Exploring the Impact of Nanotherapeutics on Histone H3 and H4 Acetylation Enrichment in Cancer Epigenome: A Systematic Scoping Synthesis
Abstract
1. Introduction
2. Results
2.1. Systematic Search
2.2. Summary of the Included Studies
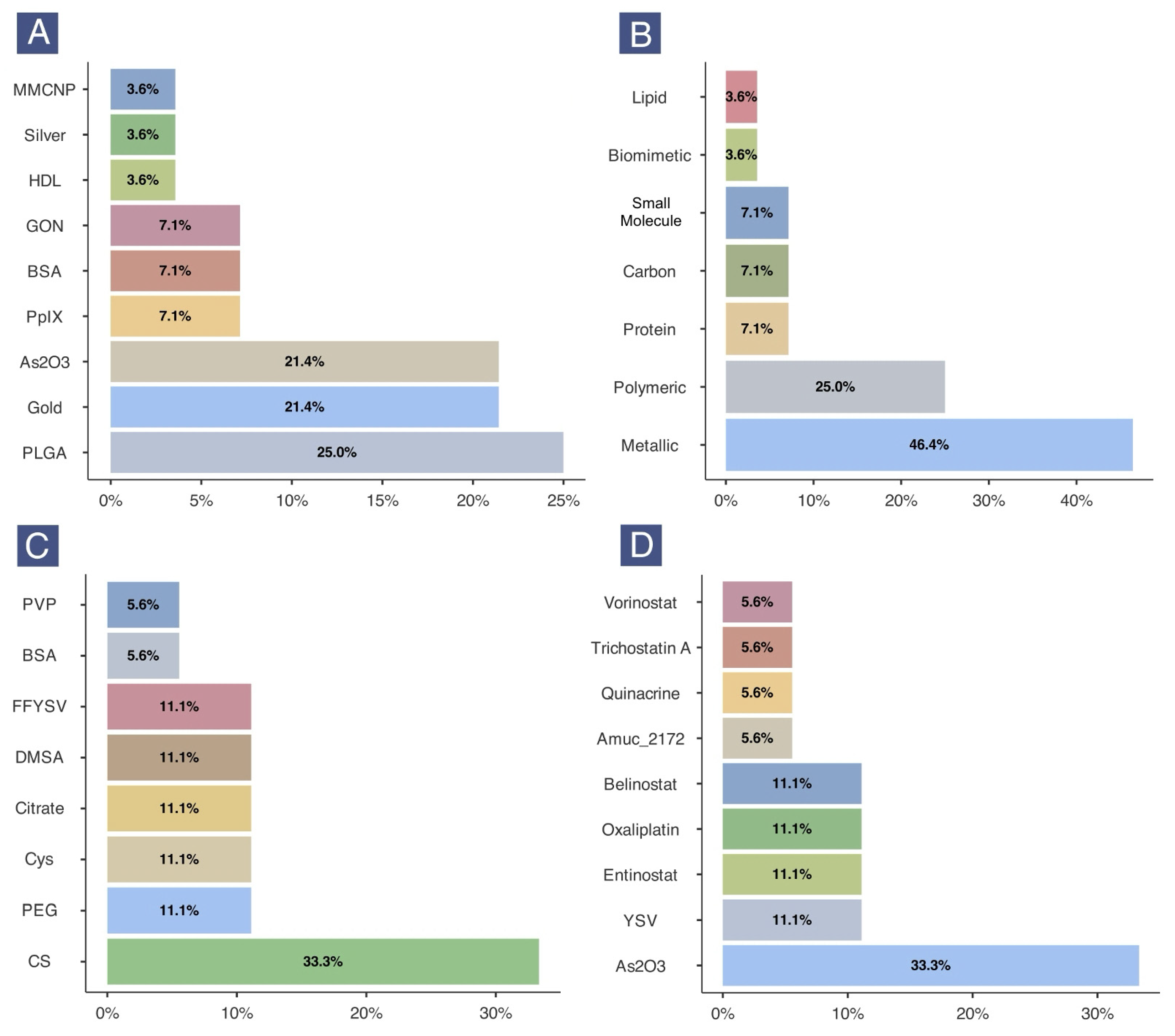
2.3. Characteristics of Nanoformulations
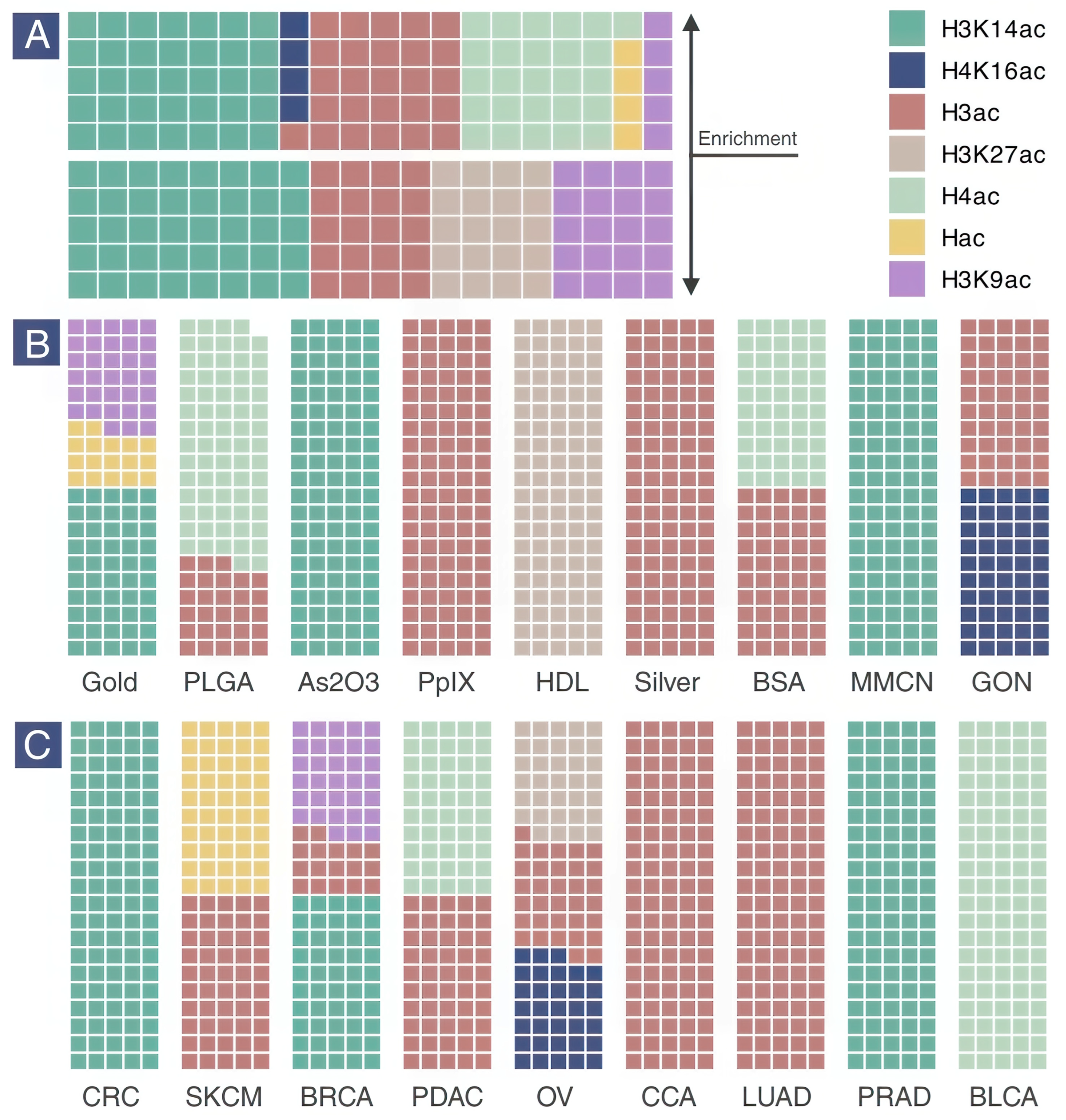
2.4. Histone Acetylation Enrichment
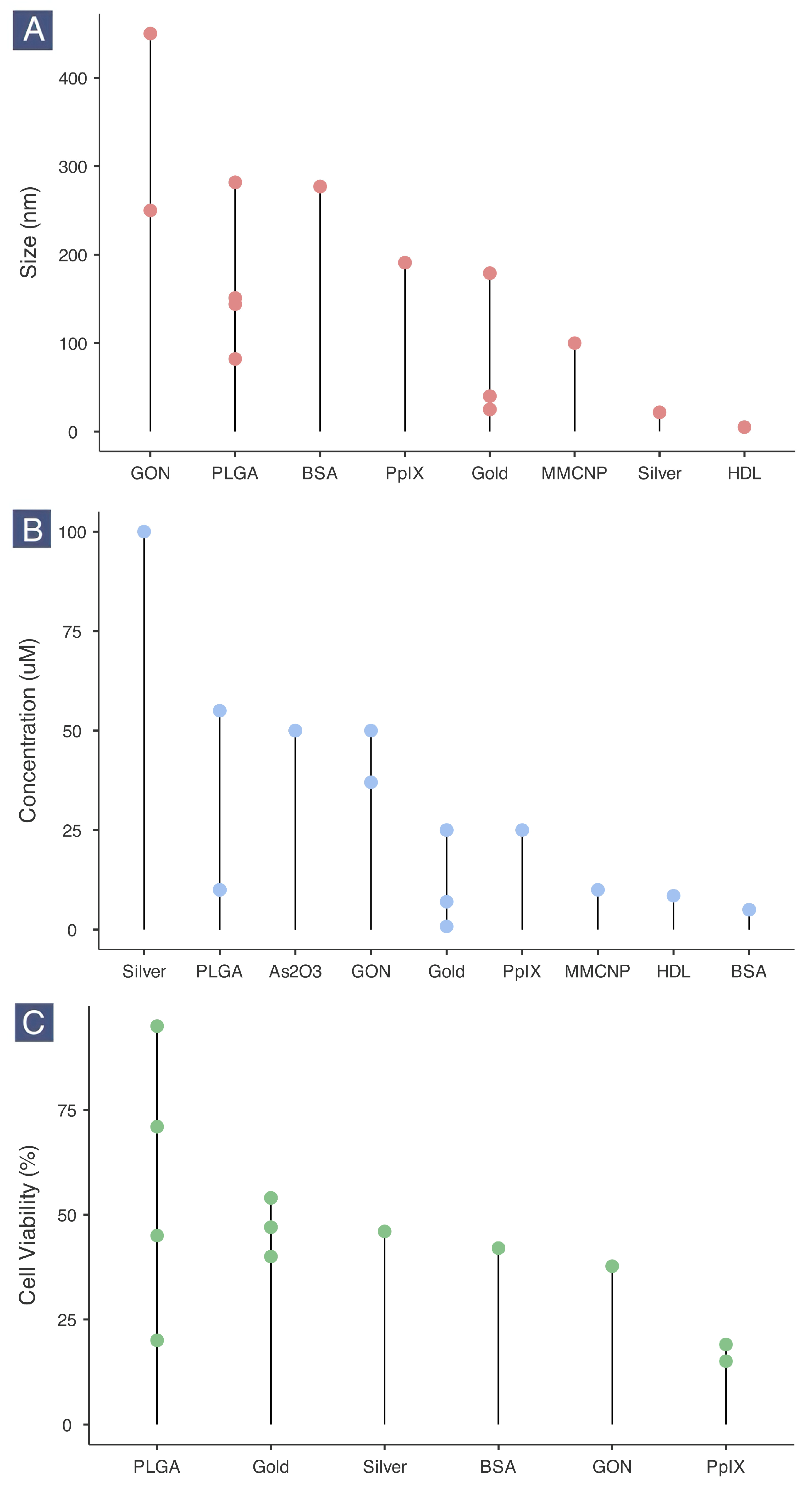
2.5. Nanotherapeutic Size, Dosage and Cancer Cell Viability
2.6. Differential Gene Expression in Cancer Cells
2.7. Anticancer Effects of Nanotherapeutics in Animal Models of Cancer
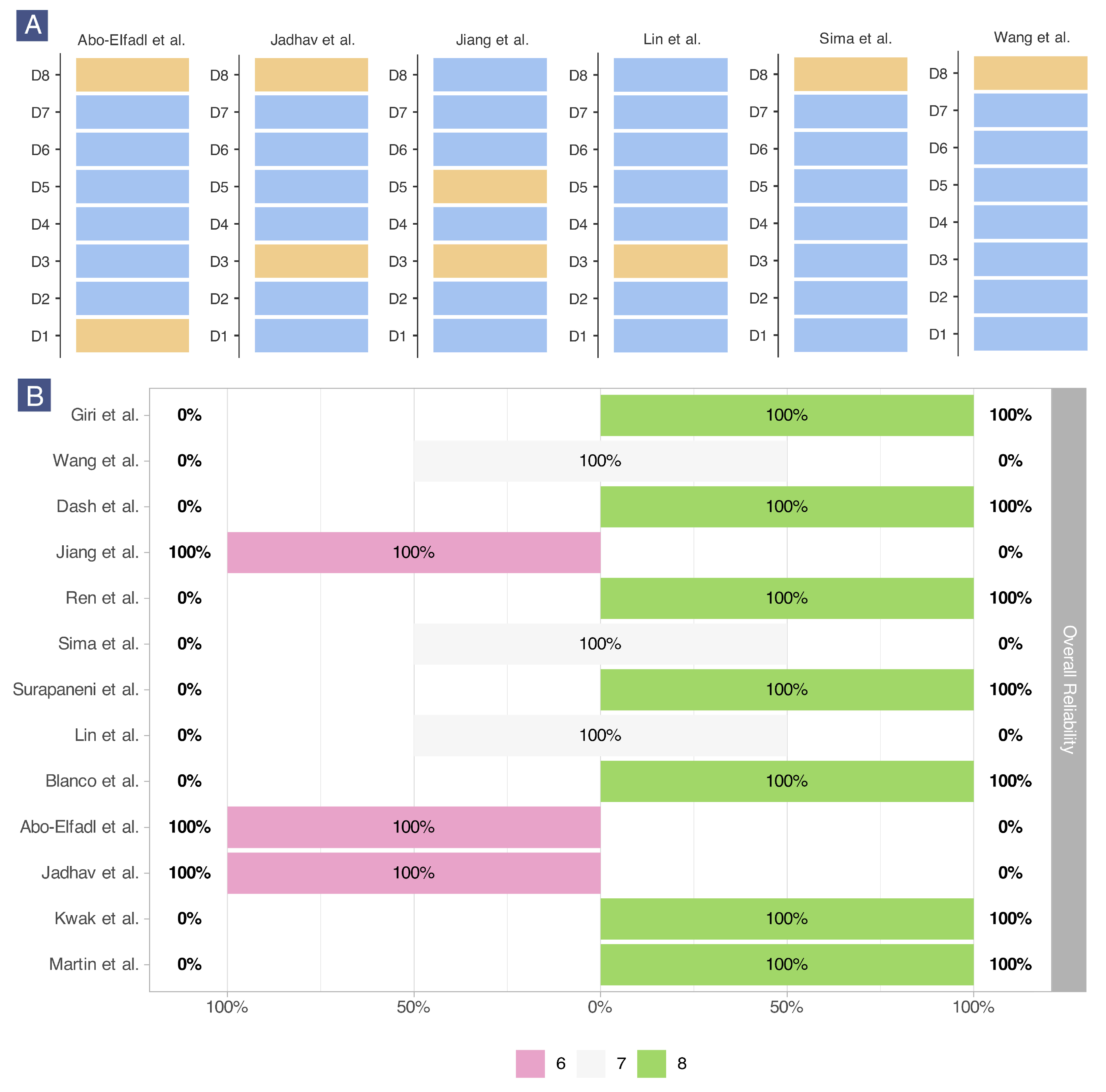
2.8. Risk of Bias Assessment
3. Discussion
Limitations
4. Materials and Methods
4.1. Protocol and Registration
4.2. Eligibility Criteria
4.3. Information Sources and Search
4.4. Selection of Sources of Evidence
4.5. Data Extraction
4.6. Critical Appraisal of Sources of Evidence
4.7. Synthesis of Evidence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNP | Silver nanoparticle |
| AsNP | Arsenic oxide nanoparticle |
| AuNP | Gold nanoparticle |
| BLCA | Bladder cancer |
| BRCA | Breast cancer |
| BSA | Bovine serum albumin |
| CCA | Cholangiocarcinoma |
| CDDP | Cisplatin |
| CRC | Colorectal cancer |
| DMSA | Dimercaptosuccinic acid |
| ENT | Entinostat |
| GON | Graphene oxide nanosheet |
| HDL | High-density lipoprotein |
| LUAD | Lung adenocarcinoma |
| MMCNP | Macrophage membrane-coated nanoparticle |
| OV | Ovarian cancer |
| OXP | Oxaliplatin |
| PEG | Polyethylene glycol |
| PDAC | Pancreatic ductal adenocarcinoma |
| PGON | poly(guanidinium oxanorbornene) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PpIX | Protoporphyrin IX |
| PVP | Polyvinylpyrrolidone |
| PRAD | Prostate adenocarcinoma |
| rINN | Vorinostat |
| SKCM | Skin cutaneous melanoma |
| TSA | Trichostatin A |
| YSV | Tyroservatide |
References
- Kushwaha, A.C.; Ayoub, M.; Sarkar, D.; Karmakar, S.; Roy Choudhury, S. Antibody Functionalized Targeted Novel Epigenetic Nanotherapy for Paediatric Neuroblastoma. Colloids Surf. B Biointerfaces 2026, 257, 115137. [Google Scholar] [CrossRef]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.-H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision Nanomedicine: Navigating the Tumor Microenvironment for Enhanced Cancer Immunotherapy and Targeted Drug Delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef]
- Elmahboub, Y.; Albash, R.; Ahmed, S.; Salah, S. The Road to Precision Nanomedicine: An Insight on Drug Repurposing and Advances in Nanoformulations for Treatment of Cancer. AAPS PharmSciTech 2025, 26, 237. [Google Scholar] [CrossRef]
- Liu, S.; Ren, Z.; Yan, M.; Ye, W.; Hu, Y. Strategies to Enhance the Penetration of Nanomedicine in Solid Tumors. Biomaterials 2025, 321, 123315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Preis, E.; Bakowsky, U.; Xia, Y. Cancer Nanomedicine: Concepts, Promises, and Challenges. Chem 2025, 11, 102706. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Gabizon-Peretz, S.; Modaresahmadi, S.; La-Beck, N.M. Thirty Years from FDA Approval of Pegylated Liposomal Doxorubicin (Doxil/Caelyx): An Updated Analysis and Future Perspective. BMJ Oncol. 2025, 4, e000573. [Google Scholar] [CrossRef] [PubMed]
- Dellapasqua, S.; Trillo Aliaga, P.; Munzone, E.; Bagnardi, V.; Pagan, E.; Montagna, E.; Cancello, G.; Ghisini, R.; Sangalli, C.; Negri, M.; et al. Pegylated Liposomal Doxorubicin (Caelyx®) as Adjuvant Treatment in Early-Stage Luminal B-like Breast Cancer: A Feasibility Phase II Trial. Curr. Oncol. 2021, 28, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, R.; Nelissen, E.; El-Shakankery, K.H.; Rogozińska, E.; Bain, E.; Veiga, S.; Morrison, J. Pegylated Liposomal Doxorubicin for Relapsed Epithelial Ovarian Cancer. Cochrane Database Syst. Rev. 2023, 2023, CD006910. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-Loaded PEG-PLGA Nanoparticles for Cancer Treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Y.; Pei, Y.; Wang, W.; Zhu, Z.; Zheng, Z.; Yang, L.; Sun, L. The Drug Release of PLGA-Based Nanoparticles and Their Application in Treatment of Gastrointestinal Cancers. Heliyon 2024, 10, e38165. [Google Scholar] [CrossRef]
- Snyder, C.S.; Repetto, T.; Burkhard, K.M.; Tuteja, A.; Mehta, G. Co-Delivery Polymeric Poly(Lactic-Co-Glycolic Acid) (PLGA) Nanoparticles to Target Cancer Stem-Like Cells. Methods Mol. Biol. 2024, 2777, 191–204. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Zhang, S. Recent Advances in PLGA Polymer Nanocarriers for Ovarian Cancer Therapy. Front. Oncol. 2025, 15, 1526718. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Kumar, V.; Goh, K.W.; Gupta, G.; Alsayari, A.; Wahab, S.; Sahebkar, A. PEGylated PLGA Nanoparticles: Unlocking Advanced Strategies for Cancer Therapy. Mol. Cancer 2025, 24, 205. [Google Scholar] [CrossRef]
