Tele-Exercise in Non-Hospitalized versus Hospitalized Post-COVID-19 Patients
Abstract
1. Introduction
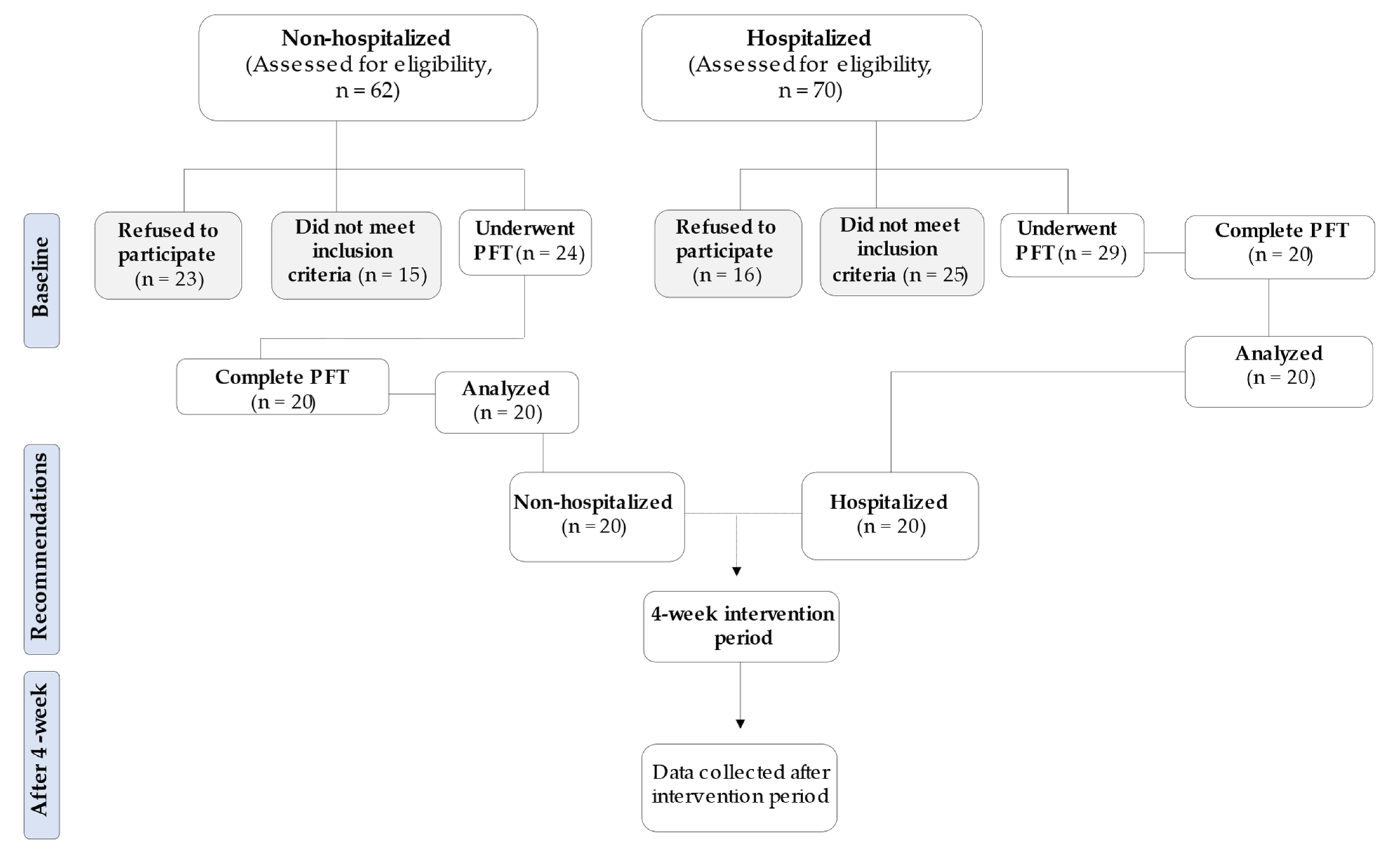
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Tele-Exercise Program
- ∘
- Warm-up: 5 min mobility exercises (child’s pose/prayer stretch, doorway stretch, quadriceps stretch) for upper and lower limbs, 2 sets for 20 s each exercise with 20 s rest;
- ∘
- Aerobic exercise: a 30 min continuous walk outdoors (flat and hard surface), and every five minutes patients checked their heart rate and oxygen saturation and subsequently recorded the total distance covered. The intensity was calculated according to HRpeak during 6MWT (approximately on 90 to 110% of HRpeak) and patients self-reported feelings of dyspnea and leg fatigue according to CR-10 Borg scales (approximately on 5 to 6 score, respectively);
- ∘
- Strength exercise: 20 min multi-joint strength exercises with body weight (chair lunges, side lateral raises, seated leg raises and squats), 3 sets for 8–12 repetitions with 40 s rest. The intensity was calculated according to the Borg scale (approximately on a 5 to 6 score from feelings of dyspnea and on a 4 to 5 score from feeling leg fatigue);
- ∘
- Cool-down: 5 min mobility exercises (child’s pose/prayer stretch, doorway stretch, quadriceps stretch) for upper and lower limbs, 2 sets for 20 s each exercise with 20 s rest.
