“Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review
Abstract
:1. Introduction
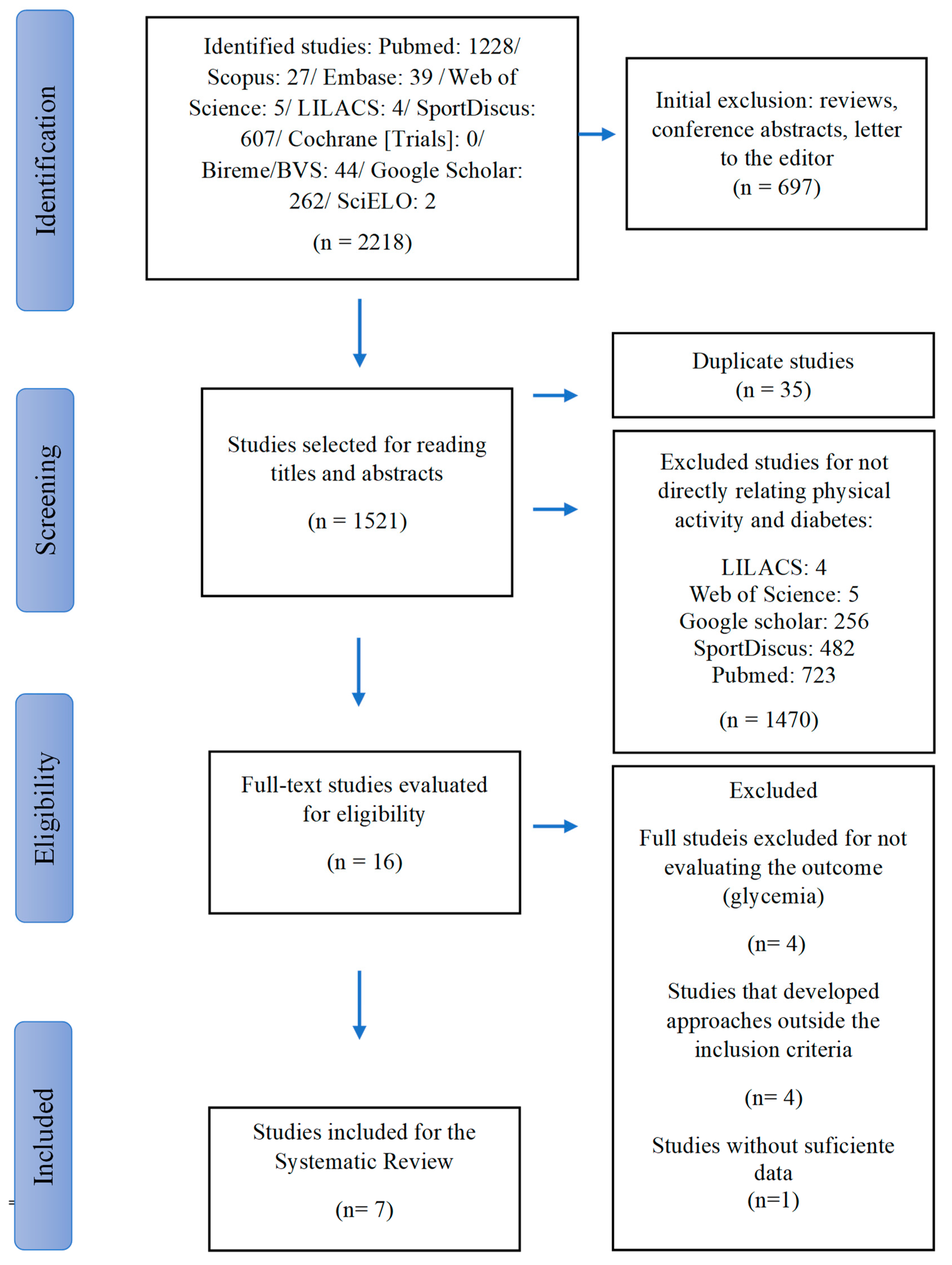
2. Materials and Methods
2.1. Study Design, Sources, and Search Strategy
2.2. Search Strategies
2.3. Inclusion of Studies
2.4. Inclusion and Exclusion Criteria
2.5. Procedure
2.6. Data Extraction
2.7. Assessment of the Quality of Individual Studies
3. Results
3.1. Characteristics of Eligible Studies and Population/Sample Details
3.2. Characterization of DM Patients Affected by COVID-19: Type of DM, Plasma Glucose Concentration and Administration of Exogenous Insulin during the Pandemic
3.3. Characterization of Studies Regarding Levels of Physical Activity, Plasma Glucose, Body Weight, and HbA1c in DM Patients during Restrictions of the COVID-19 Pandemic
3.4. Quality of Studies
4. Discussion
4.1. Maintenance of Adequate Levels of Physical Activity for Glycemic Control and Maintenance of HbA1c Concentration in Diabetic Patients during the COVID-19 Pandemic
4.2. Level of Physical Activity and Its Relationship with Maintenance and Reduction of Body Weight and BMI in DM Patients during the COVID-19 Pandemic
4.3. Restrictive COVID-19 Measures and Glycemic and HbA1c
4.4. COVID-19 and Risks for Patients Diagnosed with Diabetes
4.5. Weaknesses Detected during Article Analysis and Suggestions for Future Studies
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. COVID-19 Dashboard; World Health Organization: Geneva, Switzerland, 2020; Available online: https://covid19.who.int/ (accessed on 14 August 2023).
- Guo, J.; Lin, W.H.W.; Zucker, J.E.; Nandakumar, R.; Uhlemann, A.C.; Wang, S.; Shivakoti, R. Inflammation and Mortality in COVID-19 Hospitalized Patients with and without Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1961–1968. [Google Scholar] [CrossRef]
- Stefan, N. Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat. Rev. Endocrinol. 2022, 18, 75–76. [Google Scholar] [CrossRef]
- Müller-Wieland, D.; Marx, N.; Dreher, M.; Fritzen, K.; Schnell, O. COVID-19 and Cardiovascular Comorbidities. Exp. Clin. Endocrinol. Diabetes 2022, 130, 178–189. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Ye, S.; Lian, H.; Wang, H.; Ye, J. Obesity and COVID-19: Mechanistic Insights From Adipose Tissue. J. Clin. Endocrinol. Metab. 2022, 107, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Chen, T.; Wang, S.I.; Hung, Y.M.; Chen, H.Y.; Wei, C.J. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine 2023, 56, 101783. [Google Scholar] [CrossRef]
- Smati, S.; Tramunt, B.; Wargny, M.; Gourdy, P.; Hadjadj, S.; Cariou, B. COVID-19 and Diabetes Outcomes: Rationale for and Updates from the CORONADO Study. Curr. Diab. Rep. 2022, 22, 53–63. [Google Scholar] [CrossRef]
- Norouzi, M.; Norouzi, S.; Ruggiero, A.; Khan, M.S.; Myers, S.; Kavanagh, K.; Vemuri, R. Type-2 diabetes as a risk factor for severe covid-19 infection. Microorganisms 2021, 9, 121. [Google Scholar] [CrossRef]