- Turkmen Koc, S.N.; Rezaei Benam, S.; Aral, I.P.; Shahbazi, R.; Ulubayram, K. Gold Nanoparticles-Mediated Photothermal and Photodynamic Therapies for Cancer. Int. J. Pharm. 2024, 655, 124057. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiao, Y.; Wu, R.; Lei, J.; Li, T.; Zheng, Y. Aggregable Gold Nanoparticles for Cancer Photothermal Therapy. J. Mater. Chem. B 2024, 12, 8048–8061. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold Nanoparticles-Based Photothermal Therapy for Breast Cancer. Photodiagnosis Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef]
- Liu, S.; Phillips, S.; Northrup, S.; Levi, N. The Impact of Silver Nanoparticle-Induced Photothermal Therapy and Its Augmentation of Hyperthermia on Breast Cancer Cells Harboring Intracellular Bacteria. Pharmaceutics 2023, 15, 2466. [Google Scholar] [CrossRef]
- Melo, R.M.; Albuquerque, G.M.; Monte, J.P.; Pereira, G.A.L.; Pereira, G. Recent Advances in the Application of Silver Nanoparticles for Enhancing Phototherapy Outcomes. Pharmaceuticals 2025, 18, 970. [Google Scholar] [CrossRef]
- Nedelcu, A.; Mocan, T.; Sabau, L.I.; Matea, C.T.; Tabaran, F.; Pop, T.; Delcea, C.; Mosteanu, O.; Mocan, L. In Vitro Photothermal Therapy of Pancreatic Cancer Mediated by Immunoglobulin G-Functionalized Silver Nanoparticles. Sci. Rep. 2024, 14, 14417. [Google Scholar] [CrossRef]
- Xenodochidis, C.; Hristova-Panusheva, K.; Kamenska, T.; Santhosh, P.B.; Petrov, T.; Stoychev, L.; Genova, J.; Krasteva, N. Graphene Oxide Nanoparticles for Photothermal Treatment of Hepatocellular Carcinoma Using Low-Intensity Femtosecond Laser Irradiation. Molecules 2024, 29, 5650. [Google Scholar] [CrossRef]
- Haddad, M.A.; Zare Mehrabadi, F.; Hosseini Motlagh, N.S.; Zarei Mahmoudabadi, M.; Sardari Zarchi, M.; Haghiralsadat, B.F. Graphene Oxide-Enhanced Photothermal Therapy: Laser Parameter Optimization and Temperature Modeling for Hela Cancer Cell Mortality. Lasers Med. Sci. 2025, 40, 50. [Google Scholar] [CrossRef]
- Gospodinova, Z.; Hristova-Panusheva, K.; Kamenska, T.; Antov, G.; Krasteva, N. Insights into Cellular and Molecular Mechanisms of Graphene Oxide Nanoparticles in Photothermal Therapy for Hepatocellular Carcinoma. Sci. Rep. 2025, 15, 15541. [Google Scholar] [CrossRef]
- Báez, D.F. Graphene-Based Nanomaterials for Photothermal Therapy in Cancer Treatment. Pharmaceutics 2023, 15, 2286. [Google Scholar] [CrossRef]
- Hsu, W.H.; Ku, C.L.; Lai, Y.R.; Wang, S.S.S.; Chou, S.H.; Lin, T.H. Developing Targeted Drug Delivery Carriers for Breast Cancer Using Glutathione-Sensitive Doxorubicin-Coupled Glycated Bovine Serum Albumin Nanoparticles. Int. J. Biol. Macromol. 2023, 249, 126114. [Google Scholar] [CrossRef]
- Aly, G.A.B.; Sabra, S.A.; Haroun, M.; Helmy, M.W.; Moussa, N. Bovine Serum Albumin Nanoparticles Encapsulating Dasatinib and Celecoxib for Oral Cancer: Preparation, Characterization, and In-Vitro Evaluation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025, 398, 9291–9306. [Google Scholar] [CrossRef]
- Masoumi, S.; Aleyasin, S.A.; Faghihi, S. Albumin Nanoparticles-Mediated Doxorubicin Delivery Enhances the Anti-Tumor Efficiency in Ovarian Cancer Cells through Controlled Release. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 6885–6900. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, L.; Wang, Y.; Tian, Y.; Wu, S.; Zhou, B.; Dong, C.; Zhao, B.; Yang, Y.; Xie, D.; et al. A Dendrimer Peptide (KK2DP7) Delivery System with Dual Functions of Lymph Node Targeting and Immune Adjuvants as a General Strategy for Cancer Immunotherapy. Adv. Sci. 2023, 10, e2300116. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, C.; Poellmann, M.J.; Zhu, Z.; Hong, S. The Role of Dendrimers in Improving Antibody and Peptide Biologics for Cancer Treatment. Langmuir 2025, 41, 17322–17334. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, J.W.; Rawding, P.A.; Iida, M.; Kim, C.; Kostecki, K.L.; Poellmann, M.J.; Crossman, B.; Liu, A.S.; Kim, Y.S.; et al. Dendrimer Conjugates with PD-L1-Binding Peptides Enhance In Vivo Antitumor Immune Response. Adv. Healthc. Mater. 2025, 14, e2500551. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Hashemi, M.; Farzadnia, M.; Zarei-Ghanavati, S.; Malaekeh-Nikouei, B. Development of Targeted Gene Delivery System Based on Liposome and PAMAM Dendrimer Functionalized with Hyaluronic Acid and TAT Peptide: In Vitro and In Vivo Studies. Biotechnol. Prog. 2022, 38, e3278. [Google Scholar] [CrossRef]
- Zhao, Y.; Pei, L.; Liu, B.; Mao, Z.; Niu, Y.; Li, S.; Yang, M.; Liu, W.; Hai, H.; Luo, Y.; et al. Macrophage Membrane-Coated Nanomedicine Enhances Cancer Immunotherapy by Activating Macrophages and T Cells. Mol. Pharm. 2025, 22, 2402–2412. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, J.; Zhou, Z.; Zhang, E.; Yu, Y.; Krishnan, N.; Silva-Ayala, D.; Fang, R.H.; Griffiths, A.; Gao, W.; et al. Extending the In Vivo Residence Time of Macrophage Membrane-Coated Nanoparticles through Genetic Modification. Small 2023, 19, e2305551. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Zhou, Y.; Zheng, X.; Fu, Y.; Liu, H.Y.; Wan, Z.; Zhao, Y. Intelligent Nanoplatform Integrating Macrophage and Cancer Cell Membrane for Synergistic Chemodynamic/Immunotherapy/Photothermal Therapy of Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 59117–59133. [Google Scholar] [CrossRef]