2.4. Statistical Analysis
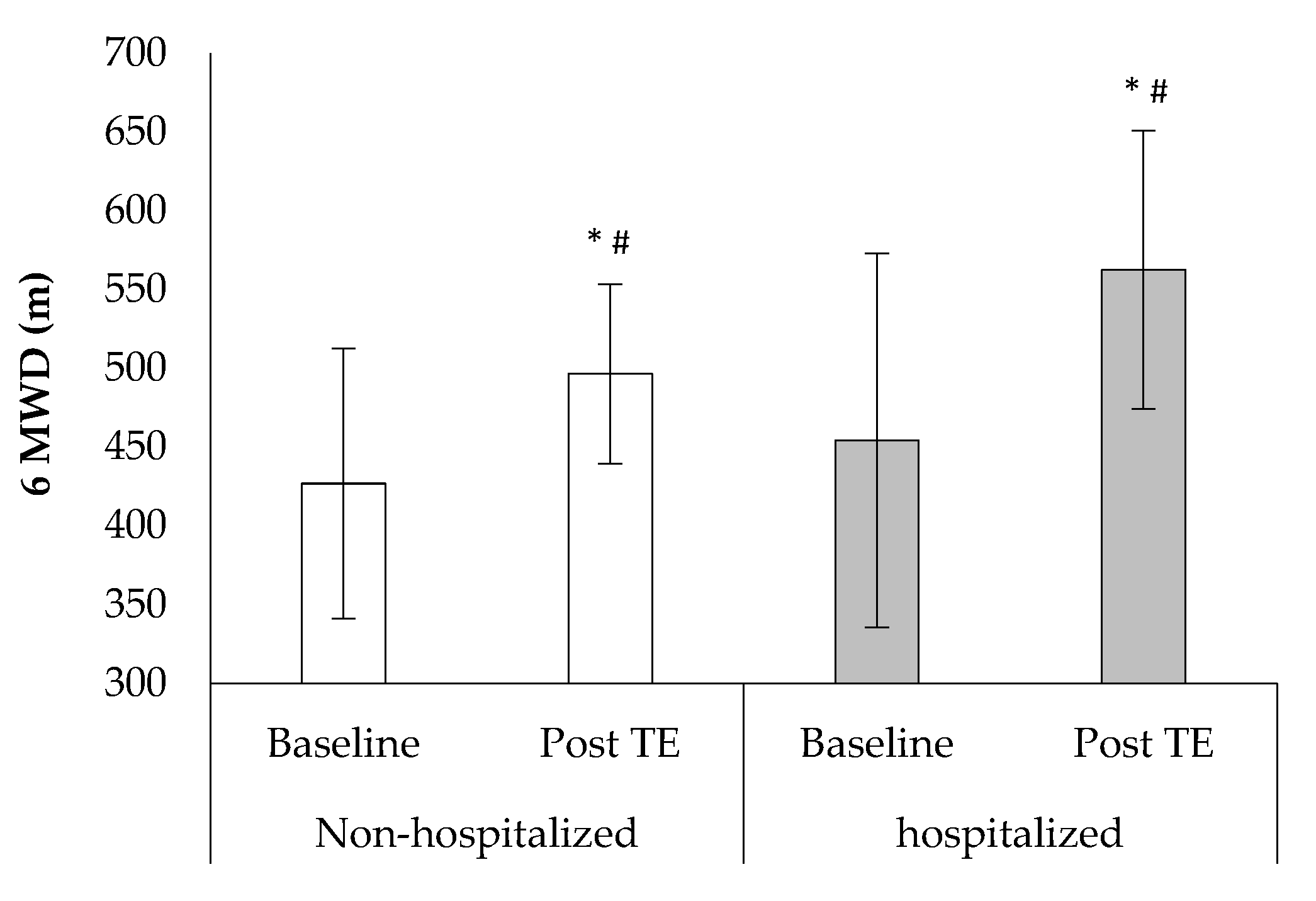
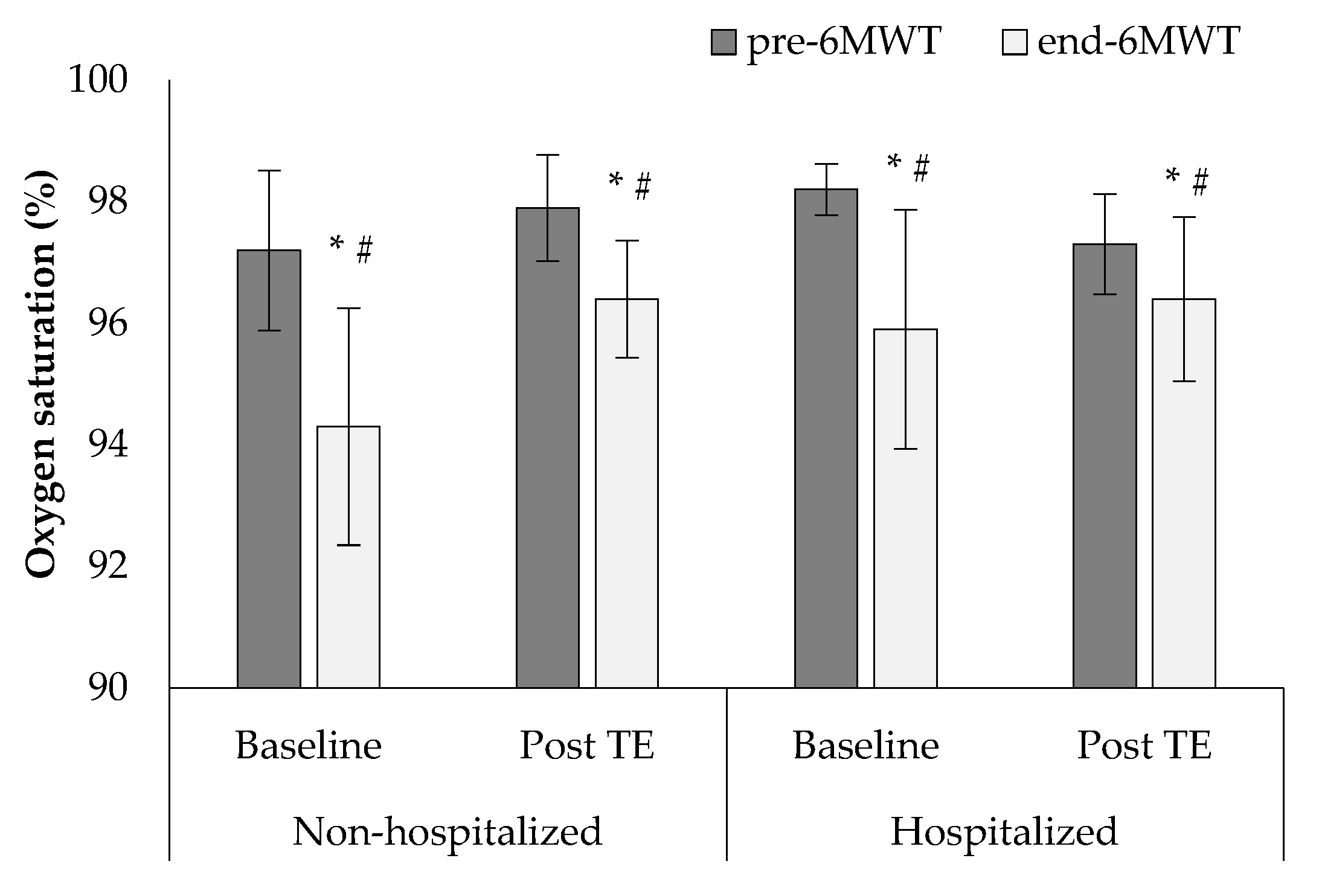
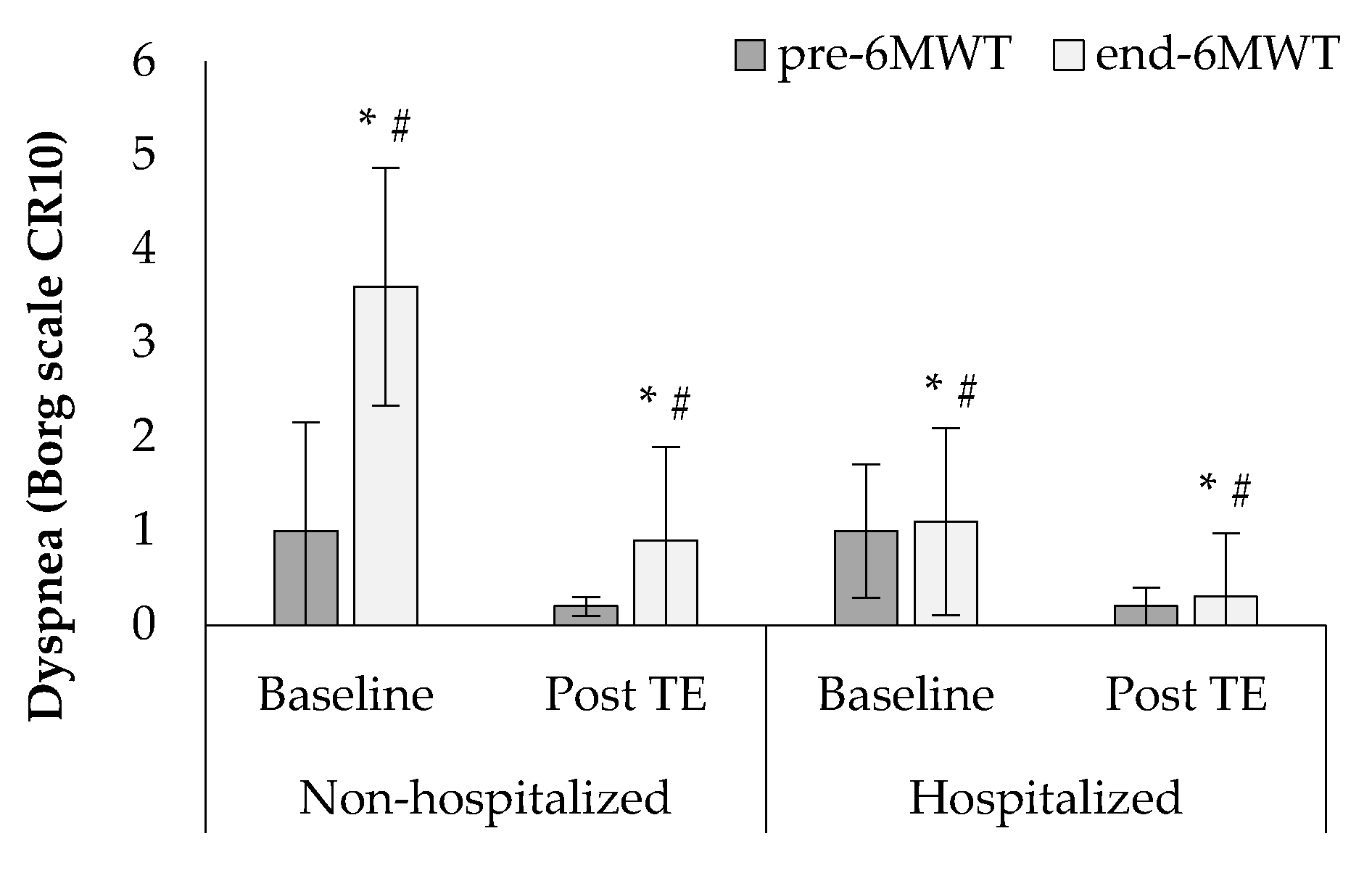
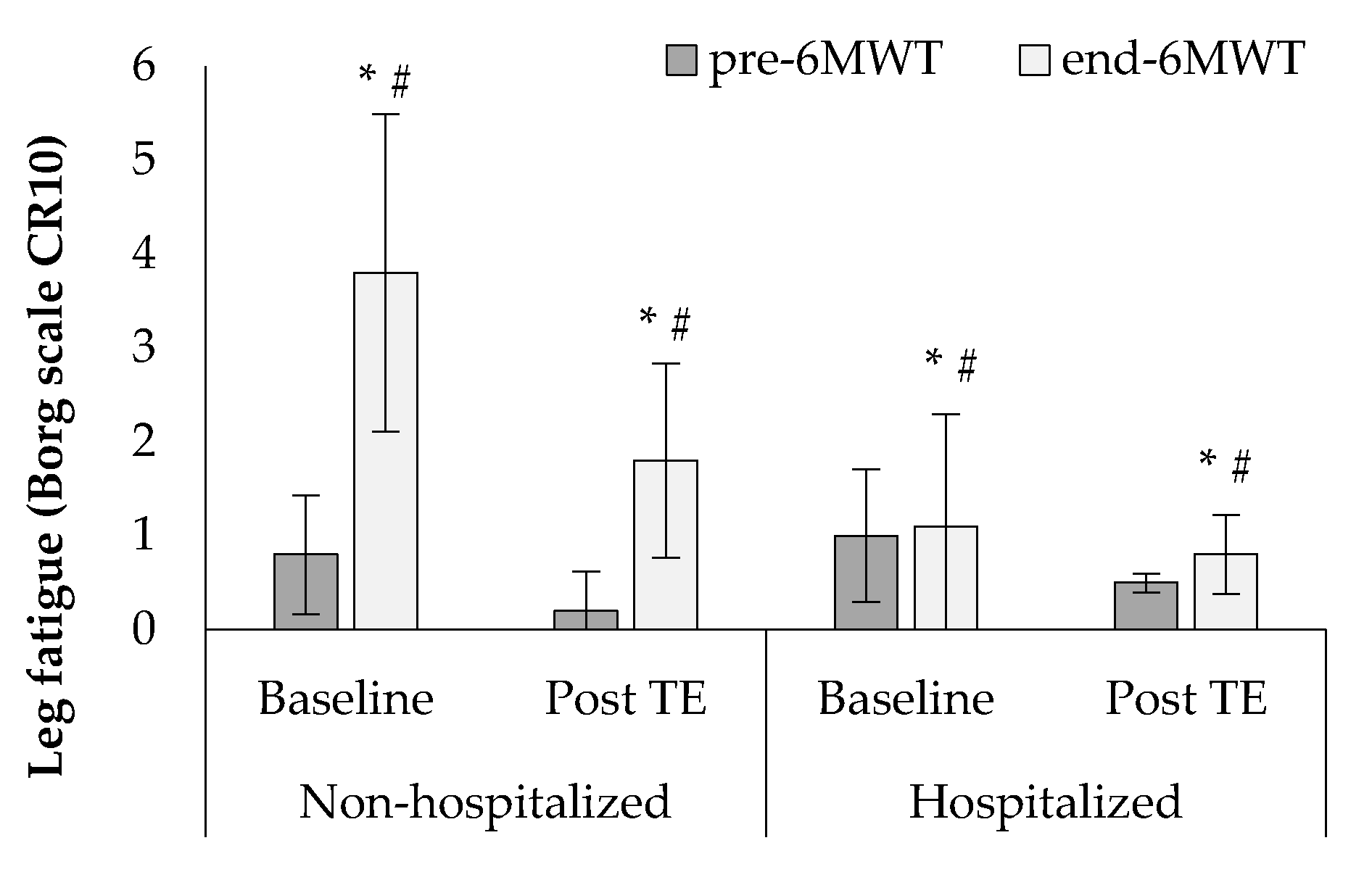
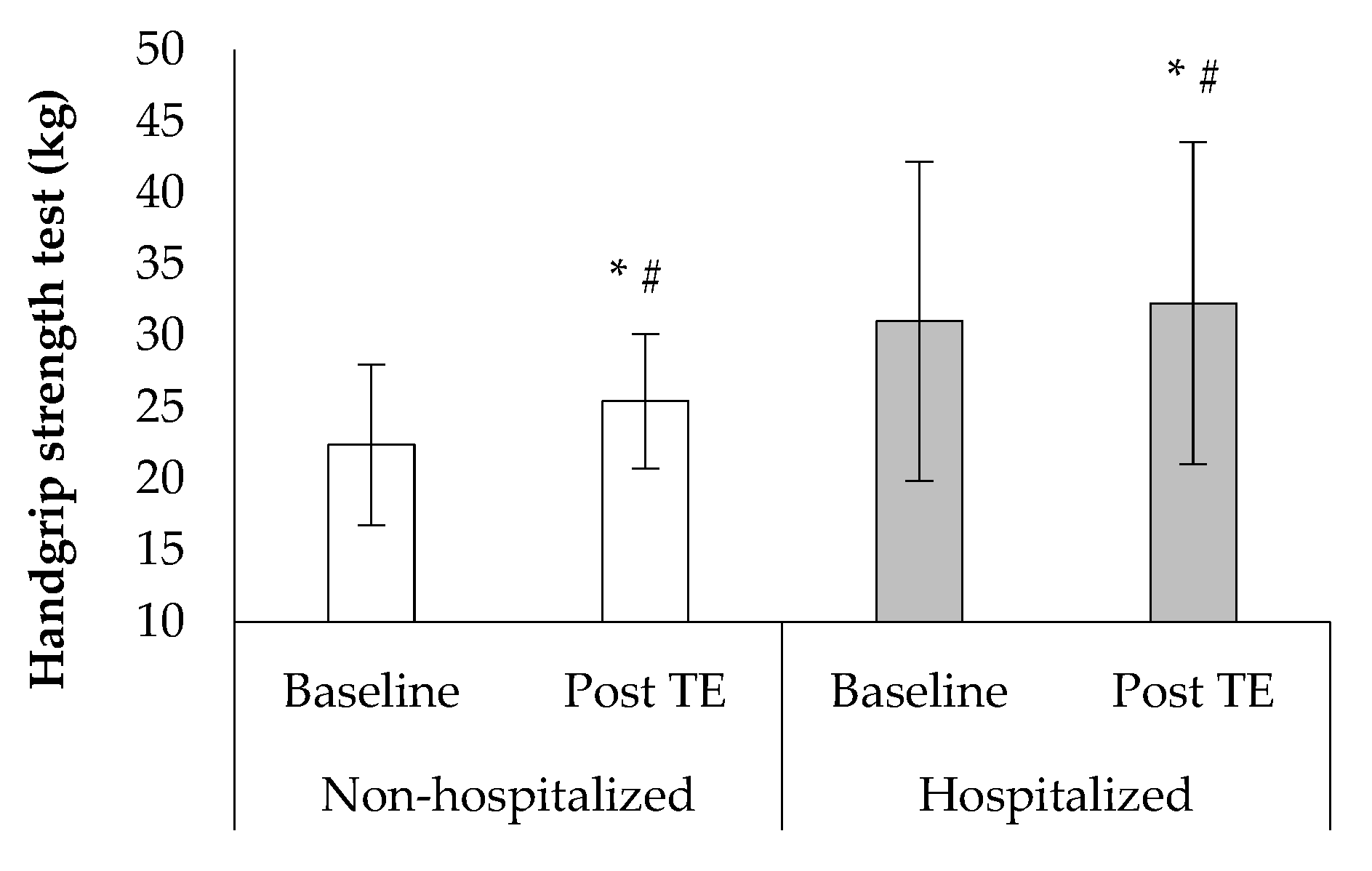
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef]
- Freuer, D.; Linseisen, J.; Meisinger, C. Impact of body composition on COVID-19 susceptibility and severity: A two-sample multivariable Mendelian randomization study. Metabolism 2021, 118, 154732. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yano, K.; Ikari, K.; Okazaki, K. Effects of the COVID-19 pandemic on body composition among patients with rheumatoid arthritis. Mod. Rheumatol. 2022, 32, 452–454. [Google Scholar] [CrossRef]
- Gloeckl, R.; Marinov, B.; Pitta, F. Practical recommendations for exercise training in patients with COPD. Eur. Respir. Rev. 2013, 128, 178–186. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Papaioannou, A.I.; Kaltsakas, G.; Louvaris, Z.; Chynkiamis, N.; Spetsioti, S.; Kortianou, E.; Genimata, S.A.; Palamidas, A.; Kostikas, K.; et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur. Respir. J. 2017, 49, 1602129. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Griziotis, M.; Vavougios, G.D.; Raptis, D.G.; Bardaka, F.; Karetsi, E.; Kyritsis, A.; Daniil, Z.; Tsarouhas, K.; Triposkiadis, F.; et al. Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era. J. Funct. Morphol. Kinesiol. 2021, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, M.; Papaioannou, A.I.; Chynkiamis, N.; Vasilogiannakopoulou, T.; Spetsioti, S.; Louvaris, Z.; Kortianou, E.; Kocsis, O.; Tsopanoglou, A.; Feridou, C.; et al. Effectiveness of home tele-rehabilitation on functional capacity and daily physical activity in COPD patients. Eur. Respir. J. 2015, 46, OA273. [Google Scholar]
- Zengin Alpozgen, A.; Kardes, K.; Acikbas, E.; Demirhan, F.; Sagir, K.; Avcil, E. The effectiveness of synchronous tele-exercise to maintain the physical fitness, quality of life, and mood of older people—A randomized and controlled study. Eur. Geriatr. Med. 2022, 13, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Tourlakopoulos, K.N.; Vavougios, G.D.; Papayianni, E.; Kiribesi, K.; Maggoutas, S.; Nikolaidis, K.; Fradelos, E.C.; Dimeas, I.; Daniil, Z.; et al. Eight Weeks Unsupervised Pulmonary Rehabilitation in Previously Hospitalized of SARS-CoV-2 Infection. J. Pers. Med. 2021, 11, 806. [Google Scholar] [CrossRef]
- Desveaux, L.; Janaudis-Ferreira, T.; Goldstein, R.; Brooks, D. An international comparison of pulmonary rehabilitation: A systematic review. COPD 2015, 12, 144–153. [Google Scholar] [CrossRef]
