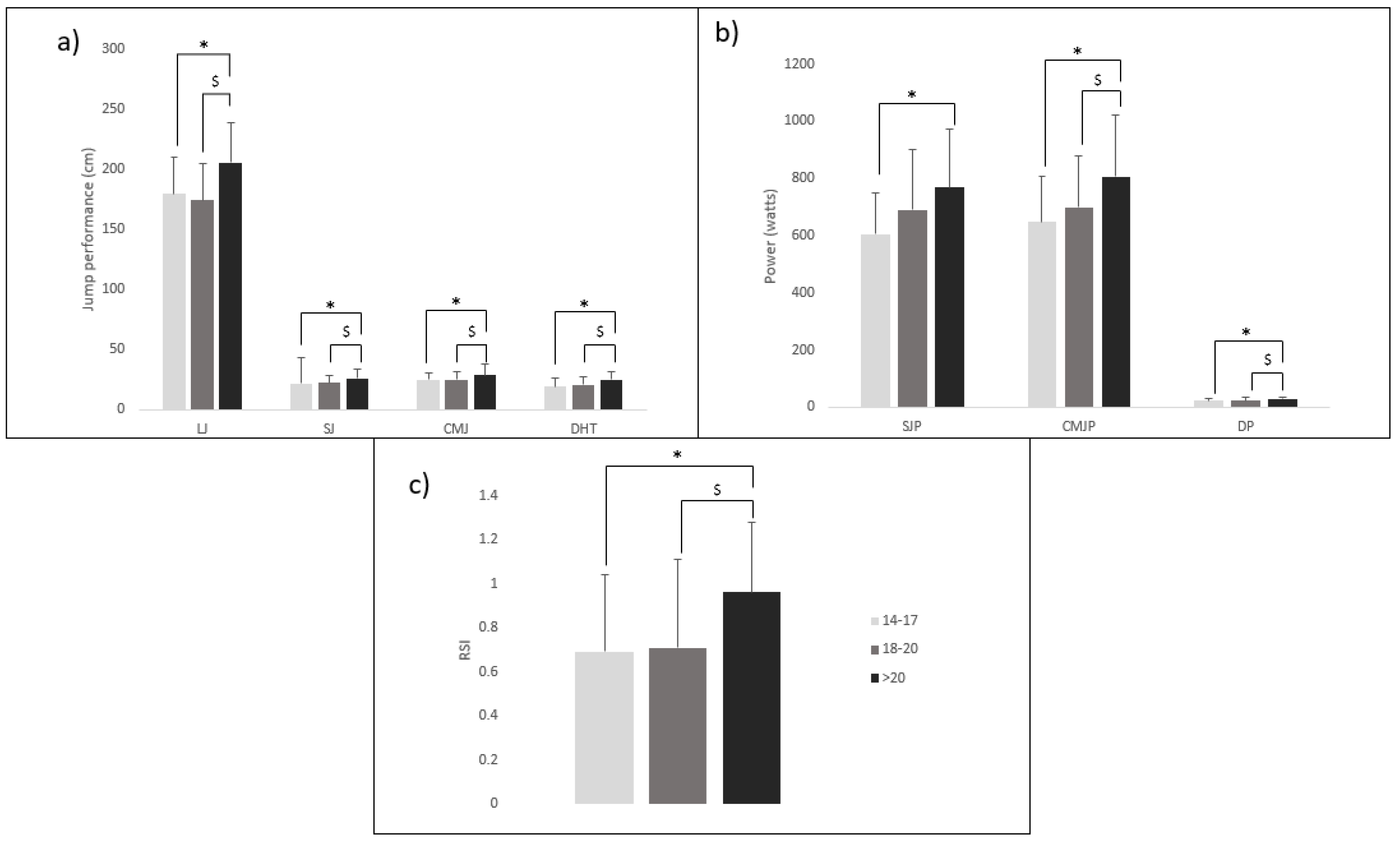
| Variables | Male Advantage (%) |
|---|---|
| Limb length | ~12 |
| Cardiovascular function | ~27 |
| Muscle Mass | ~37 |
| Muscle Strength | ~55 |
| Elite Sport | Male Advantage (%) |
|---|---|
| Swimming, Rowing, Running | ~12 |
| Jumping | ~20 |
| Upper body dominant | ~20 |
| Combat | ≥30 |
| Weightlifting | ≥30 |
| Throwing | ≥40 |
| Females | Males | p Value | |
|---|---|---|---|
| Height (cm) | 167.9 ± 6.2 | 177.6 ± 8.9 | 0.001 |
| Body mass (Kg) | 57.6 ± 7.0 | 70.4 ± 11.9 | 0.001 |
| Arm span (cm) | 168.8 ± 7.2 | 181.8 ± 9.7 | 0.001 |
| Leg length (cm) | 81.7 ± 4.3 | 88.9 ± 6.4 | 0.001 |
| BMI | 20.5 ± 2.1 | 22.2 ± 3.0 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tidmas, V.; Halsted, C.; Cohen, M.; Bottoms, L. The Participation of Trans Women in Competitive Fencing and Implications on Fairness: A Physiological Perspective Narrative Review. Sports 2023, 11, 133. https://doi.org/10.3390/sports11070133
Tidmas V, Halsted C, Cohen M, Bottoms L. The Participation of Trans Women in Competitive Fencing and Implications on Fairness: A Physiological Perspective Narrative Review. Sports. 2023; 11(7):133. https://doi.org/10.3390/sports11070133
Chicago/Turabian StyleTidmas, Victoria, Clare Halsted, Mary Cohen, and Lindsay Bottoms. 2023. "The Participation of Trans Women in Competitive Fencing and Implications on Fairness: A Physiological Perspective Narrative Review" Sports 11, no. 7: 133. https://doi.org/10.3390/sports11070133
APA StyleTidmas, V., Halsted, C., Cohen, M., & Bottoms, L. (2023). The Participation of Trans Women in Competitive Fencing and Implications on Fairness: A Physiological Perspective Narrative Review. Sports, 11(7), 133. https://doi.org/10.3390/sports11070133