- Liu, J.; Wu, M.; Lyu, Q.; Yang, C.; Fan, N.; Chen, K.; Wang, W. IR783-Stabilized Nanodrugs Enhance Anticancer Immune Response by Synergizing Oxidation Therapy and Epigenetic Modulation. Adv. Sci. 2025, 12, e2415684. [Google Scholar] [CrossRef]
- Ahmad, U.; Islam, A.; Khan, M.M.; Akhtar, J. Nanotechnology-Driven Epigenetic Cancer Therapy: Precision Delivery and Sustained Release of DNA Methylation Modulators. Yale J. Biol. Med. 2025, 98, 227–235. [Google Scholar] [CrossRef]
- Shan, G.; Wang, W.; Li, X.; Zhai, Z.; Liu, M.; Peng, R.; Sun, Q.; Zhang, H.; Guo, T.; He, X. Epigenetic-Targeted Biomimetic Nanomedicine Modulates Epithelial Mesenchymal Transition to Enhance Chemosensitivity in Heterogeneous Tumors. Biomaterials 2026, 324, 123529. [Google Scholar] [CrossRef]
- Chongloi, J.; Jaiswal, M.; Srivastava, A.; Ravi, R.; Nirala, R.K.; Pradhan, B.; Pattanaik, K.P. Recent Advancement in the Epigenetic Mediated Nanotechnology Approach: Potential Implication in Cancer Therapy. In Nanomaterials in Biological Milieu: Biomedical Applications and Environmental Sustainability; Arakha, M., Pradhan, A.K., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2025; pp. 143–160. ISBN 9789815313260. [Google Scholar]
- Mitsuhashi, R.; Sato, K.; Kawakami, H. Novel Epigenetics Control (EpC) Nanocarrier for Cancer Therapy Through Dual-Targeting Approach to DNA Methyltransferase and Ten-Eleven Translocation Enzymes. Epigenomes 2025, 9, 6. [Google Scholar] [CrossRef]
- Pogribna, M.; Word, B.; Lyn-Cook, B.; Hammons, G. Effect of Titanium Dioxide Nanoparticles on Histone Modifications and Histone Modifying Enzymes Expression in Human Cell Lines. Nanotoxicology 2022, 16, 409–424. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, L.; Lin, H.; Wang, X. Histone Modification Changes upon Exposure of Human Lung Adenocarcinoma Cells to Nanoparticles. Sci. Rep. 2025, 15, 20724. [Google Scholar] [CrossRef]
- Hoseini, Z.S.; Rezaee, Z.; Derakhshani, A.; Zhang, S.; SH Tehrani, S.; Taleb, M.; Ghanbari, H. Nanoparticles and Their Impact on Epigenetic Mechanisms: Insights and Implications. Adv. Ther. 2025, 8, 2500006. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Yu, X.; Ma, C.; Li, X.; Chen, G.; Yuan, G.; Lin, S.; Cui, R. Epigenetic and Metabolic Reprogramming via Nanotechnology: A Synergistic Approach to Cancer Vaccination in Lung Tumors. Front. Genet. 2025, 16, 1666561. [Google Scholar] [CrossRef]
- Shirvaliloo, M. Differences in Histone Modifications between Individuals. In Personalized Epigenetics, 2nd ed.; Tollefsbol, T.B.T.-P.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 41–70. ISBN 978-0-12-420135-4. [Google Scholar]
- Martin, B.J.E.; Brind’Amour, J.; Kuzmin, A.; Jensen, K.N.; Liu, Z.C.; Lorincz, M.; Howe, L.A.J. Transcription Shapes Genome-Wide Histone Acetylation Patterns. Nat. Commun. 2021, 12, 210. [Google Scholar] [CrossRef]
- Nitsch, S.; Zorro Shahidian, L.; Schneider, R. Histone Acylations and Chromatin Dynamics: Concepts, Challenges, and Links to Metabolism. EMBO Rep. 2021, 22, e52774. [Google Scholar] [CrossRef]
- Pal, D.; Patel, M.; Boulet, F.; Sundarraj, J.; Grant, O.A.; Branco, M.R.; Basu, S.; Santos, S.D.M.; Zabet, N.R.; Scaffidi, P.; et al. H4K16ac Activates the Transcription of Transposable Elements and Contributes to Their Cis-Regulatory Function. Nat. Struct. Mol. Biol. 2023, 30, 935–947. [Google Scholar] [CrossRef]
- Beacon, T.H.; Delcuve, G.P.; López, C.; Nardocci, G.; Kovalchuk, I.; van Wijnen, A.J.; Davie, J.R. The Dynamic Broad Epigenetic (H3K4me3, H3K27ac) Domain as a Mark of Essential Genes. Clin. Epigenetics 2021, 13, 138. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, Y.W.; Kang, J.; Kim, A.R. Histone H3K4me1 and H3K27ac Play Roles in Nucleosome Eviction and ERNA Transcription, Respectively, at Enhancers. FASEB J. 2021, 35, e21781. [Google Scholar] [CrossRef]
- Urdinguio, R.G.; Lopez, V.; Bayón, G.F.; Diaz De La Guardia, R.; Sierra, M.I.; García-Toraño, E.; Perez, R.F.; García, M.G.; Carella, A.; Pruneda, P.C.; et al. Chromatin Regulation by Histone H4 Acetylation at Lysine 16 during Cell Death and Differentiation in the Myeloid Compartment. Nucleic Acids Res. 2019, 47, 5016–5037. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, M.; Gan, X.L.; Ren, Y.; Yang, Y.; Weng, Z.; Zhang, X.; Guan, J.; Tang, L.; Ren, Z. Combined Low Levels of H4K16ac and H4K20me3 Predicts Poor Prognosis in Breast Cancer. Int. J. Clin. Oncol. 2023, 28, 1147–1157. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Zhang, Y. CBP/P300 and HDAC Activities Regulate H3K27 Acetylation Dynamics and Zygotic Genome Activation in Mouse Preimplantation Embryos. EMBO J. 2022, 41, e112012. [Google Scholar] [CrossRef]