- Hill, N.S. Pulmonary rehabilitation. Proc. Am. Thorac. Soc. 2006, 3, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Griziotis, M.; Raptis, D.; Bardaka, F.; Karetsi, E.; Kiritsis, A.; Daniil, Z.; Tsarouhas, K.; Triposkiadis, F.; Gourgoulianis, K.I.; et al. Eight Weeks of Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Preliminary Report. Proceedings 2019, 25, 37. [Google Scholar]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Seitanidis, G.; Daniil, Z.; Gourgoulianis, K.I. The effect of physical strain on breeders patients with obstructive sleep apnea syndrome. Respir. Physiol. Neurobiol. 2019, 260, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. 4 weeks exercise in obstructive sleep apnea syndrome patient with type 2 diabetes mellitus and without continuous positive airway pressure treatment: A case report. Sleep Med. Res. 2019, 10, 54–57. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Astara, K.; Daniil, Z.; Gourgoulianis, K.I.; Kalabakas, K.; Karagiannis, D.; Basdekis, G. The reciprocal association between fitness indicators and sleep quality in the context of recent sport injury. Int. J. Environ. Res. Public Health 2020, 17, 4810. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Vavougios, G.D.; Boutlas, S.; Tourlakopoulos, K.N.; Papayianni, E.; Astara, K.; Stavrou, I.T.; Daniil, Z.; Gourgoulianis, K.I. Physical Fitness Differences, Amenable to Hypoxia-Driven and Sarcopenia Pathophysiology, between Sleep Apnea and COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 669. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- American Thoracic Society (ATS). Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Borg, E.; Borg, G.; Larsson, K.; Letzter, M.; Sundblad, B.M. An index for breathlessness and leg fatigue. Scand. J. Med. Sci. Sports 2010, 20, 644–650. [Google Scholar] [CrossRef]
- Ogoh, S.; Fadel, P.J.; Nissen, P.; Jans, Ø.; Selmer, C.; Secher, N.H.; Raven, P.B. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J. Physiol. 2003, 550, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Vavougios, G.D.; Stavrou, V.T.; Konstantatos, C.; Sinigalias, P.C.; Zarogiannis, S.G.; Kolomvatsos, K.; Stamoulis, G.; Gourgoulianis, K.I. COVID-19 Phenotypes and Comorbidity: A Data-Driven, Pattern Recognition Approach Using National Representative Data from the United States. Int. J. Environ. Res. Public Health 2022, 19, 4630. [Google Scholar] [CrossRef] [PubMed]
- Essam, H.; Abdel Wahab, N.H.; Younis, G.; El-Sayed, E.; Shafiek, H. Effects of different exercise training programs on the functional performance in fibrosing interstitial lung diseases: A randomized trial. PLoS ONE 2022, 17, e0268589. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Zakynthinos, G.; Andrianopoulos, V. Mechanisms of physical activity limita-tion in chronic lung diseases. Pulm. Med. 2012, 2012, 634761. [Google Scholar] [CrossRef]