- Leite, N.J.C.; Raimundo, A.M.M.; Mendes, R.D.C.; Marmeleira, J.F.F. Impact of COVID-19 Pandemic on Daily Life, Physical Exercise, and General Health among Older People with Type 2 Diabetes: A Qualitative Interview Study. Int. J. Environ. Res. Public. Health 2022, 19, 3986. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Spence, A.L.; Mayerle, M.; Sardana, N.; Clay, C.M.; Eng, M.R.; Luedi, M.M.; Carroll Turpin, M.A.; Urman, R.D.; Cornett, E.M. Impact of COVID-19 infection on the cardiovascular system: An evidence-based analysis of risk factors and outcomes. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Naous, E.; Boulos, M.; Sleilaty, G.; Achkar, A.A.; Gannagé-Yared, M.H. Quality of life and other patient-reported outcomes in adult Lebanese patients with type 2 diabetes during COVID-19 pandemic. J. Endocrinol. Investig. 2022, 45, 763–772. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Care in Diabetes-2023 Abridged for Primary Care Providers. Clin. Diabetes 2023, 41, 4–31. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [PubMed]
- Hernández-Beltrán, V.; Espada, M.C.; Santos, F.J.; Ferreira, C.C.; Gamonales, J.M. Documents Publication Evolution (1990–2022) Related to Physical Activity and Healthy Habits, a Bibliometric Review. Healthcare 2023, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Khajehlandi, M.; Siahkuhian, M.; Akbarnejad, A.; Khoramipour, K.; Suzuki, K. Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus. Sports 2022, 10, 167. [Google Scholar] [CrossRef]
- Amin, M.; Kerr, D.; Atiase, Y.; Aldwikat, R.K.; Driscoll, A. Effect of Physical Activity on Metabolic Syndrome Markers in Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sports 2023, 11, 101. [Google Scholar] [CrossRef]
- American Heart Association. Recommendations for Physical Activity in Adults and Kids. In Physical Activity Guidelines Advisory Committee Scientific Report and the Physical Activity Guidelines for Americans, 2nd ed.; Available online: https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults (accessed on 2 August 2021).
- Delgado-floody, P.; Izquierdo, M.; Ramírez-Vélez, R.; Caamaño-Navarrete, F.; Moris, R.; Jerez-Mayorga, D.; Andrade, D.C.; Álvarez, C. Effect of High-Intensity Interval Training on Body Composition, Cardiorespiratory Fitness, Blood Pressure, and Substrate Utilization during Exercise among Prehypertensive and Hypertensive Patients with Excessive Adiposity. Front. Physiol. 2020, 11, 1171. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Wan, Q.; Shen, F.; Xu, M.; Zhao, Z.; Lu, J.; Gao, Z.; Chen, G.; Wang, T.; et al. Individual and combined associations of modifiable lifestyle and metabolic health status with new-onset diabetes and major cardiovascular events: The China cardiometabolic disease and cancer cohort (4C) study. Diabetes Care 2020, 43, 1929–1936. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid. Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, 65–94. [Google Scholar] [CrossRef]