- Giambartolomei, C.; Seo, J.H.; Schwarz, T.; Freund, M.K.; Johnson, R.D.; Spisak, S.; Baca, S.C.; Gusev, A.; Mancuso, N.; Pasaniuc, B.; et al. H3K27ac HiChIP in Prostate Cell Lines Identifies Risk Genes for Prostate Cancer Susceptibility. Am. J. Hum. Genet. 2021, 108, 2284–2300. [Google Scholar] [CrossRef]
- Liu, S.; Shi, C.; Hou, X.; Tian, X.; Li, C.; Ma, X.; Wang, X.; Gao, P. Transcriptional and H3K27ac Related Genome Profiles in Oral Squamous Cell Carcinoma Cells Treated with Metformin. J. Cancer 2022, 13, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, A.; Bourova-Flin, E.; Derakhshan, S.; Aminishakib, P.; Goudarzi, A. High Levels of Histone H3 K27 Acetylation and Tri-Methylation Are Associated with Shorter Survival in Oral Squamous Cell Carcinoma Patients. BioMedicine 2023, 13, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef]
- Hai, R.; Yang, D.; Zheng, F.; Wang, W.; Han, X.; Bode, A.M.; Luo, X. The Emerging Roles of HDACs and Their Therapeutic Implications in Cancer. Eur. J. Pharmacol. 2022, 931, 175216. [Google Scholar] [CrossRef]
- Parveen, R.; Harihar, D.; Chatterji, B.P. Recent Histone Deacetylase Inhibitors in Cancer Therapy. Cancer 2023, 129, 3372–3380. [Google Scholar] [CrossRef]
- Ruzic, D.; Djoković, N.; Srdić-Rajić, T.; Echeverria, C.; Nikolic, K.; Santibanez, J.F. Targeting Histone Deacetylases: Opportunities for Cancer Treatment and Chemoprevention. Pharmaceutics 2022, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Lian, Z.; Ma, S.; Liang, Z.; Ma, X.; Wen, X.; Wang, Y.; Liu, R. Combination with Vorinostat Enhances the Antitumor Activity of Cisplatin in Castration-Resistant Prostate Cancer by Inhibiting DNA Damage Repair Pathway and Detoxification of GSH. Prostate 2023, 83, 470–486. [Google Scholar] [CrossRef]
- Quinn, D.I.; Tsao-Wei, D.D.; Twardowski, P.; Aparicio, A.M.; Frankel, P.; Chatta, G.; Wright, J.J.; Groshen, S.G.; Khoo, S.; Lenz, H.J.; et al. Phase II Study of the Histone Deacetylase Inhibitor Vorinostat (Suberoylanilide Hydroxamic Acid; SAHA) in Recurrent or Metastatic Transitional Cell Carcinoma of the Urothelium—An NCI-CTEP Sponsored: California Cancer Consortium Trial, NCI 6879. Investig. New Drugs 2021, 39, 812–820. [Google Scholar] [CrossRef]
- Peter, R.M.; Sarwar, M.S.; Mostafa, S.Z.; Wang, Y.; Su, X.; Kong, A.N. Histone Deacetylase Inhibitor Belinostat Regulates Metabolic Reprogramming in Killing KRAS-Mutant Human Lung Cancer Cells. Mol. Carcinog. 2023, 62, 1136–1146. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Khalid, A.; Albratty, M.; Abdalla, A.N.; Lee, L.H.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Anticancer Clinical Efficiency and Stochastic Mechanisms of Belinostat. Biomed. Pharmacother. 2023, 165, 115212. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Hu, X.; Li, Q.; Sun, T.; Li, W.; Ouyang, Q.; Wang, J.; Tong, Z.; Yan, M.; et al. Entinostat, a Class I Selective Histone Deacetylase Inhibitor, plus Exemestane for Chinese Patients with Hormone Receptor-Positive Advanced Breast Cancer: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Acta Pharm. Sin. B 2023, 13, 2250–2258. [Google Scholar] [CrossRef]
- Solta, A.; Boettiger, K.; Kovacs, I.; Lang, C.; Megyesfalvi, Z.; Ferk, F.; Misík, M.; Hoetzenecker, K.; Aigner, C.; Kowol, C.R.; et al. Entinostat Enhances the Efficacy of Chemotherapy in Small Cell Lung Cancer Through S-Phase Arrest and Decreased Base Excision Repair. Clin. Cancer Res. 2023, 29, 4644–4659. [Google Scholar] [CrossRef]
- Pojani, E.; Barlocco, D. Romidepsin (FK228), A Histone Deacetylase Inhibitor and Its Analogues in Cancer Chemotherapy. Curr. Med. Chem. 2020, 28, 1290–1303. [Google Scholar] [CrossRef]
- Mayr, C.; Kiesslich, T.; Erber, S.; Bekric, D.; Dobias, H.; Beyreis, M.; Ritter, M.; Jäger, T.; Neumayer, B.; Winkelmann, P.; et al. HDAC Screening Identifies the HDAC Class I Inhibitor Romidepsin as a Promising Epigenetic Drug for Biliary Tract Cancer. Cancers 2021, 13, 3862. [Google Scholar] [CrossRef]
- Abu Alsamen, D.R.; Zakaraya, Z.Z.; Abed, A.; Alsa’d, A.A.; Alnajdawi, M.R. Synergistic Anticancer Effects of ABT-199 and Vorinostat Encapsulated in PLGA Nanoparticles: Formulation, Characterization, and Antiproliferative Effects against Colorectal Cancer Cells. PLoS ONE 2025, 20, e0334427. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Taymouri, S.; Rostami, M.; Mirian, M. Development of Folic Acid Functionalized Bilosomes for Delivery of Vorinostat to Breast Cancer Cells: Characterizatio Characteristics of VOR-Bilosomes n and Cytocidal Effects on MCF-7 and 4T1 Breast Cancer Cell Lines. Adv. Pharm. Bull. 2025, 15, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Pradyuth, K.S.; Salunkhe, S.A.; Singh, A.K.; Chitkara, D.; Mittal, A. Belinostat Loaded Lipid-Polymer Hybrid Nanoparticulate Delivery System for Breast Cancer: Improved Pharmacokinetics and Biodistribution in a Tumor Model. J. Mater. Chem. B 2023, 11, 10859–10872. [Google Scholar] [CrossRef] [PubMed]
