Nine Months of Hybrid Intradialytic Exercise Training Improves Ejection Fraction and Cardiac Autonomic Nervous System Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Hybrid Intradialytic Exercise Program
2.3. Hemodialysis Procedure
2.4. Echocardiography
2.5. Heart Rate Variability Assessment
2.6. Blood Chemistry
2.7. Statistical Analysis
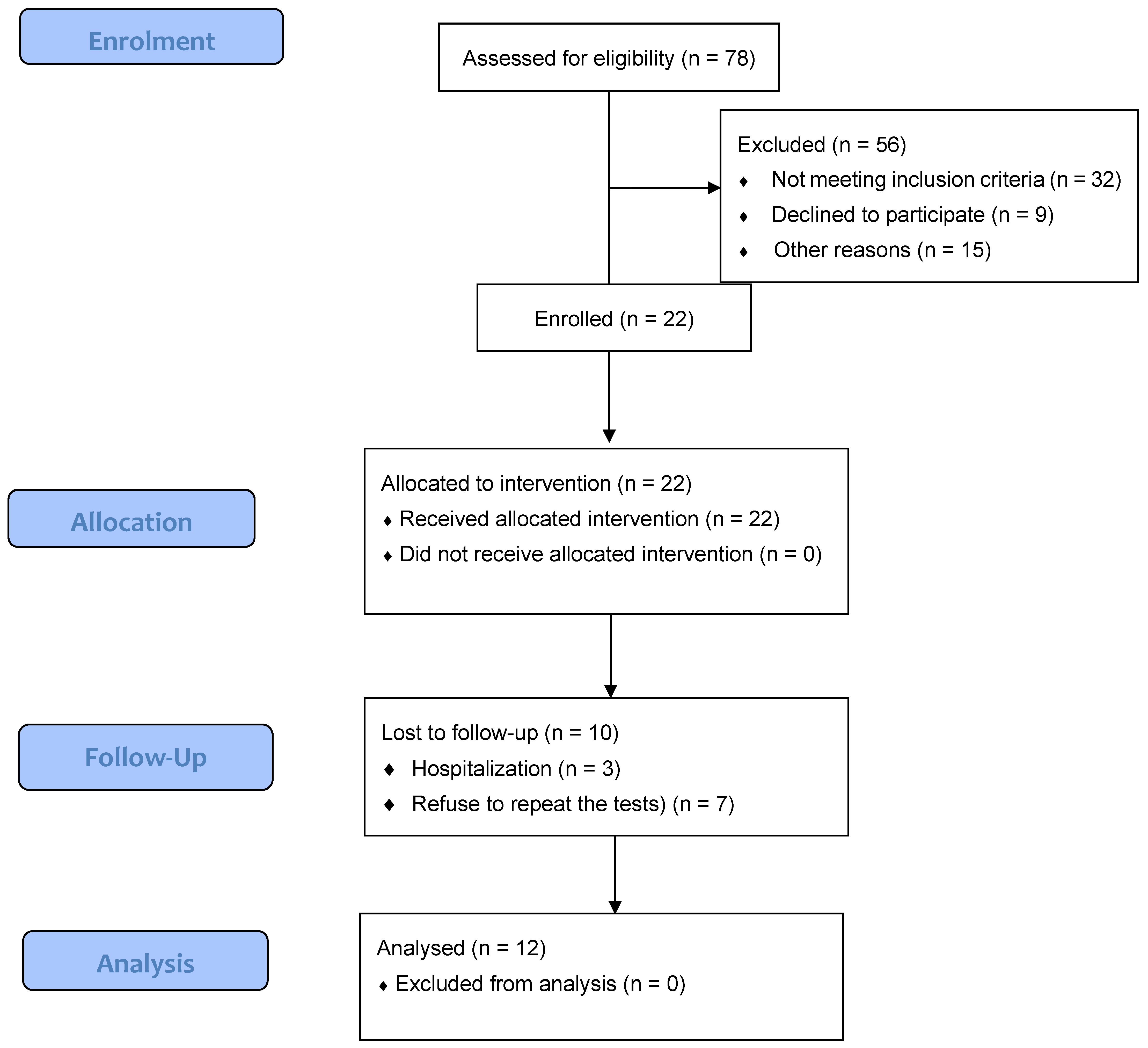
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Ahmadmehrabi, S.; Tang, W.H.W. Hemodialysis-induced cardiovascular disease. Semin. Dial. 2018, 31, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Salman, I.M. Cardiovascular Autonomic Dysfunction in Chronic Kidney Disease: A Comprehensive Review. Curr. Hypertens. Rep. 2015, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Painter, P. Determinants of exercise capacity in CKD patients treated with hemodialysis. Adv. Chronic Kidney Dis. 2009, 16, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Robertson, C.E.; Addison, P.S. Heart rate variability measurements and the prediction of ventricular arrhythmias. QJM Mon. J. Assoc. Physicians 2005, 98, 87–95. [Google Scholar] [CrossRef]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef]
- McIntyre, C.W. Haemodialysis-induced myocardial stunning in chronic kidney disease—A new aspect of cardiovascular disease. Blood Purif. 2010, 29, 105–110. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, W.; Zhang, H. The Role of Physical Activity and Mortality in Hemodialysis Patients: A Review. Front. Public Health 2022, 10, 818921. [Google Scholar] [CrossRef]
- Wilund, K.R.; Thompson, S.; Viana, J.L.; Wang, A.Y. Physical Activity and Health in Chronic Kidney Disease. Contrib. Nephrol. 2021, 199, 43–55. [Google Scholar] [CrossRef]
- Martins, P.; Marques, E.A.; Leal, D.V.; Ferreira, A.; Wilund, K.R.; Viana, J.L. Association between physical activity and mortality in end-stage kidney disease: A systematic review of observational studies. BMC Nephrol. 2021, 22, 227. [Google Scholar] [CrossRef]
- Clarkson, M.J.; Bennett, P.N.; Fraser, S.F.; Warmington, S.A. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: A systematic review and meta-analysis. Am. J. Physiol. Ren. Physiol. 2019, 316, F856–F872. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, G.K.; Giannaki, C.D.; Karatzaferi, C.; Maridaki, M.; Koutedakis, Y.; Hadjigeorgiou, G.M.; Stefanidis, I. Current trends in the management of uremic restless legs syndrome: A systematic review on aspects related to quality of life, cardiovascular mortality and survival. Sleep Med. Rev. 2015, 21, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wilund, K.R.; Viana, J.L.; Perez, L.M. A Critical Review of Exercise Training in Hemodialysis Patients: Personalized Activity Prescriptions Are Needed. Exerc. Sport Sci. Rev. 2020, 48, 28–39. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Andrade, F.P.; Leal, D.V.; Oliveira, J.S.; Wilund, K.R.; Viana, J.L. How is exercise being prescribed for patients on hemodialysis? A scoping review. J. Nephrol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Larsen, A.I.; Gjesdal, K.; Hall, C.; Aukrust, P.; Aarsland, T.; Dickstein, K. Effect of exercise training in patients with heart failure: A pilot study on autonomic balance assessed by heart rate variability. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 162–167. [Google Scholar] [CrossRef]
- Momeni, A.; Nematolahi, A.; Nasr, M. Effect of intradialytic exercise on echocardiographic findings in hemodialysis patients. Iran J. Kidney Dis. 2014, 8, 207–211. [Google Scholar] [PubMed]
- Deligiannis, A.; Kouidi, E.; Tassoulas, E.; Gigis, P.; Tourkantonis, A.; Coats, A. Cardiac effects of exercise rehabilitation in hemodialysis patients. Int. J. Cardiol. 1999, 70, 253–266. [Google Scholar] [CrossRef]
- Graham-Brown, M.P.M.; March, D.S.; Young, R.; Highton, P.J.; Young, H.M.L.; Churchward, D.R.; Dungey, M.; Stensel, D.J.; Bishop, N.C.; Brunskill, N.J.; et al. A randomized controlled trial to investigate the effects of intra-dialytic cycling on left ventricular mass. Kidney Int. 2021, 99, 1478–1486. [Google Scholar] [CrossRef]
- Johansen, K.L.; Painter, P. Exercise in individuals with CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2012, 59, 126–134. [Google Scholar] [CrossRef]
- Vogiatzaki, E.; Michou, V.; Liakopoulos, V.; Roumeliotis, A.; Roumeliotis, S.; Kouidi, E.; Deligiannis, A. The effect of a 6-month intradialytic exercise program on hemodialysis adequacy and body composition: A randomized controlled trial. Int. Urol. Nephrol. 2022, 54, 2983–2993. [Google Scholar] [CrossRef]
- Bronas, U.G. Exercise training and reduction of cardiovascular disease risk factors in patients with chronic kidney disease. Adv. Chronic Kidney Dis. 2009, 16, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Deligiannis, A.; D’Alessandro, C.; Cupisti, A. Exercise training in dialysis patients: Impact on cardiovascular and skeletal muscle health. Clin. Kidney J. 2021, 14, ii25–ii33. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S.; Horton, E.J.; Renshaw, D.; Chan, K.; Jimenez, A.; Maddock, H.; Krishnan, N.; McGregor, G. Cardiac stunning during haemodialysis: The therapeutic effect of intra-dialytic exercise. Clin. Kidney J. 2021, 14, 1335–1344. [Google Scholar] [CrossRef]