- Souza, E.; Meneses, D.; Marçal, A. Physical Activity in Diabetic Patients during the COVID-19 Pandemic. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022365123 (accessed on 24 May 2023).
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club. 1995, 123, 12–13. [Google Scholar] [CrossRef]
- Rayyan Intelligent Systematic Rewies. Available online: https://www.rayyan.ai/ (accessed on 14 April 2023).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Al Agha, A.E.; Alharbi, R.S.; Almohammadi, O.A.; Yousef, S.Y.; Sulimani, A.E.; Alaama, R.A. Impact of COVID-19 lockdown on glycemic control in children and adolescents. Saudi Med. J. 2021, 42, 44–48. [Google Scholar] [CrossRef]
- Munekawa, C.; Hosomi, Y.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Osaka, T.; Okada, H.; Majima, S.; et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: A cross-section and retrospective cohort study. Endocr. J. 2021, 68, 201–210. [Google Scholar] [CrossRef]
- Ruissen, M.M.; Regeer, H.; Landstra, C.P.; Schroijen, M.; Jazet, I.; Nijhoff, M.F.; Pijl, H.; Ballieux, B.E.P.B.; Dekkers, O.; Huisman, S.D.; et al. Increased stress, weight gain and less exercise in relation to glycemic control in people with type 1 and type 2 diabetes during the COVID-19 pandemic. BMJ Open Diabetes Res. Care 2021, 9, e002035. [Google Scholar] [CrossRef]
- Assaloni, R.; Pellino, V.C.; Puci, M.V.; Ferraro, O.E.; Lovecchio, N.; Girelli, A.; Vandoni, M. Coronavirus disease (COVID-19): How does the exercise practice in active people with type 1 diabetes change? A preliminary survey. Diabetes Res. Clin. Pract. 2020, 166, 108297. [Google Scholar] [CrossRef]
- Dalmazi, G.; Maltoni, G.; Bongiorno, C.; Tucci, L.; di Natale, V.; Moscatiello, S.; Laffi, G.; Pession, A.; Zucchini, S.; Pagotto, U. Comparison of the effects of lockdown due to COVID-19 on glucose patterns among children, adolescents, and adults with type 1 diabetes: CGM study. BMJ Open Diabetes Res. Care 2020, 8, e001664. [Google Scholar] [CrossRef] [PubMed]
- Minuto, N.; Bassi, M.; Montobbio, C.; Vinci, F.; Mercuri, C.; Perri, F.N.; Cabri, M.; Calevo, M.G.; d’Annunzio, G.; Maghnie, M. The Effect of Lockdown and Physical Activity on Glycemic Control in Italian Children and Young Patients With Type 1 Diabetes. Front. Endocrinol. 2021, 12, 690222. [Google Scholar] [CrossRef] [PubMed]
- Tornese, G.; Ceconi, V.; Monasta, L.; Carletti, C.; Faleschini, E.; Barbi, E. Glycemic Control in Type 1 Diabetes Mellitus during COVID-19 Quarantine and the Role of In-Home Physical Activity. Diabetes Technol. Ther. 2020, 22, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Strelitz, J.; Lawlor, E.R.; Wu, Y.; Estlin, A.; Nandakumar, G.; Ahern, A.L.; Griffin, S.J. Association between weight change and incidence of cardiovascular disease events and mortality among adults with type 2 diabetes: A systematic review of observational studies and behavioural intervention trials. Diabetologia 2022, 65, 424–439. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- Yajuan, W.; Eldin, D.; Roberta, J.; Tamer, F.; Scott, B.; Stefanie, P.; Madan, A.; Shah, B.R. Association of physical activity on blood glucose in individuals with type 2 diabetes. Transl. Behav. Med. 2022, 12, 448–453. [Google Scholar]