- Gamberoni, F.; Borgese, M.; Pagiatakis, C.; Armenia, I.; Grazù, V.; Gornati, R.; Serio, S.; Papait, R.; Bernardini, G. Iron Oxide Nanoparticles with and without Cobalt Functionalization Provoke Changes in the Transcription Profile via Epigenetic Modulation of Enhancer Activity. Nano Lett. 2023, 23, 9151–9159. [Google Scholar] [CrossRef]
- Koprinarova, M.; Garry, D.; Hristov, D.R.; Dimova, I.; Dawson, K.A. Induction of Epigenetic Response to Amino-Modified Polystyrene Nanoparticles in Human Cells. Comptes Rendus L’Acade’mie Bulg. Sci. 2018, 71, 1342–1349. [Google Scholar] [CrossRef]
- Giri, P.M.; Kumar, A.; Salu, P.; Sathish, V.; Reindl, K.; Mallik, S.; Layek, B. Nanocarrier Mediated Entinostat and Oxaliplatin Combination Therapy Displayed Enhanced Efficacy against Pancreatic Cancer. Biomed. Pharmacother. 2024, 175, 116743. [Google Scholar] [CrossRef]
- Wang, Y.; Calvert, A.E.; Cardenas, H.; Rink, J.S.; Nahotko, D.; Qiang, W.; Ndukwe, C.E.; Chen, F.; Keathley, R.; Zhang, Y.; et al. Nanoparticle Targeting in Chemo-Resistant Ovarian Cancer Reveals Dual Axis of Therapeutic Vulnerability Involving Cholesterol Uptake and Cell Redox Balance. Adv. Sci. 2024, 11, e2305212. [Google Scholar] [CrossRef]
- Dash, S.R.; Das, C.; Das, B.; Jena, A.B.; Paul, S.; Sinha, S.; Tripathy, J.; Kundu, C.N. Near Infrared-Responsive Quinacrine–Gold Hybrid Nanoparticles Deregulate HSP-70/P300-Mediated H3K14 Acetylation in ER/PR+ Breast Cancer Stem Cells. Nanomedicine 2024, 19, 581–596. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Zheng, C.; Ye, L.; Jiang, P.; Malik, S.; Xu, G.; Zhou, Q.; Zhang, M. Acetyltransferase from Akkermansia Muciniphila Blunts Colorectal Tumourigenesis by Reprogramming Tumour Microenvironment. Gut 2023, 72, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, Z.; Zhang, X.; Gao, J.; Gao, Y.; Zhang, Y.; Liu, J.; Yang, C.; Liu, J. Construction of All-in-One Peptide Nanomedicine with Photoacoustic Imaging Guided Mild Hyperthermia for Enhanced Cancer Chemotherapy. Chem. Eng. J. 2021, 405, 127008. [Google Scholar] [CrossRef]
- Sima, L.E.; Chiritoiu, G.; Negut, I.; Grumezescu, V.; Orobeti, S.; Munteanu, C.V.A.; Sima, F.; Axente, E. Functionalized Graphene Oxide Thin Films for Anti-Tumor Drug Delivery to Melanoma Cells. Front. Chem. 2020, 8, 184. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold Nanoparticles-Induced Cytotoxicity in Triple Negative Breast Cancer Involves Different Epigenetic Alterations Depending upon the Surface Charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, M.W.; Hsu, M.N.; Yu-Chen, G.; Chao, Y.C.; Tuan, H.Y.; Chiang, C.S.; Hu, Y.C. Graphene Oxide Sensitizes Cancer Cells to Chemotherapeutics by Inducing Early Autophagy Events, Promoting Nuclear Trafficking and Necrosis. Theranostics 2018, 8, 2477–2487. [Google Scholar] [CrossRef]
- Blanco, J.; Lafuente, D.; Gómez, M.; García, T.; Domingo, J.L.; Sánchez, D.J. Polyvinyl Pyrrolidone-Coated Silver Nanoparticles in a Human Lung Cancer Cells: Time- and Dose-Dependent Influence over P53 and Caspase-3 Protein Expression and Epigenetic Effects. Arch. Toxicol. 2017, 91, 651–666. [Google Scholar] [CrossRef]
- Abo-Elfadl, M.T.; Gamal-Eldeen, A.M.; Elshafey, M.M.; Abdalla, G.M.; Ali, S.S.; Ali, M.R.K.; Zawrah, M.F.M. Photothermal Therapeutic Effect of PEGylated Gold Nano-Semicubes in Chemically-Induced Skin Cancer in Mice. J. Photochem. Photobiol. B Biol. 2016, 164, 21–29. [Google Scholar] [CrossRef]
- Jadhav, V.; Ray, P.; Sachdeva, G.; Bhatt, P. Biocompatible Arsenic Trioxide Nanoparticles Induce Cell Cycle Arrest by P21WAF1/CIP1 Expression via Epigenetic Remodeling in LNCaP and PC3 Cell Lines. Life Sci. 2016, 148, 41–52. [Google Scholar] [CrossRef]
- Kwak, T.W.; Kim, D.H.; Jeong, Y.-I.; Kang, D.H. Antitumor Activity of Vorinostat-Incorporated Nanoparticles against Human Cholangiocarcinoma Cells. J. Nanobiotechnol. 2015, 13, 60. [Google Scholar] [CrossRef]
- Martin, D.T.; Hoimes, C.J.; Kaimakliotis, H.Z.; Cheng, C.J.; Zhang, K.; Liu, J.; Wheeler, M.A.; Kelly, W.K.; Tew, G.N.; Saltzman, W.M.; et al. Nanoparticles for Urothelium Penetration and Delivery of the Histone Deacetylase Inhibitor Belinostat for Treatment of Bladder Cancer. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1124–1134. [Google Scholar] [CrossRef]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Tanimowo Aiyelabegan, H.; Ghanbari, H. Biocompatibility and Nanostructured Materials: Applications in Nanomedicine. Artif. Cells Nanomed. Biotechnol. 2017, 45, 833–842. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of Nanomaterials and Their Immunological Properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef]