- Penny, J.D.; Salerno, F.R.; Brar, R.; Garcia, E.; Rossum, K.; McIntyre, C.W.; Bohm, C.J. Intradialytic exercise preconditioning: An exploratory study on the effect on myocardial stunning. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2019, 34, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, S.S.; Krase, A.A.; Karatzaferi, C.; Giannaki, C.D.; Lavdas, E.; Mitrou, G.I.; Bloxham, S.; Stefanidis, I.; Sakkas, G.K. Long-term intradialytic hybrid exercise training on fatigue symptoms in patients receiving hemodialysis therapy. Int. Urol. Nephrol. 2021, 53, 771–784. [Google Scholar] [CrossRef]
- Grigoriou, S.S.; Giannaki, C.D.; George, K.; Karatzaferi, C.; Zigoulis, P.; Eleftheriadis, T.; Stefanidis, I.; Sakkas, G.K. A single bout of hybrid intradialytic exercise did not affect left-ventricular function in exercise-naive dialysis patients: A randomized, cross-over trial. Int. Urol. Nephrol. 2022, 54, 201–208. [Google Scholar] [CrossRef]
- Heyward, V. Advanced Fitness Assessment and Exercise Prescription, 3rd ed.; Heyward, Human Kinetics Publishers Inc.: Champaign, IL, USA, 1997. [Google Scholar]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Berthoin, S.; Bosquet, L. Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med. Sci. Sports Exerc. 2006, 38, 887–893. [Google Scholar] [CrossRef]
- Dinas, P.C.; Koutedakis, Y.; Flouris, A.D. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int. J. Cardiol. 2013, 163, 109–115. [Google Scholar] [CrossRef]
- Kouidi, E.J. Central and peripheral adaptations to physical training in patients with end-stage renal disease. Sports Med. 2001, 31, 651–665. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Stefanidis, I.; Karatzaferi, C.; Liakos, N.; Roka, V.; Ntente, I.; Sakkas, G.K. The effect of prolonged intradialytic exercise in hemodialysis efficiency indices. ASAIO J. 2011, 57, 213–218. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Sakkas, G.K.; Hadjigeorgiou, G.M.; Karatzaferi, C.; Patramani, G.; Lavdas, E.; Liakopoulos, V.; Koutedakis, Y.; Stefanidis, I. Non-pharmacological management of periodic limb movements during hemodialysis session in patients with uremic restless legs syndrome. ASAIO J. 2010, 56, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.R.; Jung, H.H.; Kim, S.B.; Park, J.S.; Yang, W.S. Effects of regular exercise on anxiety, depression, and quality of life in maintenance hemodialysis patients. Ren. Fail. 2002, 24, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, G.K.; Hadjigeorgiou, G.M.; Karatzaferi, C.; Maridaki, M.D.; Giannaki, C.D.; Mertens, P.R.; Rountas, C.; Vlychou, M.; Liakopoulos, V.; Stefanidis, I. Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: A pilot study. ASAIO J. 2008, 54, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Kouidi, E.; Karagiannis, V.; Grekas, D.; Iakovides, A.; Kaprinis, G.; Tourkantonis, A.; Deligiannis, A. Depression, heart rate variability, and exercise training in dialysis patients. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 160–167. [Google Scholar] [CrossRef]