- Benis, A.; Banker, M.; Pinkasovich, D.; Kirin, M.; Yoshai, B.E.; Benchoam-Ravid, R.; Ashkenazi, S.; Seidmann, A. Reasons for Utilizing Telemedicine during and after the COVID-19 Pandemic: An Internet-Based International Study. J. Clin. Med. 2021, 10, 5519. [Google Scholar] [CrossRef] [PubMed]
- Reimers, A.K.; Knapp, G.; Reimers, C.D. Effects of Exercise on the Resting Heart Rate: A Systematic Review and Meta-Analysis of Interventional Studies. J. Clin. Med. 2018, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Mroszczyk-McDonald, A.; Savage, P.D.; Ades, P.A. Handgrip strength in cardiac rehabilitation: Normative values, interaction with physical function, and response to training. J. Cardiopulm. Rehabil. Prev. 2007, 5, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Bellet, R.N.; Adams, L.; Morris, N.R. The 6-minute walk test in outpatient cardiac rehabilitation: Validity, reliability and responsiveness—A systematic review. Physiotherapy 2012, 98, 277–286. [Google Scholar] [CrossRef]
- Piquet, V.; Luczak, C.; Seiler, F.; Monaury, J.; Martini, A.; Ward, A.B.; Gracies, J.M.; Motavasseli, D. Covid Rehabilitation Study Group. Do Patients With COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Arch Phys. Med. Rehabil. 2021, 102, 1067–1074. [Google Scholar] [CrossRef]
| Non-Hospitalized | Hospitalized | ||
|---|---|---|---|
| Age | yrs | 44.3 ± 12.2 | 48.9 ± 8.1 |
| Gender (M) | n (%) | 10 (50%) | 10 (50%) |
| Body mass index | kg/m2 | 25.5 ± 5.3 | 29.0 ± 2.9 |
| Body surface area | m2 | 1.7 ± 0.4 | 2.0 ± 0.2 |
| Body fat | % | 25.3 ± 7.2 | 31.9 ± 11.5 |
| Total body water | % | 57.5 ± 6.3 | 53.1 ± 1.9 |
| Lean body mass | kg | 55.3 ± 8.2 | 60.4 ± 3.9 |
| Δchest | cm | 3.6 ± 0.5 | 4.6 ± 2.8 |
| Physical activity | min/week | 45.6 ± 9.2 | 49.6 ± 12.4 |
| FEV1 | % of predicted | 101.0 ± 8.0 | 97.9 ± 7.1 |
| DLCO(SB) | % of predicted | 81.3 ± 3.1 | 79.2 ± 1.4 |
| Non-Hospitalized | Hospitalized | |||||
|---|---|---|---|---|---|---|
| Baseline | Post TE | Baseline | Post TE | |||
| Systolic blood pressure | mmHg | Pre 6MWT | 115.6 ± 17.3 | 119.0 ± 9.5 * | 138.1 ± 18.8 # | 126.5 ± 4.7 *#† |
| Post 6MWT | 129.5 ± 18.7 | 124.8 ± 9.1 * | 158.8 ± 14.9 # | 153.5 ± 9.7 *#† | ||
| Diastolic blood pressure | mmHg | Pre 6MWT | 81.8 ± 18.8 | 79.8 ± 15.0 * | 83.6 ± 14.3 | 84.0 ± 4.6 |
| Post 6MWT | 85.5 ± 18.2 | 89.2 ± 11.6 | 89.0 ± 11.0 | 89.0 ± 5.2 | ||
| Mean arterial pressure | mmHg | Pre 6MWT | 93.1 ± 18.0 | 92.9 ± 12.5 | 101.8 ± 15.1 # | 98.2 ± 3.4 *#† |