- Liu, C.; Xu, M.; Li, W.; Cao, X.; Wang, Y.; Chen, H.; Zhang, T.; Lu, M.; Xie, H.; Chen, Y. Quantitative Pattern of HPTMs by Mass Spectrometry-Based Proteomics with Implications for Triple-Negative Breast Cancer. J. Proteome Res. 2024, 23, 1495–1505. [Google Scholar] [CrossRef]
- Peña-Hernández, R.; Aprigliano, R.; Carina Frommel, S.; Pietrzak, K.; Steiger, S.; Roganowicz, M.; Lerra, L.; Bizzarro, J.; Santoro, R. BAZ2A-mediated Repression via H3K14ac-marked Enhancers Promotes Prostate Cancer Stem Cells. EMBO Rep. 2021, 22, e53014. [Google Scholar] [CrossRef]
- Liao, L.; Alicea-Velázquez, N.L.; Langbein, L.; Niu, X.; Cai, W.; Cho, E.A.; Zhang, M.; Greer, C.B.; Yan, Q.; Cosgrove, M.S.; et al. High Affinity Binding of H3K14ac through Collaboration of Bromodomains 2, 4 and 5 Is Critical for the Molecular and Tumor Suppressor Functions of PBRM1. Mol. Oncol. 2019, 13, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Shi, J.; Yuan, C.; Zhao, D.; Jiang, S.; Lyu, J.; Wang, X.; Li, H.; Wen, H.; Li, W.; et al. Recognition of Histone Acetylation by the GAS41 YEATS Domain Promotes H2A.Z Deposition in Non-Small Cell Lung Cancer. Genes Dev. 2018, 32, 58–69. [Google Scholar] [CrossRef]
- Revenko, A.S.; Kalashnikova, E.V.; Gemo, A.T.; Zou, J.X.; Chen, H.-W. Chromatin Loading of E2F-MLL Complex by Cancer-Associated Coregulator ANCCA via Reading a Specific Histone Mark. Mol. Cell. Biol. 2010, 30, 5260–5272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Li, Y.-X.; Li, M.; Song, S.; Ge, Y.; Jin, J.; Li, X.; Tan, X.; Ye, J. The Ras-ERK1/2 Signaling Pathway Regulates H3K9ac through PCAF to Promote the Development of Pancreatic Cancer. Life Sci. 2020, 256, 117936. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; Kolben, T.; Meister, S.; Kolben, T.M.; Schmoeckel, E.; Mayr, D.; Mahner, S.; Jeschke, U.; Ditsch, N.; Beyer, S. Expression of H3K4me3 and H3K9ac in Breast Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 2017–2027. [Google Scholar] [CrossRef]
- Webber, L.P.; Wagner, V.P.; Curra, M.; Vargas, P.A.; Meurer, L.; Carrard, V.C.; Squarize, C.H.; Castilho, R.M.; Martins, M.D. Hypoacetylation of Acetyl-Histone H3 (H3K9ac) as Marker of Poor Prognosis in Oral Cancer. Histopathology 2017, 71, 278–286. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Wang, X.; Zhang, L.; Huang, P. Expression of SIRT1, H3K9me3, H3K9Ac and E-Cadherin and Its Correlations with Clinicopathological Characteristics in Gastric Cancer Patients. Int. J. Clin. Exp. Med. 2016, 9, 17219–17231. [Google Scholar]
- Zhen, L.; Gui-Lan, L.; Ping, Y.; Jin, H.; Ya-Li, W. The Expression of H3K9Ac, H3K14Ac, and H4K20TriMe in Epithelial Ovarian Tumors and the Clinical Significance. Int. J. Gynecol. Cancer 2010, 20, 82–86. [Google Scholar] [CrossRef]
- Nunes, S.P.; Morales, L.; Rubio, C.; Munera-Maravilla, E.; Lodewijk, I.; Suárez-Cabrera, C.; Martínez, V.G.; Pérez-Escavy, M.; Pérez-Crespo, M.; Alonso Sánchez, M.; et al. Modulation of Tumor Microenvironment by Targeting Histone Acetylation in Bladder Cancer. Cell Death Discov. 2024, 10, 1. [Google Scholar] [CrossRef]
- Miziak, P.; Baran, M.; Borkiewicz, L.; Trombik, T.; Stepulak, A. Acetylation of Histone H3 in Cancer Progression and Prognosis. Int. J. Mol. Sci. 2024, 25, 10982. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhan, Z.; Gan, L.; Bai, O. Mechanisms of HDACs in Cancer Development. Front. Immunol. 2025, 16, 1529239. [Google Scholar] [CrossRef]
- Alp, E.; Damkaci, F.; Guven, E.; Tenniswood, M. Starch Nanoparticles for Delivery of the Histone Deacetylase Inhibitor CG-1521 in Breast Cancer Treatment. Int. J. Nanomed. 2019, 14, 1335–1346. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Gogoi, B.; Dhan, S.S.; Subramani, R.; Pushparaj, C.; Mahesh, A. Improving Therapeutic Potential in Breast Cancer via Histone Deacetylase Inhibitor Loaded Nanofibrils. Drug Dev. Res. 2024, 85, e22172. [Google Scholar] [CrossRef]
- Edwards, K.; Yao, S.; Pisano, S.; Feltracco, V.; Brusehafer, K.; Samanta, S.; Oommen, O.P.; Gazze, S.A.; Paravati, R.; Maddison, H.; et al. Hyaluronic Acid-Functionalized Nanomicelles Enhance SAHA Efficacy in 3D Endometrial Cancer Models. Cancers 2021, 13, 4032. [Google Scholar] [CrossRef]
- Kaur, J.; Jakhmola, S.; Singh, R.R.; Joshi, B.; Jha, H.C.; Joshi, A. Ultrasonic Atomizer-Driven Development of Biocompatible and Biodegradable Poly(d,l-Lactide- Co-Glycolide) Nanocarrier-Encapsulated Suberoylanilide Hydroxamic Acid to Combat Brain Cancer. ACS Appl. Bio Mater. 2021, 4, 5627–5637. [Google Scholar] [CrossRef]