- Deligiannis, A.; Kouidi, E.; Tourkantonis, A. Effects of physical training on heart rate variability in patients on hemodialysis. Am. J. Cardiol. 1999, 84, 197–202. [Google Scholar] [CrossRef]
- Cashion, A.K.; Holmes, S.L.; Arheart, K.L.; Acchiardo, S.R.; Hathaway, D.K. Heart rate variability and mortality in patients with end stage renal disease. Nephrol. Nurs. J. 2005, 32, 173–184. [Google Scholar]
- Ghafourifard, M.; Mehrizade, B.; Hassankhani, H.; Heidari, M. Hemodialysis patients perceived exercise benefits and barriers: The association with health-related quality of life. BMC Nephrol. 2021, 22, 94. [Google Scholar] [CrossRef]
- Reboredo Mde, M.; Pinheiro Bdo, V.; Neder, J.A.; Avila, M.P.; Araujo, E.R.M.L.; de Mendonca, A.F.; de Mello, M.V.; Bainha, A.C.; Dondici Filho, J.; de Paula, R.B. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J. Bras. Nefrol. Orgao Of. Soc. Bras. E Lat. Am. Nefrol. 2010, 32, 367–373. [Google Scholar]
| Variables | Pre | Post 9 Months |
|---|---|---|
| N | 12 | 12 |
| Female/Male | 2/10 | |
| Age (year) | 56 ± 19 | 57 ± 17 |
| Dry Weight (kg) | 73.2 ± 16.4 | 75.4 ± 16.9 |
| Height (m) | 1.69 ± 0.10 | 1.69 ± 0.10 |
| BMI (kg/m2) | 26.1 ± 5.2 | 26.9 ± 5.5 |
| Months in dialysis | 40 ± 44 | |
| WHR | 1.02 ± 0.12 | 1.00 ± 0.1 |
| CRP (mg/dL) | 3.7 ± 5.6 | 0.8 ± 0.5 |
| HCT | 34.7 ± 4.0 | 34.2 ± 3.2 |
| Hb(g/dL) | 11.2 ± 1.3 | 10.8 ± 1.0 |
| Iron(μg/dL) | 58.2 ± 30.8 | 51.7 ± 27.2 |
| Ferritin (ng/mL) | 1377.3 ± 1170.4 | 754.3 ± 518.7 |
| Parameter | Scenario | Pre HD | During HD | Post HD |
|---|---|---|---|---|
| Standard Echocardiographic Indices | ||||
| IVSTd(mm) | Pre | * 11.9 ± 2.2 | 11.1 ± 2.3 | 10.4 ± 1.8 |
| Post 9 months | * 9.9 ± 2.3 | 11.0 ± 4.2 | 9.9 ± 2.5 | |
| Cohens’s d | 0.88 | 0.02 | 0.22 | |
| LVPWTd (mm) | Pre | 11.0 ± 2.4 | 10.4 ± 2.4 | 9.9 ± 1.8 |
| Post 9 months | 9.9 ± 2.4 | 9.3 ± 2.5 | 9.8 ± 2.1 | |
| Cohens’s d | 0.45 | 0.44 | 0.05 | |
| LVIDd(mm) | Pre | 45.5 ± 4.6 | 28.8 ± 4.0 | 44.8 ± 4.5 |
| Post 9 months | 48.0 ± 6.2 | 45.6 ± 5.7 | 46.9 ± 5.6 | |
| Cohens’s d | −0.45 | −3.41 | −0.41 | |
| LV mass(g) | Pre | 57.8 ± 9.0 | 54.2 ± 10.7 | 51.7 ± 6.9 |
| Post 9 months | 55.4 ± 10.2 | 56.5 ± 15.9 | 55.1 ± 10.2 | |
| Cohens’s d | 0.24 | −0.16 | −0.39 | |