- Belanger, M.J.; Rao, P.; Robbins, J.M. Exercise, Physical Activity, and Cardiometabolic Health: Pathophysiologic Insights. Cardiol. Rev. 2022, 30, 134–144. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Hulett, N.A.; Rebecca, L.S.; Jane, E.B.R. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 3, 647. [Google Scholar] [CrossRef]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; Van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A.; et al. Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhao, M.; Voilquin, L.; Jung, Y.; Aikio, M.A.; Sahai, T.; Dou, F.Y.; Roche, A.M.; Carcamo-Orive, I.; Knowles, J.W.; et al. Isthmin-1 is an adipokine that promotes glucose uptake and improves glucose tolerance and hepatic steatosis. Cell Metab. 2021, 33, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Emadi, A.; Shab-Bidar, S. Dose-Dependent Effect of Supervised Aerobic Exercise on A1C in Patients with Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Sports Med. 2022, 52, 1919–1938. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ezzatvar, Y.; Huerta-Uribe, N.; Alonso-Martínez, A.M.; Chueca-Guindulain, M.J.; Berrade-Zubiri, S.; Izquierdo, M.; Ramírez-Vélez, R. Effects of exercise training on glycaemic control in youths with type 1 diabetes: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Sport Sci. 2022, 23, 1056–1067. [Google Scholar] [CrossRef]
- Nair, R.; Meadows, E.; Sheer, R.; Lipkovich, I.; Poon, J.L.; Zhao, Z.; Benneyworth, B.; Pasquale, M. Activation, physical activity, and outcomes among individuals with T2D. Am. J. Manag. Care 2022, 28, 374–380. [Google Scholar]
- Huerta-Uribe, N.; Ramírez-Vélez, R.; Izquierdo, M.; García-Hermoso, A. Association Between Physical Activity, Sedentary Behavior and Physical Fitness and Glycated Hemoglobin in Youth with Type 1 Diabetes: A Systematic Review and Meta-analysis. Sports Med. 2022, 53, 111–123. [Google Scholar] [CrossRef]
- Shorey, S.; Ng, E.D.; Law, E.C.; Wong, J.; Loke, K.Y.; Tam, W. Physical Activity and Nutrition Interventions for Type 1 Diabetes: A Meta-analysis. Pediatrics 2022, 150, e2022056540. [Google Scholar] [CrossRef]
- Zhao, Q.; Khedkar, S.V.; Johnson, K.C. Weight Loss Interventions and Skeletal Health in Persons with Diabetes. Curr. Osteoporos. Rep. 2022, 20, 240–248. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Melo, X.; Correia, I.R.; Ribeiro, R.T.; Raposo, J.; Dores, H.; Bicho, M.; Sardinha, L.B. Effects of combined training with different intensities on vascular health in patients with type 2 diabetes: A 1-year randomized controlled trial. Cardiovasc. Diabetol. 2019, 18, 34. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise training modulates gut microbiota profile and improves endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.L.; Vaccarino, V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch. Intern. Med. 2002, 162, 1286–1292. [Google Scholar] [CrossRef]