- Jiang, T.; Xie, L.; Zhou, S.; Liu, Y.; Huang, Y.; Mei, N.; Ma, F.; Gong, J.; Gao, X.; Chen, J. Metformin and Histone Deacetylase Inhibitor Based Anti-Inflammatory Nanoplatform for Epithelial-Mesenchymal Transition Suppression and Metastatic Tumor Treatment. J. Nanobiotechnol. 2022, 20, 394. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Salunke, S.; Wei, T.T.; Tang, Y.A.; Wang, Y.C. Fluorescent Nanohybrids from ZnS/CdSe Quantum Dots Functionalized with Triantennary, N-Hydroxy-p-(4-Arylbutanamido)Benzamide/Gallamide Dendrons That Act as Inhibitors of Histone Deacetylase for Lung Cancer. ACS Appl. Bio Mater. 2021, 4, 2475–2489. [Google Scholar] [CrossRef]
- Igaz, N.; Szőke, K.; Kovács, D.; Buhala, A.; Varga, Z.; Bélteky, P.; Rázga, Z.; Tiszlavicz, L.; Vizler, C.; Hideghéty, K.; et al. Synergistic Radiosensitization by Gold Nanoparticles and the Histone Deacetylase Inhibitor SAHA in 2D and 3D Cancer Cell Cultures. Nanomaterials 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Syed, V. TGF-β Signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Wang, X.; Eichhorn, P.J.A.; Thiery, J.P. TGF-β, EMT, and Resistance to Anti-Cancer Treatment. Semin. Cancer Biol. 2023, 97, 1–11. [Google Scholar] [CrossRef]
- Yue, X.; Kong, Y.; Zhang, Y.; Sun, M.; Liu, S.; Wu, Z.; Gao, L.; Liang, X.; Ma, C. SREBF2–STARD4 Axis Confers Sorafenib Resistance in Hepatocellular Carcinoma by Regulating Mitochondrial Cholesterol Homeostasis. Cancer Sci. 2023, 114, 477–489. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Sun, J.; Tang, J.; Zhou, J.; Dong, M. Investigating the Diagnostic and Therapeutic Potential of SREBF2-Related Lipid Metabolism Genes in Colon Cancer. Onco Targets Ther. 2023, 16, 1027–1042. [Google Scholar] [CrossRef]
- Wang, Q.; Bode, A.M.; Zhang, T. Targeting CDK1 in Cancer: Mechanisms and Implications. npj Precis. Oncol. 2023, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Wang, J.; Nie, X.; Ji, J.; Liu, X.; Tian, H.; Li, C. Cyclin-Dependent Kinase 1 (CDK1) in Cancers: From Upstream Regulation to Downstream Substrates and Therapeutic Inhibitors. Eur. J. Med. Chem. 2025, 299, 118090. [Google Scholar] [CrossRef]
- Jayaraj, G.G.; Hipp, M.S.; Hartl, F.U. Functional Modules of the Proteostasis Network. Cold Spring Harb. Perspect. Biol. 2020, 12, a033951. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Database Syst. Rev. Implement. Rep. 2019, 18, 2127–2133. [Google Scholar] [CrossRef]
- Segal, D.; Makris, S.L.; Kraft, A.D.; Bale, A.S.; Fox, J.; Gilbert, M.; Bergfelt, D.R.; Raffaele, K.C.; Blain, R.B.; Fedak, K.M.; et al. Evaluation of the ToxRTool’s Ability to Rate the Reliability of Toxicological Data for Human Health Hazard Assessments. Regul. Toxicol. Pharmacol. 2015, 72, 94–101. [Google Scholar] [CrossRef]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a New Tool to Assess the Reliability of Toxicological Data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Ito, K.; Murphy, D. Tutorial: Application of Ggplot2 to Pharmacometric Graphics. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e79. [Google Scholar] [CrossRef]
- Wickham, H. Modelling for Visualization. In ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; pp. 221–240. ISBN 978-3-319-24275-0. [Google Scholar]
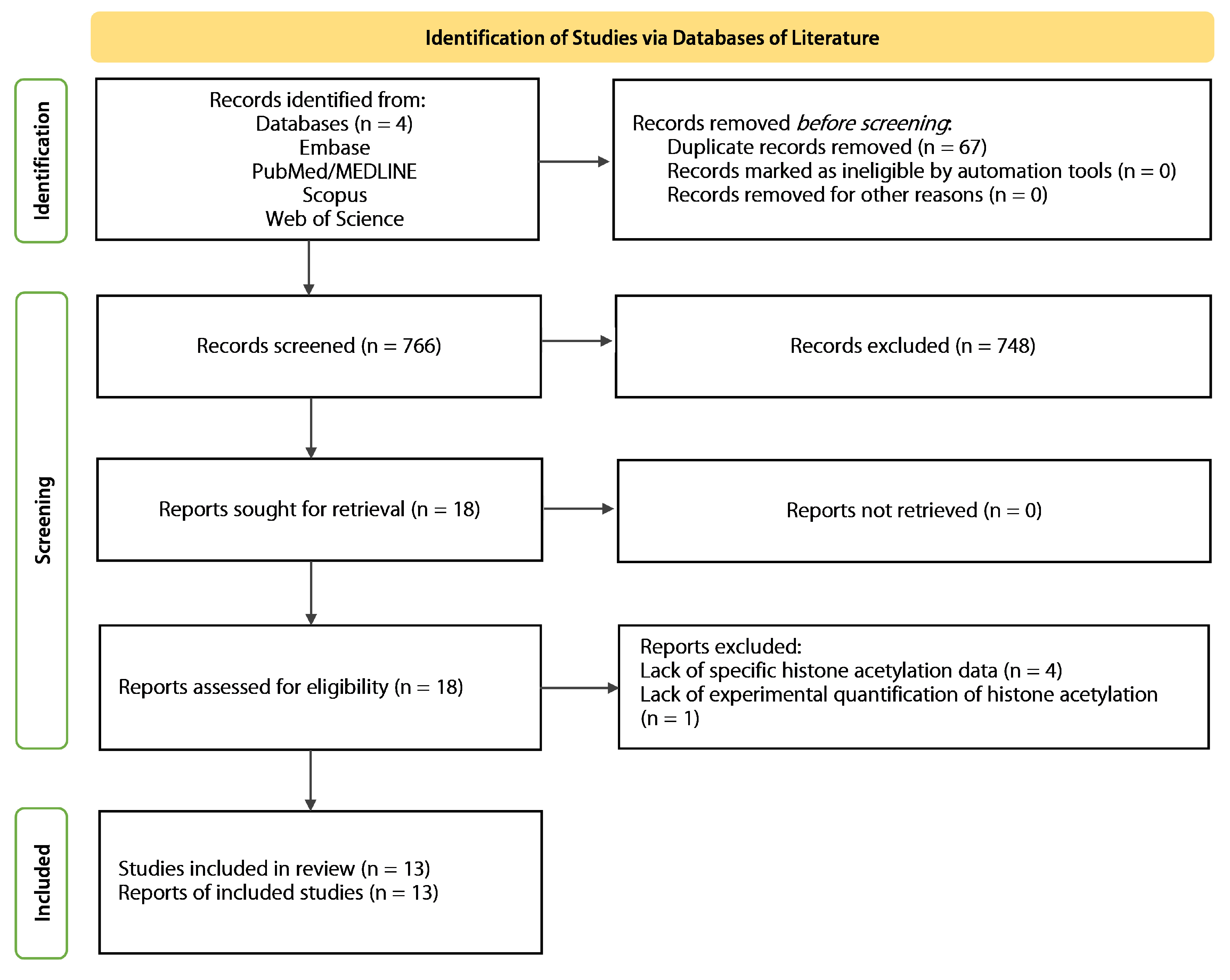



| Study | Year | Country | Design | Experimental Entries | Cancer | Ref. | |
|---|---|---|---|---|---|---|---|
| Type * | Cell Model | ||||||
| Giri et al. | 2024 | US | In vitro | 4 | PDAC | PANC-1 KPC | [72] |
| Wang et al. | 2024 | US | In vitro In vivo | 1 | OV | OVCAR5 | [73] |
| Dash et al. | 2024 | India | In vitro In vivo | 1 | BRCA | MCF-7 T47D | [74] |
| Jiang et al. | 2023 | China | In vitro In vivo | 1 | CRC | CT26 | [75] |
| Ren et al. | 2021 | China | In vitro In vivo | 2 | BRCA | 4T1 | [76] |
| OV | A2780/Taxol | ||||||
| Sima et al. | 2020 | Romania | In vitro | 1 | SKCM | SKmel23 | [77] |
| Surapaneni et al. | 2018 | India | In vitro | 4 | BRCA | MDA-MB-231 | [78] |
| Lin et al. | 2018 | Taiwan | In vitro | 1 | OV | SK-OV-3 | [79] |
| Blanco et al. | 2017 | Spain | In vitro | 1 | LUAD | A549 | [80] |
| Abo-Elfadl et al. | 2016 | Egypt | In vitro In vivo | 1 | SKCM | Skmel28 | [81] |
| Jadhav et al. | 2016 | India | In vitro | 6 | PRAD (AR+) | LNCaP | [82] |
| PRAD (AR−) | PC-3 | ||||||
| Kwak et al. | 2015 | Korea | In vitro In vivo | 1 | CCA | HuCC-T1 | [83] |
| Martin et al. | 2013 | US | In vitro In vivo | 4 | BLCA (RB1wt) | T-24 | [84] |
| BLCA (RB1mut) | UM-UC-3 | ||||||
| Study | Nanotherapeutic | Nanomaterial | Surface Functionalization | Size (nm) | Drug Encapsulation | Anticancer Agent | Ref. |
|---|---|---|---|---|---|---|---|
| Giri et al. | OXP@BSA-NPs | BSA | None | 277.1 | Yes | Oxaliplatin | [72] |
| ENT@PLGA-NPs | PLGA | 281.8 | Entinostat | ||||
| Wang et al. | HDL-NPs | HDL | None | 5.0 | No | HDL-NPs + CDDP * | [73] |
| Dash et al. | QAuNPs | Gold | None | 179.0 | Yes | Quinacrine | [74] |
| Jiang et al. | Amuc_2172@MMCNPs | MMCNP | None | ≈100.0 | Yes | Amuc_2172 | [75] |
| Ren et al. | PpIX-FFYSV | PpIX | FF-YSV | 191.0 | No | PpIX | [76] |
| Sima et al. | TSA@BSA-GONs | GON | BSA | 250.0 | Yes | Trichostatin A | [77] |