| LV mass/BSA(g/m2) | Pre | 31.7 ± 3.8 | 26.7 ± 10.0 | 28.2 ± 4.3 |
| Post 9 months | 29.7 ± 4.4 | 30.0 ± 5.8 | 29.3 ± 5.5 | |
| Cohens’s d | 0.48 | −0.40 | −0.22 | |
| LV mass/height27 | Pre | 14.7 ± 2.2 | 12.3 ± 4.8 | 12.9 ± 2.1 |
| Post 9 months | 14.1 ± 2.9 | 14.0 ± 3.7 | 13.6 ± 3.1 | |
| Cohens’s d | 0.23 | −0.39 | −0.26 | |
| EF (%) | Pre | * 48.7 ± 11.1 | 52.8 ± 10.1 | * 50.0 ± 13.4 |
| Post 9 months | * 58.8 ± 6.5 | 60.4 ± 10.1 | * 56.1 ± 3.4 | |
| Cohens’s d | −1.11 | −0.75 | −0.61 | |
| Doppler Mitral Inflow Indices | ||||
| E (mm/s) | Pre | 0.8 ± 0.2 | 0.6 ± 0.13 | 0.7 ± 0.1 |
| Post 9 months | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | |
| Cohens’s d | 0 | 0 | 0 | |
| A (mm/s) | Pre | 0.9 ± 0.2 | 0.8 ± 0.3 | 0.9 ± 0.3 |
| Post 9 months | 0.9 ± 0.3 | 0.8 ± 0 3 | 0.8 ± 0.3 | |
| Cohens’s d | 0 | 0 | 0.33 | |
| E/A | Pre | 0.9 ± 0.2 | 0.9 ± 0.3 | 1.0 ± 0.4 |
| Post 9 months | 1.1 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.3 | |
| Cohens’s d | −0.63 | 0 | 0.28 | |
| DT (ms) | Pre | ** 250.9 ± 48.0 | 255.3 ± 70.2 | ** 261.1 ± 61.9 |
| Post 9 months | ** 192.3 ± 41.4 | 228.5 ± 43.1 | ** 215.0 ± 50.5 | |
| Cohens’s d | 1.30 | 0.46 | 0.81 | |
| IVRT(ms) | Pre | 62.1 ± 12.4 | 56.6 ± 12.6 | 63.8 ± 13.2 |
| Post 9 months | 73.4 ± 13.3 | 69.4 ± 17.8 | 71.6 ± 14.7 | |
| Cohens’s d | −0.87 | −0.83 | −0.55 | |
| Tissue Doppler Myocardial Velocities Indices | ||||
| E’ (mm/s) | Pre | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Post 9 months | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| Cohens’s d | 0 | 0 | 0 | |
| A’ (mm/s) | Pre | 0.9 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Post 9 months | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | |
| Cohens’s d | 0 | 0 | 0 | |
| E’/A’ | Pre | 1.0 ± 0.4 | 0.9 ± 0.5 | 0.8 ± 0.4 |
| Post 9 months | 1.3 ± 1.1 | 0.8 ± 0.3 | 1.1 ± 0.7 | |
| Cohens’s d | −0.36 | 0.24 | −0.52 | |
| E/E’ | Pre | 10.2 ± 3.5 | 8.1 ± 2.7 | 10.0 ± 4.1 |
| Post 9 months | 8.4 ± 3.4 | 8.6 ± 3.7 | 7.5 ± 3.7 | |
| Cohens’s d | 0.52 | −0.15 | 0.64 | |
| Pre HD | 1 | 2 | 3 | 4 | End of HD | ||
|---|---|---|---|---|---|---|---|
| SDNN(ms) | Pre Post 9 months | 64.03 ± 44.1 48.4 ± 19.8 | 74.5 ± 32.2 55.7 ± 22.9 | 58.8 ± 32.9 62.9 ± 35.2 | 55.1 ± 35.1 63.8 ± 38.4 | 53.8 ± 30.9 96.7 ± 79.6 | 52.5 ± 31.5 164.8 ± 241.0 |
| Cohens’s d | 0.45 | 0.70 | −0.12 | −0.23 | −0.71 | −0.65 | |
| mean RR interval (ms) | Pre Post 9 months | * 838.6 ± 93.3 802.7 ± 70.8 | 800.1 ± 103.6 805.9 ± 79.6 | 763.2 ± 121.4 811.3 ± 96.9 | 711.0 ± 154.5 807.4 ± 88.0 | 730.4 ± 156.1 805.3 ± 99.3 | 718.7 ± 155.6 799.8 ± 103.7 |
| Cohens’s d | 0.43 | −0.06 | −0.43 | −0.76 | −0.57 | −0.61 | |