- Papagianni, G.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Antonopoulos, C.; Tachmatzidis, D.; Didangelos, T.; Lambadiari, V.; Kadoglou, N.P.E. The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: A systematic review and meta-analysis. Cytokine 2023, 164, 156157. [Google Scholar] [CrossRef]
- Peng, Y.; Ou, Y.; Wang, K.; Wang, Z.; Zheng, X. The effect of low volume high-intensity interval training on metabolic and cardiorespiratory outcomes in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2023, 13, 1098325. [Google Scholar] [CrossRef]
- Long, C.; Jingkang, W.; Hongyu, D.; Yuhui, D.; Yongcheng, A.; Lu, S.; Yinglan, L.; Huimin, L.; Chen, W.; Quantao, M.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar]
- Tan, A.; Thomas, R.L.; Campbell, M.D.; Prior, S.L.; Bracken, R.; Churm, R. Effects of exercise training on metabolic syndrome risk factors in post-menopausal women—A systematic review and meta-analysis of randomised controlled trials. Clin. Nutr. 2023, 42, 337–351. [Google Scholar] [CrossRef]
- Igarashi, Y. Effects of Differences in Exercise Programs with Regular Resistance Training on Resting Blood Pressure in Hypertensive Adults: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2023, 37, 253–263. [Google Scholar] [CrossRef]
- Jansson, A.K.; Chan, L.X.; Lubans, D.R.; Duncan, M.J.; Plotnikoff, R.C. Effect of resistance training on A1C in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: A systematic review and meta-analysis. BMJ Open Diabetes Res. Care 2022, 10, e002595. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Monedero-Carrasco, S.; Bizzozero-Peroni, B.; Garrido-Miguel, M.; Mesas, A.E.; Martínez-Vizcaíno, V. Effectiveness of Resistance Exercise on Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Systematic Review with Meta-Analysis. Diabetes Metab. J. 2023, 47, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.A.; Menezes Júnior, F.; Celli, L.R.; França, S.N.; Cordeiro, G.R.; Mascarenhas, L.P.G.; Leite, N. Effects of resistance training on the glycemic control of people with type 1 diabetes: A systematic review and meta-analysis. Arch. Endocrinol. Metab. 2022, 66, 533–540. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J. Sport Health Sci. 2023, 12, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Uribe, N.; Hormazábal-Aguayo, I.A.; Izquierdo, M.; García-Hermoso, A. Youth with type 1 diabetes mellitus are more inactive and sedentary than apparently healthy peers: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2023, 200, 110697. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, A.; Maiya, A.G.; Shastry, B.A.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef]