| Post 6MWT | 100.2 ± 17.3 | 101.1 ± 10.2 | 112.3 ± 11.3 # | 110.5 ± 4.9 # | ||
| Heart rate | BPM | Pre 6MWT | 79.6 ± 7.2 | 74.5 ± 6.9 * | 79.6 ± 16.8 | 77.5 ± 10.4 * |
| Post 6MWT | 135.0 ± 17.9 | 123.0 ± 8.7 * | 118.5 ± 19.3 # | 115.2 ± 18.7 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrou, V.T.; Astara, K.; Ioannidis, P.; Vavougios, G.D.; Daniil, Z.; Gourgoulianis, K.I. Tele-Exercise in Non-Hospitalized versus Hospitalized Post-COVID-19 Patients. Sports 2022, 10, 179. https://doi.org/10.3390/sports10110179
Stavrou VT, Astara K, Ioannidis P, Vavougios GD, Daniil Z, Gourgoulianis KI. Tele-Exercise in Non-Hospitalized versus Hospitalized Post-COVID-19 Patients. Sports. 2022; 10(11):179. https://doi.org/10.3390/sports10110179
Chicago/Turabian StyleStavrou, Vasileios T., Kyriaki Astara, Pavlos Ioannidis, George D. Vavougios, Zoe Daniil, and Konstantinos I. Gourgoulianis. 2022. "Tele-Exercise in Non-Hospitalized versus Hospitalized Post-COVID-19 Patients" Sports 10, no. 11: 179. https://doi.org/10.3390/sports10110179
APA StyleStavrou, V. T., Astara, K., Ioannidis, P., Vavougios, G. D., Daniil, Z., & Gourgoulianis, K. I. (2022). Tele-Exercise in Non-Hospitalized versus Hospitalized Post-COVID-19 Patients. Sports, 10(11), 179. https://doi.org/10.3390/sports10110179