| LF (ms2) | Pre Post 9 months | # 67.2 ± 16.5 58.9 ± 20.1 | 68.0 ± 12.9 67.8 ± 17.5 | 69.2 ± 10.8 67.2 ± 70.7 | 68.4 ± 18.6 67.2 ± 16.4 | 67.6 ± 22.8 62.2 ± 17.3 | 71.3 ± 22.6 66.1 ± 25.2 |
| Cohens’s d | 0.45 | 0.01 | 0.03 | 0.06 | 0.26 | 0.21 | |
| HF (ms2) | Pre Post 9 months | # 32.8 ± 16.5 41.1 ± 20.1 | 32.1 ± 12.9 32.2 ± 17.5 | 30.8 ± 10.8 32.8 ± 16.4 | 31.6 ± 18.6 32.8 ± 16.4 | 32.4 ± 22.8 37.8 ± 17.3 | 28.7 ± 22.6 33.9 ± 25.2 |
| Cohens’s d | −0.45 | −0.00 | −0.14 | −0.06 | −0.26 | −0.21 | |
| LF/HF ratio | Pre Post 9 months | 2.7 ± 1.5 2.1 ± 1.7 | 2.7 ± 1.5 2.9 ± 1.8 | 2.7 ± 1.5 2.9 ± 2.4 | 3.5 ± 2.7 5.3 ± 7.0 | 3.6 ± 2.6 2.2 ± 1.3 | 5.2 ± 5.2 8.2 ± 17.6 |
| Cohens’s d | 0.37 | −0.12 | −0.09 | −0.33 | 0.68 | −0.23 | |
| rMSSD(ms) | Pre Post 9 months | 42.7 ± 67.2 31.1 ± 17.2 | 32.0 ± 23.3 42.6 ± 34.3 | 24.0 ± 12.1 52.3 ± 56.9 | 20.5 ± 17.5 49.7 ± 58.8 | 31.8 ± 37.3 76.7 ± 102.2 | 28.8 ± 31.1 79.8 ± 79.7 |
| Cohens’s d | 0.23 | −0.36 | −0.68 | −0.67 | −0.58 | −0.84 | |
| pNN50% | Pre Post 9 months | 7.7 ± 11.4 10.3 ± 13.2 | # 5.8 ± 4.3 6.3 ± 7.4 | 4.5 ± 3.9 10.0 ± 15.1 | 6.6 ± 12.3 10.0 ± 15.1 | 9.1 ± 14.8 10.3 ± 15.8 | 8.1 ± 12.1 9.5 ± 13.7 |
| Cohens’s d | −0.21 | −0.08 | −0.49 | −0.24 | −0.07 | −0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannaki, C.D.; Grigoriou, S.S.; George, K.; Karatzaferi, C.; Zigoulis, P.; Lavdas, E.; Chaniotis, D.; Stefanidis, I.; Sakkas, G.K. Nine Months of Hybrid Intradialytic Exercise Training Improves Ejection Fraction and Cardiac Autonomic Nervous System Activity. Sports 2023, 11, 79. https://doi.org/10.3390/sports11040079
Giannaki CD, Grigoriou SS, George K, Karatzaferi C, Zigoulis P, Lavdas E, Chaniotis D, Stefanidis I, Sakkas GK. Nine Months of Hybrid Intradialytic Exercise Training Improves Ejection Fraction and Cardiac Autonomic Nervous System Activity. Sports. 2023; 11(4):79. https://doi.org/10.3390/sports11040079
Chicago/Turabian StyleGiannaki, Christoforos D., Stefania S. Grigoriou, Keith George, Christina Karatzaferi, Paris Zigoulis, Eleftherios Lavdas, Dimitrios Chaniotis, Ioannis Stefanidis, and Giorgos K. Sakkas. 2023. "Nine Months of Hybrid Intradialytic Exercise Training Improves Ejection Fraction and Cardiac Autonomic Nervous System Activity" Sports 11, no. 4: 79. https://doi.org/10.3390/sports11040079
APA StyleGiannaki, C. D., Grigoriou, S. S., George, K., Karatzaferi, C., Zigoulis, P., Lavdas, E., Chaniotis, D., Stefanidis, I., & Sakkas, G. K. (2023). Nine Months of Hybrid Intradialytic Exercise Training Improves Ejection Fraction and Cardiac Autonomic Nervous System Activity. Sports, 11(4), 79. https://doi.org/10.3390/sports11040079