- Gal, J.J.; Li, Z.; Willi, S.M.; Riddell, M.C. Association between high levels of physical activity and improved glucose control on active days in youth with type 1 diabetes. Pediatr. Diabetes 2022, 23, 1057–1063. [Google Scholar] [CrossRef]
- Si, K.; Hu, Y.; Wang, M.; Apovian, C.M.; Chavarro, J.E.; Sun, Q. Weight loss strategies, weight change, and type 2 diabetes in US health professionals: A cohort study. PLoS Med. 2022, 19, e1004094. [Google Scholar] [CrossRef]
- Lashkarbolouk, N.; Mazandarani, M.; Pourghazi, F.; Eslami, M.; Khonsari, N.M.; Ghonbalani, Z.N.; Ejtahed, H.S.; Qorbani, M. How did lockdown and social distancing policies change the eating habits of diabetic patients during the COVID-19 pandemic? A systematic review. Front. Psychol. 2022, 13, 1002665. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Highton, P.J.; Kudlek, L.; Morgan, J.; Lynch, R.; Schofield, E.; Sreejith, N.; Kapur, A.; Otunla, A.; Kerneis, S.; et al. The impact of the COVID-19 pandemic on glycaemic control in people with diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2022, 24, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Wafa, I.A.; Pratama, N.R.; Sofia, N.F.; Anastasia, E.S.; Konstantin, T.; Wijaya, M.A.; Wiyono, M.R.; Djuari, L.; Novida, H. Impact of COVID-19 Lockdown on the Metabolic Control Parameters in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. J. 2022, 46, 260–272. [Google Scholar] [CrossRef]
- Ojo, O.; Wang, X.H.; Ojo, O.O.; Orjih, E.; Pavithran, N.; Adegboye, A.R.A.; Feng, Q.Q.; McCrone, P. The Effects of COVID-19 Lockdown on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1095. [Google Scholar] [CrossRef]
- Muniyappa, R.; Gubbi, S. COVID-19 pandemic, corona viruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef]
- Abiri, B.; Ahmadi, A.R.; Hejazi, M.; Amini, S. Obesity, Diabetes Mellitus, and Metabolic Syndrome: Review in the Era of COVID-19. Clin. Nutr. Res. 2022, 11, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Choi, J.; Gwon, J.G. Metabolic syndrome and the risk of COVID-19 infection: A nationwide population-based case-control study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2596–2604. [Google Scholar] [CrossRef]
- Hejazi, K.; Wong, A. Effects of exercise training on inflammatory and cardiometabolic health markers in overweight and obese adults: A systematic review and meta-analysis of randomized controlled trials. J. Sports Med. Phys. Fit. 2023, 63, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour World Health Organization 2020 guidelines on physical activity and sedentary behaviour. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar]
- Dennis, J.M.; Mateen, B.A.; Sonabend, R.; Thomas, N.J.; Patel, K.A.; Hattersley, A.T.; Denaxas, S.; McGovern, A.P.; Vollmer, S.J. Type 2 diabetes and covid-19– related mortality in the critical care setting: A national cohort study in England, March–July 2020. Diabetes Care 2021, 44, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf (accessed on 14 August 2023).