| Surapaneni et al. | Cit-AuNPs | Gold | Citrate | ≈40.0 | No | AuNPs | [78] |
| Cys-AuNPs | Cysteamine | ≈25.0 | |||||
| Lin et al. | GONs | GON | None | 450.0 | No | GONs + CDDP * | [79] |
| Blanco et al. | PVP-AgNPs | Silver | PVP | 21.74 | No | AgNPs | [80] |
| Abo-Elfadl et al. | PEG-AuNPs | Gold | PEG | 25.0 | No | AuNPs | [81] |
| Jadhav et al. | AsNPs | As2O3 | None | − | No | As2O3 | [82] |
| CS-AsNPs | Chitosan | ||||||
| DMSA-AsNPs | DMSA | ||||||
| Kwak et al. | rINN@PEG/PLGA-NPs | PLGA | PEG | 82.12 | Yes | Vorinostat | [83] |
| Martin et al. | Bel@PGON/PLGA-NPs | PLGA | PGON Avidin | 151.0 | Yes | Belinostat | [84] |
| PGON/PLGA-NPs | 144.0 | − |
| Study | Treatment | Therapeutic Outcome | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Nanotherapeutic | Optimal Concentration (µM) | Histone Modification | Antitumor Effect | ||||
| Acetylation | Enrichment * | Viability (%) | Apoptosis (%) | ||||
| Giri et al. | ENT@PLGA-NPs | 10 | H3ac | ↑ | 71 | 18.1 | [72] |
| OXP@BSA-NPs | 5 | H4ac | ↑ | 42 | 25.4 | ||
| Wang et al. | HDL-NPs | 8.5 | H3K27ac | ↓ | ↓ | ↑ | [73] |
| Dash et al. | QAuNPs | 0.8 | H3K14ac | ↓ | ≈47 | ↑ | [74] |
| Jiang et al. | Amuc_2172@MMCNPs | 10 | H3K14ac | ↑ | ↓ | ↑ | [75] |
| Ren et al. | PpIX-FFYSV | 25 | H3ac | ↑ | ≈35–48 | ↑ | [76] |
| Sima et al. | TSA@BSA-GONs | 37 | H3ac | ↑ | ↓ | ↑ | [77] |
| Surapaneni et al. | Cys-AuNPs | 25 | H3K9ac | ↑ | ≈47 | ≈18 | [78] |
| Cit-AuNPs | 25 | ↓ | ≈54 | ≈13 | |||
| Cys-AuNPs | 25 | H3K14ac | ↑ | ≈47 | ≈18 | ||
| Cit-AuNPs | 25 | ↓ | ≈54 | ≈13 | |||
| Lin et al. | GONs (+CDDP) | 50 (+200) | H4K16ac | ↑ | 37.7 | 17.0 | [79] |
| Blanco et al. | AgNPs | 100 | H3ac | ↓ | ≈46 | 14.5 | [80] |
| Abo-Elfadl et al. | PEG-AuNPs | 7.0 | Hac | ↑ | ≈40 | ↑ | [81] |
| Jadhav et al. | AsNPs | 50 | H3K14ac | ↑ | ↓ | ↑ | [82] |
| CS-AsNPs | 50 | ||||||
| DMSA-AsNPs | 50 | ||||||
| Kwak et al. | rINN@PEG/PLGA-NPs | 55 | H3ac | ↑ | ≈45 | ↑ | [83] |
| Martin et al. | Bel@PGON/PLGA-NPs | 10 | H4ac | ↑ | ≈20 | ↑ | [84] |
| PGON/PLGA-NPs | 10 | ≈95 | Negligible | ||||
| Study | Anticancer Agent | Histone Modifier | Histone Enrichment | Differential Gene Expression | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Enzyme Class | Activity * | Mark | Enrichment | Gene | Expression | |||
| Wang et al. | CDDP | EP300/CBP | ↓ | H3K27ac | ↓ | SREBF2 | ↓ | [73] |
| Dash et al. | Quinacrine | EP300/CBP | ↓ | H3K14ac | ↓ | TGFB | ↓ | [74] |
| Jiang et al. | Amuc_2172 | GCN5 | ↑ | H3K14ac | ↑ | HSPA1 | ↑ | [75] |
| Jadhav et al. | As2O3 | − | − | H3K14ac | ↑ | CDKN1A | ↑ | [82] |
| Kwak et al. | rINN | HDAC | ↓ | H3ac | ↑ | CDKN1A | ↑ | [83] |
| Study | Nanotherapeutic | Cancer * | Treatment | Outcome † | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TC | TS | BW | ||||||||
| Type | Animal Model | Dosage | Duration (wk) | Route | ||||||
| Wang et al. | HDL-NPs | OV | Foxn1nu female mice | 1 μM | 4 | IP | ↓ | ↓ | NS ‡ | [73] |
| Dash et al. | QAuNPs | BRCA | BALB/c female mice | 15 μg/kg | 4 | Oral | − | ↓ | ↑ | [74] |
| Jiang et al. | Amuc_2172@MMCNPs | CRC | Apcmin/+ mice | 150 μg/kg | 2 | IP | ↓ | ↓ | ↑ | [75] |
| Ren et al. | PpIX-FFYSV | BRCA | BALB/c female mice | 20 mg/kg | 2 | IV | − | ↓ | NS | [76] |
| Abo-Elfadl et al. | PEG-AuNPs | SKCM | CD1 female mice | 4 μg/kg | 5 | IT | − | ↓ | − | [81] |
| Kwak et al. | rINN@PEG/PLGA-NPs | CCA | Foxn1nu male mice | 50 mg/kg (1 mg rINN) | 4 | SC | − | ↓ | NS | [83] |
| Martin et al. | Bel@PGON/PLGA-NPs | BLCA | Foxn1nu female mice | 5 mg/kg | 4 | IT | − | ↓ | − | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirvaliloo, M.; Khoee, S.; Khoei, S.; Sheervalilou, R.; Mohammad Hosseini, P.; Afzalipour, R.; Shirvalilou, S. Exploring the Impact of Nanotherapeutics on Histone H3 and H4 Acetylation Enrichment in Cancer Epigenome: A Systematic Scoping Synthesis. Epigenomes 2025, 9, 44. https://doi.org/10.3390/epigenomes9040044
Shirvaliloo M, Khoee S, Khoei S, Sheervalilou R, Mohammad Hosseini P, Afzalipour R, Shirvalilou S. Exploring the Impact of Nanotherapeutics on Histone H3 and H4 Acetylation Enrichment in Cancer Epigenome: A Systematic Scoping Synthesis. Epigenomes. 2025; 9(4):44. https://doi.org/10.3390/epigenomes9040044
Chicago/Turabian StyleShirvaliloo, Milad, Sepideh Khoee, Samideh Khoei, Roghayeh Sheervalilou, Parisa Mohammad Hosseini, Reza Afzalipour, and Sakine Shirvalilou. 2025. "Exploring the Impact of Nanotherapeutics on Histone H3 and H4 Acetylation Enrichment in Cancer Epigenome: A Systematic Scoping Synthesis" Epigenomes 9, no. 4: 44. https://doi.org/10.3390/epigenomes9040044
APA StyleShirvaliloo, M., Khoee, S., Khoei, S., Sheervalilou, R., Mohammad Hosseini, P., Afzalipour, R., & Shirvalilou, S. (2025). Exploring the Impact of Nanotherapeutics on Histone H3 and H4 Acetylation Enrichment in Cancer Epigenome: A Systematic Scoping Synthesis. Epigenomes, 9(4), 44. https://doi.org/10.3390/epigenomes9040044