| Author, Year of Publication and Country of Origin | Al Agha et al., 2021 Saudi Arabia [31] | Assolani et al., 2020 Italy [34] | Dalmazi et al., 2020 Italy [35] | Ruissen et al., 2021 The Netherlands [33] | Tornese et al., 2020 Italy [37] | Minuto et al., 2021 Italy [36] | Munekawa et al., 2021 Japan [32] |
|---|---|---|---|---|---|---|---|
| Place and/or region | King Abdulaziz University Hospital (KAUH) in Jeddah | Unspecified | Policlinico S. Orsola-Malpighi” in Bologna | Leiden University Medical Center, in Leiden, Holanda | Diabetes Pediatric Unit of the Institute for Maternal and Child Health ‘‘Burlo Garofolo’’, in province of Trieste | G. Gaslini Hospital, Regional Diabetes Center IRCCS Istituto Giannina Gaslini, University of Genova | Department of Endocrinology and Metabolism, Kyoto Prefectural University of Medicine, Kyoto |
| Study Design | Descriptive study: cross-sectional | Cohort study: observational | Cohort study: observational | Cohort study: observational | Cohort study: retrospective | Cohort study: cross-sectional and retrospective | Cohort study: cross-sectional and retrospective |
| Sample size and sex | 48 (♂) 102 (♀) | 71 (♂) 83 (♀) | 30 children (13 ♀) 24 adolescents (9 ♀) 76 adults (37 ♀) | 252 (♂) 183(♀) | 5 (♂) 8(♀) | 107 (♂) 95(♀) | 126 (♂) 77(♀) |
| Sample age | 8–16 years | 32–44 years | 18–47 years | 52–65 years | 11–14 years | 6–39 years | 56–65 years |
| Mean age | 12.4 | 44.8 | 8.8 (7.7–10.6) 15.6 (14.2–16.8) 45.0 (29.0–58.1) | 56.3 | 14.2 | 18.3 | 67.4 |
| Type of diabetes | T1D | T1D | T1D | T1D and T2D | T1D | T1D | T2D |
| Age at diagnosis (years) | T1D 8.23 ± 5.34 | - | 4.2 (2.3–6.5) 7.2 (5.1–9.5) 22.0 (14.3–30.8) | - | - | The median duration of the disease was 9 years. | - |
| Insulin administration | 15.3%—twice a day 50.7%— three times a day 24.7% to 32.7%—> four times a day, during confinement. 9.3%—by insulin pump. | - | Mean insulin dose (U/kg/day) 0.8 (0.6–0.8) 0.8 (0.7–1) 0.5 (0.4–0.6) | - | Total daily dose (U/kg per day) 57 (42–67) (U/kg per day) 0.9 (0.8–1.1) Basal amount (%) 57 (49–63) Basal amount (%) 50 (37–53) | 168 (83.2%) CSII 34 (16.8%) MDI | 135—Did not use insulin 68—Used insulin (unspecified dosages) |
| BMI (average) | 20.6 kg/m2 | 24.7 kg/m2 | −0.2 (−0.5–0.4) 21.3 (19.8–23.1) 24.7 (22.1–26.8) | 27.5 kg/m2 | - | - | 28.4 kg/m2 |
| Outcome | Change in lifestyle and eating habits during confinement ↑ predisposition to uncontrolled blood glucose | ↓ PA ↓ number of steps ↓ PE ↑ Mean blood glucose (during 7 days of continuous CGM monitoring) | - | = HbA1c | = HCL in adolescents with T1D ↑ HCL associated to PA practice during pandemic period | ↑ TIR in patients aged 14 years ↓ AF during confinement | ↓ PE ↑ Total diet ↑ HCFI ↑ body weight ↑ HbA1c (men only) ↑ Stress |
| Impact | Physical activity patterns and diabetes control habits | Need for recommendations for exercise during periods of confinement | - | ↑ Stress ↑ Anxiety ↑ weight gain ↓ PE ↓ Glycemic control without being associated with its deterioration | Regular PA and routine exercise in the home environment is an essential strategy for healthy living during the COVID-19 crisis, especially for young individuals with T1D | ↑ Lifestyle ↓ Mean blood glucose (during 7 days of continuous CGM monitoring) in young patients with T1D A healthier lifestyle and Improved glycemic control | ↑ stress levels caused by isolation ↓ PE ↑ total caloric intake ↑ body weight in T2D patients |
| Study | Group | Glucose (mg/dL)—Before | Glucose (mg/dL)— During | Glucose—Before vs. After (p Value) | HbA1c (mmol/mol; %)—Before | HbA1c (mmol/mol; %)—After | HbA1c— Before vs. After (p Value) | BMI (Mean) | Levels of Physical Activity (PA)—Before | Levels of Physical Activity (PA)—After | PA Before vs. After (p Value) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al Agha et al., 2021 [31] | T1D | 182.2 ± 76.6 | 200.45 ± 79.97 | p < 0.007 | 7.45 ± 1.67% | 7.40 ± 1.54 | 0.765 | 20.6 kg/m2 | 40.5% <30 min 28.0% <60 min 27.4% Inactive | ↓ 66.1% ↑ 19.0% Did not affect 14.9% | p < 0.001 |
| Assolani et al., 2020 [34] | T1D | 142.1 ± 25.4 | 150.8 ± 29.4 mg/dL | p < 0.001 | 52.0 ± 0.9 6.9 ± 0.9% | - | - | 24.7 kg/m2 | Minutes: 66 ± 42 | Minutes: 38 ± 31 | p < 0.001 |
| Dalmazi et al., 2020 [35] | T1D | - | - | - | 57 (51–62) 51 (46–57) 56 (49–64) | - | - | −0.2 (Cr) 21.3 (Ad) 24.7 (Id) | IPAQ 1440 1018 1680 | - | - |
| Ruissen et al., 2021 [33] | T1D | - | - | No impact | T1D 8%—12% T2D 8%—12% | - | No impact | 27.5 kg/m2 | - | >45.7% | p < 0.001 |
| Tornese et al., 2020 [37] | T1D | 155 (152–168) | 152–168 | - | - | - | - | - | 76% regular PA | - | - |
| Minuto et al., 2021 [36] | T1D and T2D | 176.16 ± 29.87 | 170.18 ± 30.14 | p < 0.001 | 7.76 ± 1.04 | 7.56 ± 1.05 | p < 0.001 | - | Sport (h/week) 4.64 ± 4.24 | Sport (h/week) 2.46 ± 3.22 | p < 0.001 |
| Munekawa et al., 2021 [32] | T2D | - | - | - | 7.5 (±1.0)% | 7.5 (1.0)% a 7.6 (1.1)% | p = 0.001 | 28.4 kg/m2 | 133 without habit 70 with habit | Lower PA > 50% | p < 0.001 |
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al Agha et al., 2021 [31] | y | y | y | n | n | n | y | y | abs | n | abs | y | y | 7 |
| Munekawa et al., 2021 [32] | y | y | y | n | n | n | y | n | abs | y | abs | y | y | 8 |
| Ruissen et al., 2021 [33] | y | y | y | n | n | n | y | n | abs | y | abs | y | y | 7 |
| Assolani et al., 2020 [34] | y | y | y | n | n | n | y | n | abs | y | abs | y | y | 7 |
| Dalmazi et al., 2020 [35] | y | y | y | n | n | n | y | n | abs | y | abs | y | y | 7 |
| Minuto et al., 2021 [36] | y | y | y | n | n | n | y | y | abs | y | abs | y | y | 8 |
| Tornese et al., 2020 [37] | y | y | y | n | n | n | y | y | abs | y | abs | y | y | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, E.d.; Meneses-Santos, D.; Santos, J.C.; Aidar, F.J.; Carvalho, C.R.d.O.; Santos, J.L.d.; Marçal, A.C. “Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review. Sports 2023, 11, 192. https://doi.org/10.3390/sports11100192
Souza Ed, Meneses-Santos D, Santos JC, Aidar FJ, Carvalho CRdO, Santos JLd, Marçal AC. “Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review. Sports. 2023; 11(10):192. https://doi.org/10.3390/sports11100192
Chicago/Turabian StyleSouza, Erivaldo de, Daniela Meneses-Santos, Josué Cruz Santos, Felipe J. Aidar, Carla Roberta de Oliveira Carvalho, Jymmys Lopes dos Santos, and Anderson Carlos Marçal. 2023. "“Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review" Sports 11, no. 10: 192. https://doi.org/10.3390/sports11100192
APA StyleSouza, E. d., Meneses-Santos, D., Santos, J. C., Aidar, F. J., Carvalho, C. R. d. O., Santos, J. L. d., & Marçal, A. C. (2023). “Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review. Sports, 11(10), 192. https://doi.org/10.3390/sports11100192









